Abstract
Background:
Tissue transplantation plays a pivotal role in the treatment of diseases. Pancreatic beta cell transplantation is the best way to obtain normal blood glucose in patients with diabetes type 1. However, it is not clear how long endocrine pancreas cells can be used for transplantation after the donor's death. The present study was conducted to analyze the performance and viability of pancreatic islet cells after death.
Materials and Methods:
Pancreas was separated from Balb/c mice at different times (0, 1, 4, 6, 12, and 24 h after death) at temperatures of 4°C and 23°C, and was cultured in Roswell_Park_Memorial_Institute (RPMI) 1640. Insulin shock, MTT assay, aldehyde fuchsin staining, dithizone staining, and florescence microscopy methods were applied to analyze the performance of beta cells, cell viability, islets’ diagnosis, islet cells’ diagnosis, and viable and necrotic cells diagnosis, respectively.
Results:
Islets of Langerhans and beta cells were diagnosed. By increasing the temperature and time, the viability and performance of beta cells decreased significantly (P < 0.05).
Conclusion:
The best condition for keeping the islets of Langerhans in terms of viability and performance is 4 h after death at temperature of 4°C.
Keywords: Cell viability, Langerhans islets, performance, temperature, time
INTRODUCTION
In recent decades, transplantation of different tissues has played a significant role in the treatment of various diseases, and lack of volunteer donors has increased the tendency to use more favorable methods such as transplantation of tissue components such as islets of Langerhans.[1] In the treatment of diabetes type 1, the islet transplantation is more advantageous than the whole pancreas transplantation, including possibility of saving islets before transplantation, increasing the acceptance potential of transplantation,[2] lack of lifetime use of immunosuppressive drugs, solving the problem of insufficient transplant organs, decreasing the mortality resulting from major surgeries of organ transplantation and analyzing the quality of islets and their performance outside the body by researchers before transplantation.[3] Pancreas is both an endocrine and an exocrine gland.[4] The exocrine acini of pancreas drain their secretions into duodenum, and endocrine cells called Langerhans islets, function in the metabolism of carbohydrates.[5] The endocrine component comprises 1%–% of pancreas.[6] Islets of Langerhans are compressed masses of epithelial cells through which a labyrinth of capillaries penetrate.[7] There are different types of cells in the islets, the most important of which are alpha (present in the periphery of islets and secrete glucagon hormone) and beta cells (existing in the center of islets and secrete insulin hormone).[8] In recent years, separation of islets has been one of the most applicable cases of pancreas cell separation for the purpose of transplantation to treat human diabetes type 1.[9] After separating the islets, it is possible to change the immunogenicity of cells, to increase their immune functions and to isolate immune cells by cellular coverage.[10,11] However, recent studies have indicated that the most important method of obtaining normal blood glucose in diabetic patients is transplantation of pancreatic beta cells.[12,13] The individuals who die from accidents are considered a good source of pancreas cells for transplantation. In principle, between the time of death and organ donation, different tissues can survive in normal conditions for a specific period of time; however, it is not definite how long the cells can be used for transplantation after the death. Thus, the present study was aimed to investigate the viability and performance of islet cells in various conditions after death in order to evaluate the optimum conditions in terms of time, place, and temperature to preserve pancreas tissue and cells for the follow-up use such as organ transplantation.
MATERIALS AND METHODS
Experimental animals and groups
Forty-eight male Balb/c mice with the weight range of 25–30 g were purchased from Pasteur institute (Tehran, Iran). The animals were kept in the temperature of 24 ± 2°C under controlled environmental conditions, 12/12 h light/dark cycle and free access to water and food.[14] The mice were classified into three groups: Group A: The mice were sacrificed immediately after cutting the spinal cord (control). Group B: The mice were kept in laboratory conditions (23°C) for 1, 4, 6, 12, and 24 h after cutting the spinal cord and then were sacrificed. Group C: After cutting the spinal cord, the mice were kept in refrigerator (4°C) for 1, 4, 6, 12, and 24 h and were then sacrificed.
Separation and culture of Langerhans islets cells
The animals were sacrificed immediately after cutting the spinal cord (cervical dislocation). The tissue separated from the animal was placed in the sterile Petri dish, the lipid and lymph glands were separated by stereomicroscope (CETI), and part of the tissue was removed for aldehyde fuchsin staining. The other sections of the tissue were cut into 2 mm segments by a surgical blade and 10 mL collagenase type H (Roche) with concentration of 4 mg/mL was added to it and kept in incubator for 20 min. The undigested segments were separated by Pasture pipette and washed several times with HBSS containing 20% fetal calf serum (Gibco) and centrifuged for 1 min in 200 g each time. The obtained solution was cultured for 24 h in RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma) and 1% penicillin–streptomycin (Sigma), and incubated at 37°C and in humidified atmosphere containing 5% CO2.[15]
Dithizone staining method
Dithizone staining method was used to confirm Langerhans islets. This method is based on the formation of the color complex obtained from Zn2+ ion connection within islets and dithizone. After separation and purification of the islets of Langerhans from pancreas, 20 μL of the stain stock (Sigma) was prepared for each milliliter of RPMI-containing islets and incubated at a temperature of 37°C for 30 min. After washing with FBS, the islets were analyzed by an inverted microscope.[16]
Aldehyde fuchsin staining method
This method was used to diagnose the islets of Langerhans and to determine cell death in the tissue. Accordingly, 5 μm sections were prepared from pancreas. Tissue processing was performed and tissue was blue by oxidation of potassium permanganate 0.5% (Merck) and sulfuric acid 0.05% (Merck). Also, bleaching by 2.5% sodium thiosulfate at 10 min and washing with water and ethanol 70% (Merck) were carried out. Furthermore, aldehyde fuchsin (Merck) staining, washing with water and ethanol 96% (Merck), Celestine blue staining (Merck), differentiation with acid ethanol and orange G (Merck) and light green (Merck) staining were performed as contrast. Moreover, washing with glacial acetic acid (Merck), ascending percentages of ethanol, clearing (Zylol (Merck), and imaging with light microscope (B × 50-Olympus) were carried out. The percentage of cell viability was obtained through dividing the number of live cells by total number of cells ×100.[17]
Glucose shock test
To analyze the performance of the separated beta cells, the insulin secretion level was measured by insulin enzyme-linked immunosorbent assay (ELISA) kit (DRG-Germany) according to the manufacturers’ protocol. Accordingly, 100 μL cell suspension was added to 900 μL glucose solutions (2.8 molar) and incubated for 45 min. The above procedures were repeated using glucose solution (16.7 molar) at 4°C. Other procedures were carried out according to the kit's protocol. At the end, absorbance was measured using an ELISA reader (Hyperion, Germany) at 490 nm. All experiments were carried out in triplicate.[18]
MTT assay
The viability of cells is assessed by the MTT assay. At first, the medium of each well is removed, rinsed with PBS, and replaced with 400 μL medium (serum free) and 40 μL MTT (Sigma) solutions. Next, it is incubated at 37°C, 5% CO2 for 4 h. The medium is discarded and 400 μL DMSO (Sigma) is added to each well and is incubated in dark for 2 h. Hundred microliters of the solution is transferred to 96-well plate and absorbance of each well is read at 570 nm with ELISA reader (Hyperion, Germany). The assays are performed in triplicate.[19]
Acridine orange/ethidium bromide assay
This method was used to diagnose the live and necrosis cells. In this method, 50 μL acridine orange (Sigma) solution was added to 5 μL ethidium bromide. Propidium iodide (Sigma) and acridine orange were used as florescent markers. The obtained solution was added to 400 μL culture solution containing pancreatic islets and analyzed by Florescent Microscope (Eclipse TS 100- Nikon) and G filter. The percentage of cell viability was determined via dividing the live cells (green color) by total number of cells × 100.[20]
Statistical analysis
All the quantitative data were presented as mean ± standard deviation. One-way analysis of variance and least significant difference (LSD) post hoc tests were performed to determine the statistical significance between different groups using SPSS software package 16.0. Significance was accepted at the level of P < 0.05.
RESULTS
0Dithizone staining
The findings indicated those 24 h after cell culture at both temperatures, the cells separated from islets of Langerhans joined each other on the Petri dish floor and formed new masses the same as islets of Langerhans. Following dithizone staining, the islets were purple-colored in the pink background observed under inverted microscope [Figure 1].
Figure 1.
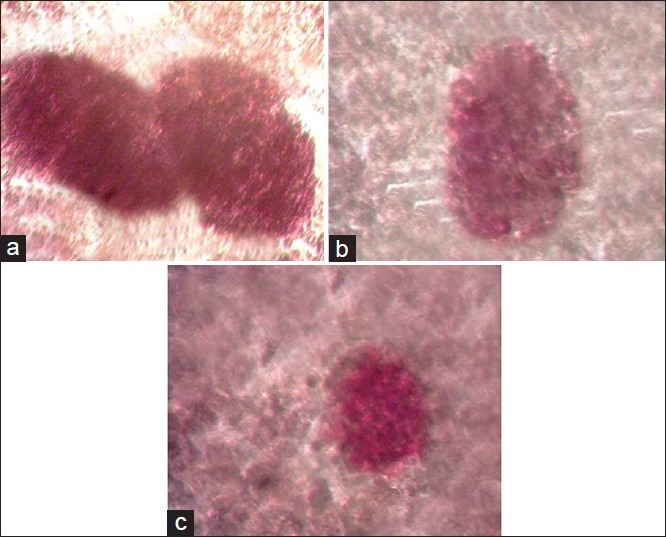
Dithizone staining of islets of Langerhans, (a) immediately after death, 24 h after culture, (b) 1 h after death at 4°C 24 h after culture, (c) 1 h after death at 23°C 24 h after culture (reverted microscope, 100×)
Aldehyde fuchsin staining
The dead cells in the live tissue were observed as eosinophilic and clear, segmented cell membrane and partly vacuolar in the groups investigated. Islets of Langerhans were separated from exocrine pancreas and islet cells (purple beta cells and dark-colored alpha cells) were separated from each other [Figure 2].
Figure 2.
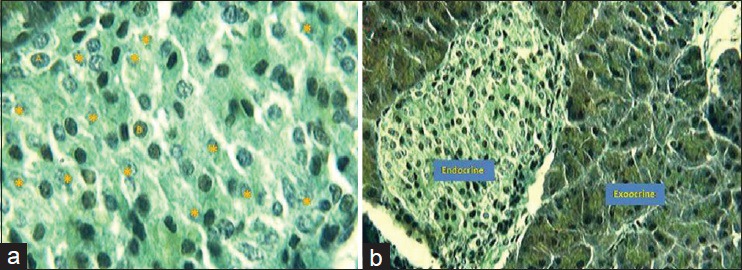
Aldehyde fuchsin staining, (a) differentiation of islets of Langerhans 1 h after death, (A: alpha, B: beta and *: apoptotic cells, optic microscope, 100×), (b) differentiation of islets from exocrine pancreas 1 h after death (optic microscope, 40×)
Insulin shock
The findings of insulin shock indicated that at intervals of 1 and 4 h after death at 4°C, 70% of beta cells were able to secrete insulin, whereas 1 h after death at 23°C, only 50% of the cells demonstrated this potential.
Cells viability (MTT)
The finding obtained showed that by increasing the time at 4°C after the mice death, the viability increased significantly compared with control group (P < 0.05), reaching 12% in the 24-h period [Figure 3]. By increasing the time after the mice death at 23°C, the viability of cells decreased significantly in comparison with the control group (P < 0.05), reaching zero in the 24-h period. The cell viability was significantly higher at all times at 4°C compared with 23°C (P < 0.05) [Figure 3].
Figure 3.
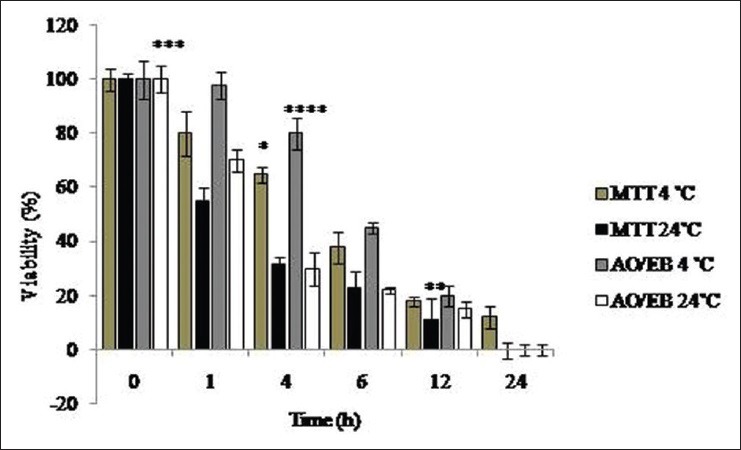
Results for the viability of separated cells in different periods at 4°C and 24°C after the animal's death by acridine orange/ethidium bromide staining and MTT. *Significant decrease of viability in all groups of study compared with control group (P< 0.05). **Significant decrease of viability in all groups of study compared with control group (P< 0.05). ***Significant decrease of viability in all groups of study compared with control group (P< 0.05). ****Significant decrease of viability in all groups of study except control group in the 1-h group (P< 0.05)
Acridine orange/ethidium bromide assay
Fluorescence staining by acridine orange/ethidium bromide depicted the live islet cells as green, and the red cells indicated ethidium bromide penetration, which resulted in cell damage [Figures 4 and 5]. The findings revealed that in the initial hour after death at 4°C, 98% of cells were healthy; in 4 h after death, 80% of cells were healthy and in other periods, less than 50% of cells were healthy [Figures 4–5]. 60% of cells were healthy at 23°C 1 h after death and less than 30% of cells were healthy in other periods [Figure 4]. All cells were dead 24 h after death in both groups [Figures 4–5].
Figure 4.
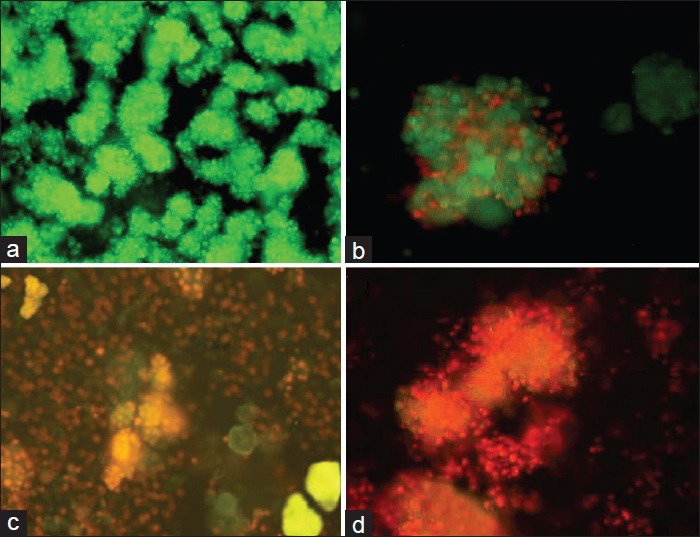
Acridine orange/ethidium bromide staining of the cells separated from islets, (a) control, (b) 1 h after death, (c) 6 h after death, (d) 24 h after death at 4°C (fluorescence microscopy, 40×)
Figure 5.
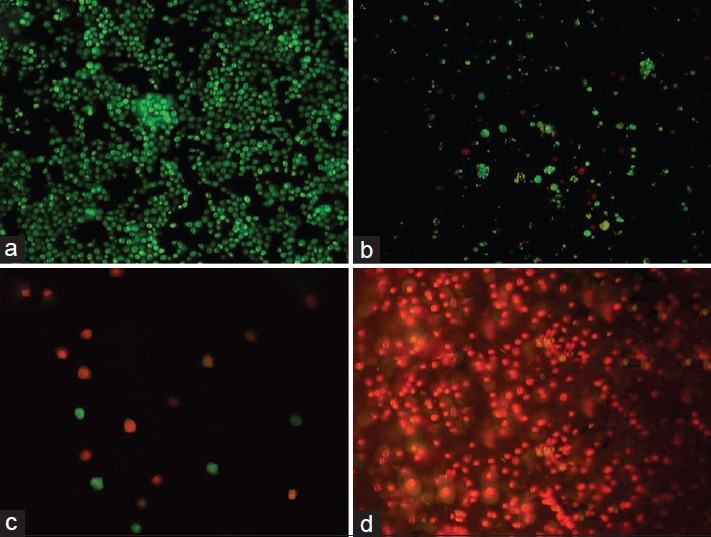
Acridine orange/ethidium bromide staining of islets, (a) control, (b) 1 h after death, (c) 6 h after death, (d) 24 h after death at 4°C (fluorescence microscopy, 40×)
DISCUSSION
In the present study, the viability and performance of islets of Langerhans were investigated at different times and temperatures after the animals’ death. Diabetes is the most important disease of endocrine pancreas.[21] In recent years, transplantation of islets of Langerhans has had many more advantages in the treatment of diabetes type 1 than transplantation of the whole pancreas, which has consequently resulted in the increasing chances of amelioration in diabetic patients.[22] Standardization and optimization of the conditions for separation and purification of islets of Langerhans is one of the crucial stages in the transplantation process.[23] In this study, dithizone staining indicated that the cell suspensions joined each other 24 h after culture and reformed the islets of Langerhans. It seems that in the present study in the separation and purification of cells, these results are due to lack of using substances such as ficoll and histopaque, which damage islets, although they perform the best separation of pancreatic cells.[24]
In the study conducted by Lag et al., the islet cells were damaged due to using sucrose concentration slope for purification of islets as well as high osmolarity, and the obtained results are in contrast with the findings of the present study.[24] In the present study, the viability of cells was analyzed by aldehyde fuchsin staining and performance of insulin secretion was evaluated by beta cells and insulin shock. Based on the obtained results and given the protein structure of insulin, it seems that temperature increase can enhance the hydrolysis velocity due to the breakdown of peptide chains in this hormone. On the other hand, the temperature fall can have reverse effects on this hormone.[25,26] The finding of the present study confirm the results of the study carried out by Kinasiewicz et al. in which they introduced the time between pancreas removal from the donor and performing laboratory process as cold ischemia time.[27] Moreover, the findings indicated that by increasing the time and temperature, the viability of islet cells was significantly reduced. It seems that the temperature fall in the environment affects the enzymatic reactions, causes dysfunction, and impedes the release of many detrimental enzymes in lysosomes and other cell vacuoles, and decreases the activity of external microorganisms that influence the cell destruction trend.[28]
In the present study, MTT test and fluorescence method were applied to analyze the viability of cells. The results of both methods showed that, increase the time was a factor for viability decline. It seems that by increasing the time, the detrimental effects of temperature rise, microorganisms, apoptosis level, necrosis level, and so on are increased.[29] Tao-Laigian et al. analyzed the viability of cells of Langerhans and used 2.8 and 16.7 molar osmosis shock to confirm the insulin secretion. The findings obtained in this study are compatible with the results of the present study.[18] In another study, Yukari et al. investigated the changes of exocrine pancreas at different times and reported more resistance for exocrine pancreas than for endocrine counterpart, which is in line with the results of the present study.[30]
The findings of the present study, however, were in contrast with the results obtained by Senn et al., which they reported to have no significant difference between viability of stem cells of inner ear and increasing the preservation time.[31] In general, given the fact that there are approximately one million islets of Langerhans in the pancreas of an adult human,[32] it seems that it is possible to save 50% of the cells in terms of viability and performance for transplantation by proper preservation of pancreas and separation techniques.
CONCLUSION
The findings of the present study indicated that 80% of the islets of Langerhans were healthy and active for 4 h after death at 4°C, whereas 60% of islet cells were alive and active 1 h after the animal's death.
ACKNOWLEDGMENT
We sincerely and gratefully thank the Kermanshah University of Medical Sciences for providing financial support for this project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Fisher JE, Lillegard JB, Mckenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl. 2013;19:328–35. doi: 10.1002/lt.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddis JS, Hanson MS, Cravens J, Qian D, Olack B, Antler M, et al. Standardized transportation of human islets: An islet cell resource center study of more than 2,000 shipments. Cell Transplant. 2013;22:1101–11. doi: 10.3727/096368912X653219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. AnnSurg. 2000;231:269–75. doi: 10.1097/00000658-200002000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junqueira LC, Carneiro J, Kelley RO. 11th ed. New York: Appleton and Lang; 2010. Basic Histology; p. 773.p. 775. [Google Scholar]
- 5.Fawcett DW. 12th ed. New York: Chapman and Hail; 1994. Bloom and Fawcett: A Textbook of Histology; pp. 603–604. [Google Scholar]
- 6.Danilenko DM, Nambiar PR, Cesta MF. A brief overview of the 32nd Annual STP Symposium on the Toxicologic Pathology of the Digestive Tract and Pancreas. Toxicol Pathol. 2014;42:45–8. doi: 10.1177/0192623313502402. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald MJ, Langberg EC, Tibell A, Sabat G, Kendrick MA, Szweda LI, et al. Identification of ATP synthase as a lipid peroxide protein adduct in pancreatic islets from humans with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E727–31. doi: 10.1210/jc.2012-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Margalet V, Lucas M, Goberna R. Pancreastatin: Further evidence for its consideration as a regulatory peptide. J Mol Endocrinol. 1996;16:1–8. doi: 10.1677/jme.0.0160001. [DOI] [PubMed] [Google Scholar]
- 9.Markmann JF, Deng S, Huang X, Desai NM, Velidedeoglu EH, Lui C, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237:741–50. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weimar B, Rauber K, Brendel MD, Bretzel RG, Rau WS. Percutaneous transhepatic catheterization of the portal vein: A combined CT -and fluoroscopy- guided technique. Cardiovasc Intervent Radiol. 1999;42:342–4. doi: 10.1007/s002709900403. [DOI] [PubMed] [Google Scholar]
- 11.Bellin MD, Beilman GJ, Dunn TB, Pruett TL, Chinnakotla S, Wilhelm JJ, et al. Islet autotransplantation to preserve beta cell mass in selected patients with chronic pancreatitis and diabetes mellitus undergoing total pancreatectomy. Pancreas. 2013;42:317–21. doi: 10.1097/MPA.0b013e3182681182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YJ, Ao Z, Kieffer TJ, Chen H, Safikhan N, Thompson DM, et al. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: Implications in type 2 diabetes and islet transplantation. Diabetologia. 2013;56:508–19. doi: 10.1007/s00125-012-2802-z. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu C. Current limitations of islet transplantation. TansplantProc. 2001;33:1707–8. doi: 10.1016/s0041-1345(00)02650-6. [DOI] [PubMed] [Google Scholar]
- 14.Jalili C, Salahshoor MR, Pourmotabbed A, Moradi S, Roshankhah SH, Shabaniz A. The effects of aqueous extract of Boswellia serrata on hippocampal region CA1 and learning deficit in kindled rats. Res Pharm Sci. 2014;9:351–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Amoli MM, Moosavizadeh R, Larijani B. Optimizing conditions for rat pancreatic islets isolation. Cytotechnology. 2005;48:75–8. doi: 10.1007/s10616-005-3586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JJ, Yi HY, Yang JW, Shin JS, Kwon JH, Kim CW. Characterization of streptozotocin-induced diabetic rats and pharmacodynamics of insulin formulations. Biosci Biotechnol Biochem. 2003;67:2396–401. doi: 10.1271/bbb.67.2396. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson J, Allsopp RH, Taylor RM, Johnston ID. Isolation and long term preservation of pancreatic islets from mouse, rat and guinea pig. Diabetologia. 1976;12:115–21. doi: 10.1007/BF00428975. [DOI] [PubMed] [Google Scholar]
- 18.Qian TL, Wang XH, Liu S, Ma L, Lu Y. Fentanyl inhibits glucose- Stimulated insulin release from beta-cells in rat pancreatic islets. World J Gastroenterol. 2009;15:4163–9. doi: 10.3748/wjg.15.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna A, Mahalingam K, Chakrabarti D, Periyasamy G. Ets-1 expression and gemcitabine chemoresistance in pancreatic cancer cells. CellMolBiolLett. 2011;16:101–13. doi: 10.2478/s11658-010-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pace CS, Sachs G. Glucose-induced proton uptake in secretory granules of beta-cells in monolayer culture. Am J Physiol. 1982;242:C382–7. doi: 10.1152/ajpcell.1982.242.5.C382. [DOI] [PubMed] [Google Scholar]
- 21.Fourcade G, Colombo BM, Grégoire S, Baeyens A, Rachdi L, Guez F, et al. Fetal pancreas transplants are dependent on prolactin for their development and prevent type 1 diabetes in syngeneic but not allogeneic mice. Diabetes. 2013;62:1646–55. doi: 10.2337/db12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anazawa T, Matsumoto S, Yonekawa Y, Loganathan G, Wilhelm JJ, Soltani SM, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation. 2011;91:508–14. doi: 10.1097/TP.0b013e3182066ecb. [DOI] [PubMed] [Google Scholar]
- 23.Fauci AS, Braunwald E, Kasper DL, Hauser SL, editors. Harrison's principles of internal medicine. 15th ed. Philadelphia: McGraw-Hill; 1998. pp. 2020–80. [Google Scholar]
- 24.Lacy PE, Kostionovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–9. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 25.von Renteln D, Quaas A, Rösch T, Denzer UW, Szyrach MN, Enderle MD, et al. A novel flexible cryoprobe for EUS-guided pancreatic biopsies. Gastrointest Endosc. 2013;77:784–92. doi: 10.1016/j.gie.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Gu J, Li W, Xiao D, Wei S, Cui W, Chen W, et al. Compound K, a final intestinal metabolite of ginsenosides, enhances insulin secretion in MIN6 pancreatic β-cells by upregulation of GLUT2. Fitoterapia. 2013;87:84–8. doi: 10.1016/j.fitote.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Kinasiewicz A, Juszczak M, Pachecka J, Fiedor P. Pancreatic islet isolation using different protocols with in situ flushing and intraductal collagenase injection. Physiol Res. 2004;53:327–33. [PubMed] [Google Scholar]
- 28.O’Gorman D, Kin T, Pawlick R, Imes S, Senior PA, Shapiro AJ. Clinical islet isolation outcomes with a highly purified neutral protease for pancreas dissociation. Islets. 2013;5:111–5. doi: 10.4161/isl.25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomita Y, Nihira M, Ohno Y, Sato S. Ultrastructural changes during in situ early postmortem autolysis in kidney, pancreas, liver, heart and skeletal muscle of rats. Legacy Med (Tokyo) 2004;6:25–31. doi: 10.1016/j.legalmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Senn P, Oshima K, Teo D, Grimm C, Heller S. Robust postmortem survival of murine vestibular and cochlear stem cells. J Assoc Res Otolaryngol. 2007;8:194–204. doi: 10.1007/s10162-007-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52:2935–42. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]


