Abstract
Background:
A group study for symptomatic cavernous malformation (CM) treated with gamma knife (GK) surgery was performed.
Methods:
A total of 298 cases collected from 23 GK centers across Japan were included. Hemorrhage was the most common manifestation, followed by seizures and neurological deficits. Most of the lesions were located in the brainstem and basal ganglia, followed by the cerebral or cerebellar hemispheres. The CMs, which had a mean diameter of 14.8 mm, were treated using GK surgery with a mean marginal dose of 14.6 Gy.
Results:
In terms of hemorrhage-free survival (HFS), a marked dissociation was confirmed between the hemorrhage and seizure groups, while no obvious difference was noted between sexes. Superficial CMs located in cerebellum or lobar regions responded to the treatment better than deeply located CMs in the basal ganglia or brainstem. No significant difference of dose-dependent response was seen for three different ranges of marginal dose: Less than 15 Gy, between 15 and 20 Gy, and more than 20 Gy. Complications were more frequent after a marginal dose of over 15 Gy and in patients with lesions more than 15 mm in diameter. The rates of annual hemorrhage were estimated to be 7.4% during the first 2 years after radiosurgery and 2.8% thereafter. The overall hemorrhage rate after radiosurgery was 4.4%/year/patient.
Conclusion:
The risk of hemorrhage is considerably reduced after GK treatment. The HFS as well as annual hemorrhage rate after GK treatment was apparently superior to that after conservative treatment for symptomatic CMs. To optimize the success of GK treatment, it is important to reduce the incidence of complications.
Keywords: Cavernous malformation, gamma knife, radiosurgery, symptomatic lesion, vascular anomaly
INTRODUCTION
Progress in neuroimaging has enabled cavernous malformations (CMs) in the brain to be easily detected using standard imaging studies, particularly magnetic resonance imaging (MRI). However, a differential diagnosis from thrombosed arteriovenous malformation (AVM), venous anomaly, or capillary hemangioectasia is not always easy. Thus the term “angiographically occult vascular malformation“ has been used in the medical literature. However, most of these lesions are believed to be CMs. A majority of CMs are clinically very silent and are often detected incidentally. Silent lesions should not be treated with surgery and should instead be managed conservatively using a so-called “wait-and-see” strategy. Some CM lesions, however, show very aggressive behaviors, with frequent bleeding, intractable seizures, or neurological deficits presenting during a short period of time.[13,14] Although these symptomatic episodes associated with CMs have been well documented, the actual natural history of such symptomatic cavernous malformations (s-CMs) has not been fully recognized. Because of this lack of information, the treatment results of surgery or radiosurgery for symptomatic lesions cannot be evaluated precisely. Despite this uncertainty, the aggressive clinical behaviors of s-CMs calls for treatment such as surgery or radiosurgery. In this article, the nation-wide results of radiosurgery for s-CMs have been summarized and compared with the natural history of s-CMs to clarify the role of radiosurgery and microsurgery.
MATERIALS AND METHODS
Since the first installation of a gamma knife (GK) facility in Japan in 1990, 55 GK centers for radiosurgery have been established. Approximately half of these centers are currently performing GK treatment for symptomatic CMs (s-CMs). With the cooperation of the Scientific Committee of the Japanese Society of Gamma Knife, this multi-institutional retrospective study was planned and conducted with the permission of the Ethics Committee of the participating institutions.
In this study, a questionnaire was distributed and the following data were collected from each GK center in Japan. Data on the initial symptoms, location and size of the lesions, and treatment methods used at the time of the first episode were collected. Then, details regarding the radiosurgery procedure including the lesion site, lesion volume, and marginal dose of radiosurgery were investigated. Data on the subsequent occurrence of symptomatic episodes and the treatment used for such episodes as well as the final neurological status and outcome, along with the available imaging studies, were also requested.
GK treatment was performed at each of the participating institutions using a GK B, 4C, or Perfexion device (ELEKTA Instruments AB). The software used for dose planning was either KULA system or GammaPlan (ELEKTA Instruments AB). All the cases treated with GK were symptomatic, chiefly with hemorrhage, and epilepsy, neurological deficits in a few cases. No asymptomatic ones were included in this series. In general, whole lesions including the low-intensity rim were covered for supratentorial and cerebellar CMs, whereas only the core of the lesion was covered for lesions located in the brainstem or basal ganglia. After radiosurgery, follow-up studies using MRI were performed every 3 months during the first year and at least every 6 months thereafter. Finally, the radiological findings at the last follow-up were described in terms of complete remission (CR), partial remission (PR), minor response (MR), no change (NC) or progression (PG). Hemorrhage was defined as a new area of blood density on CT imaging in association with or without a neurological symptom or sign. New foci of high signal intensity on T1-weighted MR images, volume expansion of the lesions and edematous changes of surrounding brain were also carefully studied.
The final clinical status was classified into five categories as follows:
Excellent: No symptoms;
Good: Minimal or intermittent nondisabling symptoms;
Fair: Moderate disability, independent of others;
Poor: Major disability, dependent on others, progressive symptoms;
Dismal: Death, vegetative state.
Statistical analyses were performed using Fisher's exact two-tailed test for comparison of proportions by JMP-R 9.0.2 (2010, SAS Institute INC). For hemorrhage rates after diagnosis and treatment, survival analysis technique were used to compare Kaplan–Meier curves with log-rank test. Statistical significance was defined as P < 0.05. The annual hemorrhage rate (AHR) was defined as number of hemorrhages divided by total follow-up years.
RESULTS
Results of questionnaire and sample acquired
A total of 298 cases were collected from 23 GK centers located across Japan, including 23 GK institutions, consisting of 42% of all sites in Japan. They were treated with GK from 1991 to 2012, and with a sufficient follow-up period at least more than 12 months. The patient characteristics, such as their age and sex, the location of the lesion, and the mean lesion size, are listed in [Table 1]. Overall, 173 males and 125 females ranging in age from 7 to 73 years (mean age, 38.6 years) were treated. Most of the lesions were located in the brainstem, followed by the lobar region, cerebellum, thalamus, and basal ganglia [Figure 1]. They were separated into deep (brainstem, basal ganglia, thalamus) and superficial locations (cerebellar and lobar).
Table 1.
Characteristics of cases with symptomatic cavernous malformation
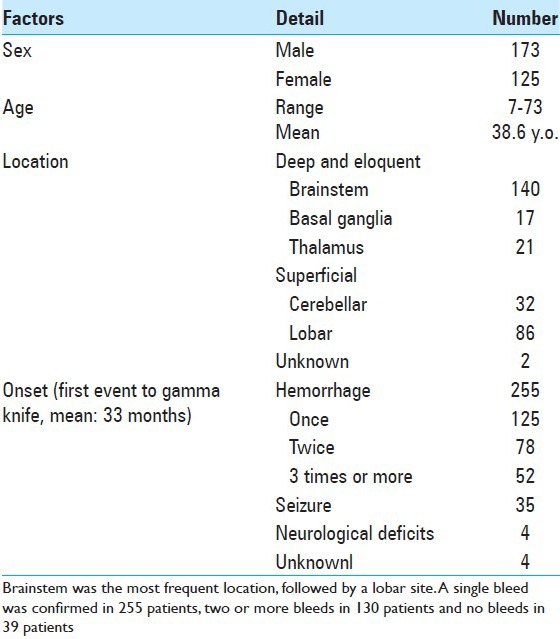
Figure 1.
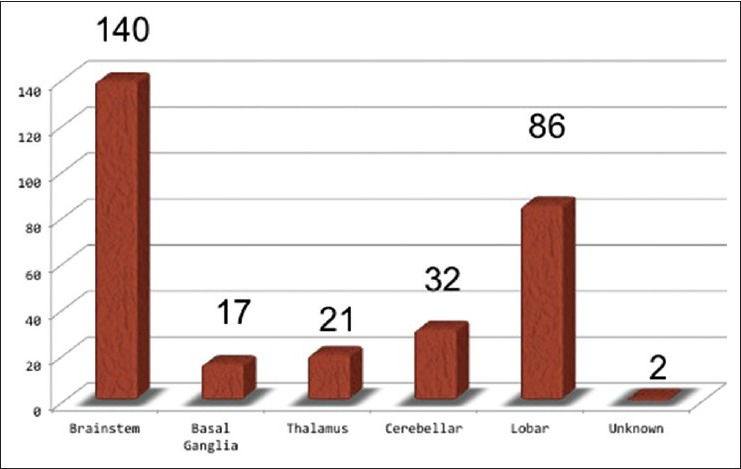
Location of the lesions treated with radiosurgery. Almost a half of lesions are located in brainstem
The majority of cases presented with hemorrhage (255 cases) prior to radiosurgery. Among them, a single hemorrhage occurred in 125 cases, two hemorrhages occurred in 78 cases, and three or more hemorrhages occurred in 52 cases. Thirty-five cases initially presented with seizure, and four cases presented with neurological deficits [Table 1]. The mean interval from the first events to GK treatment was 33 months (median: 7 months). When CMs were multiple in the brain, only the symptomatic lesions were treated.
Radiosurgery with gamma knife in all centers
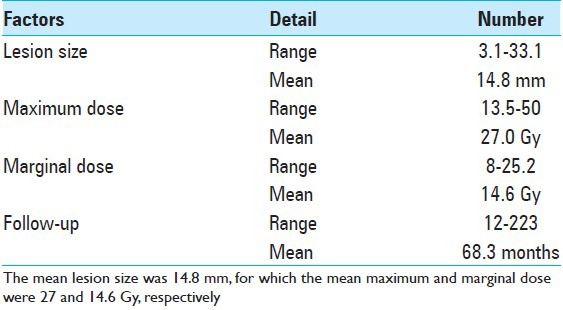
The lesion diameter at the time of radiosurgery ranged from 3.1 to 33.1 mm with a mean diameter of 14.8 mm. These lesions were treated using GK surgery with a mean marginal dose of 14.6 Gy [Table 2]. In general, only the cores of the lesion were incorporated within the target volume, and T2-high signal area surrounding them were not. However, for small supratentorial lesions in association with intractable seizures T2-high signal area were occasionally included as a target. The follow-up period ranged from 12 to 223 months with a mean of 68.3 months.
Table 2.
Radiosurgery for symptomatic cavernous malformations
Results after treatment
Radiological changes after radiosurgery
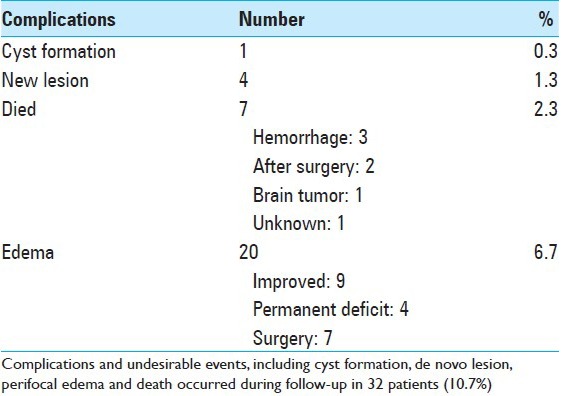
Almost half of the lesions showed some shrinkage, but the remaining lesions showed no change in size over a long time period. A few lesions increased in size mostly because of overt bleeding or minor hemorrhage after radiosurgery [Figure 2]. Perifocal edema with neurological signs was confirmed in 20 cases. De novo CM lesions were confirmed in four cases during the follow-up period [Table 3], which were not recognized at the radiosurgery.
Figure 2.
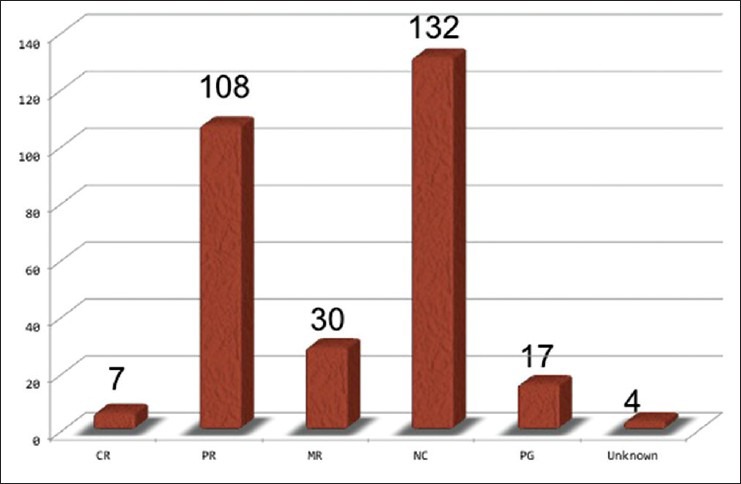
Radiological responses of CMs to radiosurgery. Approximately a half of them showed a shrinkage, and the others were unchanged
Table 3.
Complications following radiosurgery
Hemorrhage-free survival
After radiosurgery, rebleeding or new bleeding occurred in 61 cases (18.3%). Regarding the onset patterns, further bleeding occurred in the cases that had initially presented with hemorrhage. The frequency of hemorrhage before radiosurgery, either single, two or more, did not significantly influence the hemorrhage-free survival (HFS) after radiosurgery (P = 0.1605) [Figure 3a]. In relation to the locations, CMs in the deep lesions were more hemorrhagic than those in the superficial ones (P = 0.0061) [Figure 3b]. HFS was somehow correlated with the marginal dose in a dose-dependent manner, but it was not statistically significant (P = 0.2309) [Figure 3c]. No remarkable differences in HFS were seen between males and females (P = 0.2704) [Figure 3d]. The definite rate of annual hemorrhage is defined as times of bleeding divided by follow-up years. The hemorrhage rates after radiosurgery are summarized in [Table 4]. The AHR since birth was estimated to be 3.9%/year/case including the first event, and 21.4% during first event to radiosurgery with GK. The hemorrhage rate between the 1st event and GK was extremely high.
Figure 3.
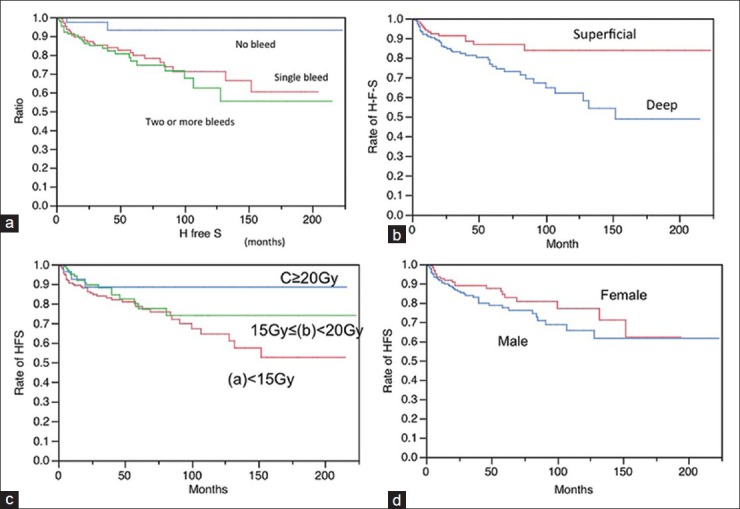
Hemorrhage-free survival (HFS) following radiosurgery according to various factors. (a) Onset, a: Single hemorrhage b: Two or more hemorrhages c: No hemorrhage (a vs b: Not significant, a vs c: Significant, b vs c: Significant) (b)Location the HFS was reasonably long in cases with superficial locations of the lesions, such as lobar and cerebellar lesions. In contrast, it was apparently worse in cases with deep locations of the lesions, such as the basal ganglia, thalamus, and brainstem. Superficial: Lobar, Cerebellar Deep: Brainstem, Basal ganglia, Thalamus (P =0.0077) (d) Marginal dose (a) <15 Gy 15 Gy< (b)<20 (c)<20 Gy (P =0.2309) (d) Sex: Male and female (P =0.2704)
Table 4.
Definite annual rates of hemorrhage in 298 cases after radiosurgery, and stratified by locations and number of bleeds
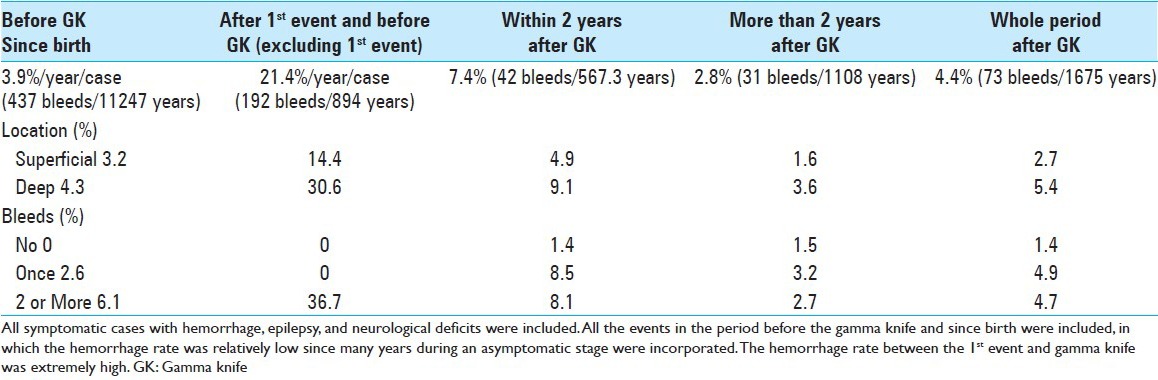
When stratified this by locations and bleeding times, the deeply located lesions and the ones of multiple bleedings demonstrated higher hemorrhage rates before GK treatment. The rates estimated to be 7.4%/year/case within 2 years after radiosurgery and 2.8%/year/case thereafter, resulting in a rate of 4.4% year/case for the entire follow-up period after radiosurgery Table 4.
When only the 255 cases presenting with hemorrhage as a first episode were analyzed, the hemorrhage rate from the birth (including first episode) was 4.4%/year/case. However, the rate increased to 28.7% during first episode to radiosurgery, in which the first episodes were excluded from the calculation. After radiosurgery, the rate dropped down to 8.4% during the first 2 years, and 3.0% thereafter. Overall hemorrhage rate after radiosurgery was 4.8%/year/case [Figure 4c].
Figure 4.
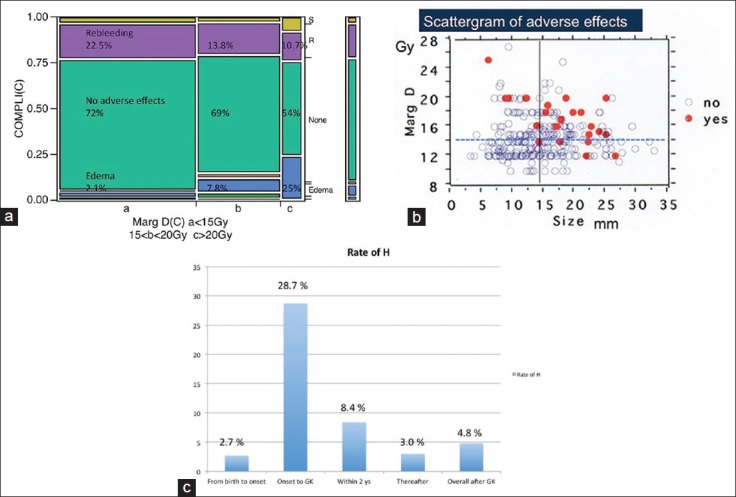
(a)Undesirable events and rebleeding occurring after gamma knife treatment and relation to the marginal dose variations during radiosurgery. (b) Complications occurred more frequently among cases more than 15 mm in diameter and cases treated with more than 15 Gy. (c) Changes of annual hemorrhage rate (AHR). Although AHR since birth to first episode was low, it jumped up to 28.7% during first episode and radiosurgery. The rate gradually dropped down to 8.4% in the first 2 years after radiosurgery and 3.0% thereafter. Overall AHR after radiosurgery was 4.8%/year/case
Seizure control
Seizure outcome data were estimated using Engel's classification. Among 27 cases with sufficient follow-up period of 83 months, 13 of them (48%) were seizure-free (Engel I), but the others remained in class II (26%) or III (26%). Two types of seizure cases were observed: Cases with s-CMs associated with repetitive minor hemorrhages, and cases with s-CMs surrounded by hemosiderin-stained brain tissue without recognizable hemorrhage. Typically, the seizures associated with the former cases were not difficult to control, whereas latter cases were often refractory to treatment. Further treatments, such as surgical resection were thus required in four cases, resulting in excellent seizure control.
Complications
Radiation-induced edema in and around the CM with accompanying neurological signs was the most common complication. In this series, there were 20 cases of symptomatic radiation-induced edema (6.7%) in the surrounding brain tissue. Among them, nine cases were improved and four had permanent deficits. The other seven cases were surgically treated at the time of radiation-induced edema. De novo lesions (1.3%) and/or cyst formation (0.3%) were observed [Table 3].
Rebleeding
Rebleeding occurred more frequently in the cases treated with the marginal dose less than 15 Gy. Conversely, perifocal edema occurred more frequently in the cases treated with more than 20 Gy [Figure 4a]. The complication rate was apparently higher among cases treated with a higher marginal dose [Figure 4b]. During the long follow-up period (mean: 65.4 months), seven cases died because of either rebleeding in three, after surgery in two cases, or by unrelated tumor and unknown cause.
DISCUSSION
There has been a long-term discussion on how radiosurgery can contribute for the treatment of s-CMs. This study is believed to be the largest series of s-CMs treated with GK and reported in the literature.
Natural history as described in medical literature
Bleedings or seizures caused by intracranial CM seem to be relatively rare, and a majority of such lesions are believed to be asymptomatic over time. In two early reports describing the natural history of CM, Curling et al.[6] estimated the AHR to be 0.25%/person-year, while Robinson [19] estimated a rate of 0.7%/person-year. Curling et al.[6] also reported a seizure rate of 1.51%/person-year. Therefore, surgery is not always necessary for all CMs, and conservative treatment or a wait-and-see strategy is commonly adopted.
Natural history of symptomatic cavernous malformations
The natural history of s-CMs is totally different from that of asymptomatic lesions, as frequent hemorrhages or seizures occur after the first event.[8,13,14] The natural history of s-CMs is very important since only the patients with symptomatic ones are currently indicated for various treatments. However, accurate information on the natural history of s-CMs is not presently available, and asymptomatic lesions were involved more or less in the past studies of natural history. In 1995, Konziolka et al.[13] reported that bleeding rates are 0.6%/year for individuals without prior hemorrhage, 4.5% after a single hemorrhage and 32% after two or more hemorrhages. However, to determine the definite timing of the transition from a asymptomatic CM to a s-CM is extremely difficult. If only the data of the subsequent events occurring after the first bleeding or first event are collected, the natural history of s-CMs can be estimated. Since reports on the natural history of silent CMs have described hemorrhage rates of less than 1%/year/case, the actual incidence of hemorrhage is much higher than silent CMs. Our personal data indicate that the trend of high hemorrhage rate surprisingly lasts at least for 5 years after an initial event. Recently, a word of “temporary clustering” was introduced by several investigators, [2,26] not only demonstrating an aggressive behavior after the first event of CM, but also indicating the return to the reset condition. The aggressiveness of s-CM, however, was not always transient in our study, and a high hemorrhagic rate was persistent for at least more than several years. These data can be compared with the results of surgery, radiosurgery, or conservative treatment.
Surgery for s-CMs
Several studies examining surgery for symptomatic lesions associated with hemorrhage or intractable seizure have been reported in the literature.[3,5,7,9,20,21,22,25] Surgical resection of the lesion appears to be the best approach for preventing further bleeding, if a complete resection of the CM can be performed safely. Unfortunately these lesions often occupy eloquent regions, such as the brainstem or basal ganglia. Surgical trials have been recently performed even for symptomatic brainstem lesions. The indications for surgery in these cases were moderately biased, since most of the lesions were exophytic and were located just under the brain or brainstem surface. Nevertheless, CM resection is not always complete, and only a partial resection can be performed in some cases, resulting in almost same annual rate of hemorrhage as conservative treatment. Complications associated with brainstem surgery are not rare, even when the procedures are performed by skillful microneurosurgeons.
Gamma knife treatment for s-CM
With the realization of successful AVM radiosurgery, CMs are now initially treated with GK surgery. However, because of the relatively high complication rate and occasional bleeding even after treatment, such trials have always been controversial and limited.[1,4,10,11,12,15,16,17,24,27] In the past, several trials were reported as being successful because of a considerable reduction in bleeding and seizures [Table 5]. The AHR has been estimated as 15–32.5%/year/case before radiosurgery, with a reduction to 5–15% within 2 years and 1–5%/year/case thereafter. Unfortunately, because of the lack of precise information on the natural history of symptomatic lesions, some researchers [23] were suspicious that this reduction of hemorrhage rate might be a reset condition after the temporary clustering of hemorrhage as a natural course.
Table 5.
Current reports on radiosurgery for cavernous malformations
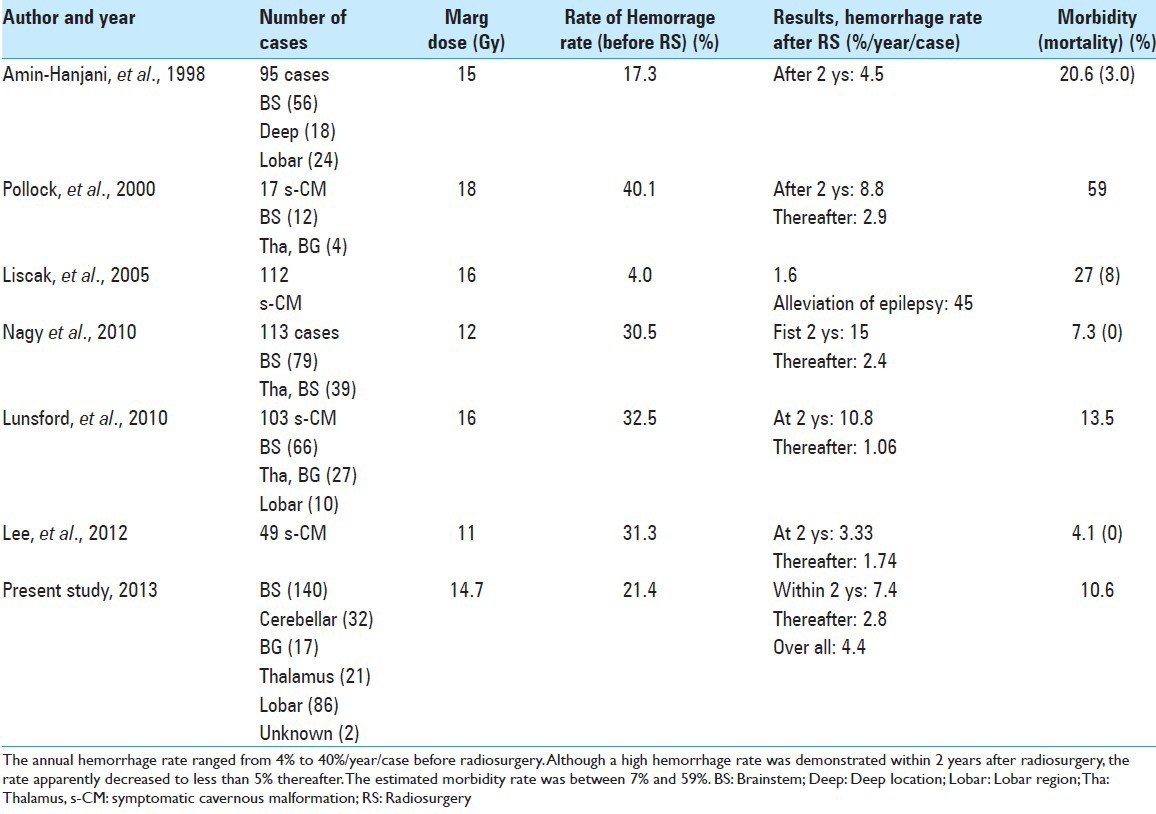
Other investigators have described negative results of radiosurgery, [10,18] mainly because of further bleeding and/or frequent complications. The complication rate was reportedly 10–30%, if the marginal dose was more than 15 Gy at the margin. Recently, the doses delivered to the margin have been reduced to less than 15 Gy and the complication rate has been less than 10%. In this report, a significant decrease in the rate of hemorrhage after GK treatment, compared with conservative treatment, was demonstrated. However, the adverse effects may increase accordingly to the higher marginal dose. Therefore to establish careful methods for preventing radiation injury following radiosurgery is mandatory.
Although randomized trial for s-CMs is required, it is difficult to accomplish the study because s-CMs are not common. Moreover, it is not adequate to watch the patients suffering from repetitive episode of hemorrhage for long time with conservative treatment.
Surgery and radiosurgery
In order to share light into this controversial dispute, a concurrent retrospective study on the natural history of s-CM, as well as the results of surgery was carried out here. Our data of natural history of pure s-CM as previously mentioned were compared with the results of surgery and radiosurgery. All three were almost comparable in age, sex, and onsets, and carried out with very similar study protocols [Table 6]. In terms of HFS, the best outcome is available with surgery. No significant difference of HFS was confirmed between surgery and radiosurgery [Figure 5]. The strict comparison between the results of GK and conservative treatment, in which the initial events were precisely adjusted to the same time course, indicates a significantly better outcome after radiosurgery. In contrast to the early reduction of hemorrhage after radiosurgery, the aggressive hemorrhage rate in the natural history of s-CM lasts for at least 5 years after the first event. Therefore, radiosurgery certainly suppressed the hemorrhages during 5 years after initial hemorrhage, while the temporary clustering was observed in the conservative group [Figure 6].
Table 6.
Comparison of the three groups in terms of the mean age, onset patterns, location of lesions, and mean lesion size
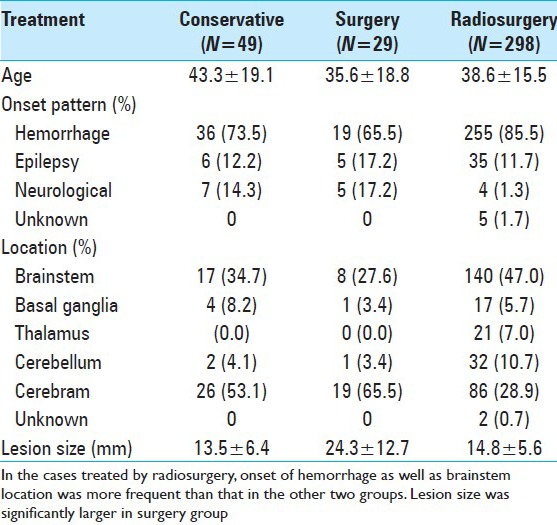
Figure 5.
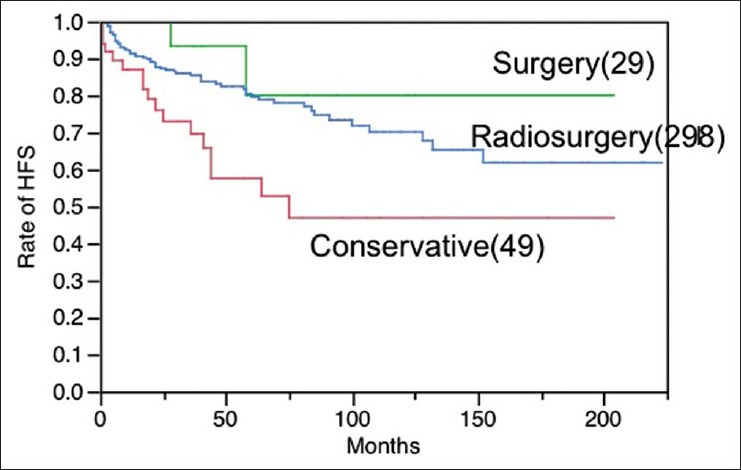
Hemorrhage-free survival after surgery (S) (29 cases), radiosurgery with gamma knife (R) (298 cases) and conservative treatment© (49 cases). The difference of HFS is statistically significant between S and C groups (P=0.0176), as well as R and C groups (P=0.0026), however, not significant between S and R groups (P=0.2489)
Figure 6.
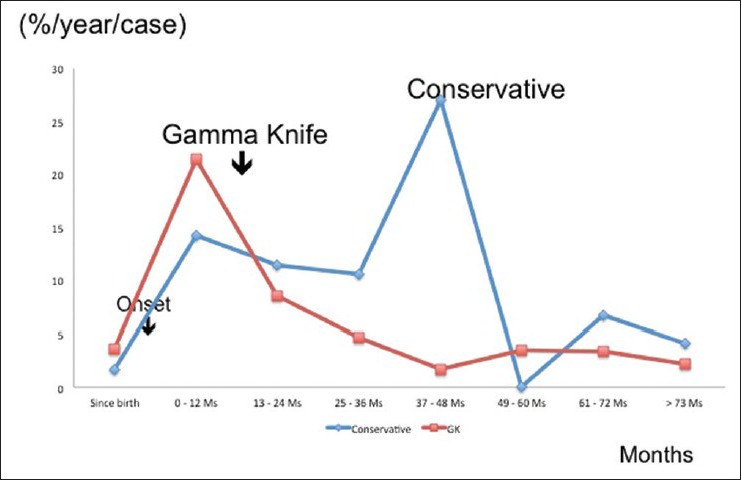
Comparison of annual hemorrhage rate between radiosurgery and conservative groups. Since the mean interval between the onset and radiosurgery was 33 months (mean: 7 months), this time differences were carefully adjusted to zero. The annual hemorrhage rate apparently decreased after radiosurgery when compared with that of conservative treatment for symptomatic cavernous malformations. In the latter (conservative group: 49 cases), hemorrhagic episodes were frequent especially within 5 years after the onset, forming peaks of high hemorrhage rate
Strategy for symptomatic cavernous malformations
Complete surgical resection is ideal for preventing rebleedings. However, the AHR is not superior to that of conservative treatment when only a partial resection is performed. Moreover, serious adverse effects of surgical intervention to the brainstem and basal ganglia may occur. Radiosurgery obviously reduces the hemorrhage rate, and can be used for lesions in the brainstem and deep brain structures. Thus, the treatment strategy for s-CM should not be simple, and a deep understanding of the natural history, neurological status, location as well as the size of the lesion is required. When a small part of CM tissue remains after surgery, radiosurgery seems to be an ideal option in order to prevent further bleedings.
CONCLUSION
Because s-CMs often show aggressive behaviors, adequate treatment is necessary. After radiosurgery, the AHR is significantly lower than that of conservative treatment. GK radiosurgery is an important option, since the adverse effects are less frequent than with surgery, especially for eloquent locations such as the brainstem or basal ganglia. Radiosurgery brings the hemorrhage rate of these lesions at least to that of natural history when safe doses are used. Early intervention with radiosurgery is preferable when a small part of the CM remains after surgery.
Author contributions to the study and manuscript preparation include following. Conception and design: Kida, Hasegawa. Acquisition of data: Kida. Analysis and interpretation of data: Kida, Hasegawa. Critically revising the article: All authors.
ACKNOWLEDGMENTS
Including seven authors, investigators in the 23 GK institutions described below from across Japan that provided the data used are gratefully acknowledged.
Chigasaki Tokusyuukai Hospital, Takai Hospital, Koyou Hospital, Shiroyama Hospital, Mito Gamma House, North Japan Neurosurgical Hospital, Mominoki Hospital, Tominaga Neurosurgical Hospital, Takashima Hospital, Yamagata Kenritu Central Hospital, Akita Cerebrovascular Research Center, Atuzi Neurosurgical Hospital, Shiokawa Hospital, Nagoya Kyoritu Hospital, South Tohoku Medical Clinic, Kotou Memorial Hospital, Saitama Gamma Knife Cente.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/6/249/157071
Contributor Information
Yoshihisa Kida, Email: yoshihisa_kida@mac.com.
Toshinori Hasegawa, Email: h-toshi@aa.cyberhome.ne.jp.
Yoshiyasu Iwai, Email: y-iwai@rc5.so-net.ne.jp.
Takashi Shuto, Email: shuto@yokohamah.rofuku.go.jp.
Manabu Satoh, Email: rakusimi@atlas.plala.or.jp.
Takeshi Kondoh, Email: comopark1958@gmail.com.
Motohiro Hayashi, Email: gkrmoto@gmail.com.
REFERENCES
- 1.Amin-Hanjani S, Ogilvy CS, Candia GJ, Lyons S, Chapman PH. Stereotactic radiosurgery for cavernous malformations: Kjellberg's experience with proton beam therapy in 98 cases at the Harvard cyclotron. Neurosurgery. 1998;42:1229–38. doi: 10.1097/00006123-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Barker FG, II, Amin-Hanjani S, Butler WE, Lyons S, Ojemann RG, Chapman PH, et al. Temporal clustering of hemorrhages from untreated cavernous malformation of the central nervous system. Neurosurgery. 2001;49:15–25. doi: 10.1097/00006123-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: Report of 26 cases. Acta Neurochir (Wien) 1991;108:91–9. doi: 10.1007/BF01418515. [DOI] [PubMed] [Google Scholar]
- 4.Chang SD, Levy RP, Adler JR, Martin DP, Krakovitz PR, Steinberg GK. Stereotactic radiosurgery of angiographically occult vascular malformations: 14-year experience. Neurosurgery. 1998;43:213–21. doi: 10.1097/00006123-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83:237–42. doi: 10.3171/jns.1995.83.2.0237. [DOI] [PubMed] [Google Scholar]
- 6.Curling OD, Kelly DL, Elster AD, Craven TE. An analysis of the natural history of cavernous angioma. J Neurosurg. 1991;75:702–8. doi: 10.3171/jns.1991.75.5.0702. [DOI] [PubMed] [Google Scholar]
- 7.Gross BA, Batjer HH, Awad IA, Bendok BR. Brainstem cavernous malformations. Neurosurgery. 2009;64:E805–18. doi: 10.1227/01.NEU.0000343668.44288.18. [DOI] [PubMed] [Google Scholar]
- 8.Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011;30:E24. doi: 10.3171/2011.3.FOCUS1165. [DOI] [PubMed] [Google Scholar]
- 9.Hauck EF, Barnett SL, White JA, Samson D. Symptomatic braistem cavernomas. Neurosurgery. 2009;64:61–70. doi: 10.1227/01.NEU.0000335158.11692.53. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson B, Kihlstrom L, Lindquist C, Ericson K, Steiner L. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293–7. doi: 10.3171/jns.1998.88.2.0293. [DOI] [PubMed] [Google Scholar]
- 11.Kida Y. Radiosurgery for cavernous malformations in basal ganglia, thalamus and brainstem. Prog Neurol Surg. 2009;22:31–7. doi: 10.1159/000163380. [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D, Lunsford D, Flickinger JC, Kestle JR. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825–31. doi: 10.3171/jns.1995.83.5.0825. [DOI] [PubMed] [Google Scholar]
- 13.Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820–4. doi: 10.3171/jns.1995.83.5.0820. [DOI] [PubMed] [Google Scholar]
- 14.Kupersmith MJ, Kalish H, Epstein F, Berenstein A, Woo H, Jafar J, et al. Natural history of braistem cavernous malformations. Neurosurgery. 2001;48:47–53. doi: 10.1097/00006123-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Lee CC, Pan DH, Chung WY, Liu KD, Yang HC, Wu HM, et al. Brainstem cavernous malformations: The role of Gamma Knife surgery. J Neurosurg. 2012;117(Suppl):164–9. doi: 10.3171/2012.8.GKS121066. [DOI] [PubMed] [Google Scholar]
- 16.Liscak R, Vladyka V, Simonova G, Vymazal J, Novotny J., Jr Gamma knife surgery of brain cavernous hemangiomas. J Neurosurg. 2005;102:207–13. doi: 10.3171/jns.2005.102.s_supplement.0207. [DOI] [PubMed] [Google Scholar]
- 17.Lunsford LD, Kahn AA, Niranjan A, Kano H, Flickinger JC, Kondziolka D. Stereotactic radiosurgery for symptomatic solotary cerebral cavernous malformations considered high rrisk for resection. J Neurosurg. 2010;113:23–9. doi: 10.3171/2010.1.JNS081626. [DOI] [PubMed] [Google Scholar]
- 18.Pollock BE, Garces YI, Stafford SL, Foote RL, Schomberg PJ, Link MJ. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987–91. doi: 10.3171/jns.2000.93.6.0987. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709–14. doi: 10.3171/jns.1991.75.5.0709. [DOI] [PubMed] [Google Scholar]
- 20.Samii M, Eghbal R, Carvalho GA, Mathies C. Surgical management of braistem cavernomas. J Neurosurg. 2001;95:825–32. doi: 10.3171/jns.2001.95.5.0825. [DOI] [PubMed] [Google Scholar]
- 21.Sindou M, Yada J, Salord F. Functional results after microsurgical resection of brainstem cavernous malformations (retrospective study of a 12 patient series and review of the recent literature) Acta Neurochir (Wien) 2000;142:843–52. doi: 10.1007/s007010070069. [DOI] [PubMed] [Google Scholar]
- 22.Sola RG, Pulido P, Pastor J, Ochoa M, Castedo J. Surgical treatment of symptomatic cavernous malformations of the brainstem. Acta Neurochir (Wien) 2007;149:463–70. doi: 10.1007/s00701-007-1113-5. [DOI] [PubMed] [Google Scholar]
- 23.Steiner L, Karlsson B, Yen CP, Torner JC, Lindquist C, Schlesinger D. Radiosurgery in cavernous malformations: Anatomy of a controversy. J Neurosurg. 2010;113:16–22. doi: 10.3171/2009.11.JNS091733. [DOI] [PubMed] [Google Scholar]
- 24.Tarnaris A, Fernandes RP, Kichen ND. Does conservative management for brain stem cavernomas have better long-term outcome? Br J Neurosurg. 2008;22:748–57. doi: 10.1080/02688690802354210. [DOI] [PubMed] [Google Scholar]
- 25.Wang CW, Liu AL, Zhang J, Sun B, Zhao Y. Surgical management of brainstem cavernous malformations: Report of 137 cases. Surg Neurol. 2003;59:444–54. doi: 10.1016/s0090-3019(03)00187-3. [DOI] [PubMed] [Google Scholar]
- 26.Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010;29:E7. doi: 10.3171/2010.5.FOCUS10149. [DOI] [PubMed] [Google Scholar]
- 27.Weil S, Tew JM, Steiner L. Comparison of radiosurgery and microsurgery for treatment of cavernous malformations of the brain stem. J Neurosurg. 1990;72:336A. [Google Scholar]


