Abstract
Background:
Considering the role of kisspeptin (KISS) in the process of puberty, this study aimed to determine the mutation of KISS1 gene among a group of patients with idiopathic central precocious puberty (ICPP).
Methods:
In this case control study, a group of children with diagnosed ICPP and a group of healthy children were selected. Genomic DNA was extracted from peripheral blood of selected population. After proving the quality and quantity of extracted DNA samples by nano-drop instrument, PCR was performed using 3 set of primers to amplify all coding exons and flanking intron region of Kiss1 gene.
Results:
In this study, 33 patients with idiopathic PP and 30 control age and sex matched children were studied. Genetic analysis indicated that there was not any polymorphism or mutation in studied participants of the control group. Among patients with ICPP, 4 single nucleotide polymorphisms within the promoter and coding regions of KISS1 gene were determined in 9 patients (5 boys and 4 girls). Among them, the c.-148 T > A was novel variant.
Conclusions:
The results of the current study identified one novel polymorphism and three reported polymorphism in KISS gene among patients with ICPP. It is recommended to design further studies for analysis other genes related to ICPP in accordance with more complementary biochemical evaluations is recommended also.
Keywords: Central precocious puberty, kisspeptin gene, mutation, polymorphism
INTRODUCTION
Puberty is considered as a complex network of coordinated biological process with multiple levels of neuroendocrine and genetic regulations.[1] Timing of puberty could influence by different environmental and genetic factors.[2,3] Precocious puberty (PP) is one of its variations which defines as appearance of physical signs of sexual development in a child prior to the earliest accepted age of sexual maturation, 7 years in girls and 9 years in boys.[4]
Evidences indicated that the age of puberty has decreased during last decades.[5] In addition reported incidence rate of PP has also been increased. The rate of PP has reported to ranges between 1 in 5000 and 1 in 10000 children with higher rate in girls than in boys. The most common cases of PP are classified as idiopathic central PP (ICPP).[6]
Central PP is characterized by premature activation of hypothalamic GnRH secreting neurons that result in precocious development of secondary sexual characteristics and acceleration of linear growth and bone age advancement. As factors that affecting the premature activation of the hypothalamic-pituitary-gonadal axis are not fully determined yet, so most cases of central PP defined as ICPP.[7,8]
Precocious puberty is associated with various short and long term complications including short adult stature, premature sexual development, and related psychosocial difficulties. Patients with PP should be monitored for early initiation of sexual life and its related risk factors such as sexually transmitted infections, early teenage pregnancies, breast, ovarian and endometrial cancers, obesity, type 2 diabetes and cardiovascular disease.[9,10,11]
Though several studies have reported the role of different genes in the pathogenesis of ICPP, the exact mechanisms and genetic background of ICPP are not well understood. It is suggested that the kisspeptin (KISS) neuropeptide, encoded by the KISS1 gene, is recognized as one of the factors involved in GnRH-dependent LH secretion and puberty onset.[12,13]
Kisspeptin that encoded by KISS gene is considered as a key gatekeeper of the onset of puberty.[14] The gene is located on chromosome 1q32-q41. It was first identified in 1996 as a tumor metastasis suppressor.[15] Later, its role as a major regulator during puberty was identified.[16]
Some mutations and polymorphisms of Kiss 1 have been identified recently. However, the results of the studies regarding the role of this gene in ICPP was not similar in different studies and populations.[12,13,17,18,19]
Given the role of KISS in the process of puberty, higher proportion of idiopathic cases of PP as well as racial and ethnical differences, this study aimed to determine the mutation of KISS gene among a group of patients with ICPP and compare it with control group. However, the results of this investigation could help us in planning proper therapeutic strategies to eliminate the burden of the disorder and its related complication.
METHODS
Study design and participants
In this case control study, children with diagnosed PP who were referred to the Endocrine Clinic of Imam Hossien Hospital, the only referral pediatric hospital of Isfahan city, affiliated to Isfahan University of Medical Sciences, were enrolled.
The protocol of the study was approved by the Regional Ethics Committee of Isfahan University of Medical Sciences and the Institutional Review Board of Child Growth and Development Research Center (research project number; 192146).
Reviewing the medical files of enrolled patients those with ICPP were selected and those with other cause of PP were excluded.
Idiopathic central PP was defined by pubertal onset before 7 years of age in girls and 9 years of age in boys.[4]
Bone age of studied population was evaluated. Magnetic resonance imaging (MRI) was performed in patients with diagnosed ICPP. Stage of puberty was determined using Tanner staging method. Pelvic ultrasonography was performed also.
Selected patients had advanced bone age (Greulich and Pyle method), and normal central nervous system MRI.
A group of age- and sex-matched children who referred to the hospital clinic for annual check-up or other acute disease was selected randomly as control group. Written informed consent was obtained from parents of all selected CH patients and control group.
All selected children in case and control groups were examined by a pediatric endocrinologist (AM), and their characteristics and information related to their disease (PP in the case group) were recorded using a questionnaire.
Venous blood samples (4 ml) were obtained from studied population and collected in ethylenediaminetetraacetic acid tubes and used for genetic analysis.
Genetic analysis
Genomic DNA was extracted from peripheral blood of the patient by salting out method. After proving the quality and quantity of extracted DNA samples by nano-drop instrument (thermo scientific), the samples were stored at −20°C. PCR was performed using 3 set of primers to amplify all coding exons and flanking intron region of Kiss1 gene. Primer sequences to amplify exon 1–3 were as follows, respectively:
EXON 1 F: GGGCTTTATAAAAGGGATGTG
EXON 1 R: CTTAGAACGGATTCCCTG
EXON 2 F: CAGATCCTGTGCCTGACCT
EXON 2 R: TTGCAACAACCCACTTGCT
EXON 3 F: GTGTTGCAAAGCCATCTTTC
EXON 3 R: TCTTTTATTGCCTCGGGTTG
In this study, all PCR reactions were performed in a 25 μl reaction containing 1.5 mM MgCl2, 200 mM of each dNTPs, I U of Taq DNA polymerase (Feldan, Germany) 150 ng DNA and 25 pmol of each primers.
The amplifications were conducted over 35 cycles, after initial denaturation at 94°C for 5 min, each cycle was performed using the following conditions; denaturation at 94°C for 1 min, annealing at 58°C (for exon 1 and 2) or 49°C (for exon 3) for 45 s, and extension at 72°C for 1 min. Additional extension at 72°C for 10 min after the last amplification cycle was performed.
PCR products were separated on 2% agarose gel electrophoresis and sequenced with ABI 3730xl (Life Technologies, USA) automated sequencer and analyzed with Chromas Lite software (Technelysium Pty Ltd, Australia; Windows).
Statistical analysis
All data were analyzed using SPSS version 20 software (SPSS Inc., Chicago, IL, USA). Continuous variables reported as mean ± standard deviation (SD) and frequencies for categorical variables reported as n (%). Mentioned variables compared in case and control groups and subjects with and without polymorphism and mutation using Chi-squared test and t-test for qualitative and quantitative variables, respectively.
RESULTS
In this study, 33 patients with idiopathic PP and 30 control children with similar age and sex were investigated. Baseline characteristics of studied population are presented in Table 1.
Table 1.
Characteristics of patients with precocious puberty and control group
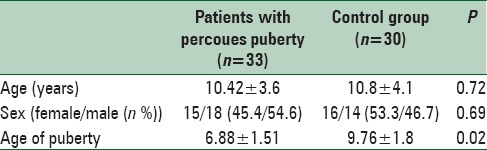
Mean ± SD (range) of weight percentile, height percentile and age of treatment in patients with PP were 77.00 ± 27.70th (15–110), 73.50 ± 27.49th (30–100) and 7.74 ± 1.5 years old (4.2–10), respectively. Stages of puberty in patients with PP at the time of diagnosis were as follows; stage II 21 (63.64%), stage III 10 (30.3%), stage IV 1 (3.03%) and stage V: 1 (3.03%).
Genetic analysis indicated that there was not any polymorphism or mutation in studied participants of the control group. Among ICPP patients, 4 single nucleotide polymorphisms (SNPs) within the coding regions of KISS1 gene were determined [Figure 1] in 9 (27.3%) patients (5 boys and 4 girls). Among them, the c.-148 T > A was novel variant. Pathogenicity of these variants is predicted by inSilico prediction softwares e.g. polyphen2, phylop and mutationtaster.
Figure 1.
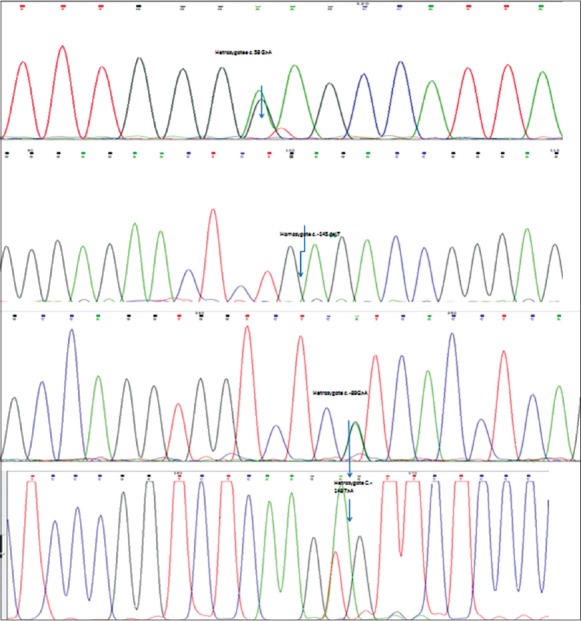
Sequencing results of detected four polymorphisms among patients with idiopathic central precocious puberty
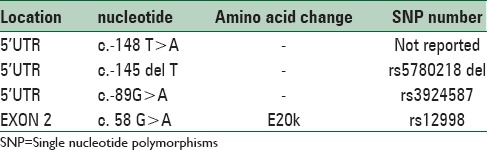
As only 3 exons of these genes were sequenced, presence of mutations outside these regions, not investigated in our study. Characteristics of detected SNPs are presented in Table 2.
Table 2.
Allelic variants identification in the kiss1 gene of patients with idiopathic central precocious puberty
Mean age of puberty in patients with and without polymorphism was 6.97 ± 0.82 and 6.85 ± 1.7 years, respectively (P = 0.83).
DISCUSSION
In this study, the mutations of KISS gene among patients with ICPP were investigated. Among four detected polymorphism in the studied population, one of them was novel. From studied population 27.7% had KISS gene polymorphism, wherase control group had not any.
The timing of puberty onset is different among subjects with various ethnicity and genetic background.[20,21] So far, the role of several genes in the pathogenesis of ICPP has been identified. One of the investigated genes is KISS 1. Though its role in ICPP has been reported in some studies, it seems that the results are not conclusive enough.[12,13,22]
There were few studies regarding the role of KISS1 gene;[12,13,17] and to the best of our knowledge, it was the first time that the polymorphisms in KISS1 gene were investigated in Iran. Previously, the role of GPR54 gene in girls with familial PP was investigated by Ghaemi et al. in Mashhad-Iran. They reported three polymorphisms in 88% of patients with ICPP.[23]
Results of previous studies regarding the role of this gene in ICPP were controversial. Some of them reported mutations of the gene in their studied patients with ICPP whereas others concluded that mutations in KISS1 are not a common cause for ICPP.[12,13,17]
In this study, we identified four polymorphisms among 9 patients with ICPP. One of them (c.-148 T > A) was a novel polymorphism. Though the reminder, three polymorphisms have been reported but not in patients with ICPP.
Luan et al. have evaluated the relationship between KISS1 gene mutations and CPP among 272 Chinese Han girls with CPP. They identified 8 polymorphisms. One of them was novel (P110T) and had significant negative relationship with the disease.[12]
Ko et al. have investigated KISS1 gene mutations or polymorphisms among 101 Korean girls with CPP. They found 8 polymorphisms (two novels) in studied population. They also reported that p.P110T polymorphism is an infrequent polymorphism in the KISS1 gene that was suggested to have a protective effect on pubertal precocity.[13]
Tomiska et al. in Finland have investigated the role of kiss 1 gene in the pathogenesis of ICPP in 30 patients. They did not find any mutation and concluded that KISS1 gene is not a common cause for ICPP.[17]
Krstevska-Konstantinova et al. in Macedonia have studied the mutation of KISS1 in 28 girls with ICPP.[18] Their results were similar to Tomiska et al.
In another study in Brazil, Silveira et al. have investigated KISS1 mutations in 83 patients with ICPP. They detected two missense variants, c. 369C > T (p.Pro74Ser) and c. 417C > G (p.His90Asp), in three unrelated children (two girls and one boy).[19]
As mentioned, the results of different studies were not similar possibly due to differences in ethnicity and genetic background.
One of the challenging findings in our study that needs more investigations was a similar frequency of detected polymorphism in boys and girls. Though in mentioned studies, most of the studied populations were girls, but in the study of Silveria, the mutations was higher in girls than boys.[16] For achieving more conclusive results, further studies with consideration of familial history of the disease and parental consanguinity are recommended.
The limitations of current study were as follows; small sample size and lack of complementary evaluation regarding the relation of studied genes with other demographic or biochemical factors of studied population.
Thus, it is recommended to design studies for evaluating the interaction of different environmental and nutritional factors with detected polymorphisms in the pathogenesis of ICPP. In addition it is recommended to study the role of one of the most potent genes in the pathogenesis of ICPP, makorin ring finger 3 (MRKN3) gene, which had identified recently (after designing of our study). Abreu et al. used whole-exome sequencing to analyze genes of 40 subjects (32 with central PP and 8 with normal puberty) from 15 families. They reported four novel heterozygous mutations in MKRN3 in five of the 15 families and concluded that the gene has an important role in familial PP.[24] The results of the current study identified one novel polymorphism and three reported polymorphism in KISS gene among patients with ICPP. Further studies should be designed with considering the limitations of the current study. More studies for analysis other genes related to ICPP in accordance with more complementary biochemical evaluations is recommended also.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cesario SK, Hughes LA. Precocious puberty: A comprehensive review of literature. J Obstet Gynecol Neonatal Nurs. 2007;36:263–74. doi: 10.1111/j.1552-6909.2007.00145.x. [DOI] [PubMed] [Google Scholar]
- 2.Nathan BM, Palmert MR. Regulation and disorders of pubertal timing. Endocrinol Metab Clin North Am. 2005;34:617–41. doi: 10.1016/j.ecl.2005.04.015. ix. [DOI] [PubMed] [Google Scholar]
- 3.Gajdos ZK, Hirschhorn JN, Palmert MR. What controls the timing of puberty? An update on progress from genetic investigation. Curr Opin Endocrinol Diabetes Obes. 2009;16:16–24. doi: 10.1097/MED.0b013e328320253c. [DOI] [PubMed] [Google Scholar]
- 4.Styne DM, Grumbach MM. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. 12th ed. Ch. 25. Philadelphia, Pa: Elsevier Saunders; 2011. p. 24. [Google Scholar]
- 5.Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: The Copenhagen Puberty Study. Pediatrics. 2009;123:e932–9. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 6.Lee PA, Kerrigan JR. Precocious puberty. In: Pescovitz OH, Eugster EA, editors. Textbook of Pediatric Endocrinology. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 316–30. [Google Scholar]
- 7.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 8.Phillip M, Lazar L. Precocious puberty: Growth and genetics. Horm Res. 2005;64(Suppl 2):56–61. doi: 10.1159/000087760. [DOI] [PubMed] [Google Scholar]
- 9.Kulik-Rechberger B. Individual and environmental conditions influencing puberty in girls. Ginekol Pol. 2008;79:697–701. [PubMed] [Google Scholar]
- 10.Shiva S, Fayyazi A, Melikian A, Shiva S. Causes and types of precocious puberty in north-west Iran. Iran J Pediatr. 2012;22:487–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–30. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 12.Luan X, Zhou Y, Wang W, Yu H, Li P, Gan X, et al. Association study of the polymorphisms in the KISS1 gene with central precocious puberty in Chinese girls. Eur J Endocrinol. 2007;157:113–8. doi: 10.1530/EJE-07-0061. [DOI] [PubMed] [Google Scholar]
- 13.Ko JM, Lee HS, Hwang JS. KISS1 gene analysis in Korean girls with central precocious puberty: A polymorphism, p. P110T, suggested to exert a protective effect. Endocr J. 2010;57:701–9. doi: 10.1507/endocrj.k10e-073. [DOI] [PubMed] [Google Scholar]
- 14.Navarro VM, Castellano JM, García-Galiano D, Tena-Sempere M. Neuroendocrine factors in the initiation of puberty: The emergent role of kisspeptin. Rev Endocr Metab Disord. 2007;8:11–20. doi: 10.1007/s11154-007-9028-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. [PubMed] [Google Scholar]
- 16.West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1) Genomics. 1998;54:145–8. doi: 10.1006/geno.1998.5566. [DOI] [PubMed] [Google Scholar]
- 17.Tommiska J, Sørensen K, Aksglaede L, Koivu R, Puhakka L, Juul A, et al. LIN28B, LIN28A, KISS1, and KISS1R in idiopathic central precocious puberty. BMC Res Notes. 2011;4:363. doi: 10.1186/1756-0500-4-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krstevska-Konstantinova M, Jovanovska J, Tasic VB, Montenegro LR, Beneduzzi D, Silveira LF, et al. Mutational analysis of KISS1 and KISS1R in idiopathic central precocious puberty. J Pediatr Endocrinol Metab. 2014;27:199–201. doi: 10.1515/jpem-2013-0080. [DOI] [PubMed] [Google Scholar]
- 19.Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–80. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JH, Yoo HW. Control of puberty: Genetics, endocrinology, and environment. Curr Opin Endocrinol Diabetes Obes. 2013;20:62–8. doi: 10.1097/MED.0b013e32835b7ec7. [DOI] [PubMed] [Google Scholar]
- 21.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JS. The genes associated with gonadotropin-releasing hormone-dependent precocious puberty. Korean J Pediatr. 2012;55:6–10. doi: 10.3345/kjp.2012.55.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaemi N, Asl SN, Abbaszadegan MR, Ghahraman M, Vakili R. GPR54 gene mutations in Iranian girls with central familial precocious puberty. Iran J Obstetr Gynecol Infertil. 2012;15:1–8. [Google Scholar]
- 24.Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368:2467–75. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]


