Abstract
Background
A vesicovaginal fistula (VVF) is an abnormal fistulous tract between the bladder and vagina, causing continuous loss of urine via the vagina. VVF is a relatively uncommon condition, but there is a drastically higher prevalence in the developing world. Furthermore, iatrogenic postoperative VVF is most common in developed countries, compared to mainly obstetric trauma in developing countries. In this review we focus on the development of current management techniques for VVF.
Methods
Medline was searched to identify articles related to urogenital fistulae, including VVF. Based on these reports we focus on the aetiology, clinical presentation, diagnosis and management of VVF. This in-depth review includes the optimal surgical timing, different surgical approaches (including minimally invasive techniques such as laparoscopic and robotic surgery), recommendations for postoperative care, surgical complications, and the need for further research in the use of robotic surgery to treat this condition.
Results
In all, 60 articles were identified and included in this review; eight were related to the aetiology, 12 to diagnosis, and 40 to the management of VVF. A thorough evaluation of VVF is imperative for planning the repair. Although the surgeonís experience typically influences the surgical approach, special situations will dictate the best approach.
Conclusion
The treatment of genitourinary fistulae with robotic assistance continues to develop, but further research is necessary to fully understand the use of this technology.
Abbreviations: VVF, vesicovaginal fistula; UVF, ureterovaginal fistula; LESS, laparo-endoscopic single-site surgery
Keywords: Vesicovaginal fistula, Presentation, Diagnosis, Evaluation, Management
Introduction
History
A vesicovaginal fistula (VVF) is a debilitating condition that has affected women for millennia, the first recorded reference of VVF being in 1550 BCE. In 1037, Avicenna first documented the relationship between VVF and obstructed labour, or traumatic delivery [1]. In 1923 the earliest case of VVF in a mummified body (2050 BCE) was described [2].
In 1845, a series of experimental operations on slaves was conducted in Montgomery, Alabama, USA. Based on these experiments, James Marion Sims established the foundations of VVF repair in 1852 and included: (1) Proper exposure with the knee-chest position; (2) The use of a weighted vaginal retractor; (3) The use of silver-wire sutures; (4) Tension-free closure of the defect; (5) Proper postoperative bladder drainage.
Definition and epidemiology
VVFs are epithelialised or fibrous communications between the bladder and vagina, and are relatively uncommon. Nonetheless, this is one of the most socially devastating conditions. Developing countries have a drastically higher prevalence of VVF than have developed nations [2]. Although the true incidence of VVF is unknown, it is estimated at 0.3–2% in developed countries [3]. The leading cause of VVF is iatrogenic, after surgery, in 81–91% of patients [1]. In developing countries such as Nigeria, the WHO has estimated that as of 2001, 800,000–1 million women have an unrepaired VVF. Prolonged or obstructed labour is the most prevalent cause of VVF in the large majority of developing countries (97%).
The aetiology of adult VVF
The aetiology of VVF varies geographically. In developed countries, a VVF is most commonly a result of gynaecological surgery, radiotherapy, injury during the healing process, or severe pelvic pathology. Conversely, in developing countries VVF is most commonly related to childbirth.
Iatrogenic (after surgery)
Despite the best efforts of surgeons, injury to the urinary tract occurs during the healing process after pelvic surgery or pelvic irradiation. In this scenario, VVF occurs in 81–91% of patients in developed countries in the form of:
-
(1)
Tissue necrosis after tissue ischaemia, attributed to external pressure (crush/clamp injury), kinking of urinary tract tissue (proximity to a ligated pedicle), or marked inflammation and tissue fibrosis [4].
-
(2)
Direct laceration or puncture injury to the urinary tract resulting in immediate urine leakage. Delayed injury from retroperitoneal fibrosis, tissue pressure, or partial obstruction might manifest as fistula formation and urine leakage after many days or even weeks [5].
-
(3)
Pelvic radiotherapy for malignancy has a 5% incidence of VVF formation, even after many years. Small-vessel endarteritis obliterans is the ongoing process associated with radiotherapy-related fistula, occurring years after initial radiotherapy [6]. It is hypothesised that the decreased blood supply as a result of radiotherapy leads to tissue necrosis, sloughing, and fistula formation. In all these scenarios, concurrent infection can exacerbate the problem.
Hysterectomy is the most common surgical procedure resulting in VVF formation in developed countries, with an 80% incidence [7]. Other gynaecological procedures account for up to 11% [8]. However, the incidence varies depending on the approach. The lowest is with transvaginal (0.2:1000), followed by transabdominal (1:1000), and laparoscopic procedures (2.2:1000). VVFs most commonly form above the trigone, at the level of the vaginal cuff [9].
Non-iatrogenic (obstetric)
Obstetric trauma is primarily responsible for the 98% urogenital fistula rate in sub-Saharan African nations such as Nigeria. This is primarily due to the shortage of medical care, wherein one physician will serve up to 200,000 individuals [10,11].
In Western countries, obstructed labour represents only 5% of all VVFs. Pelvic tumour and trauma, congenital anomalies, foreign body, and abscess account for <5% [9,12].
Of VVF in women in developed countries, the cause is secondary to benign gynaecological surgery in 80%, to obstetric trauma in 10%, to radiotherapy in 5%, and to gynaecological oncological procedures in 5%.
Presentation
Generally, the time to onset is 7–10 days after surgery, but can range from immediate to 6 weeks. A VVF after radiotherapy can take months to years to develop.
Symptoms
VVF are usually painless and result in the leakage of urine from the vagina, which might be either intermittent, particularly when positional, as this can be a sign of a ureterovaginal fistula (UVF), or continuous, which is more characteristic of a VVF. Intra-abdominal urinary extravasation can present with abdominal pain and ileus.
Potential secondary effects include vulvar irritation, recurrent infections or pyelonephritis leading to renal insufficiency.
Signs
On vaginal examination using the split speculum, the VVF can appear as a small, red granulated area with no visible opening or hole. Small fistulae can be located by instilling sterile milk or methylene blue through a Foley catheter into the bladder, and then asking the patient to cough or perform Valsalva manoeuvre.
A surgical grading system was described for obstetric fistulae [13], with Type I fistulae not involving the urethral-closing mechanism, Type II fistulae involving the urethral-closing mechanism, and Type III fistulae involving the ureter and other exceptional fistulae. The goal of this classification system is to allow a systematic comparison of different surgical techniques and treatment outcomes among centres.
Diagnosis
A high index of suspicion remains the most important diagnostic tool for VVF, where a thorough review of the patient’s medical and surgical history, and a careful physical examination, can reveal the pathology. Surgical notes, specifically related to intraoperative technical difficulty, including excessive bleeding and obesity for example, can be suggestive of the cause. Confirming the leakage of urine is very critical to diagnosing a VVF. Several methods are used. The present authors use the Moir test and specifically the double-dye technique that helps to differentiate between VVF and UVF. The Moir test consists of the patient first taking phenazopyridine; in the clinic, three cotton swabs are placed into the vagina and 100 mL of methylene blue solution is inserted into the bladder through the urethra [14]. After removing the catheter, a tampon is inserted into the vagina. After 2 h the tampon is inspected and if stained blue indicates a VVF; an orange stain indicates a UVF. A suspected UVF warrants further confirmation using intravenous indigo carmine or oral phenazopyridine. IVU can be used to delineate any anatomical defects present. About a quarter of patients with a VVF have hydronephrosis, while 10% have a concomitant UVF [15]. An antegrade pyelogram using a nephrostomy tube to fill the vagina with contrast medium can be used to delineate the anatomy, while cystoscopy and vaginoscopy can be used to determine the site, size, number, and location relative to the ureteric orifices [16].
Several small fistulae might be present in a supratrigonal transverse position, particularly after aggressive vaginal cuff closure. A biopsy is recommended in patients with a history of malignancy. Fluoro-urodynamic studies are used to determine bladder compliance, capacity and outlet competence, and to identify hidden fistulae. Three-dimensional (Fig. 1) CT urography provides excellent delineation of the fistula (Fig. 2).
Figure 1.
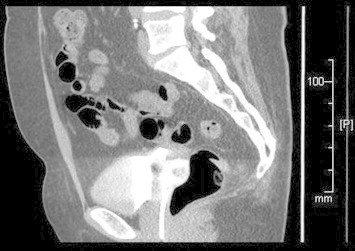
A high fistula at the vaginal cuff after laparoscopic hysterectomy. Note that the Foley catheter balloon is inflated at the vagina.
Figure 2.
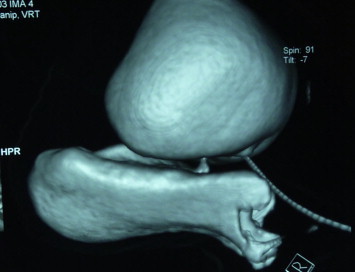
Three-dimensional CT urogram showing the fistula in mid-vagina.
Differential diagnosis
A differential diagnosis should include urinary incontinence, especially overflow incontinence, as this is continuous. Other causes of vaginal fluid include an ectopic ureter, a watery discharge from the vagina or cervix, and rarely from the uterus or Fallopian tubes.
Prevention
For iatrogenic fistulae, good surgical techniques should always be used to decrease the frequency of injury. The surgeon should take extra care to notice, diagnose and manage such injuries during surgery, if they do occur. However, fistula can still form despite the recognition and repair of an injury [17]. Some cases, such as a fistula after radiotherapy, might be impossible to prevent if tissue devascularisation causes delayed injury.
In developing countries, better access to obstetric care, and drainage of the bladder during labour, are useful preventative measures.
Management
Conservative treatment
Conservative treatment should be attempted only in small, clean, non-malignant VVF. Prolonged catheter drainage for 3–5 weeks is suggested. Anticholinergic drugs cause a relaxation of the detrusor muscle, preventing spasm. Topical oestrogens are routinely used in postmenopausal women to promote healing. In addition to our group [1], Schlunt Eilber et al. [3] used conservative therapy such as catheter drainage alone or with electrocautery for fistulae of <5 mm. The use of electrocautery through the cystoscope has been suggested to close very small (<0.5 cm) VVF tracts [18,19]. In theory, cautery destroys the fistula tract lining, allowing the bladder and vaginal tissues to reseal.
In recent years we have successfully managed small VVF with catheterisation and cystoscopic fulguration with/without fibrin glue or bulking agents. In a case report from Osaka, Japan, 1 mL of fibrin glue (Beriplast-P™, Aventis Pharma, Ltd, UK) was used successfully to treat a 1-mm VVF for a recurrent endometrial tumour at the ‘vaginal stump’ [10]. Conservative treatments, including glue products, reportedly have varying degrees of success, which depends on the cause, size and location of the fistula. Fibrin-based surgical sealants can also be used to augment a fistula repair. When an interposition is used between overlapping suture lines, the sealant can keep the area dry for long enough to allow primary healing [20,21]. However, reports of these methods are still limited.
Surgical repair
As in most surgery, the first attempt at a surgical repair is the most effective, and the most likely to succeed. The choice of surgical approach should be determined by the surgeon’s skill with the specific technique. One dose of a broad-spectrum antibiotic, e.g. 1 g of intravenous cefazolin, is usually given before surgery, for prophylaxis.
Surgical timing
The general consensus on the timing of fistula repair depends on the condition of the surrounding tissue. Healthy tissue allows an early repair, while unhealthy tissue warrants a 2–3 month delay to allow recovery from inflammation, infection, or tissue necrosis. This delay increases the opportunity for a successful repair.
Successful healing of a VVF is more likely with a proper and timely diagnosis and repair, e.g., in an uncomplicated VVF after hysterectomy, which still has evident tissue planes, a surgical repair is easier than for an obstetric fistula. In fact, excising and repairing a fistulous tract at 1–2 weeks after confirmation of leakage has become increasingly more common [22–24]. Specifically Zimmern et al. [25] did not find increased morbidity or failure after the early repair of a VVF at 2–3 weeks after injury.
Conversely, radiotherapy or obstetric surgery-related fistulae need time for adequate tissue regeneration before attempting a repair. In this case it is acceptable to delay the repair for a 3–6 month period [26]. Contrary to popular consensus, Waaldijk [27] concluded that the immediate management of fresh obstetric fistulae was highly effective in terms of closure and continence, thus preventing the patient from becoming a social outcast, with progressive downgrading medically, socially and mentally.
The surgical approach
The choice of surgical repair depends on the surgeon’s experience, the type and location of the fistula, and the patient’s specific preferences [19].
In the Western world, VVF most commonly develops at the vaginal cuff after an abdominal hysterectomy. The current trend towards a transvaginal approach, even for deep and large fistulae, has been associated with lower morbidity rates than for transabdominal repairs, and with an equally good outcome. Also, the approach to the type of vascularised interposition flap is determined by how proximal (peritoneal) or distal (Martius) the genitourinary fistula is in the vagina.
For a high vaginal cuff VVF after a hysterectomy, we prefer a Latzko procedure [28], or mini-colpocleisis (Fig. 3). This type of repair does not require excision of the fistula, as the margins provide good anchoring for the first layer of sutures. The damaged vaginal wall is then closed in layers over the fistula. The resulting vaginal shortening is nearly negligible and with no sequelae.
Figure 3.
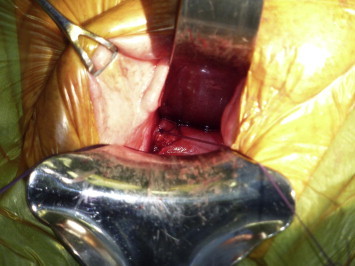
A deep VVF at the cuff after hysterectomy. Two silk sutures are placed at the edges for light traction during a Latzko repair.
Other causative factors contributing to high vaginal-vault fistulae include those secondary to radiation necrosis or a long obstructed labour with Caesarean hysterectomy. These fistulae are usually larger, with more fibrosis and tissue scarring, and might therefore require different techniques for repair.
Schlunt Eilber et al. [3] evaluated 207 women who had interposition grafts placed, over a 10-year period. The authors reported that peritoneal tissue is an excellent graft source for interposition in complex proximal VVF, with success rates similar to Martius grafts. Others have reported a clearly higher success rate with interposition grafts, regardless of type or cause of the fistula, in the transabdominal approach, in both benign and malignant VVF [23]. Success seems to depend on the condition of the vascular pedicle in the interposition graft.
The transvaginal approach
Distal fistulae require wide dissection for mobilisation and tension-free closure in non-overlapping layers. It is important to use a leak-test method. The authors use diluted methylene blue via a Foley catheter and a clean white laparotomy sponge for precise identification of any leak site. A ureteric catheter can be passed from the bladder to the vagina. In patients with previous radiotherapy or poor tissue quality, a Martius graft (in distal fistulae) or other biological graft materials can be used for reconstruction. Reported success rates are 92–96% [1,3,29]. A three-layer closure, at a minimum, is the key to the successful treatment of VVFs. The first layer consists of revised bladder mucosa or epithelialised edges of the fistula tract, followed by imbricated perivesical fascia in the second layer, and the third layer incorporates undermined vaginal epithelium or an advancement flap.
The transabdominal approach
In cases of ureteric reimplantation or augmentation or a deep and/or narrow vagina, a transabdominal approach, intraperitoneal with wide mobilisation of the bladder, is recommended. Our preference is for the O’Conor approach, which has a success rate of 85–100% [12]. The O’Conor approach provides excellent mobilisation of different layers, and omental interposition. This technique entails midline bivalving of the bladder, passing and then excising the fistula. One modification calls for a limited posterior-wall cystotomy extending to the fistula, with good success. The bladder is dissected and isolated from the fistulous tract, which is then excised. The bladder (two to three layers) and vagina (two layers) are then closed. Omental interposition is routinely used (Fig. 4).
Figure 4.
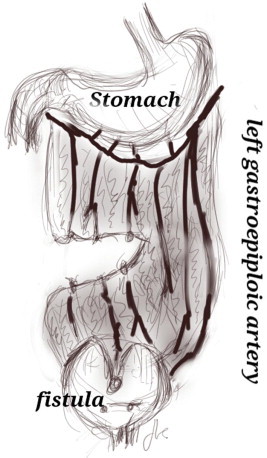
The interposition of an omental flap.
Laparoscopic repair
In 2005, Chibber et al. [12] described a laparoscopic approach to the O’Conor technique. The main reported advantages to using a laparoscopic technique are decreased morbidity and a more rapid recovery. Because maintaining a pneumoperitoneum after fistula excision can be challenging, the use of a Foley catheter is recommended. Chibber et al. [12] reported overall success in almost all patients (seven of eight). Success rates with the laparoscopic approach are reportedly as high as 93% at 26 months [25]. The use of omental J-flaps has also had reportedly good results, even after transvaginal failure using the Latzko procedure [27,30].
Several other studies have reported the effectiveness of laparoscopic techniques for the treatment of VVF. Abdel-Karim et al. [31] studied the laparoscopic transperitoneal extravesical repair of a supratrigonal VVF in 15 patients. Those with malignant, post-radiotherapy and recurrent fistulae were excluded. A laparoscopic transperitoneal extravesical repair was undertaken using 4–5 ports. The bladder was closed longitudinally, while the vagina was closed transversely with the interposition of omentum. The bladder was drained after surgery by a urethral catheter for 3 weeks. At mean (SD) follow-up of 18.9 (8.6) months, all patients were cured. There were no complications during or after surgery. The authors concluded that the laparoscopic transperitoneal extravesical repair of a VVF is safe and effective. Abdel-Karim et al. [32] also studied laparo-endoscopic single-site surgery (LESS) for the extravesical repair of a VVF. They included five patients with a supratrigonal VVF, who presented with urinary leakage via the vagina after obstetric and gynaecological procedures. The authors concluded that the LESS extravesical repair of VVF is a technically feasible and effective procedure that adheres to the principles of transabdominal open surgical repair. This technique has a significantly lower morbidity rate but requires advanced laparoscopic skills.
In limited case series with few patients, some authors [32–35] reported that a laparoscopic repair can be effective in managing VVF. This approach is safe and provides all the advantages of minimally invasive surgery, including a shorter hospital stay and recovery time, which have been shown to have a positive effect on the patients’ well-being.
Robotic-assisted repair
The laparoscopic repair of a VVF with robotic reconstruction is the most recent technology used in the treatment of VVF. Melamud et al. [36] were among the first to report the robotic reconstruction of a VVF using the DaVinci™ system (Intuitive Surgical, Sunnyvale, CA, USA). They reported an operative duration of 280 min and an estimated blood loss of 50 mL. The 16-week follow-up confirmed the cure of a post-hysterectomy proximal VVF [36]. Other authors have also reported excellent outcomes [25,37–41]. Despite the good results, robotic repairs will need further evaluation, as well as randomised controlled trials, to confirm their success. The disadvantages of robotic VVF repairs include an increased learning curve, time, cost, and availability, as well as surgeon experience.
The transvesical approach
The transvesical approach is less popular due to reportedly higher morbidity rates. This approach is indicated for loculated fistulae and those in which the ureteric orifices are cannulated. The fistulous tract is incised, leaving the vaginal wall intact, with circumferential dissection for 1–2 cm. Overlapping suture lines must be avoided at all costs; transverse and perpendicular suture lines should be used during close, if feasible.
Special cases of VVF
Large VVFs after radiotherapy or hysterectomy
These types of VVFs can be managed by ileocystoplasty. Although this technique is associated with a high morbidity rate, it has been effective in a selected group of women with a large VVF. To ensure a successful outcome it is crucial to use a well-vascularised portion of the ileum, which has not been irradiated [42]. The only other alternative with end-stage bladder disease and multiple failed procedures is urinary diversion.
Obstetric fistulae
These fistulae are classified according to their anatomical location and the repair is tailored to each type.
Suburethral or juxta-urethral
VVFs are among the most common obstetric fistulae seen in some parts of Africa. If total urethral transection with retropubic fixation of the fistula has not been used, simple vaginal tissue mobilisation with a layered closure has a 95% successful closure rate.
If retropubic fixation of the fistula edges has been used, then anterior bladder wall mobilisation through the space of Retzius, using the vaginal route, will bring the bladder wall edges to the severed urethra. Placing a Martius graft might also improve the closure in these instances, but type II stress urinary incontinence has been noted in at least 10–20% of these repairs [43,44].
Midvaginal or massive VVF
These types of VVF are most common in Western Africa, and require bilateral wide tissue mobilisation into the paravaginal spaces to facilitate bladder closure. Preservation of vaginal depth using full-thickness Martius grafts for vaginal closure has reduced the incidence of stenosis after this repair [45].
Juxtacervical VVF
These VVFs are typically located directly above or adjacent to the anterior cervical lip. They are best repaired by a combined vaginal-abdominal approach, with insertion of an omental graft between the bladder and cervix [46].
Fistulae with total urethral loss
These are difficult to manage and the repair involves mobilisation of the vulva and labial tissue to create a neourethra [47,48]. With severe scarring, the authors’ preference is mobilisation of the anterior bladder wall through the vagina, using an anterior bladder-wall flap to create a urethra [49]. Elevation of the neo-urethrovesical neck is mandatory to reduce the rate of type III stress urinary incontinence associated with this repair, even after successful closure of the VVF [50]. Patients who develop stress urinary incontinence might be candidates for a mid-urethral sling.
Other genitourinary fistulae
UVF
These types of VVFs occur after gynaecological surgery, most often an attempted repair of a urethral diverticulum, an anterior repair, and forceps rotation. UVF are usually closed by wide mobilisation into the lateral periurethral spaces and placing a tension-free vertical layer, with/without a Martius graft.
Ureterovaginal fistula
The timing of a ureterovaginal fistula repair is controversial [51,52]. Ureteric injuries can be repaired using reimplantation or anastomotic procedures; ureteric stenting can relieve an obstruction and preserve renal function. Rarely there can be spontaneous healing. Case series have been reported related to a robot-assisted repair of these types of fistula [53–55]. Laungani et al. [56] reported on three patients, after abdominal hysterectomy, who had a robot-assisted repair with ureteric re-implantation. The authors suggested an early repair with robotic assistance to decrease the morbidity. In addition, Kumar et al. [57] reported a case, describing the successful treatment of a uretero-uterine fistula, a rare complication, using laparoscopic ureteroneocystostomy.
Postoperative care
Postoperative care is as crucial for a successful repair as the procedure itself. A vaginal pack is used until the morning after surgery. Hydration, a high-volume bladder flow, drains and catheters will ensure constant fluid flow through the bladder. This will help to prevent any catheter blockage due to blood clots, which causes bladder distension and disruption of the repair.
Prophylactic antibiotics to prevent UTI can be useful, administered for 1–2 weeks after surgery, depending on the duration of catheter drainage. A cystogram is used to assess a bladder fistula repair, and IVU can be used to evaluate ureteric repairs. A cystogram through the suprapubic tube is obtained 3 weeks after the procedure, just before the tube is removed.
The authors’ preference is for suprapubic catheter drainage for 2–3 weeks and urethral catheter drainage for 5 days after surgery. Anticholinergics are used to prevent early suture tension as a result of bladder spasm. Other measures include minimising an increase in abdominal pressure, the use of laxatives, minimal pelvic examination, and pelvic rest (by avoiding using tampons, or coitus) for 6 weeks.
Complications
Complications include frequency, urgency, urge incontinence, recurrence, stress urinary incontinence, ureteric obstruction, and bowel obstruction. Failed repairs require a complete bladder, ureter and kidney evaluation before planning subsequent surgery. The repair often fails in the form of a ‘pinpoint fistula’ seen at the lateral corners of the previous repair. Adequate exposure and lighting, and a good measure of patience, are useful when re-evaluating patients with continued leaks after a repair.
Infection should be promptly addressed and treated. Repeated repairs will often require the use of soft-tissue grafts. Stenting or percutaneous drainage can be used to allow further healing, or for drainage of a urinoma or abscess.
Conclusion
A thorough evaluation of a VVF is of utmost importance when planning a repair. Although the surgeon’s experience influences the surgical approach in most cases, some special situations dictate the best approach, as discussed above. The treatment of genitourinary fistula with robotic assistance continues to develop, but further research is necessary to fully understand the use of this technology in treating these disorders [58].
Conflict of interest
None.
Source of funding
None.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Ghoniem G.M., Khater U.M. Vesicovaginal fistula. Springer-Verlag; London: 2006. (Pelvic floor dysfunction). [Google Scholar]
- 2.Riley V.J. Vesicovaginal fistula. EMed WebMD. 2006 June 25. [Google Scholar]
- 3.Schlunt Eilber K., Kavalier E., Rodriguez L., Rosenblum N., Raz S. Ten-year experience with transvaginal vesicovaginal fistula repair using tissue interposition. J Urol. 2003;169:1033–1036. doi: 10.1097/01.ju.0000049723.57485.e7. [DOI] [PubMed] [Google Scholar]
- 4.Cohn J.N. Blood pressure measurement in shock. Mechanism of inaccuracy in ausculatory and palpatory methods. JAMA. 1967;199:118–122. doi: 10.1001/jama.199.13.118. [DOI] [PubMed] [Google Scholar]
- 5.Symmonds R.E. Ureteral injuries associated with gynecologic surgery. Prevention and management. Clin Obstet Gynecol. 1976;19:623–644. doi: 10.1097/00003081-197609000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Zoubek J., McGuire E.J., Noll F., DeLancey J.O. The late occurrence of urinary tract damage in patients successfully treated by radiotherapy for cervical carcinoma. J Urol. 1989;141:1347–1349. doi: 10.1016/s0022-5347(17)41303-6. [DOI] [PubMed] [Google Scholar]
- 7.Tancer M.L. Observations on prevention and management of vesicovaginal fistula. J Urol. 1980;123:839–840. doi: 10.1016/s0022-5347(17)56155-8. [DOI] [PubMed] [Google Scholar]
- 8.McKay H.A., Hanlon K. Vesicovaginal fistula after cervical cerclage. Repair by transurethral suture cystorrhaphy. J Urol. 2003;169:1086–1087. doi: 10.1097/01.ju.0000047516.32699.aa. [DOI] [PubMed] [Google Scholar]
- 9.Harkki-Siren P., Sjoberg J., Tiitinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113–118. doi: 10.1016/s0029-7844(98)00146-x. [DOI] [PubMed] [Google Scholar]
- 10.Waaldijk K. The surgical management of bladder fistula in 775 women in Northern Nigeria. Nymegen: Benda BV; 1989.
- 11.Wall L.L., Karshima J.A., Kirschner C., Arrowsmith S.D. The obstetric vesicovaginal fistula. Characteristics of 899 patients from Jos, Nigeria. Am J Obstet Gynecol. 2004;190:1011–1019. doi: 10.1016/j.ajog.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Chibber P.J., Navinchandra Shah H., Jain P. Laparoscopic O’Conor’s repair for vesico-vaginal and vesico-uterine fistulae. BJU Int. 2005;96:183–186. doi: 10.1111/j.1464-410X.2005.05592.x. [DOI] [PubMed] [Google Scholar]
- 13.Waaldijk K. Surgical classification of obstetric fistulas. Int J Gynaecol Obstet. 1995;49:161–163. doi: 10.1016/0020-7292(95)02350-l. [DOI] [PubMed] [Google Scholar]
- 14.Moir J.C. Personal experiences in the treatment of vesicovaginal fistulas. Am J Obstet Gynecol. 1956;71:476–491. doi: 10.1016/0002-9378(56)90476-8. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin W.E., Scardino P.T. Vesicovaginal and ureterovaginal fistulas: a summary of 25 years experience. J Urol. 1980;123:370–374. doi: 10.1016/s0022-5347(17)55941-8. [DOI] [PubMed] [Google Scholar]
- 16.Andreoni C., Bruschini H., Truzzi J.C., Simonetti R., Srougi M. Combined vaginoscopy-cystoscopy. A novel simultaneous approach improving vesicovaginal fistula evaluation. J Urol. 2003;170:2330–2332. doi: 10.1097/01.ju.0000096343.03276.75. [DOI] [PubMed] [Google Scholar]
- 17.Tancer M.L. Observations on prevention and management of vesicovaginal fistula after total hysterectomy. Surg Gynecol Obstet. 1992;175:501–506. [PubMed] [Google Scholar]
- 18.Kursh E.D. Etiology, evaluation, and endoscopic management of vesicovaginal fistulas. In: Kursh E.D., McGuire E.J., editors. Female urology. J.B. Lippincott; Philadelphia: 1994. p. 359. [Google Scholar]
- 19.Latzko W. Postoperative vesicovaginal fistulas. Genesis and therapy. Am J Surg. 1992;48:211. [Google Scholar]
- 20.Kanaoka Y., Hurai K., Ishiko O., Ogita S. Vesicovaginal fistula treated with fibrin glue. Int J Gynecol Obstet. 2001;73:147–149. doi: 10.1016/s0020-7292(00)00381-7. [DOI] [PubMed] [Google Scholar]
- 21.Evans L.A., Ferguson K.H., Foley J.P., Rozanski T.A., Morey A.F. Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications. J Urol. 2003;169:1360–1362. doi: 10.1097/01.ju.0000052663.84060.ea. [DOI] [PubMed] [Google Scholar]
- 22.Witters S., Cornelissen M., Vereecken R. Iatrogenic ureteral injury: aggressive or conservative treatment. Am J Obstet Gynecol. 1986;155:582–584. doi: 10.1016/0002-9378(86)90283-8. [DOI] [PubMed] [Google Scholar]
- 23.Collins C.G., Pent D., Jones F.B. Results of early repair of vesicovaginal fistula with preliminary cortisone treatment. Am J Obstet Gynecol. 1960;80:1005–1012. doi: 10.1016/0002-9378(60)90480-4. [DOI] [PubMed] [Google Scholar]
- 24.Waaldijk K. The immediate surgical management of fresh obstetric fistulas with catheter and/or early closure. Int J Gynaecol Obstet. 1994;45:11–16. doi: 10.1016/0020-7292(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 25.Zimmern P.E., Hadley H.R., Staskin D.R., Raz S. Genitourinary fistulae. Vaginal approach for repair of vesico-vaginal fistulae. Urol Clin North Am. 1985;12:361–367. [PubMed] [Google Scholar]
- 26.Falk H.C., Orkin L.A. Nonsurgical closure of vesicovaginal fistulas. Obstet Gynecol. 1957;9:538–541. [PubMed] [Google Scholar]
- 27.Waaldijk K. The immediate management of fresh obstetric fistulas. Am J Obstet Gynecol. 2004;191:795–799. doi: 10.1016/j.ajog.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Ghoniem GM. Transvaginal repair of recurrent vesicovaginal fistula utilizing suburethral sling and Martius grafts. Video-Urology Times 1992: 5. Program 4.
- 29.Sundaram B.M., Kalidasan G., Hemal A. Robotic repair of vesicovaginal fistula: case series of five patients. Urology. 2005;11:970–973. doi: 10.1016/j.urology.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Miklos J.R., Sobolewski C., Lucente V. Laparoscopic management of recurrent vesicovaginal fistula. Int Urogynecol J. 1999;10:116–117. doi: 10.1007/s001920050029. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Karim A.M., Mousa A., Hasouna M., Elsalmy S. Laparoscopic transperitoneal extravesical repair of vesicovaginal fistule. Int Urogynecol J. 2011;22:693–697. doi: 10.1007/s00192-010-1334-7. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Karim A.M., Mousa A., Elsalmy S. Laparoendoscopic single-site surgery extravesical repair of vesicovaginal fistula: early experience. Urology. 2011;78:567–571. doi: 10.1016/j.urology.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Gozen A.S., Teber D., Canda A.E., Rassweiler J. Transperitoneal laparoscopic repair of iatrogenic vesicovaginal fistulas: heilbronn experience and review of the literature. J Endourol. 2009;23:475–479. doi: 10.1089/end.2008.0236. [DOI] [PubMed] [Google Scholar]
- 34.Lee J.H., Choi J.S., Lee K.W., Han J.S., Choi P.C., Hoh J.K. Immediate laparoscopic nontransvesical repair without omental interposition for vesicovaginal fistula developing after total abdominal hysterectomy. J Soc Laparoendosc Surg. 2010;14:187–191. doi: 10.4293/108680810X12785289143918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizvi S.J., Gupta R., Patel S., Trivedi A., Trivedi P., Modi P. Modified laparoscopic abdominal vesico-vaginal fistula repair; ‘Mini-O’Conor’ vesicotomy. J Laparoendosc Adv Surg Technol. 2010;20:13–15. doi: 10.1089/lap.2009.0176. [DOI] [PubMed] [Google Scholar]
- 36.Melamud O., Eicheel B., Turbow B., Shanberg A. Laparoscopic vesicovaginal fistula repair with robotic reconstruction. Urology. 2005;65:163–166. doi: 10.1016/j.urology.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 37.Shimpf M.O., Morgenstern J.H., Tulikangas P.K., Wagner J.R. Vesicovaginal fistula repair without intentional cystotomy using laparoscopic robotic approach: a case report. JSLS. 2007;11:378–380. [PMC free article] [PubMed] [Google Scholar]
- 38.Hemal A.K., Shrma N., Mukherjee S. Robotic repair of complex vesicouterine fistula with and without hysterectomy. Urol Int. 2009;82:411–415. doi: 10.1159/000218529. [DOI] [PubMed] [Google Scholar]
- 39.Kurz M., Horstmann M., John H. Robot-assisted laparoscopic repair of high vesicovaginal fistulae with peritoneal flap inlay. Eur Urol. 2012;61:229–230. doi: 10.1016/j.eururo.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Gupta N.P., Mishra S., Hemal A.K., Mishra A., Seth A., Dogra P.N. Comparative analysis of outcome between open and robotic surgical repair of recurrent supra-trigonal vesico-vaginal fistula. J Endourol. 2010;24:1779–1782. doi: 10.1089/end.2010.0049. [DOI] [PubMed] [Google Scholar]
- 41.Shah S.J. Laparoscopic transabdominal transvesical vesicovaginal fistula repair. J Endourol. 2009;23:1135–1137. doi: 10.1089/end.2009.0080. [DOI] [PubMed] [Google Scholar]
- 42.Tabakov I.D., Slavchev B.N. Large post-hysterectomy and post-radiation vesicovaginal fistulas: repair by ileocystoplasty. J Urol. 2004;171:272–274. doi: 10.1097/01.ju.0000101801.95459.54. [DOI] [PubMed] [Google Scholar]
- 43.Hassim A.M., Lucas C. Reduction in the incidence of stress incontinence complicating fistula repair. Br J Surg. 1974;61:461–465. doi: 10.1002/bjs.1800610612. [DOI] [PubMed] [Google Scholar]
- 44.Schleicher D., Ojengbede O.H.A., Elkins T.E. Urodynamic evaluation of patients after successful closure of vesicovaginal fistulas. Int Urogynecol J. 1993;4:262–265. [Google Scholar]
- 45.Margolis T., Elkins T.E., Seffah J., Oparo-Addo H.S., Fort D. Full-thickness Martius grafts to preserve vaginal depth as an adjunct in the repair of large obstetric fistulas. Obstet Gynecol. 1994;84:148–152. [PubMed] [Google Scholar]
- 46.Kiricuta I., Goldstein A.M. The repair of extensive vesicovaginal fistulas with pedicled omentum: a review of 27 cases. J Urol. 1972;108:724–727. doi: 10.1016/s0022-5347(17)60851-6. [DOI] [PubMed] [Google Scholar]
- 47.Hamlin R.H., Nicholson E.C. Reconstruction of urethra totally destroyed in labour. Br Med J. 1969;2:147–150. doi: 10.1136/bmj.2.5650.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symmonds R.E., Hill L.M. Loss of the urethra: a report on 50 patients. Am J Obstet Gynecol. 1978;130:130–138. doi: 10.1016/0002-9378(78)90354-x. [DOI] [PubMed] [Google Scholar]
- 49.Tanagho E.A. Bladder neck reconstruction for total urinary incontinence: 10 years experience. J Urol. 1981;125:321–326. doi: 10.1016/s0022-5347(17)55024-7. [DOI] [PubMed] [Google Scholar]
- 50.Elkins T.E., Ghosh T.S., Tagoe G.A., Stocker R. Transvaginal mobilization and utilization of the anterior bladder wall to repair vesicovaginal fistulas involving the urethra. Obstet Gynecol. 1992;79:455–460. doi: 10.1097/00006250-199203000-00026. [DOI] [PubMed] [Google Scholar]
- 51.Hoch W.H., Kursh E.D., Persky L. Early, aggressive management of intraoperative ureteral injuries. J Urol. 1975;114:530–532. doi: 10.1016/s0022-5347(17)67075-7. [DOI] [PubMed] [Google Scholar]
- 52.O’Quinn AG, Degefu S, Batson HK, et al. Early repair of vesicovaginal fistula following preliminary corticosteroid. Presented at the society of pelvic surgeons, New Orleans, November 1984.
- 53.Shao-Chun R., Chang Jackson M.D., Uchenna C., Acholonu M.D., Jr., Farr R., Nezhat M.D. Robotic-assisted laparoscopic repair of a vesicouterine fistula. JSLS. 2011;15:339–342. doi: 10.4293/108680811X13071180407438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perveen K., Gupta R., Al-Badr A., Hemal A.K. Robot-assisted laparoscopic repair of rare post-cesarean section vesicocervical and vesicouterine fistula: a case series of a novel technique. Urology. 2012;80:477–482. doi: 10.1016/j.urology.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Tarhan F., Erbay E., Penbegül N., Kuyumcuoglu U. Minimal invasive treatment of vesicouterine fistula: a case report. Int Urol Nephrol. 2007;39:791–793. doi: 10.1007/s11255-006-9076-7. [DOI] [PubMed] [Google Scholar]
- 56.Laungani R., Patil N. Robotic-assisted uteterovaginal fistula repair. Report of efficacy and feasibility. J Laparoendosc Adv Surg Tech A. 2008;18:731–734. doi: 10.1089/lap.2008.0037. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S., Barapatre Y.R., Ganesamoni R., Nanjappa B., Barwal K., Singh S.K. Laparoscopic management of a rare urogenital fistula. J Endourol. 2011;25:603–606. doi: 10.1089/end.2010.0179. [DOI] [PubMed] [Google Scholar]
- 58.Swan K., Advincula A.P. Advances in urogynaecological robotic surgery. BJU Int. 2011;108:1024–1027. doi: 10.1111/j.1464-410X.2011.10557.x. [DOI] [PubMed] [Google Scholar]



