Abstract
Study Objectives:
To test the hypothesis that enlarged Virchow-Robin space volumes (VRS) are associated with objective measures of poor quality sleep.
Design:
Retrospective cross-sectional study.
Setting:
Sunnybrook Health Sciences Centre.
Patients:
Twenty-six patients being evaluated for cerebrovascular disease were assessed using polysomnography and high-resolution structural magnetic resonance imaging.
Measurements and Results:
Regionalized VRS were quantified from three-dimensional high-resolution magnetic resonance imaging and correlated with measures of polysomnography-derived sleep parameters while controlling for age, stroke volume, body mass index, systolic blood pressure, and ventricular cerebrospinal fluid volume. Sleep efficiency was negatively correlated with total VRS (rho = −0.47, P = 0.03) and basal ganglia VRS (rho = −0.54, P = 0.01), whereas wake after sleep onset was positively correlated with basal ganglia VRS (rho = 0.52, P = 0.02). Furthermore, VRS in the basal ganglia were negatively correlated with duration of N3 (rho = −0.53, P = 0.01).
Conclusions:
These preliminary results suggest that sleep may play a role in perivascular clearance in ischemic brain disease, and invite future research into the potential relevance of Virchow-Robin spaces as an imaging biomarker for nocturnal metabolite clearance.
Citation:
Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI. Virchow-Robin spaces: correlations with polysomnography-derived sleep parameters. SLEEP 2015;38(6):853–858.
Keywords: basal ganglia, metabolite clearance, MRI, perivascular space, polysomnography, sleep, small vessel disease, stroke, Virchow-Robin, white matter
INTRODUCTION
Virchow-Robin perivascular spaces (VRS) are interstitial fluid-filled channels that surround the brain's smaller arteries and veins. Since the brain lacks the lymphatic vessels found in peripheral tissue, these perivascular spaces act as a protolymphatic system and play a role in interstitial clearance.1 Enlarged VRS, visible on magnetic resonance imaging (MRI),2 are associated with age, hypertension,3,4 white matter hyper-intensities, lacunar stroke subtype,5,6 dementia, and cognitive dysfunction.7–10
Additionally, some studies suggest that the anatomical distribution of VRS may indicate different pathological mechanisms; those found in the basal ganglia may be a marker for hypertensive arteriopathy, whereas those found in the white matter may reflect cerebral amyloid angiopathy.6–8,11 Although there is no consensus on the exact mechanisms involved, VRS may be related to blockage of interstitial fluid (ISF) along these channels.12 Recent findings indicate that ISF clearance in the mouse brain occurs primarily during sleep, suggesting a homeostatic function of sleep through the removal of waste generated from neuronal metabolism, possibly occurring primarily during slow wave sleep.13
The relationship between VRS and sleep has not been previously studied in humans. In the current study, using in vivo MRI obtained from a sample of patients being evaluated for cerebrovascular disease, we examined the relationships between VRS volumes in the basal ganglia and white matter, and various polysomnography-derived sleep measures. We postulated that poor quality sleep may disrupt the removal of neurotoxins, interrupt the drainage of ISF, and possibly result in enlargement of the perivascular space. Specifically, we tested the hypothesis that increased VRS volumes would be correlated with markers of poor sleep quality.
METHODS
Study Sample
We retrospectively assessed all patients being evaluated for cerebrovascular disease who underwent high-resolution MRI and polysomnography from November 2011 to April 2014 at our institution. All patients provided informed consent for retrospective analyses of their data. These patients were recruited from the Sunnybrook Stroke Prevention Clinic or inpatient ward (n = 28). All patients included in our analysis underwent evaluation by a stroke neurologist. Transient ischemic attack (TIA) was diagnosed in seven, acute stroke in four, subacute/ chronic stroke in four, other cerebrovascular diagnoses in six: retinal artery occlusion (n = 2), central nervous system vasculitis (n = 2), subarachnoid hemorrhage (n = 1), and carotid dissection not associated with stroke (n = 1). Migraine was diagnosed in one patient, and non-neurological events were suspected in the remaining three patients. Each patient underwent a brain MRI and overnight polysomnography. One patient was removed due to motion artefact and another was identified as a univariate outlier for VRS volumes in the white matter and as a bivariate outlier for white matter VRS and various sleep measures; these patients were subsequently removed from the analysis, leaving a total sample of n = 26 (Table 1).
Table 1.
Demographics, volumetric, and polysomnography data.

MRI Protocol and Assessment
All imaging was acquired on a 3 Tesla Philips Achieva (Holland) at Sunnybrook Health Sciences Centre, Toronto, Canada. Imaging sequences included: a three-dimensional sagittal T1-weighted (echo time [TE] = 2.3 ms, repetition time [TR] = 9.5 ms, number of excitations [NEX] = 1, flip angle = 8°, acquisition matrix = 240 × 240 mm, in-plane voxel size = 1 × 1 mm [interpolated in scanner to 0.5 × 0.5mm], slice thickness = 1 mm) and an axial T2-weighted MRI (TE = 16ms, TR = 760 ms, NEX = 1, flip angle = 18°, acquisition matrix = 256 × 256 mm, percent phase field of view = 0.8, in-plane voxel size = 0.9 × 0.9 [interpolated in scanner to 0.45 × 0.45mm], slice thickness = 6 mm).
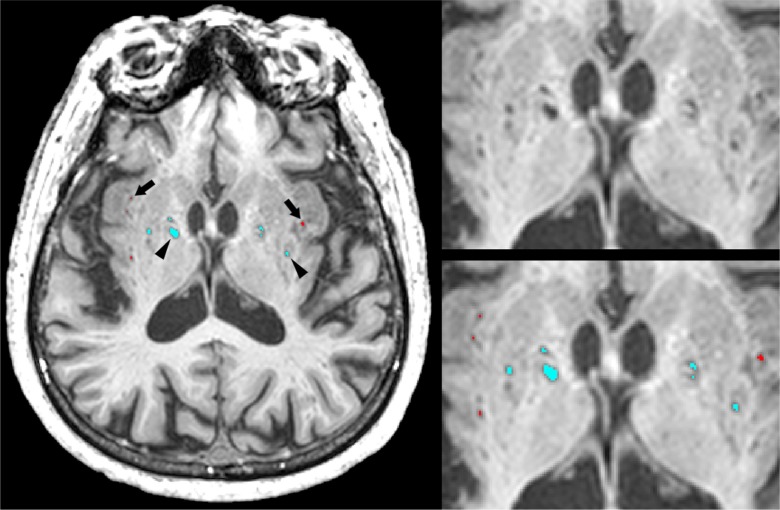
VRS volumetrics were generated from a modified version of a previously published in-house neuroimaging pipeline that was validated against two commonly used VRS visual rating scales: the Patankar VRS9 and Wardlaw EPVS (http://www.bric.ed.ac.uk/documents/epvs-rating-scale-user-guide.pdf) rating scales.7,14,15 VRS were defined using the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria.2 In brief, cerebrospinal fluid (CSF) intense VRS voxels on T1 were manually relabelled into white matter VRS (WMVRS) and basal ganglia VRS (BG-VRS) (Figure 1). For this study, BG-VRS were defined as those located within the region limited by the lateral edge of the lentiform nucleus, the posterior edge of the thalamus, and the anterior edge of the caudate nucleus. In contrast, WM-VRS were those located within the remaining supratentorial cerebral white matter. Stroke volumes were manually traced using T1-based tissue segmentation and all tracings were confirmed by a research neuroradiologist.
Figure 1.
Enlarged Virchow-Robin space (VRS) segmentation with white matter VRS in red (arrows) and basal ganglia VRS in blue (arrowheads) overlaid on an axial T1-weighted MRI.
Polysomnography
Level 1, technologist-monitored, in-hospital polysomnography (Compumedics Neuroscan, Australia), using standard recording and scoring methods,16 was obtained an average of 102 days from imaging, blinded to imaging results. Sleep was manually staged according to criteria from the American Academy of Sleep Medicine.16 All studies were interpreted by a diplomate of the American Board of Sleep Medicine and scored by a registered polysomnographic technologist. Using current scoring rules,16 sleep efficiency (SE) was defined as the total sleep time divided by the total time in bed (i.e. “lights out” to “lights on”) multiplied by 100. The apneahypopnea index (AHI) was defined as the total number of apneas and hypopneas per hour of sleep. Wake after sleep onset (WASO) was defined as time spent awake after the first sleep episode and before final awakening. We also assessed the absolute duration of time spent in each sleep stage (nonrapid eye movement sleep— N1, N2, N3, and rapid eye movement sleep [REM]).
Statistical Analysis
Data were tested for outliers using z-scores (univariate) and the Mahalanobis test (bivariate). Due to the non-normal distribution of the data, a nonparametric partial Spearman's rank-order correlation was used. Total VRS, WM-VRS, and BG-VRS volumes were correlated with SE, AHI, WASO, and duration of N1, N2, N3, and REM sleep, respectively. Age, stroke volume, body mass index (BMI), systolic blood pressure, and ventricular cerebrospinal fluid (vCSF) volumes were entered as covariates in the analysis because of their potential influence on both sleep and VRS.5,6,17–20 Finally, WM-VRS volumes were compared with BG-VRS volumes using a Wilcoxon signed rank test. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 20.0 IBM Corp., Armonk, NY). Due to the exploratory nature of this study, a statistical threshold of P < 0.05 was used (uncorrected for multiple comparisons).
RESULTS
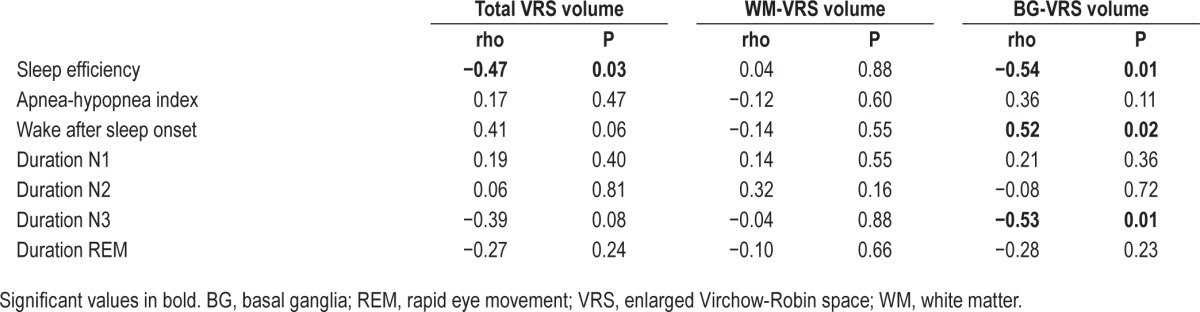
Demographics and basic volumetrics are summarized in Table 1. Correlation results are summarized in Tables 2 and 3. One patient was identified as a univariate outlier for WM-VRS volume (z-score = 4.48, P < 0.001) and as a bivariate outlier for SE and WM-VRS (Mahalanobis distance = 20.57, P < 0.001) and WASO and WM-VRS (Mahalanobis distance = 20.31, P < 0.001). Additionally, this patient was identified as a bivariate outlier for each sleep stage and was subsequently removed from further analyses. After accounting for age, stroke volume, BMI, systolic blood pressure, and vCSF volume, SE was negatively correlated with total VRS (rho = −0.47, P = 0.03) and BG-VRS (rho = −0.54, P = 0.01). Furthermore, WASO was positively correlated with BG-VRS (rho = 0.52, P = 0.02). In addition, duration of N3 was negatively correlated with BG-VRS (rho = −0.53, P = 0.01). Correlations between VRS volumes and AHI, duration N1, duration N2, and duration REM were not significant. Finally, a Wilcoxon signed rank test revealed significantly higher BG-VRS volumes compared to WM-VRS volumes (Z = −3.11, P < 0.05).
Table 2.
Partial Spearman's rank order correlation results unadjusted for covariates.
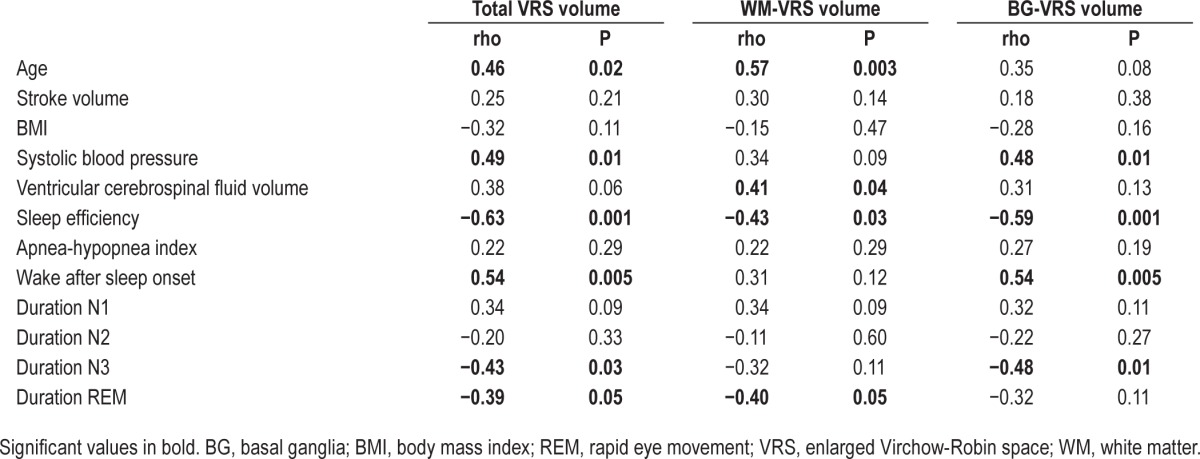
Table 3.
Partial Spearman's rank order correlation results controlling for age, stroke volume, body mass index, systolic blood pressure, and ventricular cerebrospinal volume.
DISCUSSION
Perivascular spaces are thought to play an important role in maintaining neural homeostasis in an environment with high metabolic activity, yet lacking a true lymphatic system.1 Based on in vivo tracer studies, it is believed that the brain's ‘protolymphatic’ system begins with the production of CSF in the choroid plexus, which then travels into the subarachnoid space. From the subarachnoid space, the sulcal CSF is either cleared through the arachnoid granulations or it enters the parenchyma via the perivascular space, where it combines with interstitial fluid prior to exiting the brain.12,21 Although there is no consensus regarding the directionality of drainage along these channels, some studies suggest that perivascular drainage follows the normal direction of blood flow beginning with the penetrating arteries and arterioles to reach the terminal capillary beds and exiting along the paravenous routes,21 whereas other studies suggest that drainage occurs in the reverse direction to the flow of blood.12,22 This perivascular drainage system may allow for the clearance of toxic metabolites within the parenchyma and possibly plays a role in the brain's immunological response.12,23
Although it is important to note that the VRS visible on MRI are not necessarily pathological, they have been shown to be associated with age-related disorders and vascular risk factors.2–10 Furthermore, they are thought to be the result of perivascular blockage, which can be exacerbated by beta-amyloid (Aβ) deposition around blood vessels24 and/or hardening of the artery wall resulting in decreased arterial pulsatility.12 Venous collagenosis may also contribute to poor drainage along the perivascular space.25 Furthermore, WM-VRS and BG-VRS are believed to reflect differential pathological mechanisms. Specifically, WM-VRS may reflect cerebral amyloid angiopathy, whereas BG-VRS may indicate hypertensive arteriopathy.11 In elderly and Alzheimer's disease patient samples, WM-VRS volumes were found to be greater than BG-VRS.7 Conversely, in the current study, which examined a younger vascular patient sample, the opposite was found (i.e., BGVRS > WM-VRS). The preponderance of VRS to be distributed mainly in the basal ganglia is likely indicative of a more vascular sample, compared to a higher distribution in the white matter, which is more likely indicative of higher cerebral amyloid angiopathy in an elderly and/or Alzheimer's disease patient sample. Findings from a recent study of stroke and TIA patients suggest that VRS in the basal ganglia are specifically related to white matter hyperintensities, hypertension, and lacunar strokes.6 These studies may explain the larger BG-VRS volumes relative to WM-VRS in our stroke clinic sample.
One explanation for these differences in the regional distribution of VRS may be related to the anatomy of the perivascular space. Postmortem work using electron microscopy showed distinct differences in the structural anatomy of the perivascular space in the basal ganglia relative to those in the cortex.26 More specifically, in the basal ganglia, arterial perivascular spaces are surrounded by an inner and an outer coating of leptomeninges, whereas those found in the cortex have only a single outer coating. It was speculated that this anatomical variability may account for differences in clearance efficiency along these drainage pathways.
Using real-time two-photon microscopy, a recent study examined the clearance rates of exogenous (tetramethylammonium) and endogenous (Aβ) tracers in awake, asleep, and anesthetized mice.13 This study found that the rate of clearance in mice was greatest during sleep. When mice were awake, tracer influx into the perivascular space was decreased by 95% relative to natural and anesthesia-induced sleep. Similarly, Aβ was also cleared twice as quickly in the sleeping mice. These results were explained by a 60% increase in interstitial space volume fraction during sleep, indicating decreased tissue resistance, allowing for greater fluid influx/efflux. This sleep-derived increase in interstitial space volume may be modulated by changes in sleep related neurotransmitters, simultaneously modulating sleep onset and metabolite clearance. Although some may argue that the cerebral protolymphatic drainage system is fundamentally different between humans and non-human mammals,12 our preliminary results lend support to the hypothesis that sleep may play a role in metabolite clearance in the human brain, possibly along the perivascular channels.
In our study, we found that VRS were negatively correlated with SE, and duration of N3 sleep, and positively correlated with WASO, particularly in the basal ganglia. A normal SE is approximately 85% or higher.16 Greater VRS volumes were correlated with lower SE scores, suggesting that a lower percentage of time spent asleep may be associated with inefficient perivascular drainage, potentially leading to enlargement of the perivascular space.
In keeping with this, the WASO measure is a temporal measure accounting for the number of minutes spent awake after sleep has been initiated, indicating trouble staying asleep. Our study demonstrated a positive correlation with BG-VRS and WASO, as further support for the notion that VRS may be a marker for suboptimal perivascular clearance during poor quality sleep.
However, a caveat of the SE and WASO measures is that they are general metrics that do not account for sleep stage distribution. In order to address this limitation, we further examined whether VRS volumes were related to sleep stage duration. Our study found a significant negative correlation with BG-VRS and duration of time spent in N3, a relationship that was not significant for N1, N2, or REM sleep. These results may provide additional support for the theory that ISF clearance occurs primarily during slow wave sleep. This is consistent with the study by Xie and colleagues,13 who demonstrated increased tracer clearance during slow wave sleep in mice.
Although the main strength of the current study is its use of volumetric quantification of VRS, one limitation to this technique is the requirement of a high resolution three-dimensional T1-weighted MRI, which was only available in a limited number of patients with polysomnography. Also, this was a retrospective analysis; future prospective studies examining the relationship between sleep and VRS would ideally include multispectral MRI, polysomnography, and a larger sample of patients with a control group for comparison. Additionally, polysomnography measures obtained in a hospital setting may not be a true representation of an individual's normal sleep pattern. Future technological advances may allow for these measurements to be acquired at home in a more natural setting. Furthermore, as there is no noninvasive, reliable way to discriminate between arteries and veins in vivo, future work using arterial spin labeling, susceptibility weighted imaging, and examinations from postmortem neuropathological analyses using alkaline phosphatase (arterioles and capillaries) and trichrome staining (venules),25 may provide additional insight into the pathogenesis of VRS.
Despite these limitations, our study provides preliminary evidence to support the notion that sleep may play an important role in protolymphatic clearance along the perivascular Virchow-Robin channels in humans. VRS may be a useful imaging biomarker for inefficient protolymphatic clearance, particularly those found in the basal ganglia of stroke/TIA patients with poor sleep quality. Furthermore, cerebral clearance of Aβ in Alzheimer's disease27 could potentially be mediated by sleep deprivation or prolonged wakefulness,28 and diurnal patterns.29 Future examinations with the inclusion of different patient populations may provide a better understanding of how the brain clears potentially neurotoxic metabolites and its relationship with the restorative effects of sleep.
DISCLOSURE STATEMENT
The authors gratefully acknowledge financial and salary support (Courtney Berezuk, Dr. Ramirez, Christopher Scott, Dr. Gao) from the Canadian Institutes of Health Research (MT#13129), the Linda C. Campbell Foundation, Heart & Stroke Foundation Canadian Partnership for Stroke Recovery. Additionally, we would like to graciously thank the Sunny-brook Health Sciences Centre, Sunnybrook Research Institute, Brill Chair Neurology, and the University of Toronto for financial and salary support (Dr. Black). Dr. Swartz is supported by a Heart and Stroke Foundation of Canada New Investigator Award and Henry Barnett Award and receives operating grant funding (unrelated to this project) from the Canadian Institutes of Health Research. During the study, Dr. Boulos was supported by a Focus on Stroke 2010 Research Fellowship, which was funded by the Heart and Stroke Foundation of Canada, the Canadian Stroke Network and the Canadian Institutes of Health Research; Dr. Boulos also received fellowship support from the Canadian Partnership for Stroke Recovery. Primary Institution: Sunnybrook Health Sciences Centre. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- Aβ
beta-amyloid
- AHI
apnea-hypopnea index
- BG-VRS
basal ganglia enlarged Virchow-Robin space
- BMI
body mass index
- CSF
cerebrospinal fluid
- ISF
interstitial fluid
- MRI
magnetic resonance imaging
- N1
nonrapid eye movement stage 1
- N2
nonrapid eye movement stage 2
- N3
nonrapid eye movement stage 3
- REM
rapid eye movement
- SE
sleep efficiency
- vCSF
ventricular cerebrospinal fluid
- VRS
enlarged Virchow-Robin space
- WASO
wake after sleep onset
- WM-VRS
white matter enlarged Virchow-Robin space
Footnotes
A commentary on this article appears in this issue on page 845.
REFERENCES
- 1.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–52. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–90. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 4.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255:692–6. doi: 10.1007/s00415-008-0777-y. [DOI] [PubMed] [Google Scholar]
- 5.Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–81. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry. 2014;85:522–5. doi: 10.1136/jnnp-2013-305815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 2015;43:415–24. doi: 10.3233/JAD-132528. [DOI] [PubMed] [Google Scholar]
- 8.Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med. 2003;9:112–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–20. [PMC free article] [PubMed] [Google Scholar]
- 10.MacLullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–23. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013;84:624–9. doi: 10.1136/jnnp-2012-304434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 13.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez J, Gibson E, Quddus A, et al. Lesion Explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963–73. doi: 10.1016/j.neuroimage.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez J, Scott CJ, McNeely AA, et al. Lesion Explorer: a video-guided, standardized protocol for accurate and reliable MRI-derived volumetrics in Alzheimer's disease and normal elderly. J Vis Exp. 2014 Apr 14;(86) doi: 10.3791/50887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 17.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YC, Dufouil C, Mazoyer B, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011;32:709–13. doi: 10.3174/ajnr.A2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palomaki H, Berg A, Meririnne E, et al. Complaints of poststroke insomnia and its treatment with mianserin. Cerebrovasc Dis. 2003;15:56–62. doi: 10.1159/000067127. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg JF, Knvistingh NA, Tulen JH, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32:1083–90. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 21.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238:962–74. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 25.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 26.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191:337–46. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep. 2013;36:1027–32. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–7. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 29.Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging. 2014;35:S29–34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]