Abstract
Objectives:
There is limited research on racial/ethnic variation in sleep disturbances. This study aimed to quantify the distributions of objectively measured sleep disordered breathing (SDB), short sleep duration, poor sleep quality, and self-reported sleep disturbances (e.g., insomnia) across racial/ethnic groups.
Design:
Cross-sectional study.
Setting:
Six US communities.
Participants:
Racially/ethnically diverse men and women aged 54–93 y in the Multi-Ethnic Study of Atherosclerosis Sleep Cohort (n = 2,230).
Interventions:
N/A.
Measurements and Results:
Information from polysomnography-measured SDB, actigraphy-measured sleep duration and quality, and self-reported daytime sleepiness were obtained between 2010 and 2013. Overall, 15.0% of individuals had severe SDB (apnea-hypopnea index [AHI] ≥ 30); 30.9% short sleep duration (< 6 h); 6.5% poor sleep quality (sleep efficiency < 85%); and 13.9% had daytime sleepiness. Compared with Whites, Blacks had higher odds of sleep apnea syndrome (AHI ≥ 5 plus sleepiness) (sex-, age-, and study site-adjusted odds ratio [OR] = 1.78, 95% confidence interval [CI]: 1.20, 2.63), short sleep (OR = 4.95, 95% CI: 3.56, 6.90), poor sleep quality (OR = 1.57, 95% CI: 1.00, 2.48), and daytime sleepiness (OR = 1.89, 95% CI: 1.38, 2.60). Hispanics and Chinese had higher odds of SDB and short sleep than Whites. Among non-obese individuals, Chinese had the highest odds of SDB compared to Whites. Only 7.4% to 16.2% of individuals with an AHI ≥ 15 reported a prior diagnosis of sleep apnea.
Conclusions:
Sleep disturbances are prevalent among middle-aged and older adults, and vary by race/ethnicity, sex, and obesity status. The high prevalence of sleep disturbances and undiagnosed sleep apnea among racial/ethnic minorities may contribute to health disparities.
Citation:
Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). SLEEP 2015;38(6):877–888.
Keywords: apnea-hypopnea index, body mass index, daytime sleepiness, obesity, polysomnography, race/ethnicity, sleep disordered breathing, sleep disturbance, sleep duration, sleep quality
INTRODUCTION
The prevalence of sleep disordered breathing (SDB), short sleep duration, and insomnia has increased substantially over the past two decades.1,2 Epidemiologic studies have linked sleep disturbances to adverse health outcomes including obesity,3 hypertension,3 cardiovascular disease (CVD),3,4 premature mortality,5,6 impaired quality of life,7 and economic burden.8 An understanding of the distributions of sleep disturbances across sociodemographic groups may inform public health efforts aimed at reducing health and economic burdens of sleep disturbances.9
Previous research has indicated that SDB and other sleep disturbances are common among men and older individuals, and may vary across racial/ethnic groups.2,9–14 Few population-based studies, however, have quantified differences in sleep disturbances across multiple racial/ethnic groups, and even fewer have included Asian Americans.13 Prior epidemiologic studies have often relied on self-reported sleep information,9,10,12,15 which have limitations in accuracy.16 Polysomnography (PSG) is the gold-standard method for the assessment of SDB.17 Wrist actigraphy is a valid and reliable method for assessing sleep duration and quality in individuals' home environment.16,18 Few population-based studies have objectively measured sleep disturbances with both PSG and actigraphy, and those that have, had limited racial/ethnic diversity.14,19,20
This study sought to quantify the distributions of objectively measured SDB, short sleep duration, poor sleep quality, and self-reported sleep disturbances (e.g., insomnia) across racial/ ethnic groups represented in the Multi-Ethnic Study of Atherosclerosis (MESA). Because obesity is frequently co-morbid with sleep disturbances,14,21 we also examined the extent to which racial/ethnic differences in sleep disturbances were explained by differences in body mass index (BMI).
METHODS
Study Design and Sample
The study design for MESA has been published.22 Briefly, MESA is a multisite prospective study designed to investigate the prevalence and progression of subclinical CVD and to identify risk factors for incident CVD in a racially/ethnically diverse sample. Between July 2000 and August 2002, a total of 6,814 men and women who identified themselves as White, Black/African American, Hispanic, or Chinese aged 45–84 y and free of clinically apparent CVD were recruited from six US communities: Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN.
At MESA Exam 5 (2010–2013), 10 y after the initial examination, all MESA participants other than those reporting regular use of oral devices, nocturnal oxygen, or nightly positive airway pressure (PAP) devices were invited to participate in the MESA Sleep Ancillary Study, which consisted of PSG, actigraphy, and sleep questionnaire data collected during an in-home examination. Of 4,077 participants approached, 147 (6.5%) were ineligible (95 due to a history of the PAP use (2%); 4 due to use of an oral appliance; and 4 due to oxygen use) and 141 participants lived too far away to participate. Of the remaining 3,789 participants, 2,261 participated in the sleep exam (59.7%). Comparison of characteristics between sleep study participants and those who did not participate in the sleep examination showed that nonparticipants were slightly more likely to be White, older, current/ex-smokers, and to have hypertension and chronic obstructive pulmonary disease. However, there was no statistically significant difference in the prevalence of self-report doctor-diagnosed sleep apnea (Table S1, supplemental material). In total, 2,060 participants had successful PSG data, 2,156 had actigraphy data, and 2,240 participants completed sleep questionnaires.
Institutional Review Board approval was obtained at each study site and written informed consent was obtained from all participants.
Measures
PSG-Measured SDB
PSG was conducted using a 15-channel monitor (Compumedics Somte System; Compumedics Ltd., Abbotsville, Australia). The recording montage included electroencephalography (EEG), bilateral electrooculograms, a chin electromyography (EMG), bipolar electrocardiography (ECG), thoracic and abdominal respiratory inductance plethysmography, airflow measured by thermocouple and nasal pressure cannula, finger pulse oximetry, and bilateral limb movements. PSG provided quantitative assessments of levels of overnight hypoxemia, apneas and hypopneas, and sleep stage distributions. Sleep stages and EEG (cortical) arousals were scored according to published guidelines23 adapted from the Sleep Heart Health Study.24,25
Apneas were scored when the thermocouple signal flattened or nearly flattened for greater than 10 sec. Hypopneas were scored when the amplitude of the sum of the abdominal and thoracic inductance signals or the nasal pressure flow signal decreased by 30% or more for greater than or equal to 10 sec. Events were classified as either “central” or “obstructive” according to the presence of absence of respiratory effort. Specialized software link apnea and hypopnea with data from the oxygen saturation and EEG signals, allowing each event to be characterized according to the degree of associated desaturation and arousal. Apnea-hypopnea index (AHI) was calculated based on the average number of all apneas plus hypopneas associated with a 4% desaturation per hour of sleep. SDB was defined as an AHI ≥ 5 events/h, and further categorized as: mild (AHI = 5–14), moderate (AHI = 15–29), and severe (AHI ≥ 30).
Actigraphy-Measured Sleep Duration and Quality
Actigraphy was performed using the Actiwatch Spectrum wrist actigraph (Philips Respironics, Murrysville, PA) worn on participants' nondominant wrists for 7 consecutive days, while participants completed a sleep diary over the same period.26 Actigraphic data during 30-sec epochs were scored as sleep or wake by Actiware-Sleep version 5.59 analysis software (Mini-Mitter Co, Inc, Bend, OR). A validated algorithm was used in which activity counts recorded during the measured epoch were modified by the level of activity in the surrounding 2-min time period (e.g., ± 2 min) to yield the final activity count for each epoch.27 Sleep duration and sleep quality (sleep maintenance efficiency) were estimated for each day. Short sleep duration was defined as an average of sleep duration < 6 h and 6–7 h. Long sleep duration was defined as ≥ 8 h, which represented approximately the highest 12% of the study sample. The choice of sleep duration (7–8 h) as the reference was based on the literature.6,12 Poor sleep quality was defined as having sleep maintenance efficiency (percentage of sleep time after sleep onset/sleep period) < 85%.28
Sleep Questionnaires
Information pertaining to doctor-diagnosed sleep apnea, habitual snoring, insomnia, and excessive daytime sleepiness was collected using validated instruments.29–31
Doctor-Diagnosed Sleep Apnea: Information on doctor-diagnosed sleep apnea was obtained from the sleep questionnaire survey: “Have you ever been told by a doctor that you had sleep apnea (a condition in which breathing stops briefly during sleep)?” Those who answered ‘yes’ were defined as having doctor-diagnosed sleep apnea.
Habitual Snoring: Participants were asked: (a) “Have you ever snored?” and, if yes, (b) “How often do you snore?” Habitual snoring was considered if participants who reported snoring sometimes (3–5 nights/w) or always/almost always (6–7 nights/w).
Insomnia: A validated five-item Women's Health Initiative Insomnia Rating Scale (WHIIRS) was used to measure insomnia.30 The WHIIRS assesses insomnia symptoms including long sleep latency, sleep maintenance insomnia, early morning awakening, and poor sleep quality over the past 4 w. The score ranges from 0 to 4 for each item (0 to 20 total score range). Insomnia was defined as a WHIIRS score > 10.
Excessive Daytime Sleepiness: The Epworth Sleepiness Scale (ESS)31 was applied to define excessive daytime sleepiness using eight scenarios scored on a four-point Likert scale from 0 to 3. The total score of the ESS ranged from 0 to 24. Those with an ESS score > 10 were defined as having excessive daytime sleepiness.
Sleep apnea syndrome (SAS) was defined as having an AHI ≥ 5 plus an ESS score > 10.2
Covariates
Participants were grouped into three age categories: 54–64 y, 65–74 y, and 75–93 y. Race/ethnicity was self-identified and categorized as: non-Hispanic White, non-Hispanic Black/ African American, Hispanic, and Chinese. Measured weight and height were used to calculate BMI, and categorized as follows: < 25, 25–29.9, and ≥ 30 kg/m2, to represent normal, overweight, and obese individuals for all analyses.32 We also defined normal, overweight, and obese categories for Chinese participants as follows: < 23, 23–24, and ≥ 25 kg/m2 based on the International Association for the Study of Obesity and the International Obesity Task Force guidelines for Asians.33
Statistical Analysis
Frequency distributions of sociodemographic characteristics and sleep disturbances were determined by performing cross-tabulations across racial/ethnic groups. Analysis of variance was used to evaluate unadjusted mean differences for continuous variables. Age-adjusted means of AHI and sleep duration were estimated from general linear models. Chi-square tests were used to evaluate the differences in the distributions of categorical variables. Univariate and multivariable logistic regression procedures were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of sleep disturbances according to racial/ethnic groups. Sex, age, study site, and BMI were included in final models.
Multinomial logistic regression analyses were conducted for SDB (reference: normal (AHI < 5); mild, moderate, and severe SDB) and sleep duration (short: < 6, 6–7 h; reference: 7–8 h; long sleep: ≥ 8 h). Analyses were repeated after stratification by participants' sex, age, and BMI categories. We conducted sensitivity analysis by using the Asian criteria of overweight (BMI: 23–24 kg/m2) and obesity (BMI ≥ 25 kg/m2). For subgroup analyses, we defined SDB using AHI ≥ 15 (moderate or severe SDB) versus AHI < 15; short sleep duration was defined as having sleep < 6 versus ≥ 6 h. We further adjusted for BMI in analyses stratified by sex and age. In addition, we conducted sensitivity analysis by considering 95 individuals who were excluded from participating in the sleep examination due to active PAP use as having “moderate or severe” SDB. Those 95 PAP users were not, however, included in multivariable analyses that required measurement of sleep traits. We also examined the relative probabilities of self-reported doctor-diagnosed sleep apnea for Blacks, Hispanics, and Chinese as compared with Whites given a PSG-measured SDB. We also conducted sensitivity analyses by adjusting for one sleep disturbance (e.g., insomnia, SAS) for another (e.g., sleep duration, sleep quality) in related logistic regression models. Statistical analyses were performed using Statistical Analysis Software (version 9.3; SAS Institute, Cary, NC). The significance levels were set at P < 0.05 for two-sided analyses.
RESULTS
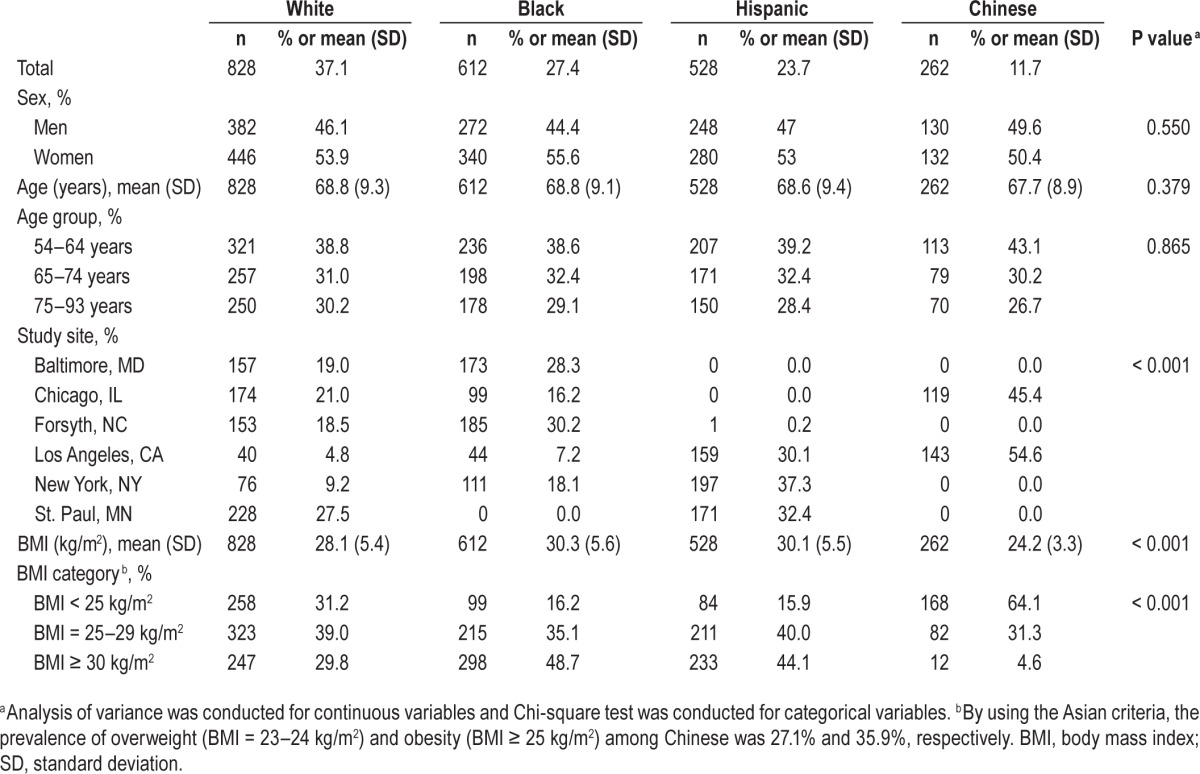
Of the 2,230 participants aged 54–93 y included in the study, 37.1% were White, 27.4% were Black, 23.7% were Hispanics, and 11.7% were Chinese. The distributions of sex and age were similar across racial/ethnic groups (Table 1). The mean of BMI was the highest among Blacks and Hispanics, and was the lowest among Chinese.
Table 1.
Sociodemographic characteristics of study participants across racial/ethnic groups.
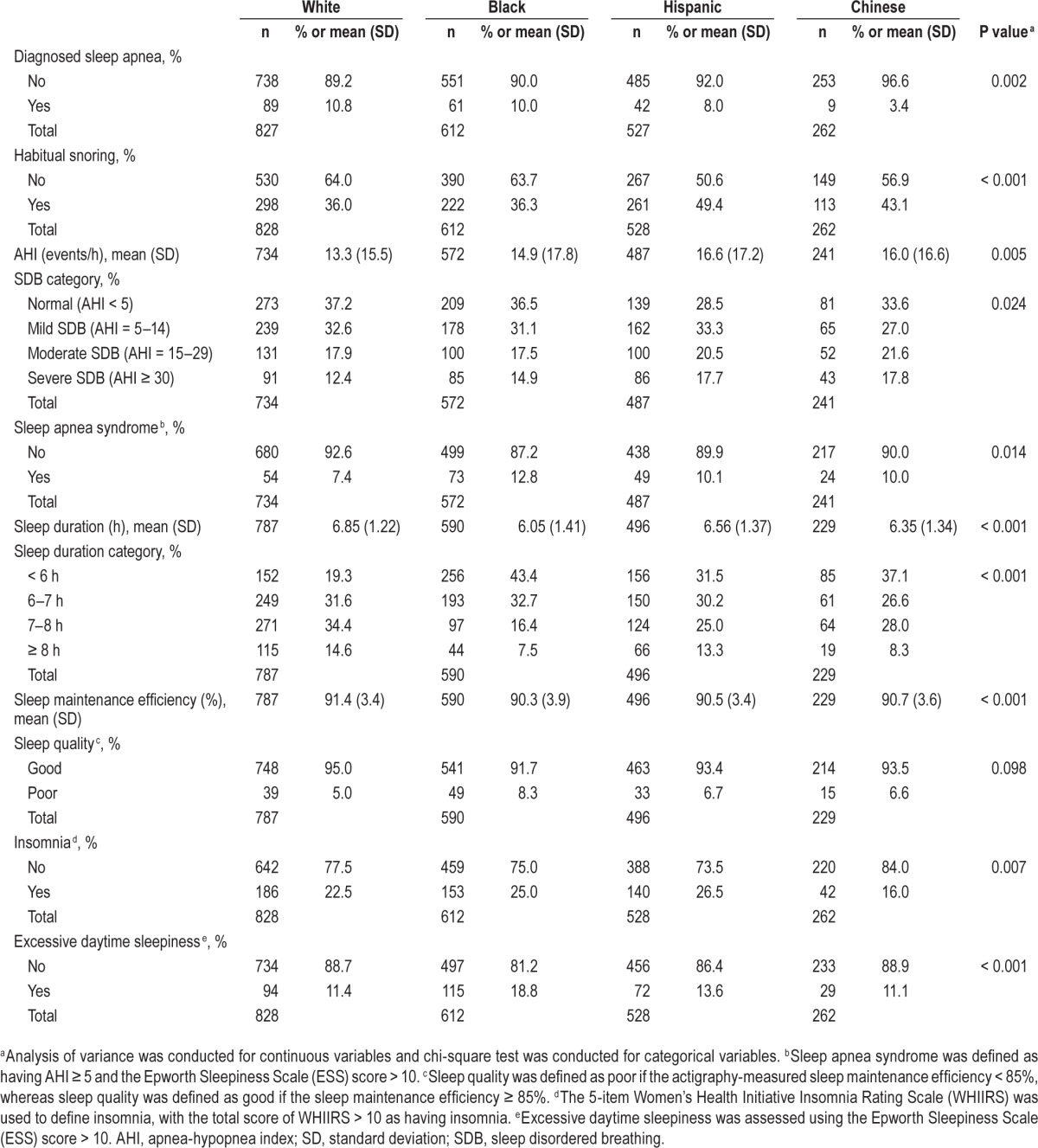
Overall, 9.0% of participants reported doctor-diagnosed sleep apnea. PSG showed that 33.8% had moderate or severe SDB (AHI ≥ 15), 15.0% had severe SDB (AHI ≥ 30), and 9.8% had SAS. Actigraphy showed that 30.9% had short sleep and 6.5% low sleep efficiency. Self-reported insomnia (23.4%) and daytime sleepiness (13.9%) were prevalent. Sleep disturbances varied across racial/ethnic groups (Table 2). For instance, doctor-diagnosed sleep apnea was uncommon among Chinese, whereas habitual snoring and PSG-measured SDB were most common among Hispanics and Chinese; SAS was most prevalent among Blacks. The prevalence of short sleep varied from 43.4% (among Blacks) to 19.3% (among Whites). Chinese had the lowest prevalence of insomnia. Daytime sleepiness was the most common among Blacks and Hispanics, whereas it was similar for Whites and Chinese.
Table 2.
Sleep disturbances of study participants across racial/ethnic groups.
Across race/ethnic groups, men had age-adjusted mean AHI values that were 60–98% higher than values observed among women (Figure S1, supplemental material). Age-adjusted mean sleep duration varied by both race/ethnicity and sex, with the shortest sleep duration observed among Black men (5.76 h) and Black women (6.29 h) and the longest sleep among White women (7.01 h) (Figure S2, supplemental material).
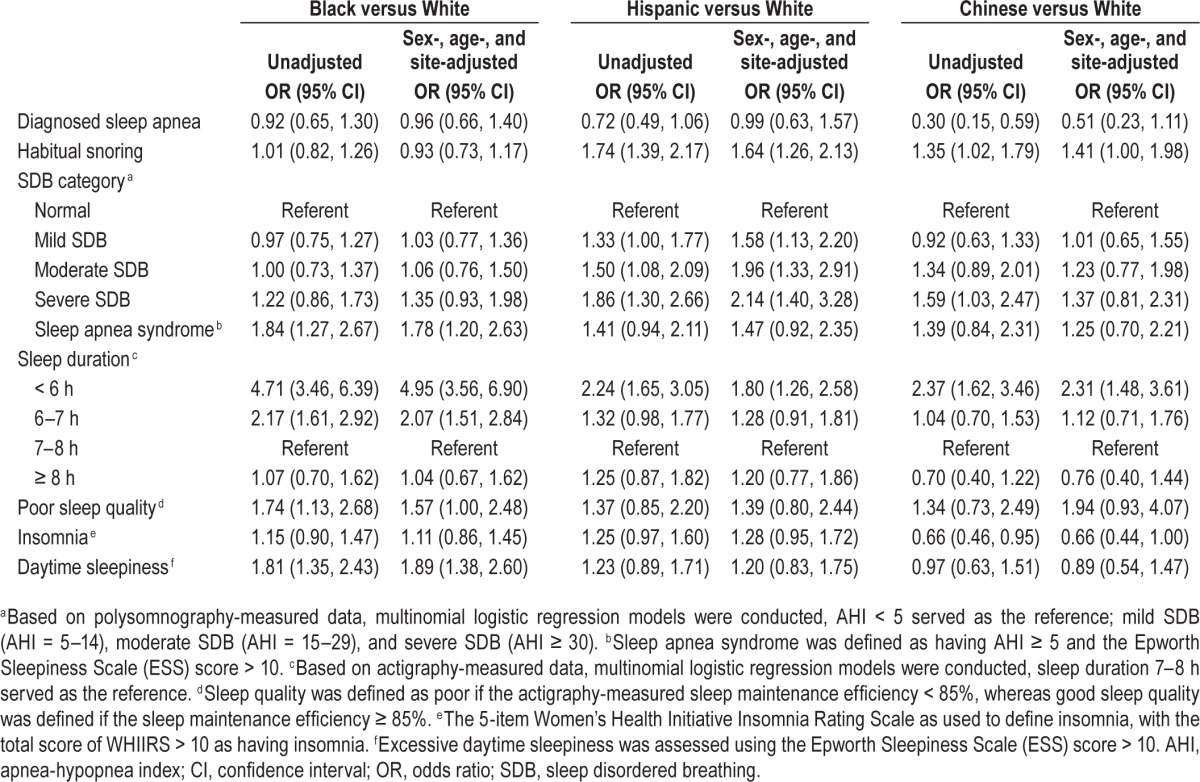
Compared with Whites, Blacks had higher sex-, age-, and study site-adjusted odds of SAS (OR = 1.78; 95% CI: 1.20, 2.63), short sleep (OR = 4.95; 95% CI: 3.56, 6.90), poor sleep quality (OR = 1.57; 95% CI: 1.00, 2.48), and daytime sleepiness (OR = 1.89; 95% CI: 1.38, 2.60) (Table 3). Hispanics were 1.64 times as likely to report habitual snoring, 2.14 times as likely to have severe SDB, and 1.80 times as likely to have short sleep as compared with Whites. Although Chinese were 49% less likely to report doctor-diagnosed sleep apnea and 34% less likely to report insomnia, they were 37% more likely to have severe SDB and 131% more likely to have short sleep than Whites. Additional analyses of race/ethnicity variation in insomnia and SAS showed that additional adjustment for sleep duration and for sleep efficiency did not appreciably influence the variation in insomnia or SAS by race/ethnicity (data not shown in tables). For example, adjusting for sleep duration did not substantially alter the increased odds of SAS among Blacks as compared with Whites. The sex-, age-, and site-adjusted OR changed from 1.78 (95% CI: 1.20, 2.63) to 1.52 (95% CI: 1.01, 2.29) when sleep duration was included in the model.
Table 3.
Logistic regression models: associations between race/ethnicity and sleep disturbances.
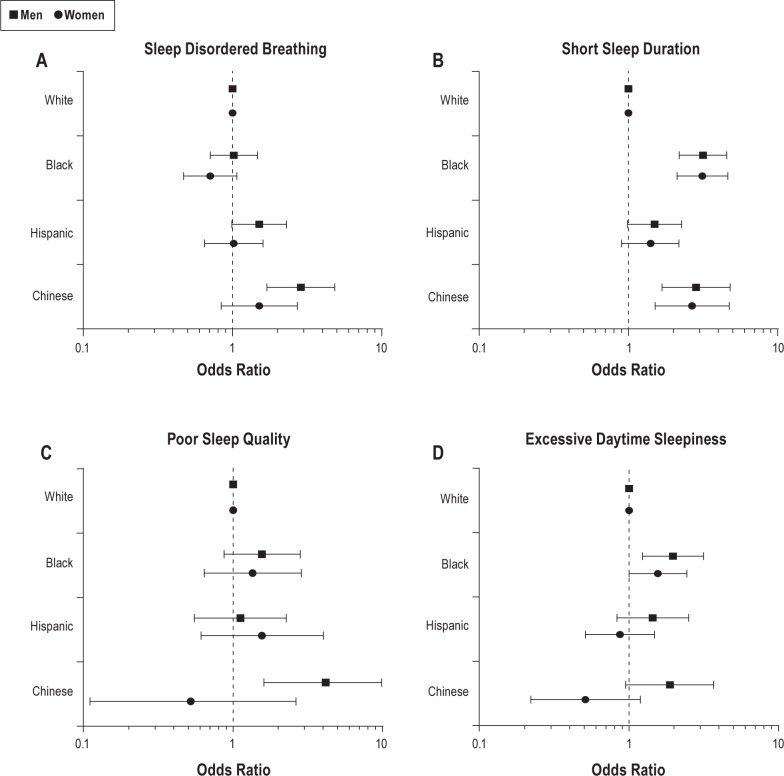
Age-, study site-, and BMI-adjusted ORs of SDB were statistically significantly greater for Chinese men and women compared to their White sex-specific counterparts (Figure 1A). The adjusted odds of short sleep was elevated for Black, Hispanic, and Chinese men and women, as compared with their White counterparts (Figure 1B). Chinese men had elevated odds of poor sleep quality as compared with White men (Figure 1C). Black men and Chinese men had elevated odds of daytime sleepiness compared with White men (Figure 1D). Black women had elevated odds of sleepiness as compared with White women. Overall, we observed no evidence of statistically significant effect modification by sex (P values for interaction terms were > 0.05).
Figure 1.
Age-, study site-, and body mass index-adjusted logistic regression models for associations between race/ethnicity and sleep disturbances, stratified by sex: the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. (A) Sleep disordered breathing: apnea-hypopnea index (AHI) ≥ 15 (reference: AHI < 15). (B) Short sleep duration: < 6 h versus ≥ 6 h. (C) Poor sleep quality (sleep efficiency < 85% versus ≥ 85%). (D) Excessive daytime sleepiness (the Epworth Sleepiness Scale score > 10 versus score ≤ 10).
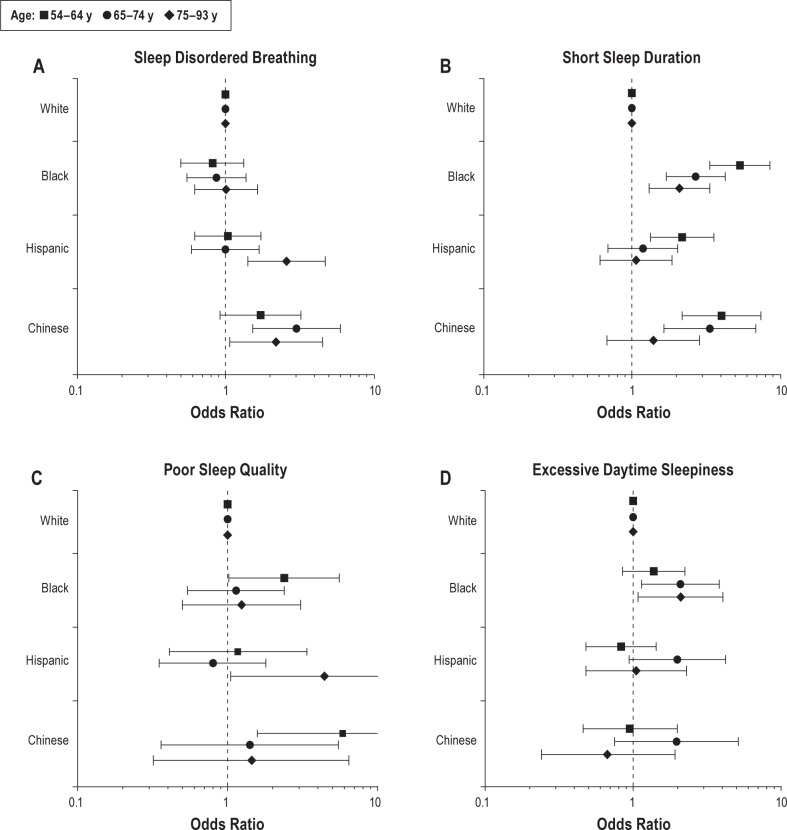
We repeated sex-, study site-, and BMI-adjusted analyses stratified by age (Figure 2). Higher odds of SDB among Chinese (Figure 2A) and short sleep among Blacks (Figure 2B) were observed across all age groups. Compared with Whites, Hispanics aged 75–93 y tended to have elevated odds of SDB, although the interaction term was not statistically significant. There was no evidence of elevated odds of SDB among Hispanics in younger age groups. Racial/ethnic differences in short sleep duration were more pronounced among younger individuals. Middle-aged Hispanics and Chinese had higher odds of short sleep than Whites; this was not true for the oldest group (P value for interaction = 0.045). The odds of poor sleep quality was elevated among middle-aged Blacks and Chinese when compared with their White counterparts (Figure 2C). Among Hispanics, the odds of poor sleep quality tended to be the highest in the oldest group. Across age groups, the odds of daytime sleepiness was higher among Blacks, but was similar for Hispanics and Chinese, as compared to their White counterparts (Figure 2D).
Figure 2.
Sex-, study site-, and body mass index-adjusted logistic regression models for the associations between race/ethnicity and sleep disturbances, stratified by age groups: the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. (A) Sleep disordered breathing: apnea-hypopnea index (AHI) ≥ 15 (reference: AHI < 15). (B) Short sleep duration: < 6 h versus ≥ 6 h. (C) Poor sleep quality (sleep efficiency < 85% versus ≥ 85%). (D) Excessive daytime sleepiness (the Epworth Sleepiness Scale score > 10 versus score ≤ 10).
We conducted sex-, age-, and study site-adjusted analyses stratified by BMI categories (Figure 3). Chinese had higher odds of SDB than Whites irrespective of BMI categories (Figure 3A). Among non-obese individuals, Chinese had the highest odds of SDB compared to Whites. Blacks, Hispanics, and Chinese had higher odds of short sleep than Whites irrespective of BMI categories (Figure 3B). Blacks with normal weight or obesity had higher odds of poor sleep quality than their White counterparts (Figure 3C). These variations in short sleep duration and poor sleep quality by race/ethnicity persisted with further adjustment for SDB and insomnia (data not shown). Within the stratum BMI of ≥ 30 kg/m2, Blacks had higher odds of sleepiness than Whites (Figure 3D), although the interaction terms did not reach statistical significance. Sensitivity analyses using the Asian cut-points of obesity showed similar results (data not shown).
Figure 3.
Sex-, age-, and study site-adjusted logistic regression models for the associations between race/ethnicity and sleep disturbances, stratified by body mass index (BMI): the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. (A) Sleep disordered breathing: apnea-hypopnea index (AHI) ≥ 15 (reference: AHI < 15). (B) Short sleep duration: < 6 h versus ≥ 6 h. (C) Poor sleep quality (sleep efficiency < 85% versus ≥ 85%). The Chinese group had no data for the obese group. (D) Excessive daytime sleepiness (the Epworth Sleepiness Scale score > 10 versus score ≤ 10). Normal weight: BMI < 25 kg/m2; Overweight: BMI = 25–29 kg/m2. Obese: BMI ≥ 30 kg/m2.
Among MESA participants, a total of 95 individuals (58 Whites, 14 Blacks, 21 Hispanics and 2 Chinese) were excluded from participating in the sleep examination due to their active PAP use. When including these individuals in the sensitivity analysis, classifying them as having “moderate or severe” SDB, we found that the prevalence of moderate or severe SDB increased from 30.3% to 35.1% for Whites, 32.3% to 34.1% for Blacks, 38.2% to 41.0% for Hispanics, and 39.4% to 39.9% for Chinese, respectively. When considering these 95 individuals as having “severe” SDB, the prevalence of severe SDB was increased from 12.4% to 18.5% for Whites, 14.9% to 17.0% for Blacks, 17.7% to 21.4% for Hispanics, and 17.8% to 18.5% for Chinese, respectively (Table S2, supplemental material). The multinomial logistic regression models showed that after including the 95 prior PAP users in the “severe SDB” category, the relative odds of SDB prevalence increased among Hispanics compared to Whites (sex-, age-, and site-adjusted OR = 1.76; 95% CI: 1.20, 2.57). However, assigning PAP users as having severe SDB and including these in the analytical dataset attenuated the associations related to Chinese race (sex-, age-, and site-adjusted OR = 1.18; 95% CI: 0.72, 1.95), although the OR was significantly elevated after adjustment for BMI (sex-, age-, site-, and BMI-adjusted OR = 3.13; 95% CI: 1.80, 5.44) (Table S3, supplemental material).
As shown in Table S4 (supplemental material), approximately 10.0% of Whites with PSG-measured SDB (AHI ≥ 15) reported having a doctor-diagnosed SDB. The corresponding conditional probabilities for self-reported doctor-diagnosed SDB (given MESA PSG-measured SDB) among Blacks, Hispanics, and Chinese were 16.2%, 14.5%, and 7.4%, respectively. In other words, 84–93% of MESA participants with PSG-measured moderate or more severe SDB did not report a doctor-diagnosed sleep apnea. The percent of doctor-diagnosed sleep apnea among individuals with severe SDB (AHI ≥ 30) was slightly higher, ranging from 9.3% (Chinese) to 18.8% (Blacks). Compared to Whites with PSG-measured SDB, Blacks and Hispanics were more likely to have a doctor-diagnosed sleep apnea, whereas Chinese were less likely to have a doctor-diagnosed sleep apnea, although these associations were not statistically significant.
DISCUSSION
To our knowledge, this is the first study that has systematically evaluated objective measures of SDB, short sleep duration, and poor sleep quality, as well as subjective measures of habitual snoring, insomnia, and daytime sleepiness in a multi-ethnic US sample that includes Chinese Americans. Findings from this direct multiple comparison of sleep disturbances showed a high prevalence of sleep disturbances and undiag-nosed sleep apnea among racial/ethnic minority groups, as well as significant variation in SDB, short sleep duration, SAS, sleep quality, and sleepiness across racial/ethnic groups. Hispanics and Chinese had higher odds of measured SDB, with Chinese differences most evident after adjusting for BMI. However, Chinese had the lowest prevalence of doctor-diagnosed sleep apnea. Short sleep duration was significantly more prevalent among Blacks, Hispanics, and Chinese as compared with Whites. Overall, Blacks had the shortest sleep duration and also had higher odds of SAS, poor sleep quality, and daytime sleepiness as compared with Whites. A higher prevalence of sleepiness among Blacks was not explained by differences in sleep duration or SDB. Notably, these associations remained evident after adjustment for sex, age, study site, and BMI. These findings indicate that sleep disturbances such as short sleep duration and SDB are more frequent among racial/ethnic minorities than Whites, and suggest that sleep disturbances may contribute to health disparities among US adults.
Sleep Disordered Breathing (SDB)
SDB is influenced by a complex array of risk factors that affect airway patency, some of which may reflect environmental influences, genetic factors, and others.34 Family and pediatric studies indicate that among children and young adults, SDB is more common among Blacks than among Whites,35–37 with racial/ethnic differences partly explained by exposures to factors that aggregate in disadvantaged neighborhoods, including tobacco smoke. SDB is also highly heritable.38 Obesity is a strong risk factor for SDB, although more than half of the genetic factors associated with SDB are estimated to be independent of obesity.39,40 Data from Asia also point to a high prevalence of SDB among Chinese, Japanese, Korean, and Indian populations.41–44 Several potential mechanisms may link race/ethnicity to SDB, including variation in craniofacial anatomy and body fat distribution. Asians have more pronounced craniofacial skeletal abnormalities than Whites for a given degree of SDB severity.45,46 Blacks may be predisposed to SDB through the influence of obesity and enlarged upper airway soft tissues,21 as well as through exposures to adverse social and physical environments.
Few studies have directly compared SDB prevalence across multiple racial/ethnic groups. Although some investigators have reported no racial/ethnic difference in SDB,47 others report that Blacks and Hispanics have a high prevalence of severe SDB.29,48,49 In MESA, where SDB was comprehensively assessed using PSG, we observed a high prevalence of moderate or more severe SDB among all groups, which was significantly elevated among Chinese (39.4%) and Hispanics (38.2%) as compared with Whites. Although consideration of differences in obesity attenuated some of racial/ethnic variation, the odds of SDB among Chinese increased after adjustment for BMI. Furthermore, among participants with BMI ≥ 25 kg/m2, the SAS prevalence was higher among Blacks, Hispanic, and Chinese than Whites. As SDB has been implicated as a risk factor for CVD, stroke, diabetes, and premature mortality,3,4,6 our findings underscore the need to consider unrecognized sleep apnea in middle-aged and older adults, with potential value in developing new strategies to screen and treat Chinese group.
Blacks had the highest prevalence of SAS (12.8%), but did not have a higher prevalence of doctor-diagnosed sleep apnea or PSG-measured SDB than Whites. This finding likely reflects the high prevalence of daytime sleepiness (18.8%) among Blacks. Whether Blacks with even low levels of AHI (5 or higher) are more susceptible to sleepiness than other racial/ethnic groups, and thus have a true higher prevalence of SAS, or if instead the sleepiness associated with low AHI levels reflects insufficient sleep due to factors other than SDB, is unclear. However, adjusting for sleep duration did not substantially alter the increased odds of SAS prevalence among Blacks.
The prevalence of moderate or severe SDB was as much as tenfold higher than reported doctor-diagnosed sleep apnea. In fact, between 84% and 93% of individuals with AHI levels moderately or severely elevated (and thus candidates for sleep apnea treatment), were undiagnosed. Although data from the past 15 y indicate that there is a high prevalence of undiag-nosed and untreated sleep apnea,8 these recent data collected in a cohort receiving regular health feedback information through MESA examinations suggest that this problem persists, and is particularly salient for some groups, such as middle-aged and older Chinese, who may not be routinely recognized to be at risk for SDB. In MESA, those who participated versus not participated in the sleep study did not differ in regards to likelihood of having doctor-diagnosed sleep apnea; however the pattern did vary by racial/ethnic groups. There were discrepancies between self-reported sleep apnea and polysomno-graphically measured sleep apnea (SDB), which were the most prominent for Chinese participants.
Sleep Duration
Short sleep duration has been identified as a risk factor for obesity, hypertension, diabetes, and CVD.50,51 In MESA, 32% of participants had actigraphy-measured sleep with < 6 h per night. We found that objectively measured short sleep duration was more frequent in all three minority groups and was observed in both men and women and across age and BMI strata. We also found that racial/ethnic differences in short sleep duration were more pronounced among younger individuals. The shortest sleep duration was observed among Black men, who slept on average 75 min less than White women. Black women slept on average 43 min less than White women. These findings are consistent with a prior national survey using questionnaire data of Whites and Blacks aged 18–85 y.15 Our findings are also consistent with data from an actigraphy-based study of Chicago Whites and Blacks aged 38–50 y in the Coronary Artery Risk Development in Young Adults (CARDIA) study showing that sleep duration was the shortest for Black men (5.1 h) and the longest for White women (6.7 h).20 Our data further extend these results by showing that short sleep duration also occurred in an older sample of Chinese and Hispanics and was not explained by insomnia or SAS. Among Blacks, we showed that an increased prevalence of short sleep also occurred across all age groups, and that Blacks had a high prevalence of poor sleep quality and sleepiness. There is a need for research that examines factors that may contribute to reduced sleep duration, such as family burden, employment status, stress, and environmental disturbances.
Long sleep duration, usually identified through self-report, has been associated with numerous adverse health outcomes.50 With the use of 7-day actigraphy, we found a relatively low prevalence of longer sleep (≥ 8 h), and little evidence for racial/ethnic variation. Although in the general population ≥ 8 h of sleep is usually considered as normal sleep duration, this middle-aged and older population in MESA had few participants (2%) with longer sleep duration ≥ 9 h. Further research is needed to understand differences between self-reported as compared to actigraphy-based sleep assessments and their association with health outcomes, especially among racial/ethnic minority populations.
Poor Sleep Quality
Poor sleep quality (reduced sleep efficiency) reflects an increased time awake after sleep onset. This may be due to any number of factors that disturb sleep, such as physiological arousals or environmental disturbances. We found that Black and Chinese men were more likely to have poor sleep quality than their White counterparts, even after adjustment for BMI, SDB, and insomnia. A finding of higher odds of poor sleep quality in racial/ethnic minorities is of clinical significance given prior reports linking poor sleep quality to elevated blood pressure,52 metabolic disturbances,53 and cognitive deficits.54
Insomnia
Insomnia is common in the general population, especially among women and older adults.55 Approximately 48% of Americans have insomnia occasionally, whereas 22% experience insomnia every or almost every night.55 Insomnia and poor sleep quality have been associated with stress, anxiety, and depression.56,57 Telephone survey data from the Behavioral Risk Factor Surveillance System found that Asian Americans reported the least sleep complaints, and Blacks and Hispanics reported fewer sleep complaints than Whites.9 We found that fewer Chinese reported insomnia symptoms and daytime sleepiness, whereas more Blacks, particularly those who were obese, reported insomnia and sleepiness than Whites. Our use of validated measures of self-reported sleep problems reduces, but did not eliminate, the likelihood of differential reporting across racial/ethnic groups. Further research is needed to address potential differences in objective and subjective measures of sleep disturbances across population groups.
Sex and Age Variation
A number of studies have reported sex and age differences in SDB and other sleep disturbances.2,11,13,14,19,41 We also explored whether racial/ethnic variation in sleep disturbances differed by sex and age groups. Our findings confirm prior research showing a higher prevalence of SDB in men than in women. Furthermore, we found that Chinese men had higher prevalence of SDB than White men despite a relatively lower prevalence of doctor-diagnosed sleep apnea. Racial/ethnic minority men may underutilize health services,58 and thus, our findings indicate that special efforts to improve screening in these groups may be warranted. Most of the racial/ethnic differences in sleep disturbances were observed across the age range of 54–93 y. This suggests that sleep health disparities exist in both middle-aged and older Americans.
Strengths and Limitations
Our study has important strengths including the relatively large sample size, the community-based sampling of a multi-ethnic cohort with considerable heterogeneity. Of note, sleep disturbances were assessed using valid objective measures including PSG- and actigraphy-based methodologies, and self-reported sleep disturbances were assessed using well-established questionnaires. The diverse study sample allowed us to assess the prevalence and odds of sleep disturbances across groups defined by race/ethnicity and within strata defined by sex, age, and BMI. In addition, we considered study site and other sleep disturbances when examining a specific sleep disturbance.
Despite these strengths, several limitations merit consideration. First, the lack of temporality makes it difficult to determine whether BMI, a covariate we adjusted for, served as a confounder or mediator of relationships of interest. Further studies are needed to examine the roles of sleep and obesity in the racial/ethnic differences using longitudinal data. Second, we cannot rule out the fact that our findings may be at least, in part, influenced by selection bias. The estimates of SDB prevalence are limited by factors that influence participation in epidemiologic studies. When the MESA cohort was first assembled (10 y prior to the sleep exam), individuals with clinically apparent CVD were excluded. Therefore, those at high risk for early onset CVD may have been underrepresented in the sleep examination. Although the participation rate in the sleep examination was relatively high, the possibility of selective participation may exist. However, sleep symptoms and health characteristics were generally similar for participants and nonparticipants in the sleep examination. Because of the difficulty associated with studying individuals on chronic PAP use, 2% of the MESA sample (n = 95) did not undergo a sleep examination. A sensitivity analysis that assumed those individuals who had moderate or severe SDB was conducted and showed overall similar prevalence rates as the primary analysis other than attenuation of the SDB risk among Chinese. Third, we did not include information on occupation (e.g., night shift work), the use of sleep medications, depressive symptoms, or urban versus rural residence in this article. This information may help identify more specific environmental (e.g., noise and types of jobs) or comorbidities that influence racial/ethnic differences in sleep. Finally, although we had an overall large sample size, we had limited statistical power for some subgroup analyses, particularly those aimed at examining the odds of SDB and poor sleep quality among Chinese participants according to BMI categories.
SUMMARY
Analyses from this well-described, community-based multi-ethnic cohort identified a high prevalence of sleep disturbances in middle-aged and older men and women. Using objective sleep testing, moderate or more severe SDB and short sleep duration each were found in more than 30% of the cohort. The largest observed racial/ethnic differences were for short sleep duration. Blacks were five times, Hispanics were 1.8 times, and Chinese were 2.3 times as likely to have short sleep duration as compared with Whites after adjustment for sex, age, and study site. SDB was more prevalent in Hispanics and in Chinese, especially Chinese-American men, a group rarely recognized to be at increased risk for SDB. In fact, sleep apnea had been diagnosed in only 7% to 16% of individuals with an elevated AHI. Our findings underscore the high prevalence of undiagnosed sleep disturbances in middle-aged and older adults, and identify racial/ethnic disparities that include differences in short sleep duration, SDB, and daytime sleepiness, as well as the likely differences in recognition of SDB. These data support a need for further research to better understand the bases for sleep health disparities that can identify opportunities for improving health across population groups.
DISCLOSURE STATEMENT
The Multi-Ethnic Study of Atherosclerosis (MESA) is supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) at the National Institutes of Health. The work presented in this paper was supported by grants from NIH 1R01HL083075-01, R01HL098433, R01 HL098433-02S1, 1U34HL105277-01, 1R01HL110068-01A1, 1R01HL113338-01, R21 HL108226, P20 NS076965, 3R01HL115941-01S1, and R01 HL109493. This work was also supported by an award from the National Center for Advancing Translational Sciences (NCATS): 8UL1TR000170-07. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- CVD
cardiovascular disease
- ECG
electrocardiogram
- EEG
electroencephalography
- EMG
electromyography
- ESS
Epworth Sleepiness Scale
- MESA
Multi-Ethnic Study of Atherosclerosis
- OR
odds ratio
- PAP
positive airway pressure
- PSG
polysomnography
- SAS
sleep apnea syndrome
- SD
standard deviation
- SDB
sleep disordered breathing
- WHIIRS
Women's Health Initiative Insomnia Rating Scale
SUPPLEMENTAL MATERIAL
Comparison of sociodemographic and health characteristics at the Multi-Ethnic Study of Atherosclerosis (MESA) exam 5 between participants with and without sleep data at exam 5.
Prevalence of polysomnography-measured sleep disordered breathing plus prior positive airway pressure use (n = 95) across racial/ethnic groups.
Multinomial logistic regression models: associations between race/ethnicity and sleep disordered breathing plus prior positive airway pressure use.
Percent and odds of study participants with self-reported diagnosed sleep apnea according to measured sleep disordered breathing across racial/ethnic groups.
Age-adjusted apnea-hypopnea index across sex and racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. Age-adjusted means of apnea-hypopnea index (AHI) with standard errors were presented. The general linear model was conducted to generate least-square means and standard errors. P values for the comparisons of the differences in AHI between groups were less than 0.001. AHI, apnea-hypopnea index.
Age-adjusted sleep duration (hours) across sex and racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. Age-adjusted means of sleep duration with standard errors were presented. The general linear model was conducted to generate least-square means and standard errors. P values for the comparisons of the differences in sleep duration between groups were less than 0.001.
REFERENCES
- 1.Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep. 2010;33:962–7. doi: 10.1093/sleep/33.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccolo RS, Yang M, Bliwise DL, Yaggi HK, Araujo AB. Racial and socioeconomic disparities in sleep and chronic disease: results of a longitudinal investigation. Ethn Dis. 2013;23:499–507. [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124:2049–51. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 5.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 6.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva GE, An MW, Goodwin JL, et al. Longitudinal evaluation of sleep disordered breathing and sleep symptoms with change in quality of life: the Sleep Heart Health Study (SHHS) Sleep. 2009;32:1049–57. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: Institute of Medicine: National Academies Press; 2006. Institute of Medicine Committee on Sleep Medicine and Research. [PubMed] [Google Scholar]
- 9.Grandner MA, Patel NP, Gehrman PR, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11:470–8. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17:948–55. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralls FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18:568–73. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 12.Jackson CL, Redline S, Kawachi I, Williams MA, Hu FB. Racial disparities in short sleep duration by occupation and industry. Am J Epidemiol. 2013;178:1442–51. doi: 10.1093/aje/kwt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagishi K, Ohira T, Nakano H, et al. Cross-cultural comparison of the sleep disordered breathing prevalence among Americans and Japanese. Eur Respir J. 2010;36:379–84. doi: 10.1183/09031936.00118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 15.Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. J Natl Med Assoc. 2008;100:317–22. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 16.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–6. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 17.Gharibeh T, Mehra R. Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep. 2010;2:233–55. doi: 10.2147/NSS.S6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 19.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep disordered breathing. JAMA. 2003;289:2230–7. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 20.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–22. doi: 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 24.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–58. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 25.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 26.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 27.Oakley NR. Validation with polysomnography of the Sleepwatch sleep/ wake scoring algorithm used by the Actiwatch activity monitoring system. Technical Report to Mini Mitter Co., Inc. 1997 [Google Scholar]
- 28.Fung MM, Peters K, Ancoli-Israel S, Redline S, Stone KL, Barrett-Connor E. Total sleep time and other sleep characteristics measured by actigraphy do not predict incident hypertension in a cohort of community-dwelling older men. J Clin Sleep Med. 2013;9:585–91. doi: 10.5664/jcsm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–44. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 32.National Institutes of Health. Washington, DC: National Institutes of Health; 1998. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report. [PubMed] [Google Scholar]
- 33.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Australia: Health Communications; 2000. The International Association for the Study of Obesity and the International Obesity Task Force. [Google Scholar]
- 34.Redline S, Strohl KP. Recognition and consequences of obstructive sleep apnea hypopnea syndrome. Clin Chest Med. 1998;19:1–19. doi: 10.1016/s0272-5231(05)70428-7. [DOI] [PubMed] [Google Scholar]
- 35.Weinstock TG, Rosen CL, Marcus CL, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37:261–9. doi: 10.5665/sleep.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 37.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 38.Buxbaum SG, Elston RC, Tishler PV, Redline S. Genetics of the apnea hypopnea index in Caucasians and African Americans: I. Segregation analysis. Genet Epidemiol. 2002;22:243–53. doi: 10.1002/gepi.0170. [DOI] [PubMed] [Google Scholar]
- 39.Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–34. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–50. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohdaira F, Nakamura K, Nakayama H, et al. Demographic characteristics of 3,659 Japanese patients with obstructive sleep apneahypopnea syndrome diagnosed by full polysomnography: associations with apnea-hypopnea index. Sleep Breath. 2007;11:93–101. doi: 10.1007/s11325-006-0087-5. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, In K, You S, et al. Prevalence of sleep disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 43.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125:127–34. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 44.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 45.Chang ET, Shiao GM. Craniofacial abnormalities in Chinese patients with obstructive and positional sleep apnea. Sleep Med. 2008;9:403–10. doi: 10.1016/j.sleep.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–93. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–83. [PMC free article] [PubMed] [Google Scholar]
- 48.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 49.Pranathiageswaran S, Badr MS, Severson R, Rowley JA. The influence of race on the severity of sleep disordered breathing. J Clin Sleep Med. 2013;9:303–9. doi: 10.5664/jcsm.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 52.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Sleep Foundation. [Accessed January 8, 2014]. http://www.sleepfoundation.org/
- 56.Bunevicius R, Liaugaudaite V, Peceliuniene J, Raskauskiene N, Bunevicius A, Mickuviene N. Factors affecting the presence of depression, anxiety disorders, and suicidal ideation in patients attending primary health care service in Lithuania. Scand J Prim Health Care. 2014;32:24–9. doi: 10.3109/02813432.2013.873604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malik S, Kanwar A, Sim LA, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014;3:18. doi: 10.1186/2046-4053-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Errickson SP, Alvarez M, Forquera R, et al. What will health-care reform mean for minority health disparities? Public Health Rep. 2011;126:170–5. doi: 10.1177/003335491112600207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of sociodemographic and health characteristics at the Multi-Ethnic Study of Atherosclerosis (MESA) exam 5 between participants with and without sleep data at exam 5.
Prevalence of polysomnography-measured sleep disordered breathing plus prior positive airway pressure use (n = 95) across racial/ethnic groups.
Multinomial logistic regression models: associations between race/ethnicity and sleep disordered breathing plus prior positive airway pressure use.
Percent and odds of study participants with self-reported diagnosed sleep apnea according to measured sleep disordered breathing across racial/ethnic groups.
Age-adjusted apnea-hypopnea index across sex and racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. Age-adjusted means of apnea-hypopnea index (AHI) with standard errors were presented. The general linear model was conducted to generate least-square means and standard errors. P values for the comparisons of the differences in AHI between groups were less than 0.001. AHI, apnea-hypopnea index.
Age-adjusted sleep duration (hours) across sex and racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis (MESA), 2010–2013. Age-adjusted means of sleep duration with standard errors were presented. The general linear model was conducted to generate least-square means and standard errors. P values for the comparisons of the differences in sleep duration between groups were less than 0.001.