Abstract
Study Objectives:
Sleep and memory are stable and heritable traits that strongly differ between individuals. Sleep benefits memory consolidation, and the amount of slow wave sleep, sleep spindles, and rapid eye movement sleep have been repeatedly identified as reliable predictors for the amount of declarative and/or emotional memories retrieved after a consolidation period filled with sleep. These studies typically encompass small sample sizes, increasing the probability of overestimating the real association strength. In a large sample we tested whether individual differences in sleep are predictive for individual differences in memory for emotional and neutral pictures.
Design:
Between-subject design.
Setting:
Cognitive testing took place at the University of Basel, Switzerland. Sleep was recorded at participants' homes, using portable electroencephalograph-recording devices.
Participants:
Nine hundred-twenty-nine healthy young participants (mean age 22.48 ± 3.60 y standard deviation).
Interventions:
None.
Measurements and results:
In striking contrast to our expectations as well as numerous previous findings, we did not find any significant correlations between sleep and memory consolidation for pictorial stimuli.
Conclusions:
Our results indicate that individual differences in sleep are much less predictive for pictorial memory processes than previously assumed and suggest that previous studies using small sample sizes might have overestimated the association strength between sleep stage duration and pictorial memory performance. Future studies need to determine whether intraindividual differences rather than interindividual differences in sleep stage duration might be more predictive for the consolidation of emotional and neutral pictures during sleep.
Citation:
Ackermann S, Hartmann F, Papassotiropoulos A, de Quervain DJF, Rasch B. No associations between interindividual differences in sleep parameters and episodic memory consolidation. SLEEP 2015;38(6):951–959.
Keywords: declarative memory, rapid eye movement sleep, sample size, sleep EEG, slow wave sleep
INTRODUCTION
Sleep and memory are stable traits. Twin studies have revealed that genetic differences account for approximately 50% of the interindividual variance in memory performance,1 as well as the duration of sleep stages including slow wave sleep (SWS), whereas genetic contribution to rapid eye movement (REM) sleep is less conclusive.2 Highest heritability values have been revealed for electroencephalographic (EEG) spectral power density as well as REM density (h2 = 64–96%).2–4 In addition, sleep is remarkably stable in the same individual across multiple nights whereas sleep largely differs between individuals.5 Importantly, these interindividual differences are remarkably robust against sleep disturbances, first night effects or prior sleep deprivation.
Memory consolidation profits from sleep after learning.6,7 In particular, SWS and its characteristic slow oscillatory activity have been implicated in plastic processes underlying the consolidation of declarative memories of events and facts.6 According to the active system consolidation account, slow oscillatory activity during SWS synchronizes spontaneously reactivated hippocampal memories with thalamocortical spindle activity, thereby facilitating plastic and integrative processes in cortical areas involved in long-term storage.7 Alternatively, the synaptic homeostasis hypothesis implicates slow wave activity (SWA) during SWS in processes of synaptic downscaling, preparing the brain for new learning the next day.8 In contrast to SWS, REM sleep and its associated theta activity have been mostly associated with the reprocessing of emotional memories.9
Although the mechanisms underlying memory consolidation processes during sleep are increasingly understood, it is still an open question whether interindividual differences in sleep parameters are predictive for memory. Several studies have reported strong associations between the amount of nonrapid eye movement (NREM) sleep, SWS, or SWA during NREM sleep and memory processes during sleep, ranging from r = 0.69 to r = 0.94.10–12 In addition, sleep spindle number and density were highly correlated with overnight retention of memories as well as general learning ability and intelligence (r = 0.56 to r = 0.68).13–15 Finally, the amount of REM sleep or REM-associated theta power was highly predictive for recall of emotional memories (r = 0.63 to r = 0.88).16 However, these correlations are not consistently observed. More importantly, almost all of these studies used very small sample sizes (range of N in the aforementioned studies: n = 6 to n = 31), raising two main issues: (1) Studies using small sample sizes typically overestimate the real effect size, as only very large correlations can reach significance because of a lack of statistical power. The problem increases when multiple sleep and memory parameters are correlated in the same study. (2) Nonsignificant correlations cannot be interpreted because of a lack of statistical power and a high chance of false negatives. The low informative value of results using small sample sizes have been emphasized just recently,17–19 calling for studies with larger sample sizes and sufficient statistical power to validate current findings.
In the current study, we rigorously investigated how inter-individual differences in sleep are related to memory performance in 929 young healthy volunteers. Participants viewed emotional and neutral pictures in the evening and freely recalled them the next day. Sleep was recorded at home using a mobile EEG recording device. We predicted that overnight retention of neutral pictures is significantly associated with the amount of SWS, power of SWA, and sleep spindle density. In addition, we hypothesized that the retention of emotional pictures is positively correlated to the amount of REM sleep and theta activity during REM sleep.
MATERIALS AND METHODS
Participants
We had complete data from 985 subjects. Fifty-six subjects had to be excluded because their measures (memory or/and sleep parameters) exceeded our outlier criterion (four standard deviations (SD) from group mean). Data from 929 healthy young women and men (633 women, 296 men) between 18 and 35 y (mean age 22.48 ± 3.60 y [SD]) were included in the analyses. EEG frequency data were available from 885 subjects; data from 44 subjects could not be analyzed because of EEG artefacts. Participants were students or employees from the Basel area and were paid for their participation. They did not take any medication (except hormonal contraceptives), and reported no neurological or mental illness. To get used to wearing a portable EEG recording device, subjects spend a night at home wearing a portable dummy EGG recording device before entering the study. The study was approved by the local ethics committee and all participants gave written informed consent prior to participation.
Procedure
The experiments were conducted on 2 consecutive days (Figure 1). On day 1, after participants had arrived electrodes were applied. Afterward, participants received instructions and were trained on the tasks. After training, participants viewed emotional and neutral pictures of the picture memory task (picture set 1). Afterward, they performed on a working memory task for 10 min (n-Back). This task was followed by an unannounced free recall test of the previously seen pictures (short-delay free recall day 1, picture set 1). The session ended with a finger-tapping task. Testing on day 1 always occurred between 15:30 and 20:00. After testing on day 1, participants spend the night at home wearing a portable EEG recording device. On day 2, testing occurred between 12:30 and 15:00. Participants came back to the laboratory and viewed another set of emotional and neutral pictures (picture set 2). Previous studies have shown that interference learning before recall makes the effect of sleep on memory consolidation more discernable.20 After 10 min performance on the working memory task, participants freely recalled pictures from both set 1 (long-delay free recall picture set 1) and set 2 (short-delay free recall day 2, picture set 2) and afterward performed on the recall phase of the finger sequence tapping task (see Figure 1 for a summary of the procedure). Participants were tested in groups of 1–6 individuals.
Figure 1.
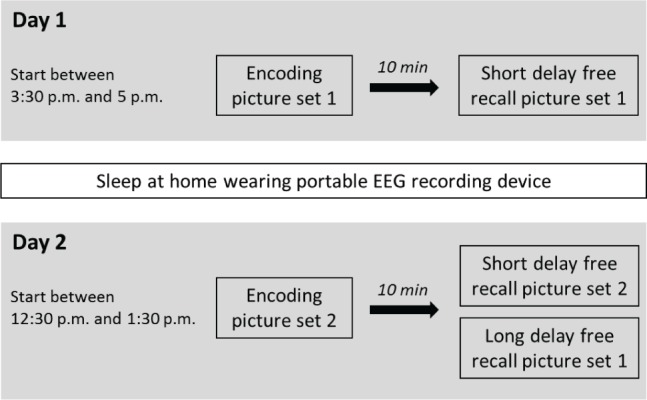
Summary of the study design and procedure. On day 1 the participants arrived between 15:30 and 17:00. First, the electroencephalography (EEG) electrodes were positioned on the participants' heads. Afterward they viewed 72 negative, positive, and neutral pictures (set 1) and rated them for valence and arousal. After 10 min during which participants performed on an n-back working memory task, they freely recalled the pictures (short-delay free recall picture set 1). Then they performed on a finger sequence-tapping task. After the experimental session, participants slept at home while sleep EEG was recorded. On day 2, participants came back to the laboratory (arrival between 12:30 and 13:30) and viewed another set of 72 pictures (set 2). After 10 min during which participants again performed on an n-back working memory task, participants freely recalled pictures from set 2 seen on day 2 (10 min short-delay recall free recall picture set 2) as well as from set 1 seen on day 1 (20 h long-delay free recall picture set 1). Finally, participants performed on the recall phase of the sequential finger-tapping task.
Picture Memory Task
The picture memory task consisted of 72 pictures taken from the International Affective Picture System (IAPS),21 as well as from in-house standardized picture sets. Stimuli consisted of two sets (picture set 1 and picture set 2) of 24 positive, 24 negative, and 24 neutral pictures interleaved with 24 scrambled pictures. In addition, four pictures showing neutral objects were presented to control for primacy and recency effects (two pictures were shown in the beginning of the presentation, the other two at the end). These pictures were not included in the analysis. Picture set 1 was presented on day 1, picture set 2 was presented on day 2. The two sets were counterbalanced for ratings of arousal and valence as well as for visual complexity and presence of humans.
The pictures were presented in a quasirandomized order so that a maximum of four pictures of the same category followed consecutively. A fixation-cross appeared for 500 ms before each picture. Then the picture was presented for 2.5 sec. After presentation of each picture, subjects rated the presented picture according to its emotional valence (negative = 1, neutral = 2, positive = 3) and arousal (low = 1, medium = 2, high = 3) on a three-point scale. Trials were separated by variable intertrial periods (9–12 sec). Participants were not told to memorize the pictures (incidental encoding).
For the free recall task, participants had to write down a short description of each picture. The participants were instructed to recall as many pictures as possible. There was no time limit for this task. Participants were not told how many pictures they saw during picture presentation; therefore, no expectation of the amount of pictures to be recalled was mentioned. Two independent and blind raters analyzed the recalled pictures and decided for each picture whether it could be recognized as one of the presented pictures. The interrater reliability added up to 0.96 (Cronbach α). Afterward, a third independent and blind rater decided on pictures, which were rated differently.
Participants recalled the pictures learned on day 1 (picture set 1) 10 min after encoding (short-delay free recall picture set 1 on day 1) as well as 20 h after encoding (long-delay free recall picture set 1 on day 2). Pictures learned on day 2 (picture set 2) were recalled 10 min after encoding (short delay free recall picture set 2 on day 2; see Figure 1). Overnight memory retention was calculated as relative retrieval performance of picture set 1 with learning performance before the retention interval (short delay recall picture set 1 on day 1) set to 100% (long delay free recall picture set 1 / short delay free recall picture set 1 * 100%).
Working Memory Task
Between picture presentation and recall, participants performed on the 0- and 2-back versions of the n-back working memory task.22 In this task, letters are presented successively in the center of the screen. In the 0-back condition, participants had to respond to the occurrence of the letter ‘x’, which is a baseline measure of general attention, concentration, and reaction time. The 2-back task requires participants to respond to a letter repetition with one intervening letter (g – S – f – s). The latter condition required both the maintenance of the last two letters in memory and updating of these remembered stimuli as each new stimulus was presented. We analyzed differences in accuracy between the 2-back and the 0-back condition as this variable represents a reliable measure of working memory.23 Complete n-back data were available for 857 subjects.
Procedural Memory Task
After memory recall, procedural memory was measured with a motor learning task.24 The participants worked on a sequential finger-tapping task using their nondominant hand (i.e., with the fingers of the left hand for a right hander and vice versa). They had to press four numeric keys on the computer keyboard, repeating the sequence “4-2-3-1-4” as quickly and accurately as possible for 30 sec, followed by a resting phase of 30 sec. The numeric sequence was displayed on the computer screen throughout the task to keep working memory demands at a minimum. Participants could only see a white star displayed on the black screen for every pressed key, but not the actual number pressed. On day 1, participants completed 12 rounds, each round lasted for 30 sec, and 30 sec rest between rounds. On day 2, they completed three rounds with the same sequence of numbers and three rounds with a new sequence of numbers. The key press responses were recorded and for each round the number of overall responses and the number of correct responses was scored. The following measures were calculated: baseline (mean of correct responses of rounds 9 to 12 [on day 1]), recall (mean of correct responses of rounds 1 to 3 [on day 2]), new sequence (mean of correct responses of rounds 4 to 6 [on day 2]), and learning (difference between recall and baseline). Data on procedural memory was available for 902 participants.
EEG Recordings, Sleep Analysis, and Spindle Count
Sleep was recorded at home using a mobile EEG recording device (Somnoscreen Neuro, Somnomedics, Germany). Six Ag-AgCl electrodes were placed according to the international 10–20 System (Fz, C3, Pz, Oz, left and right mastoid). Electrodes were physically referenced to Cz. Additionally, an electroocculogram (EOG), an electromyogram (EMG, chin) and an electrocardiogram (ECG) were recorded for standard polysomnography. Finally, an actimeter was used to monitor movements. EEG signals were recorded between 0.2–35 Hz, EOG between 0.2–35 Hz, and EMG and ECG between 1–128 Hz. The sampling rate for the EEG channels as well as for the EMG and the ECG channels was 256 Hz, and the sampling rate for the EOG channels was 128 Hz.
Sleep Scoring
Analysis of sleep data was restricted to the period between the lights-off and lights-on markers provided by the participants. If participants had forgotten these markers, sleep onset and offset were determined visually. For sleep stage analysis, data were referenced to the right mastoid. Sleep scoring of all sleep data was performed by an automatic algorithm (Somnolyzer 24 × 7) provided by the Siesta Group, Vienna, according to standard criteria.25 Scoring accuracy of the algorithm has been validated in several studies.26,27 For the total time in bed every 30-sec epoch was scored as NREM sleep stage 1, 2, 3, 4, or REM sleep with SWS defined by the sum of time spent in sleep stages 3 and 4. Sleep onset was defined by the first period in stage 1 sleep followed immediately by stage 2 sleep. SWS latency and REM sleep latency was determined with reference to sleep onset.
Frequency Analysis
For frequency analysis, EEG data was rereferenced to the averaged mastoids. Data of Cz, which was used as physical reference during data acquisition, were reinstated during re-referencing. Fz, Cz, C3, Pz, and Oz electrodes were then referenced to the averaged mastoids for frequency analyses. Then, sleep scoring data were imported and used as segmentation markers. Data were segmented to 30-sec periods of wakefulness, stage 1 sleep, NREM sleep (consisting of sleep stages 2, 3, and 4) and REM sleep. For frequency analysis, equally sized segments of EEG data consisting of 1,024 datapoints (4 sec) with 100-point overlap were created. Data quality was controlled by using an automatic artifact rejection procedure: segments were kept for further analysis when (1) the maximal difference in EMG activity was < 150 muV, (2) the maximal voltage step in each EEG channel (Fz, Cz, Pz, Oz) was < 50 muV/ms, and (3) the maximal difference in each channel was below 300 muV (500 muV during NREM sleep). Power in each frequency band was calculated in each artifact-free segment for each EEG channel using a fast Fourier transform (FFT) with a 10% Hanning window (resolution 0.25 Hz). Then, power spectra were averaged over all segments. Averaged power was calculated for the following frequency bands: slow oscillation band (0.5–0.75 Hz); delta band (0.75–4.5Hz); theta band (4.5–8 Hz); alpha band (8–11 Hz), slow spindle band (11–13 Hz); fast spindle band (13–15 Hz); beta band (15–25 Hz). Because 50-Hz artifacts were greatest for Oz, this electrode was discarded from the analysis.
Spindle Analysis
Spindles (counts and density) during NREM sleep stages were analyzed because of their well-known relationship with overnight retention of memories.15,28,29 Discrete spindles are a characteristic feature of sleep stage 2 and occur also in SWS, but are virtually absent during REM sleep. Slow (< 13 Hz) and fast spindles (> 13 Hz) were separately identified at the three selected EEG recording sites (Fz, Cz, Pz) during NREM sleep stages 2, 3, and 4, based on an algorithm adopted from previous studies.15,30 In brief, frequency power was extracted in the frequency bands of interest (10–13 Hz; 13–15 Hz), and the events were counted for which the power signal exceeded an fixed threshold (± 10 muV) for an interval lasting 0.5–3 sec. Spindles were counted separately in each channel during EEG segments free of movement artifacts (maximal EMG difference < 150 muV). Mean spindle counts were calculated by averaging spindle counts of all three channels. To calculate mean spindle density, mean spindle counts were divided by the number of analyzed 30-sec epochs. The two separate spindle bands were chosen based on previous studies that demonstrated the presence of two kinds of spindles in humans possibly linked to different aspects of cognitive function, i.e., slow spindles that prevail over the frontal cortex and show greater topographical variability than the fast spindles that concentrate over the parietal cortex.31,32
REM Analysis
Average REM density was calculated by dividing the number of 1-sec periods during REM sleep that contained REM by the total number of 1-sec REM sleep epochs.33 REM during REM sleep was detected automatically and defined as rapid signal changes in the EOG channel (> 0.8 mV/s) after movement arte-fact rejection and application of a 50-ms moving average.
Statistical Analysis and Data Reduction
Data were analyzed with bivariate Pearson correlations, partial correlations, regressions, repeated-measures analysis of variance, and t tests (SPSS Statistics 19.0). Statistical comparison of correlations coefficients was performed using the software ‘R’.34 Recalled pictures are presented as percentage of presented pictures. Overnight memory retention was calculated as relative retrieval performance, with learning performance before the retention interval set to 100%.
P < 0.05 was considered significant; where appropriate we corrected for multiple testing using Bonferroni correction. According to our 5 a priori hypotheses, we used a Bonferroni corrected significance level of pBonferroni = 0.05 (pnominal = 0.05 / 5 = 0.01) for statistical testing of the critical correlation coefficients. At this significance level, the statistical power for detecting correlations coefficients as low as r = 0.2 was 1-β > 99% in our study. Thus, in the case of nonsignificance, the nonexistence of correlations r > 0.2 can be inferred with a > 99% certainty. The significance level for all other correlations as well as for group comparisons was set to P = 0.05.
Unless indicated differently, values are presented as mean ± standard error of the mean. Because we found significant sex differences in memory recall and sleep measures, we conducted all analyses controlling for the influence of sex. We also detected a significant association of various sleep parameters with age; therefore, we additionally controlled for influences of age.
Results: Pilot Study
In a pilot study (n = 55; 18 men; mean age 24.73 y ± 3.55 [SD]), we first checked whether the used memory task is sleep dependent. Participants either encoded the pictures in the evening and recalled them after sleep (sleep group), or encoded the pictures in the morning and recalled the pictures in the evening (wake group). Sleep was not recorded and the participants did not complete any tasks other than encoding and recall of pictures. Memory retention was calculated as relative retrieval performance with learning performance before the retention interval (short-delay free recall of picture set 1) set to 100%. Participants who slept after picture encoding (n = 27) remembered significantly more pictures (75.69 ± 3.29 %) than participants being awake (n = 28; 59.68 ± 3.39 % t(53) = 3.39, P = 0.001). Importantly, the benefit of sleep on memory retention did not differ between the three valence categories (+13.78 %, +16.99 %, +19.67 %, for negative, positive, and neutral pictures, P = 0.80). In addition, we did not find any differences in learning performance of set 1 or 2 nor between learning performance of participants who slept after picture encoding and those being awake (short delay after 10 min, all P ≥ 0.28, see Table S1 (supplemental material) for descriptive values), excluding possible time of day effects. Thus, memory consolidation in this experimental paradigm clearly benefits from sleep in a valence-independent manner. However, because we used a between-subject design in our pilot study and do not have a control group that stays awake during the retention period overnight, possible effects of circadian rhythm on long delay recall cannot entirely be excluded.
Results: Main Study
Picture Memory Performance
In general, emotional pictures were significantly better remembered than neutral pictures in both the short- and the long-delay condition (all P < 0.001). However, overnight retention scores (with performance at learning set to 100%) did not differ between the three valence categories (negative pictures: 69.56 ± 0.74%, positive pictures: 69.22 ± 0.71%, neutral pictures: 68.99 ± 1.00%, P = 0.82). Compared to men, women had higher retention scores for positive pictures (P < 0.001) and marginally higher retention scores for negative pictures (P < 0.05). They did not differ with respect to the retention score for neutral pictures (P = 0.23). Age did not influence overnight retention scores (all P > 0.12). Memory variables conformed to a normal distribution (see Figure S1, supplemental material). Subjective arousal ratings of the pictures were highest for negative pictures (set 1: 2.33 ± 0.01; set 2: 2.25 ± 0.01), medium for positive (set 1: 1.84 ± 0.01; set 2: 1.76 ± 0.01), and lowest for neutral pictures (set 1: 1.37 ± 0.01; set 2: 1.31 ± 0.01, F(2,1830) = 4010.13, P < 0.001). Pictures seen on day 1 were generally rated as more arousing than pictures seen on day 2 (P < 0.001).
Sleep Parameters
The distribution of %SWS and %REM as well as fast sleep spindle density conformed to a normal distribution. The distribution of power values of SWA during NREM as well as theta during REM sleep were asymmetric and were therefore log-transformed to conform to a normal distribution (see Figure S2, supplemental material). Generally, women had a longer sleep duration than men (P < 0.001; Table S2, supplemental material) and a trend for a higher % SWS (P = 0.10). Men and women did not differ in relation to % REM sleep (P = 0.12). Furthermore, women showed higher SWA during NREM sleep, higher sleep spindle density, and higher theta activity during REM sleep than men (all P < 0.001; Table S2). Despite the rather narrow age range (i.e., 18–35 y; mean age 22.48 ± 3.60 y [SD]), older participants had significantly lower % SWS (r = −0.28, P < 0.001), lower SWA during NREM sleep (r = −0.40, P < 0.001), lower theta activity during REM sleep (r = −0.25, P < 0.001) as well as a lower spindle density during NREM sleep (r = −0.15, P < 0.001). Conversely, age correlated positively with percentage stage 2 sleep (r = 0.17, P < 0.001) and % REM sleep (r = 0.08, P = 0.01). Based on these results, all calculated associations between memory and sleep were corrected for age and sex.
Associations between Overnight Memory Consolidation and Sleep Parameters
Episodic Memory and Sleep
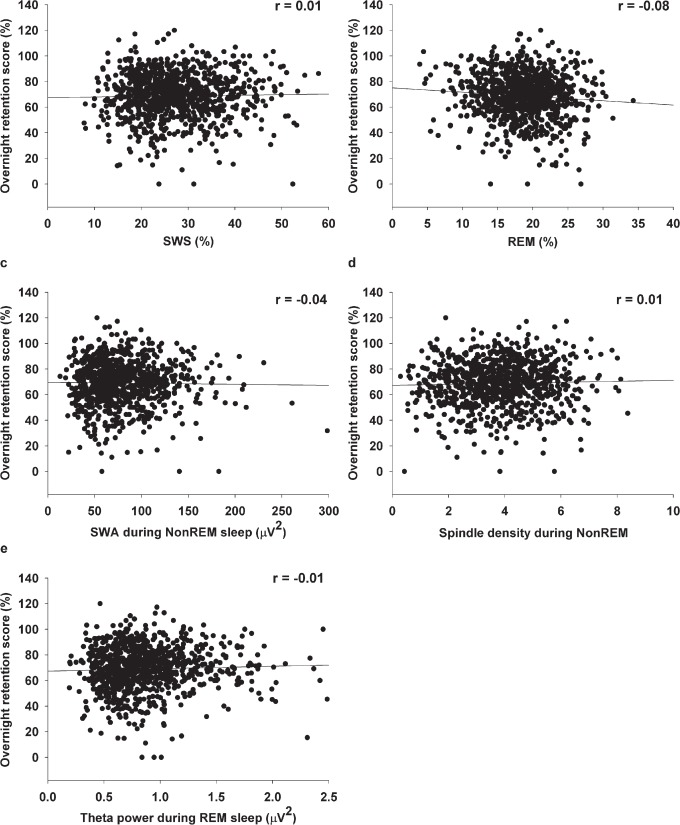
Unexpectedly, we could not confirm our hypotheses regarding an association between the overnight retention of neutral pictures and sleep parameters of NREM sleep. Neither %SWS (r = −0.01, pnominal = 0.70, pBonferroni > 0.99), SWA (r = −0.05, pnominal = 0.17, pBonferroni = 0.85) nor spindle density (r = −0.01, pnominal = 0.81, pBonferroni > 0.99; supplemental material) predicted overnight retention of neutral pictures. Similarly, no correlation was observed when using overnight retention of all pictures independently of their valence (all −0.04 ≤ r ≤ 0.01, Figure 2; Table 1). In addition, we did not observe any correlation with memory for emotional pictures and overnight theta activity during REM sleep (r = −0.02, pnominal = 0.60, pBonferroni > 0.99). We observed a nominally significant association between retention of emotional pictures and %REM sleep, which were unexpectedly negatively correlated (r = −0.60, pnominal = 0.03, pBonferroni = 0.15). A similarly directed correlation was also observed for neutral pictures (r = −0.07, pnominal = 0.05, pBonferroni = 0.25; Table S3, supplemental material) and for all pictures independent of their valence (r = −0.08, pnominal = 0.02, pBonferroni = 0.10, Figure 2, Table 1). However, these correlations did not withstand Bonferroni correction. Thus, in striking contrast to our expectation, none of the expected correlations between sleep parameters (% SWS, SWA during NREM sleep, spindle density during NREM sleep, %REM sleep, and theta activity during REM sleep) and overnight memory retention reached significance.
Figure 2.
Associations between sleep parameters and overnight memory retention (relative retrieval performance with learning performance before the retention interval (short-delay free recall picture set 1) set to 100%), independent of picture valences. (A) Association between slow wave sleep (SWS) and overnight memory retention score. (B) Association between rapid eye movement (REM) sleep and overnight memory retention score. (C) Association between slow wave activity (SWA) during nonrapid eye movement (NREM) sleep and overnight memory retention score. (D) Association between spindle density during NREM sleep and overnight memory retention score. (E) association between theta activity during REM sleep and overnight memory retention score.
Table 1.
Correlations between memory performance and sleep parameters (n = 929).
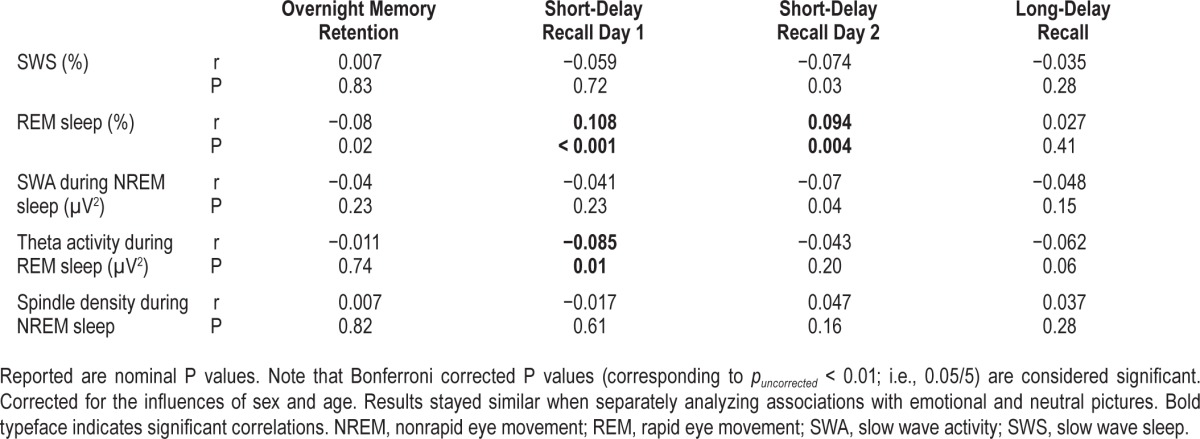
In addition, we analyzed whether sleep parameters correlate with short-delay recall (Table 1). Correlations between short-delay recall and %REM sleep reached Bonferroni-corrected significance (short-delay recall day 1: r = 0.108, pnominal < 0.001, pBonferroni < 0.005; short-delay recall day 2: r = 0.094, pnominal = 0.004, pBonferroni = 0.02). Also, the correlation between short-delay recall on day 1 and theta activity during REM sleep reached significance (r = −0.085, pnominal = 0.01, pBonferroni = 0.05).
When correcting for short-delay recall performance on day 1, the correlation between overnight memory retention and %REM sleep reached significance (r = −0.101, pnominal = 0.003, pBonferroni = 0.02). However, correlations between overnight memory retention and other sleep parameters stayed nonsignificant also when correcting for short delay recall performance on day 1.
In further exploratory analyses, neither total sleep time, nor %wake, nor %N1, nor %N2, nor SWS latency, nor REM latency, nor slow or fast spindle density during NREM sleep, nor REM density was associated with overnight memory consolidation (all pBonferroni ≥ 0.10; Table S3).
Control Measures
No correlation was observed between %SWS, %REM, %N2, SWA, theta activity during REM sleep, or spindle density during NREM sleep and overnight retention in the procedural memory task or the overnight improvement (accuracy and reaction time) in the working memory task (all −0.05 ≤ r ≤ 0.06, all pnominal > 0.05).
DISCUSSION
In the current study we show that although the picture memory task we used is clearly sleep dependent, recall performance in this task is not significantly associated with time spent in SWS or REM sleep. In fact, no associations between sleep and pictorial memory parameters reached significance after correction for multiple testing, except for a small negative correlation (only explaining roughly 1% of variance) between overnight memory retention and REM sleep when additionally correcting for short-delay recall on day 1.
Our results do not contradict the notion that sleep plays an important role for consolidating memories, and the results of our pilot study clearly replicate the improved recall of pictures after a retention period of sleep as compared to wakefulness (in a valence-independent manner). The nonfindings of sleep-memory associations in our study cannot be attributed to missing sleep dependency of the memory task we used in the current study. The type of recall, i.e., free recall, also does not explain our null findings, as beneficial effects of sleep on free recall have been repeatedly shown.7 Indeed, sleep effects on declarative memory are most pronounced for cued recall and free recall but are smaller for recognition tests.35 Moreover, using a declarative as well as a procedural memory task should not diminish the effects, as in previous studies using both tasks a clear benefit of sleep for the declarative memory task has been shown.36,37
However, our null results concerning sleep and memory associations clearly show that interindividual differences in specific sleep parameters (i.e., time spent in SWS or REM sleep, SWA during NREM sleep, theta during REM sleep, sleep spindle density) do not reliably predict interindividual differences in memory retention of emotional and neutral pictures across sleep. Please note that we did not test whether intraindividual differences in the amount of sleep stages (i.e., variations in sleep architecture across multiple nights in the same subject) are related to intraindividual variations in memory performance. The possible relation between sleep and memory measured in multiple occasions in the same participant might possibly be much more important for memory processes occurring during sleep as compared to interindividual differences and remains to be determined in future studies.
In many previous studies on interindividual correlations between sleep and memory parameters, large correlation coefficients have been found in relatively small sample sizes. With respect to declarative memory, several studies have reported correlations between different hippocampus-dependent declarative memory tasks and NREM sleep10–12 or spindle activity.13,15,38 In addition, associations between REM sleep and emotional memory have been reported.16 Furthermore, motor skill learning has been associated with stage N2 NREM sleep.28 Associations of motor skill learning with sleep spindles have been found as well.39,40 Often the reported correlations between sleep and memory parameters are large; in the aforementioned studies the reported r of significant correlations ranges from r = 0.5 and r = 0.9; explaining approximately 25% to 80% of the variance. All samples in which these correlations between memory parameters and sleep have been found are rather small (in the aforementioned studies sample sizes ranges between n = 6 and n = 31). However, in studies with small sample sizes, effects are often overestimated because of larger random variations in the sample from the true association in the population.19 Overestimation is even more likely when multiple testing is performed; for example, when comparing duration of different sleep stages (or night halves, night quarters, etc.) with several memory parameters, the likelihood of reporting false positives is increased. Unfortunately, an appropriate correction for multiple comparisons is also not always done in these studies. Finally, because of the small sample size, nonsignificant correlations cannot be reliably rejected because of a lack of statistical power.17,18 These circumstances may call into question the value of the reported effects and may to some extent explain the partially inconsistent findings seen in the current sleep and memory literature concerning correlations between different sleep stages and memory consolidation parameters. It is important to note that in most of the aforementioned studies, the reported association between sleep and memory parameters is typically not the main result of these studies, possibly explaining less rigorous testing of correlations.
In our exploratory analysis we also investigated associations between sleep time and memory parameters and did not find any significant results. Likewise, previous studies show that sleep time is not related to measures of cognitive performance nor brain size across mammalian species.41,42 As an example, guinea pigs and baboons have about the same sleep duration.41 Another example shows that bats sleep about 18 to 20 h a day whereas elephants sleep only about 3 to 4 h a day.42
Our study has some important limitations that limit the generalizability of our results. First, encoding in the current study was incidental, and our participants were not informed that they had to recall the pictures again after sleep. Previous studies have shown that effects of sleep on intentional encoded memory are larger than on incidentally encoded memory.35 Moreover, only if participants were told of the future relevance of the memories after incidental encoding, sleep benefited memory consolidation as compared to a retention period filled with wakefulness, and only then memory was correlated with SWA and spindle count during SWS.43 However, also in our study recall of pictures was improved after a retention interval filled with sleep as compared to wakefulness, excluding that the incidental encoding and lack of future relevance completely abolished the positive effect of sleep on memory in our study.
Second, our null findings are limited to memory for neutral and emotional pictures, and cannot necessarily be generalized to memory for associative stimuli (e.g. word-pairs) or spatial locations. However, free recall of pictures clearly is a declarative and episodic memory task involving hippocampal areas, and current theories on sleep and memory processes do not clearly separate episodic memory for pictures from other types of episodic memory. Future studies will need to test the existences of interindividual associations between sleep and memory parameters also for other learning tasks. Please note that we also did not find any correlations between sleep parameters and consolidation in a procedural sequential finger- tapping task.
Third, in contrast to many sleep and memory studies, study participants did not sleep right after learning. In our study, approximately 6 h passed between encoding and sleep onset, and during this time, participants were on their own and not in the laboratory. It might be possible that time spent in different sleep stages only predicts consolidation of memories that are encoded shortly before sleep. However, in our view, this would in fact strongly diminish the importance for memory consolidation processes occurring during sleep for memories encoded during the day.
Finally, we only recorded 1 night with polysomnography. Therefore, we cannot tell what effects are state effects and what effects are trait effects. However, sleep EEG is a stable marker,5 and study participants underwent an adaptation night wearing a dummy EEG recording device and therefore were used to wearing the portable EEG recording device. As mentioned previously, to be able to distinguish intraindividual and interindividual effects of sleep parameters on memory, several nights of sleep EEG should be recorded. Furthermore, a comparison of subjects showing normal sleep patterns with subjects having sleep disturbances might have shed more light on the association of sleep parameters and memory consolidation and the role of normal versus disturbed sleep.
In conclusion, we found that interindividual differences only contribute to a small extend to the effect of sleep on memory consolidation. The current results point to a stronger involvement of intraindividual differences in sleep in respect to overnight memory consolidation as compared to interindividual factors and lead to the question whether sleep in general is important for memory consolidation but not so much the various sleep stages per se.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was from grants of the University of Basel (to Dr. Rasch).; the Swiss National Science Foundation (PP00P3-123391 to Dr. de Quervain, PP00P3-114813 to Dr. Papassotiropoulos, CRSIK0_122691 to Dr. Papassotiropoulos and Dr. de Quer-vain, and PP00P1_133685 to Dr. Rasch); the European Science Foundation (EUROStress to Dr. Papassotiropoulos and Dr. de Quervain) and from the University of Zurich, clinical research priority project “Sleep and Health” (to Dr. Rasch and Dr. Erich Seifritz). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Erich Seifritz for his support through the clinical research priority project “Sleep and Health.” We thank Sami Abdel Aziz, Stefanie Bartocha, Désirée Bruttin, Tobias Egli, Karen Hellhammer, Nathalie Holz, Marietta Jäger, Carmen Kohler, Katrin Meier, Ursina Moor, Julia Rihm, Sarah Schoch, Myriam Stebler, Manuela Steinauer, Valeria Suhl, Eva Vanova, Irina Wächter, and Cedric Zeindler for their help in conducting the experiments.
ABBREVIATIONS
- ECG
electrocardiogram
- EEG
electroencephalography
- EMG
electromyogram
- EOG
electroocculogram
- IAPS
International Affective Picture System
- N2
stage 2 sleep
- REM
rapid eye movement
- SWA
slow wave activity
- SWS
slow wave sleep
SUPPLEMENTAL MATERIAL
Descriptives of memory performance in the pilot study (n = 55).
Descriptives of sleep parameters in men and women (n = 929).
Correlations between sleep parameters and memory recall (sleep stages: n = 929; frequency measures: n = 885).
Histograms of memory measures (n = 929). Short-delay free recall measures from day 1 and day 2 as well as the long-delay free recall measure are depicted as % recalled of all pictures presented. Overnight memory retention was calculated as relative retrieval performance with learning performance before the retention interval (short delay free recall day 1) set to 100%.
Histograms of sleep stages (n = 929) and sleep electroencephalography frequencies (n = 885). Because the distributions of slow wave activity (SWA) during nonrapid eye movement (NREM) sleep and theta activity during rapid eye movement (REM) sleep were asymmetric, data from those two variables was log-transformed for all analyses.
REFERENCES
- 1.Volk HE, McDermott KB, Roediger HL, Todd RD. Genetic influences on free and cued recall in long-term memory tasks. Twin Res Hum Genet. 2006;9:623–31. doi: 10.1375/183242706778553462. [DOI] [PubMed] [Google Scholar]
- 2.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8:11–3. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 3.De Gennaro L, Marzano C, Fratello F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–60. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosius U, Lietzenmaier S, Wehrle R, et al. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64:344–8. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Buckelmüller J, Landolt H-P, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 7.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner U, Kashyap N, Diekelmann S, Born J. The impact of post-learning sleep vs. wakefulness on recognition memory for faces with different facial expressions. Neurobiol Learn Mem. 2007;87:679–87. doi: 10.1016/j.nlm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep ? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Cox R, Hofman WF, Talamini LM. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012;19:264–7. doi: 10.1101/lm.026252.112. [DOI] [PubMed] [Google Scholar]
- 14.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–60. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler K. Voodoo correlations are everywhere - not only in neuroscience. Perspect Psychol Sci. 2011;6:163–71. doi: 10.1177/1745691611400237. [DOI] [PubMed] [Google Scholar]
- 19.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–16. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 20.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- 22.Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–43. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- 23.Rasch B, Spalek K, Buholzer S, et al. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service. Brain Information Institute UCLA; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human sleep. [Google Scholar]
- 26.Anderer P, Gruber G, Parapatics S, Dorffner G. Automatic sleep classification according to Rechtschaffen and Kales. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3994–7. doi: 10.1109/IEMBS.2007.4353209. [DOI] [PubMed] [Google Scholar]
- 27.Anderer P, Gruber G, Parapatics S, et al. An E-health solution for automatic sleep classification according to Rechtschaffen and Kales: validation study of the Somnolyzer 24 x 7 utilizing the Siesta database. Neuropsychobiology. 2005;51:115–33. doi: 10.1159/000085205. [DOI] [PubMed] [Google Scholar]
- 28.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Schimicek P, Zeitlhofer J, Anderer P, Saletu B. Automatic sleep-spindle detection procedure: aspects of reliability and validity. Clin Electroencephalogr. 1994;25:26–9. doi: 10.1177/155005949402500108. [DOI] [PubMed] [Google Scholar]
- 31.Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitlhofer J, Gruber G, Anderer P, Asenbaum S, Schimicek P, Saletu B. Topographic distribution of sleep spindles in young healthy subjects. J Sleep Res. 1997;6:149–55. doi: 10.1046/j.1365-2869.1997.00046.x. [DOI] [PubMed] [Google Scholar]
- 33.Ficca G, Scavelli S, Fagioli I, Gori S, Murri L, Salzarulo P. Rapid eye movement activity before spontaneous awakening in elderly subjects. J Sleep Res. 2004;13:49–53. doi: 10.1046/j.1365-2869.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 34.R Development Core Team. Vienna: R Foundation for Statistical Computing; 2012. R: a language and environment for statistical computing. [Google Scholar]
- 35.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Rasch B, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
- 37.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–4. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 39.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 40.Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–11. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mo M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–9. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptives of memory performance in the pilot study (n = 55).
Descriptives of sleep parameters in men and women (n = 929).
Correlations between sleep parameters and memory recall (sleep stages: n = 929; frequency measures: n = 885).
Histograms of memory measures (n = 929). Short-delay free recall measures from day 1 and day 2 as well as the long-delay free recall measure are depicted as % recalled of all pictures presented. Overnight memory retention was calculated as relative retrieval performance with learning performance before the retention interval (short delay free recall day 1) set to 100%.
Histograms of sleep stages (n = 929) and sleep electroencephalography frequencies (n = 885). Because the distributions of slow wave activity (SWA) during nonrapid eye movement (NREM) sleep and theta activity during rapid eye movement (REM) sleep were asymmetric, data from those two variables was log-transformed for all analyses.