Abstract
Study Objectives:
Sleep neurobiology studies use nocturnal species, mainly rats and mice. However, because their daily sleep/wake organization is inverted as compared to humans, a diurnal model for sleep studies is needed. To fill this gap, we phenotyped sleep and waking in Arvicanthis ansorgei, a diurnal rodent widely used for the study of circadian rhythms.
Design:
Video-electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG) recordings.
Setting:
Rodent sleep laboratory.
Participants:
Fourteen male Arvicanthis ansorgei, aged 3 mo.
Interventions:
12 h light (L):12 h dark (D) baseline condition, 24-h constant darkness, 6-h sleep deprivation.
Measurements and Results:
Wake and rapid eye movement (REM) sleep showed similar electrophysiological characteristics as nocturnal rodents. On average, animals spent 12.9 h ± 0.4 awake per 24-h cycle, of which 6.88 h ± 0.3 was during the light period. NREM sleep accounted for 9.63 h ± 0.4, which of 5.13 h ± 0.2 during dark period, and REM sleep for 89.9 min ± 6.7, which of 52.8 min ± 4.4 during dark period. The time-course of sleep and waking across the 12 h light:12 h dark was overall inverted to that observed in rats or mice, though with larger amounts of crepuscular activity at light and dark transitions. A dominant crepuscular regulation of sleep and waking persisted under constant darkness, showing the lack of a strong circadian drive in the absence of clock reinforcement by external cues, such as a running wheel. Conservation of the homeostatic regulation was confirmed with the observation of higher delta power following sustained waking periods and a 6-h sleep deprivation, with subsequent decrease during recovery sleep.
Conclusions:
Arvicanthis ansorgei is a valid diurnal rodent model for studying the regulatory mechanisms of sleep and so represents a valuable tool for further understanding the nocturnality/diurnality switch.
Citation:
Hubbard J, Ruppert E, Calvel L, Robin-Choteau L, Gropp CM, Allemann C, Reibel S, Sage-Ciocca D, Bourgin P. Arvicanthis ansorgei, a novel model for the study of sleep and waking in diurnal rodents. SLEEP 2015;38(6):979–988.
Keywords: Arvicanthis ansorgei, circadian rhythm, crepuscular, direct effects of light, diurnality, nocturnality, rodent, sleep deprivation, sleep homeostasis, sleep regulation
INTRODUCTION
The majority of laboratory sleep research currently focuses on nocturnal rodents, primarily mice, due to the transgenic tools available, including optogenetics. Drosophila and zebrafish, two other powerful genetic models, have been used for sleep studies, yet restrictions such as the lower complexity of neuronal networks limit their interest for research on mammalian physiology and behavior. To date, sleep has been characterized in a large range of species. Historically, sleep research was conducted on disparate animals such as cats or even rabbits, though now is all but abandoned (experiments and colonies expensive to maintain, lack of genetic and biological tools). Though mice and rat research have provided major insights into sleep neurobiology, these animals are nocturnal, underlying the need for a diurnal rodent model.
To our knowledge, a chipmunk Eutamias sibiricus and a ground squirrel Citellus spp, are the only truly diurnal rodents in which sleep has been studied using electroencephalography (EEG) recordings.1,2 Sleep and wake distribution across the 24-h day of the chipmunk showed that they sleep for about one quarter of the 12-h light period and three quarters of the 12-h dark period.1Additionally, a significant increase in the level of EEG delta power, a marker of sleep need, was seen following a longer 24-h sleep deprivation.1 Though these initial results were promising, further experimentation was stopped, probably due to the isolated nature of this research, conducted on a poorly characterized model, as well as the lack of laboratory colonies leading to difficulties in performing additional studies. Sleep regulation has also been studied in Octodon degus, a dual-phasing rodent showing crepuscular timed episodes of sustained waking evocative of a bimodal crepuscular modulation of arousal.3,4 Furthermore, selective rapid eye movement (REM) sleep deprivation resulted in consistent REM sleep rebound only after nocturnal deprivation, which suggested a unimodal promotion of nocturnal REM sleep. Inevitably, the O. degus exhibits no strong preference for sleep during the light or dark phase, and thus further sleep studies were not conducted in this species.
In the field of chronobiology, the diurnal muridae of the Arvicanthis family (Arvicanthis ansorgei and Arvicanthis niloticus) have proven to be useful models. Originally, more than 100 A. ansorgei were screened for daily patterns of wheel-running activity in our laboratory.5 The colony was then extended and maintained for 15 y, with recurrent import of new animals to reinforce the genetic diversity of the species. Most of the animals expressed a clear diurnal pattern of locomotor activity.6,7 Subsequent studies analyzed the circadian expression of clock genes (Per1, Per2, Cry2, Bmal1)8 and clock-related neuropeptides (AVP, VIP, and GRP)9 in the suprachiasmatic nucleus (SCN) where the primary clock is located. The circadian rhythmic expression of these clock genes and neuropeptides was roughly similar to what was observed in nocturnal rodents.8,9 Melatonin and its rate-limiting enzyme, arylalkylamine N-acetyltransferase (AA-NAT) are expressed in A. ansorgei during the night or dark phase, similarly to the distribution observed in human.10,11 This underlies not only a parallel to humans for translational research but also the possibility to study the melatoninergic regulation of sleep, whereas most laboratory mice strains used in research do not express melatonin (i.e. C57BL6/J, 129/Sv).12 Moreover, the phase response curve to light pulses in A. ansorgei is comparable to both nocturnal and other diurnal species,8 whereas the circadian responses to dark pulses differ from those of nocturnal rodents.13 Resetting of the circadian clock through the use of hypocaloric feeding in A. ansorgei results in phase delays of the SCN pacemaker, contrary to nocturnal rodents.14 Additionally, the proportion of the retinal photoreceptors, rods, cones and melanopsin, in the retina of the A. ansorgei, and subsequently their phototransduction systems that mediate the nonvisual effects of light, are closer to what is observed in humans.15,16 These data taken together have confirmed the relevance of A. ansorgei as a diurnal model, yet it remains essential to phenotype sleep and to characterize sleep regulatory mechanisms in this species.
In the current study we recorded sleep for the first time in A. ansorgei and sought to address whether this species is a valid diurnal model for studying the regulatory mechanisms of sleep and waking.
METHODS
Animals, Housing Conditions, and Sleep Deprivation
A. ansorgei were obtained from our animal facility, the Chronobiotron CNRS UMS 3415, housed at the Institute for Cellular and Integrative Neurosciences, France. Animals were raised under environmentally stable conditions [12-h:12-h light-dark schedule (12hL:12hD); 150 lux; 23 ± 0.5°C; food and water ad libitum] and were maintained according to the European Union guidelines for laboratory animal experimentation. Light-dark and dark-light (LD, DL) transitions switched instantly, thus not mimicking natural twilight. Sleep deprivation was performed by gentle handling as described previously.17 All experimental sleep protocols were supervised by a veterinarian and approved by the ethical committees for animal research at the University of Strasbourg and CNRS.
Wheel-Running Activity Monitoring
Prior to electrode implantation, running-wheel actimetry (DataportDP24) was performed under standard 12hL:12hD conditions using appropriate software (VitalView, Mini-Mitter). Analysis and production of the actograms were performed using Clocklab (Actimetrics) following data transformation via Matlab. Fourteen male A. ansorgei were included in the study, all of which showed a clearly diurnal profile of wheel running activity (Figure 1A).
Figure 1.
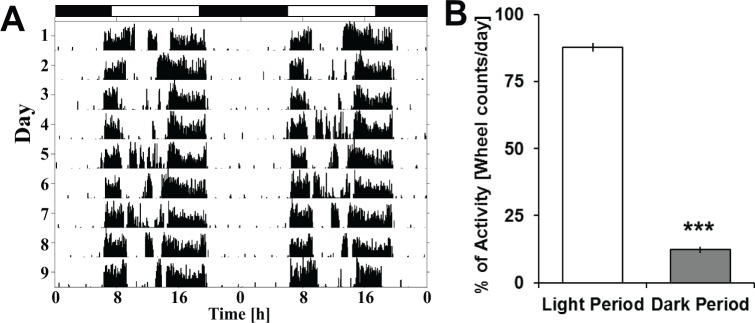
Daily wheel-running activity under a standard 12hL:12hD cycle. (A) Actimetry sample from a single animal, double-plotted and centered at ZT0 (7h00) for a total period of 11 days under standard baseline conditions. (B) Differences for total wheel counts under actimetry recording during the light versus dark period. Analysis was done using Student t test and found to be highly significant. Asterisks represents Student t test significance (P < 0.00001).
Surgery and Experiments
Surgery protocol for electrodes implantation was performed according to methods previously described17 with minor changes to adapt to the Arvicanthis species. The electrodes were implanted under deep anesthesia (intraperitoneal injection of ketamine 80 mg/kg and xylazine 7 mg/kg), then soldered to a connector and cemented to the skull before the skin was sutured. Animals were implanted with two EEG electrodes on the dura using the same coordinates as in the rat (frontal: 2.0 mm lateral to midline, 2.0 mm anterior to bregma; parietal 2.0 mm lateral to midline, 2.0 mm anterior to lambda),18 one reference electrode, two EMG electrodes inserted into the neck muscles along the back of the skull and, in a subset of rodents (n = 4), two EOG electrodes placed next to the orbital socket to record eye movements observed during waking and especially during REM sleep. Video was also recorded in these animals to verify the behavioral state in conjunction with EEG. Given the increased activity of Arvicanthis, compared to laboratory mice or rats, we designed a specific cable in order to prevent decoupling of the electrode chip as well as we reinforced it with INOX metal to prevent destruction by the animal. The cable material, however, was flexible and relatively light and the increased weight of this system was offset by the size of the A. ansorgei, which averaged 150 g at the time of implantation. All experiments were performed using male A. ansorgei, aged approximately 3 mo at the time of implantation. A minimum of 14 days was given to recover from surgery and to habituate to the baseline conditions before any protocol began. Signals were recorded for analysis using commercially available hardware and software (Micromed Macon, France; SystemPLUS Evolution version 1092). Sleep recordings were performed under several experimental conditions: (1) baseline 12hL:12hD cycle, (2) 24 h of constant darkness (24h D:D), (3) 6-h sleep deprivation starting at Zeitgeber time (ZT)12. All experiments occurred on different days and a minimum of 14 days under 12hL:12hD between each condition was used to rehabituate the animals to the control conditions. All animals were recorded simultaneously.
EEG Sleep Scoring and Power Spectrum Analysis
EEG and EMG signals were amplified, filtered, and analog-to-digital converted to 256 Hz. The vigilance states for each 4-sec epoch were then classified as waking, non-rapid eye movement (NREM) sleep, or REM sleep, using visual inspection without knowledge of the recording condition. The scoring was performed according to criteria similar to those classically used for other rodents.19 The EEG during REM sleep was characterized by a regular, low-amplitude theta (6–10 Hz) rhythm and a low EMG signal. During NREM, the EEG amplitude was larger and dominated by synchronized delta activity (0.75–4 Hz) and the EMG signal was low. Wakefulness was characterized by a higher and variable EMG and a low-amplitude EEG with both slower (delta during drowsiness) and faster (theta during exploratory behavior) components. Furthermore, as sleep and waking had not previously been categorized in these animals, initial scoring was verified in several animals using EEG/EMG/EOG in conjunction with infrared video recording (n = 4). Video recording was made using a commercially available night-vision camera (Sony HDR-CX550VE; Sony Corporation, Tokyo, Japan) mounted on a tripod to confirm concordance between behavior and EEG scoring (wake, cessation of EMG activity during NREM, cessation of EMG activity coupled with EOG measured REMs during REM sleep). The EEG signal was subjected to a rectangular discrete-Fourier transform with a 50% overlap, yielding power spectra between 0 and 128 Hz (0.25 Hz resolution) using a 4-sec window and a 50 Hz filter to remove power-line artifacts. All 4-sec epochs containing other signal artifacts were marked so they could be excluded from EEG spectral analyses. Amounts spent in each vigilance state were calculated by averaging time spent either in 5 min, 1-, 12-, and 24-h intervals. Moreover, an average spectral profile was constructed using all 4-sec epochs scored with the same vigilance state. The absolute spectral power of the delta band frequencies were calculated (0.75–4 Hz) and normalized to the period during baseline (12hL:12hD) with the lowest average value corresponding to the last 4 h of darkness (ZT20–24). Previously, this type of analysis has been performed in mice and rats; however, their lowest delta power is seen at the end of the light period (ZT8–12), consistent with their inherent nocturnality. Only continuous NREM periods of greater than 1 min were included in the analysis. To sleep deprive the animals, a starting time was chosen consistent with the animal's chronobiological diurnality (ZT12–18), in opposition to previous experiments performed in nocturnal laboratory rodents.17 Delta power analysis following this 6-h NREM sleep deprivation was performed in the same manner. Time-dependent changes in EEG power for specific frequency bands under NREM sleep were performed for delta (0.75–4 Hz), and during REM, theta (6–10 Hz), and in wake, both theta (6–10 Hz) and gamma (40–70 Hz).
Statistical Analysis
Statistical analysis was realized using Statistica (Statsoft, Tulsa, OK, USA; version 8) with graphic representations created either in SigmaPlot (Systat, San Jose, CA, USA; version 11) or Microsoft Excel (Microsoft Corporation, Redmond, WA, USA; version 2010). To measure time-dependent changes in vigilance state amounts or bout lengths, and power spectra of EEG, either one-way or repeated-measures analysis of variance were used. Specific tests are listed in each figure legend. To determine post hoc significance, testing for protected least significant difference (PLSD) was performed unless otherwise specified.
RESULTS
In chronobiology, locomotor activity is the most widely used method for identifying the daily rhythm of the biological clock in order to determine whether the studied animals are diurnal or nocturnal; however, the characterization of their sleep-wake regulation was missing. In the current study, all activity recordings confirmed a diurnal profile using wheel- running actimetry (Figures 1A and 1B).
Polysomnographic Characterization of Sleep and Waking in A. ansorgei
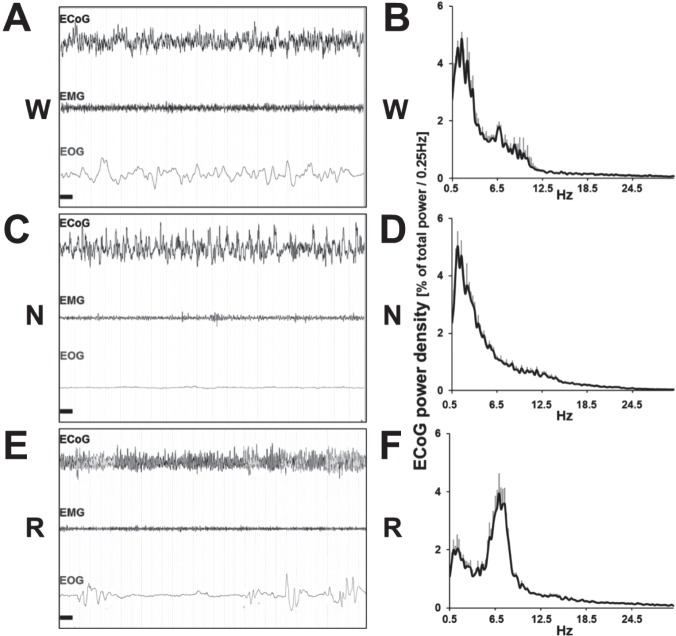
Initial scoring was verified in four animals using EEG/EMG/ EOG in conjunction with video recording (Videos 1–3, supplemental material; for details, see aforementioned methods). Epochs scored as wake (W) showed highly desynchronized EEG and high muscle activity on the EMG, as well as non-rhythmic eye movements on the EOG (Figure 2A, Video 1). The EEG spectral profile showed higher power within the theta (6–10 Hz), and delta range (0.75–4 Hz), this latter being also observed in nocturnal rodents during wake (Figure 2B). NREM sleep (N) was identified according to highly synchronized slow oscillating delta waves and lower activity of higher frequency bands, associated with low muscle tone, consistent with a resting state. Additionally no eye movements were observed (Figure 2A). The EEG power spectrum was dominated by delta frequencies (0.75–4 Hz), characteristic EEG rhythm of NREM sleep (Figure 2B, Video 2). REM or REM sleep was characterized by highly desynchronized EEG activity, coupled with complete muscle atonia, and REMs every several seconds (Figure 2C, Video 3). Note the presence of high peaks of theta waves in the absence of delta associated to muscle atonia and an EEG characteristics of this state (Figure 2C). These vigilance states were additionally verified using video monitoring of a subset of animals (n = 4). During wake states, animals were visibly active and commonly engaged in food burying. This activity increased during light-to-dark and dark-to-light transitions (Video 1). Under NREM sleep, animals remained immobile and in a similar position to that classically observed in laboratory rodents (Video 2). Finally, when animals were engaged in REM sleep, the video confirmed the lack of movements and muscle tone (Video 3).
Figure 2.
Samples of polygraphic recordings and electroencephalography (EEG) power spectrum profile in wake, non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep under baseline condition. Samples of EEG, electromyography (EMG), and electrooculography (EOG) recordings obtained during baseline 12hL:12hD condition: (A) During waking a desynchronized EEG pattern occurs in parallel with high levels of EOG and EMG activities. (B) Power spectrum analysis of all waking epochs during baseline. Peaks are noticed in both the delta (0.5–4 Hz) and theta (6–10 Hz) ranges. (C) Recordings during NREM sleep show synchronized slow-wave activity oscillations in the EEG, with a near complete suppression of EMG and EOG activity. (D) Spectral profile is dominated by delta frequencies. (E) During REM sleep a desynchronized EEG pattern emerges with a total flattening of the EMG, reflecting the atonic state of the animal. Rapid eye movements are indicated by EOG activity. (F) Power spectrum profile is dominated by theta activity. Graph represents peak relative frequencies of total power between 0.5–25 Hz and standard error of the mean. Horizontal black bars: 1 sec, window represents 20 sec of recording. N, non-rapid eye movement; R, rapid eye movement; W, wake.
Distribution of Sleep and Wakefulness during the 12hL:12hD Baseline
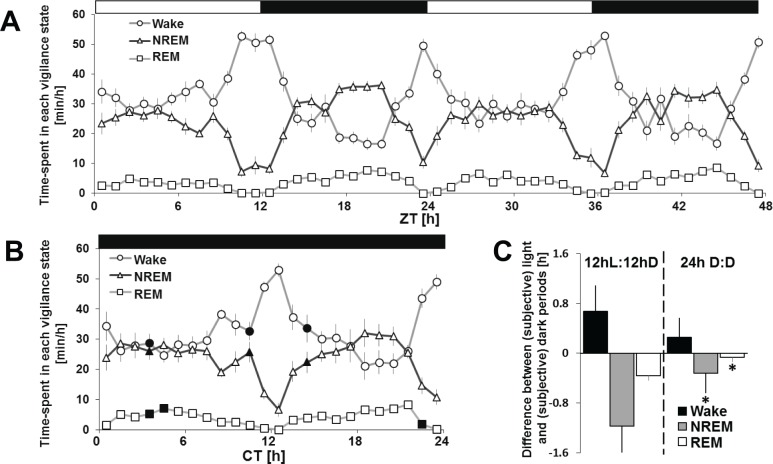
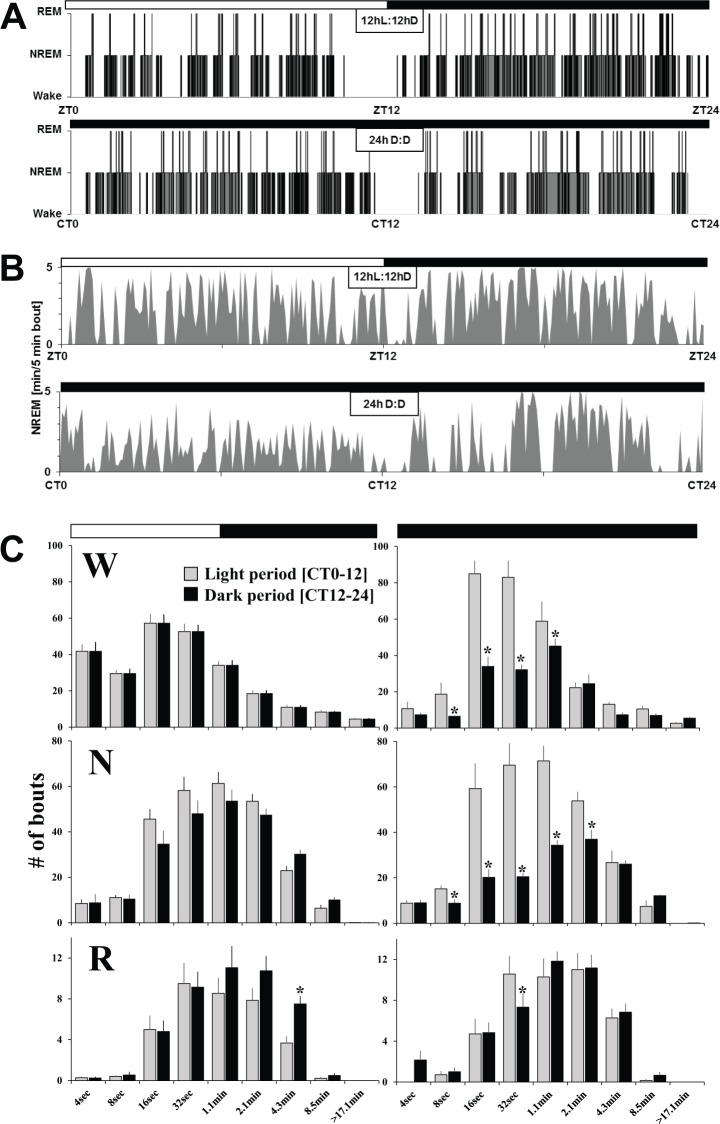
Analysis of 2 consecutive days under a standard 12hL:12hD cycle showed that average wake over 2 days accounted for 58% (7.01 h ± 0.3) during the light period compared to 50% (5.99 h ± 0.3) in the dark phase (Figures 4A and 4C; Table S1, supplemental material). There was a significant difference of time spent in NREM [37% (4.42 h ± 0.3) versus 43% (5.13 h ± 0.2)] or REM [4.7% (34.8 min ± 4.5) versus 7.3% (52.8 min ± 4.4)] between the light and dark periods. Baseline day 1 and 2 were not significantly different (Table S1). Across the 24-h period, wake and NREM sleep closely mirror each other (Figure 4A). Moreover, the length of wake and sleep bouts was not different between 12 h light and dark phases (Figure 3C). Finally, under a standard 12hL:12hD condition, the 24-h sleep and wake distribution, represented as a hypnogram, was overall inverted to that observed in rats or mice (Figure 3A). This is further confirmed by the 24-h distribution of NREM sleep expressed per 5-min bouts (Figure 3B).
Figure 4.
Time-course of sleep and waking under 12hL:12hD and 24h D:D. Vigilance states are represented as the amount of minutes per hour across 48-hours of the 12hL:12hD cycle (A) or 24h D:D (B). (C) Difference between the light and dark periods of total amounts of wake, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep during the 12hL:12hD baseline condition (left), and difference of vigilance states between the subjective light and dark periods under 24h D:D (right). All values are expressed as mean ± standard error of the mean. A two-way ANOVA for time-course and light condition showed significance for any vigilance states between baseline and constant darkness (black points- one-way ANOVA for light condition, post hoc t test, P < 0.05). Asterisks denote significant differences (P < 0.05). CT, circadian time; D, dark; L, light.
Figure 3.
Twenty-four hour distribution of sleep and waking under 12hL:12hD and 24h D:D. Examples of a hypnogram based on 4-sec epoch scoring (A) and of non-rapid eye movement (NREM) sleep per 5-min bouts (B) in two different Arvicanthis ansorgei across 12hL:12hD and 24h D:D conditions. Frequency distribution of W, NREM sleep, and REM sleep episode lengths (C) during 12hL:12hD versus 24h D:D. Vertical bars represent the number of episodes (mean + standard error of the mean) expressed as time spent during light or dark periods (left) or subjective light or dark periods (right), in each state by episode duration. Two-way analyses of variance (ANOVAs) were performed to examine bout length differences as a function of light/dark period as well as lighting condition (12hL:12hD versus 24h D:D). Significance was seen at differing points. pLight condition × L vs. D < 0.05 (W- 16 sec, 17.1 m; N- 16 sec, 32 sec, 1.1 min; R- 4 sec). pLight condition < 0.05 (W- 4 sec, 8 sec, 1.1 min; R- 4 sec). pL vs. D < 0.05 (W- 8 sec, 16 sec, 32 sec, 4.3 min, 8.5 min; N- 8 sec, 16 sec, 32 sec, 1.1 min, 8.5 min; R- 4 sec, 4.3 min). Asterisks represent significance between subjective light and dark phases (one-way ANOVAs, post hoc t tests P < 0.05). D, dark; L, light; N, non-rapid eye movement; R, rapid eye movement; W, wake; ZT, Zeitgeber time; CT, circadian time.
Circadian, Direct Photic, Crepuscular, and Homeostatic Components Regulate Sleep and Waking
Circadian and Direct Photic Noncircadian Regulation of Sleep and Waking in A. ansorgei
We analyzed sleep under a 24-h constant darkness condition to determine whether the 24-h distribution of sleep and waking observed under a standard 12hL:12hD cycle was regulated by a clock-driven mechanism and/or due to the direct influence of light on sleep.17,20 The results confirm that both sleep regulatory mechanisms play a role. Under constant darkness, sleep and waking display an overall daily distribution relatively similar to that of baseline 12hL:12hD confirming the clock-driven circadian regulation of the sleep-wake cycle (Figures 4A and 4B). The difference in sleep amounts was quantified between the light and dark phase under 12hL:12hD, which represents the amplitude of the daily sleep-wake cycle. When comparing this light/dark difference to the difference in sleep amounts between the corresponding subjective periods under 24h D:D (CT0–12, subjective light period and CT12–24, subjective dark period), we observed that the amplitude of the sleep-wake cycle is attenuated in constant darkness, due to the lack of wake promotion by light (Figure 4C). Indeed, in constant darkness we observed during the subjective light period, higher amounts of shorter wake bouts as compared to the standard 12hL:12hD condition, suggesting a light-dependent consolidation of wakefulness (Figure 3C). The longest wake bout lasted on average 57.1 (± 8.5) min during the 12hL:12hD condition, compared to 27.2 (± 2.7) min during 24-h darkness (one-way analysis of variance light condition: P = 0.007). Conversely, during the subjective dark period, we observed under constant darkness lower amounts of shorter NREM sleep bouts as compared to the standard 12hL:12hD condition. Finally, the frequency distribution of REM sleep bouts did not show any significant differences between the 12hL:12hD and 24h D:D conditions.
When the average length of sleep bouts are calculated, a significant difference is seen for wake bouts under 12hL:12hD condition (light: 9.11 ± 0.65 min versus dark: 7.24 ± 0.44 min) (Table S2, supplemental material). Under constant darkness there is a significant difference in the duration of episodes between subjective light and dark periods for both wake (subjective Light: 5.04 ± 0.18 min, sub. Dark: 7.84 min ± 0.89) and NREM sleep (subjective Light: 2.28 ± 0.18 min, subjective Dark: 3.25 ± 0.15 min) bout lengths. Interestingly, there is a significant increase in average wake bout length during the light period under 12hL:12hD (9.11 ± 0.65 min) versus subjective light under 24h D:D (5.04 ± 0.18 min), whereas no significant change is found during dark and subjective dark periods (Table S2). Average NREM sleep bout length is increased during the subjective dark period under 24h D:D (3.25 ± 0.15 min), as compared to the dark period under 12hL:12hD (2.72 ± 0.14 min) (Table S2).
Crepuscular Regulation of Sleep and Waking
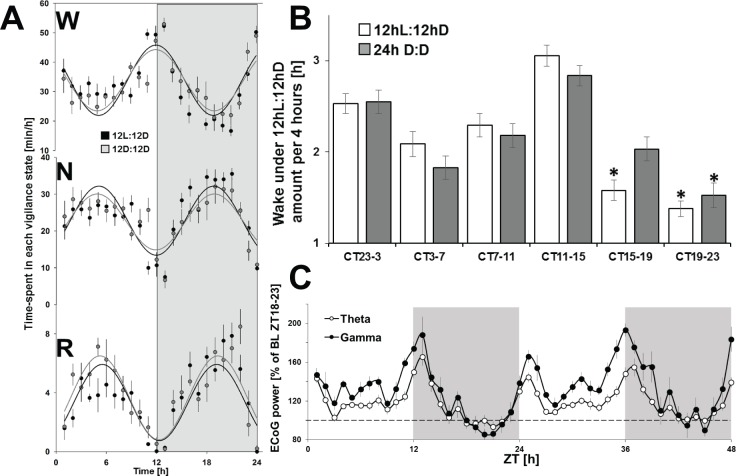
Abrupt changes in wake and sleep are observed in Arvicanthis at L-D-L transitions with two sustained periods of wake at light and dark onsets, referred to as crepuscular (Figures 4A, 5A, and 5B).4 This bimodal profile persists under constant darkness (Figures 4B, 4C, 5A, and 5B) with a maximum of waking amounts 2 h immediately before and following the light or dark onset (Figure 5B). Additionally, EEG power spectrum analysis at light-dark-light transitions showed highly increased EEG theta and gamma activities (Figure 5C), EEG correlates of cognition and exploratory behavior in rodents.21,22 Thus, this bimodal expression of waking corresponds to a cyclic pattern with a period of 12 h. Therefore, we then applied sine-wave calculations to the results obtained during baseline under 12hL:12hD and 24-h constant darkness. The time course of each vigilance state fits a sine wave curve of 12 h periodicity without significant differences between both lighting conditions (Figure 5A). These data suggest that A. ansorgei has a 12-h periodic expression of sleep and waking, resulting from a prevailing crepuscular regulatory mechanism.
Figure 5.
Crepuscular regulation of sleep and waking. (A) Sine-waves were calculated for each vigilance state to determine their mathematical profile during 12hL:12hD (gray) and 24h D:D (black). No significant difference was observed between the two conditions. (B) Amounts of wake per periods of 4 h centered on the (subjective – gray bar) D-L-D transitions show predominant wake activity at (subjective – gray bar) circadian time points (CT) and an increased wake during the (subjective – gray bar) light period outside of the (subjective – gray bar) crepuscular zone, as compared to the dark (subjective – gray bar) period outside the (subjective – gray bar) crepuscular zone. Asterisks denote significant differences (P < 0.05) by t test, between the different 4-h periods. All values are expressed as mean ± standard error of the mean. (C) Time-course of electroencephalography theta and gamma power spectrum during waking epochs expressed per hour across the two baseline days. D, dark; L, light; ZT, Zeitgeber time.
Process S is Conserved in A. ansorgei
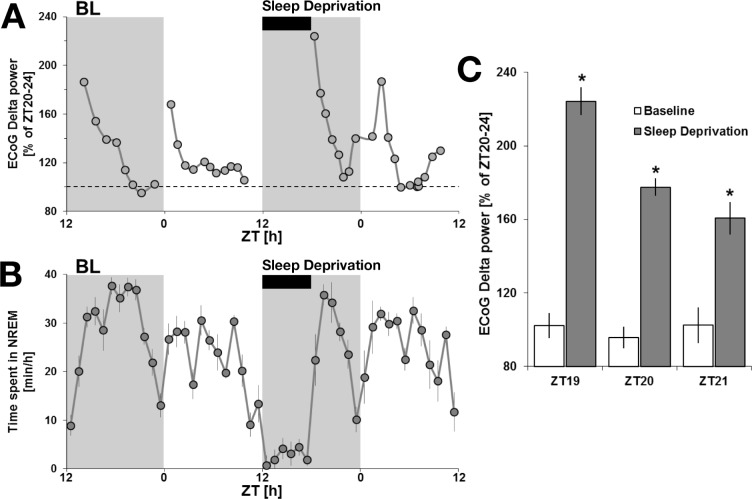
EEG delta activity is the most reliable marker of the buildup of homeostatic sleep need with time spent awake. Sleep deprivation represents the best approach to challenge the sleep homeostat.23,24 Analysis of baseline days revealed an increase in delta power following activity periods corresponding to the animal's crepuscular peaks of activity at light and dark transitions (Figure 6A). The EEG delta power exponentially decreased during subsequent NREM sleep rebound following these activity peaks, describing the classic time-course of sleep homeostasis (Figures 6A and 6B).23,24 This is consistent with the increases seen in theta markers for alertness, observed during the activity peaks preceding these bouts of NREM sleep (Figure 5C). Because of an overall inversion of the sleep-wake architecture in A. ansorgei compared to mice, animals were placed under a 6-h sleep deprivation which began at dark onset (ZT12), whereas sleep deprivation experiments in nocturnal rodents are usually performed at light onset (ZT0).17,23 Following sleep deprivation, animals showed a significant increase in the EEG delta power peak consistent with standard sleep deprivation protocols showing augmented sleep pressure (Figures 6A and 6C). Sleep rebound was similar to that seen in mice under a similar length deprivation, exponentially decreasing within 4 h to the baseline levels observed before sleep deprivation.17
Figure 6.
Non-rapid eye movement (NREM) sleep and electroencephalography (EEG) delta power under sleep deprivation at ZT12. (A) EEG delta power expressed as a percentage of ZT20-ZT24 during baseline (ZT20-ZT24 was determined to consistently be the period with the lowest sleep pressure). The 6-h sleep deprivation is displayed as well as the preceding baseline day. (B) NREM sleep amounts under baseline conditions are at the maximum level at ZT 18. The rebound of NREM following the 6-h sleep deprivation occurs at ZT 18, which more likely explains why NREM amounts following a 6-h sleep deprivation stay within the baseline range. However, the peak of EEG delta power reached after sleep deprivation, a more reliable marker of the homeostatic process, is increased compared to baseline values. (C) Histograms representing EEG delta power during the first 3 h of recovery after the sleep deprivation as compared to the same baseline ZT (n = 8). A repeated-measures analysis of variance for baseline versus sleep deprivation and time-course showed significance. Asterisks denote significant differences following post hoc protected least significant difference (P < 0.05). BL, baseline; ZT, Zeitgeber time.
DISCUSSION
Here we characterized sleep and waking in A. ansorgei. Our findings reveal that sleep and waking are is mainly regulated by a crepuscular component, with a bimodal waking distribution centered around light/dark/light transitions. The main sleep regulatory mechanisms classically described in mammals are conserved in A. ansorgei, a species that has been characterized as diurnal from extensive research in the field of chronobiology. This suggests that A. ansorgei may represent a useful model for future sleep research, especially from the perspective of deciphering the mystery of the neuroanatomical switch between diurnality and nocturnality.
Why is A. ansorgei a Model of Interest for Sleep Research?
Our comprehension of the neurobiology of sleep and wakefulness results mainly from studies performed in nocturnal rodents, mice and rats, whose sleep-wake cycle is inverted in comparison to humans. This underlines the importance for the development and characterization of a diurnal model that could satisfy the requirements for the study of sleep neurobiology. Animal research on circadian rhythms has been largely conducted on rats and mice, facing the same challenge. The field of chronobiology addresses this issue through the extensive study for more than 15 y of the rodent Arvicanthis. Two subspecies of this African grass rat have been used for investigations, the Nile A. niloticus and A. ansorgei from Mali. Interestingly, the ansorgei subtype has been maintained under laboratory conditions for over 15 years, which turned the animal behavior from wild to a behavior closer to laboratory conditions.25 Finally, Arvicanthis proved to be an appropriate model for laboratory experiments and this species has been widely utilized for the study of circadian rhythms. As a result and as described in the introduction, the species has already been well characterized and specific biological tools have been created, in addition to the development of transgenic models and other genetic tools, which is under way. However, as of this writing, sleep has never been phenotyped in Arvicanthis, although a few sleep recordings were performed in different rodent species. Despite challenges associated with this animal because of its more savage nature within the laboratory environment as compared to rats or mice, the procedure was nevertheless similar. Based on EEG, EOG, EMG, and with video support, A. ansorgei have clear differences in their vigilance states, which are nearly identical to those found in other rodents such as mice and rats.19,26 Characteristics seen in each vigilance state based upon power spectrum analysis were extremely similar to those observed in previously studied nocturnal rodents, in addition to the length of sleep and wake bouts.19,26 Moreover, the main processes known to regulate sleep and waking are conserved in A. ansorgei and differences with other rodent species are discussed next.
A. ansorgei is a Diurnal Rodent Whose Sleep is Regulated by Light and Circadian, Crepuscular, and Homeostatic Processes
A. ansorgei Display a Lack of Strong Circadian Drive in the Absence of Clock Reinforcement by External Cues Such as a Running Wheel or Photic Stimulation
In mammals, the distribution of sleep over the 24-h cycle is regulated by a circadian process generated by an endogenous clock located in the suprachiasmatic nuclei.27,28 In A. ansorgei the sleep-wake distribution under a standard 12hL:12hD condition shows a weak, but significant, diurnal circadian rhythmicity29. This circadian distribution of sleep and wakefulness is clock-driven, as it is also observed under 24 h of constant darkness. Yet, some differences are observed between the 12hL:12hD and 24h D:D conditions, more likely due to a sustained direct effect of light promoting wakefulness in diurnal species. This is supported by what is observed when light is removed using a constant darkness condition, as average wake bout length changes only under light and subjective light periods, but not during the 12hL:12hD dark period or subjective dark under 24h D:D. The direct influence of light, through melanopsin-based phototransduction, plays a major role in sleep regulation in mice, as it did here in Arvicanthis ansorgei. Therefore, further experiments are needed especially to understand by which mechanisms the direct photic regulation of sleep switches between diurnal and nocturnal animals.
Crepuscular Activity Overrides the Circadian Drive
Under standard 12hL:12hD cycles the animals display peaks of wakefulness at light-dark-light transitions that are conserved, albeit slightly altered, under constant darkness. These consolidated periods of waking are sustained, beginning about 2 h before light and dark onset, and ending about 2 h after light and dark offset. Certain animal species are incredibly active at light and dark onset, referred to as crepuscular.3,4 The crepuscular regulation of waking observed in A. ansorgei is far more pronounced than what is normally seen in laboratory rats and mice, and its persistence under constant darkness implies crepuscularity as a major sleep-wake regulatory component. This 12-h bimodal rhythm is also found for core body temperature in the absence of a running wheel7 and in corticosterone release with peak values close to light-dark and dark-light transitions.30 However, wheel-running locomotor activity recordings show a clear diurnal pattern of locomotor activity. (Figures 1A and 1B). This indicates that the species can engage its daily sleep-wake distribution from a bimodal, crepuscular pattern in the absence of external cues, to a clear diurnal pattern of sleep-wake rhythmicity in the presence of circadian Zeitgebers such as a running wheel. For A. ansorgei, several reasons might explain this crepuscular wake behavior. Primarily one must consider the environment in which the Arvicanthis ansorgei comes from. In the sub-Saharan grasslands, average temperature can reach above 40°C as the day progresses, forcing the animal to rest during certain times, such as the “siesta-like” period of NREM sleep seen between ZT5–9. Following this, activity slowly increases, maximizing around sunset and dusk, continuing after lights-off in a laboratory environment. It is likely at this time of the day, the animal searches for food and shelter before sleeping during the night. Video recording also confirms that at certain times during both the light and dark period the animal is engaged in a waking state with restricted movement, confirmed with EEG and EMG. Additional video comparison between animals showed that during the light and dark transitions most were engaged in foraging behavior, having buried their food in the preceding period. Furthermore, the repetition of this activity around light and dark onsets suggests an ultradian cycle of 12 h rather than a circadian period of 24 h. Finally, it would be of interest to study the circadian and crepuscular regulatory mechanisms under experimental conditions mimicking dawn and dusk, as suggested in flies.30
In humans, a bimodal organization of sleep and wakefulness can be observed under specific conditions. In the case of a Mediterranean climate or in the Sahel, people adapt to the afternoon heat by taking a prolonged siesta. In shift workers, a scheduled siesta during the day often compensates for sleep loss. Celebrating the Matins splits the night of cloistered monks and nuns with a biphasic core body temperature profile.31 However, it remains speculative as to whether the crepuscular regulation of the sleep-wake cycle in A. ansorgei shares any similarities with the bimodal organization of sleep and wakefulness sometimes observed in human populations under specific conditions.
The Homeostatic Regulation of Sleep in A. ansorgei
A. ansorgei is an active animal compared to laboratory rats, and increased activity is known to augment homeostatic pressure.32,33 The time-course of delta activity following the two periods of high activity suggests that sleep pressure builds up faster in A. ansorgei than in mice or rats, yet this remains to be further established. A 6-h sleep deprivation started at dark onset challenged the sleep homeostat with a significant increase in the amount of delta power at sleep rebound. As compared to delta peaks after crepuscular wake bouts under baseline, the delta peak obtained at recovery sleep was relatively low. Given that there are two peaks of activity, it may be pertinent to institute a sleep deprivation at crepuscular activity onset. A more complete analysis of all increases in EEG delta activity in relation with periods of waking needs to be examined for further integrated understanding of the interaction between the circadian, the crepuscular, and the homeostatic components.
The Sleep-Wake Rhythm Has a Weaker Circadian Organization as Compared to Wheel-Running Activity
In A. ansorgei, the amplitude of the sleep-wake circadian rhythm is weak as compared to that of wheel-running loco-motor activity. Locomotor activity is known to reinforce circa-dian rhythmicity and, indeed, in our experiments the diurnal pattern of locomotor activity in A. ansorgei is largely strengthened in presence of a running wheel. Therefore, further experiments are needed to explore whether the Arvicanthis diurnal sleep-wake rhythm might be solidified in the presence of external cues such as a running wheel.34 The influence of locomotor activity on the circadian system can even be more pronounced in certain species such as the diurnal unstriped Nile Grass rat. Indeed, this related species, A. niloticus, has been shown to switch their locomotor profile partially or totally from a diurnal to a nocturnal pattern when a wheel is available.35 However, this observation was not reproduced in A. ansorgei because no significant changes were noticed after recording animals using infrared motion detectors in the absence of a wheel,5 except for a few animals displaying a behavioral switch from “predominantly diurnal” to “predominantly nocturnal”.7 This behavioral variety is probably due to different constitutive traits of the species, even though A. niloticus and A. ansorgei share common characteristics.
In conclusion, this is the first characterization of sleep and waking, as well as of their regulatory mechanisms in the rodent A. ansorgei. This animal's diurnal sleep-wake rhythm is strongly overridden by a crepuscular regulatory process. It remains speculative as to whether this crepuscular pattern in Arvicanthis has similarities with the bimodal organization of sleep and wakefulness described in humans under specific conditions. A stronger synchronization of the diurnal sleep-wake profile might be obtained through exposure of the animals to external cues such as light-dark condition as well as the reward properties of wheel-running. Our findings suggest that A. ansorgei may represent a powerful model for further research aimed at deciphering the mystery of the diurnality/ nocturnality switch. This represents an important step for translational research from nocturnal animals to humans.
DISCLOSURE STATEMENT
This study received financial support from ADS ALSACE. The authors have indicated no financial conflicts of interest. This work was performed at the Institute of Cellular and Integrative Neurosciences, Strasbourg, France.
ACKNOWLEDGMENTS
The authors thank ADS ALSACE for financial support.
SUPPLEMENTAL MATERIAL
Daily amounts of wake (h), non-rapid eye movement sleep (h) and rapid eye movement sleep (min) in 12hL:12hD cycle and 24h D:D cycles
Average bout length in wake (min), non-rapid eye movement sleep (min), and rapid eye movement sleep (min) in 12hL:12hD cycle and 24h D:D cycles
REFERENCES
- 1.Dijk DJ, Daan S. Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J Comp Physiol A. 1989;165:205–15. doi: 10.1007/BF00619195. [DOI] [PubMed] [Google Scholar]
- 2.Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol. 1977;233:R213–21. doi: 10.1152/ajpregu.1977.233.5.R213. [DOI] [PubMed] [Google Scholar]
- 3.Kas MJ, Edgar DM. Crepuscular rhythms of EEG sleep-wake in a hystricomorph rodent, Octodon degus. J Biol Rhythms. 1998;13:9–17. doi: 10.1177/074873098128999871. [DOI] [PubMed] [Google Scholar]
- 4.Ocampo-Garces A, Hernandez F, Palacios AG. REM sleep phase preference in the crepuscular Octodon degus assessed by selective REM sleep deprivation. Sleep. 2013;36:1247–56. doi: 10.5665/sleep.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challet E, Pitrosky B, Sicard B, Malan A, Pevet P. Circadian organization in a diurnal rodent, Arvicanthis ansorgei Thomas 1910: chronotypes, responses to constant lighting conditions, and photoperiodic changes. J Biol Rhythms. 2002;1:52–64. doi: 10.1177/074873002129002339. [DOI] [PubMed] [Google Scholar]
- 6.Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–55. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- 7.Cuesta M, Clesse D, Pevet P, Challet E. From daily behavior to hormonal and neurotransmitters rhythms: comparison between diurnal and nocturnal rat species. Horm Behav. 2009;55:338–47. doi: 10.1016/j.yhbeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Caldelas I, Poirel VJ, Sicard B, Pevet P, Challet E. Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei. Neuroscience. 2003;116:583–91. doi: 10.1016/s0306-4522(02)00654-1. [DOI] [PubMed] [Google Scholar]
- 9.Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res. 2004;124:143–51. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Garidou ML, Gauer F, Vivien-Roels B, Sicard B, Pevet P, Simonneaux V. Pineal arylalkylamine N-acetyltransferase gene expression is highly stimulated at night in the diurnal rodent, Arvicanthis ansorgei. Eur J Neurosci. 2002;15:1632–40. doi: 10.1046/j.1460-9568.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- 11.Garidou-Boof ML, Sicard B, Bothorel B, Pitrosky B, et al. Environmental control and adrenergic regulation of pineal activity in the diurnal tropical rodent, Arvicanthis ansorgei. J Pineal Res. 2005;38:189–97. doi: 10.1111/j.1600-079X.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107:6412–7. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza J, Revel FG, Pevet P, Challet E. Shedding light on circadian clock resetting by dark exposure: differential effects between diurnal and nocturnal rodents. Eur J Neurosci. 2007;25:3080–90. doi: 10.1111/j.1460-9568.2007.05548.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza J, Gourmelen S, Dumont S, Sage-Ciocca D, Pevet P, Challet E. Setting the main circadian clock of a diurnal mammal by hypocaloric feeding. J Physiol. 2012;590:3155–68. doi: 10.1113/jphysiol.2012.230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobu C, Lahmam M, Vuillez P, Ouarour A, Hicks D. Photoreceptor organisation and phenotypic characterization in retinas of two diurnal rodent species: potential use as experimental animal models for human vision research. Vision Res. 2008;48:424–32. doi: 10.1016/j.visres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Karnas D, Hicks D, Mordel J, Pevet P, Meissl H. Intrinsic photosensitive retinal ganglion cells in the diurnal rodent, Arvicanthis ansorgei. PLoS One. 2013;8:e73343. doi: 10.1371/journal.pone.0073343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai JW, Hannibal J, Hagiwara G, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourgin P, Fabre V, Huitron-Resendiz S, et al. Cortistatin promotes and negatively correlates with slow-wave sleep. Eur J Neurosci. 2007;26:729–38. doi: 10.1111/j.1460-9568.2007.05696.x. [DOI] [PubMed] [Google Scholar]
- 19.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–37. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: lessons from transgenic mouse models. Sleep Med Rev. 2013;17:445–52. doi: 10.1016/j.smrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–98. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–41. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–21. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 25.Katona C, Smale L. Wheel-running rhythms in Arvicanthis niloticus. Physiol Behav. 1997;61:365–72. doi: 10.1016/s0031-9384(96)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16:467–77. [PubMed] [Google Scholar]
- 27.Ibuka N, Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res. 1975;96:76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- 28.Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6:217–33. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- 29.Verhagen LA, Pevet P, Saboureau M, et al. Temporal organization of the 24-h corticosterone rhythm in the diurnal murid rodent Arvicanthis ansorgei Thomas 1910. Brain Res. 2004;995:197–204. doi: 10.1016/j.brainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Vanin S, Bhutani S, Montelli S, et al. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–5. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 31.Arnulf I, Brion A, Pottier M, Golmard JL. Ring the bell for Matins: circadian adaptation to split sleep by cloistered monks and nuns. Chronobiol Int. 2011;28:930–41. doi: 10.3109/07420528.2011.624436. [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 33.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 34.Kohler M, Wollnik F. Locking and unlocking of running wheel affects circadian period stability differently in three inbred strains of rats. J Biol Rhythms. 1998;13:296–304. doi: 10.1177/074873098129000138. [DOI] [PubMed] [Google Scholar]
- 35.Blanchong JA, McElhinny TL, Mahoney MM, Smale L. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–77. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Daily amounts of wake (h), non-rapid eye movement sleep (h) and rapid eye movement sleep (min) in 12hL:12hD cycle and 24h D:D cycles
Average bout length in wake (min), non-rapid eye movement sleep (min), and rapid eye movement sleep (min) in 12hL:12hD cycle and 24h D:D cycles