Abstract
Background
Assessment of poststroke motor impairment has historically focused on the ability to move within and outside of abnormal synergistic motor patterns and is typically quantified by the Fugl-Meyer Assessment (FMA). However, it is unclear if the voluntary, isolated movement tasks of the FMA are appropriate for evaluating walking task-specific motor control requirements because walking is cyclical and involves considerable sensorimotor integration.
Objective
The purpose of this study is to test whether the motor impairment measured by the FMA is indicative of motor dysfunction during walking in poststroke adults.
Methods
Thirty-four individuals with chronic poststroke hemiparesis and 17 healthy controls walked for 60 seconds on an instrumented treadmill while recording electromyographic activity (EMG) from 8 lower extremity muscles. EMG recordings were also obtained during the FMA for those with hemiparesis to examine muscle activation patterns. Each participant was examined with a battery of walking-specific clinical and biomechanical assessment tools and stratified based on the FMA synergy (FMS) score. To further quantify muscle activation patterns during walking, a nonnegative matrix factorization (NNMF) determined the number of independent modules required to describe 90% of the total variance in the EMG patterns.
Results
Stratification poorly differentiated motor activation across FMA tasks as well as EMG patterns during walking. While FMS correlated with 2 of 6 walking assessments, the number of EMG modules significantly correlated with all 6 walking performance measures.
Conclusions
Voluntary, discrete activities as performed in the FMA may be inadequate to capture the complex motor behavior in walking. Conversely, walking-specific evaluations such as NNMF appear more appropriate. Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Keywords: stroke, gait, motor control, EMG
Introduction
Motor recovery poststroke is difficult to measure, and theories surrounding motor function poststroke have been dominated by the concept of progressing through predictable stages of recovery.1,2 This progression is based on the organization of reflex behavior, theorizing that severe impairments reflect a return to previously assimilated primitive reflexes. According to this theory, primitive motor pathways accessible by reflex activation provide a foundation for more complicated voluntary movements,3 and someone with nonflaccid hemiparesis (preservation of reflexes with no voluntary movement) would present with a recovery of motor function in a regular sequence in which initial voluntary movements are dependent on primitive motor pathways and synergistic movements. Patients fully recovering from stroke are thought to gradually develop more complex motor behaviors, fully integrating voluntary movement patterns outside of stereotypical abnormal synergy patterns.2 Based on this theory, Fugl-Meyer in 1975 developed a measurement instrument reflecting this hierarchy of the emergence of complex motor control behaviors to quantify recovery of motor function poststroke.3 This instrument is divided into upper-extremity and lower-extremity components, focusing on distinct constructs such as reflexes, voluntary control of isolated movement, coordination, and speed, with an additional section specific to balance recovery.3 Specifically, the Fugl-Meyer lower-extremity motor evaluation (FM-LE) consists of a total score of 34 points with 17 items scored on a 0 to 2 scale.
Since its inception, the FM-LE has been studied extensively to document stroke-related motor impairment and recovery. The FM-LE exam has undergone scrutiny via reliability and validity studies3,4 and has been used to validate other instruments.5,6 Furthermore, the FM-LE has been used to measure the efficacy of novel therapeutic approaches7 and has been included in models attempting to predict functional recovery.8,9 However, the motor control deficits that the FM measures may differ from deficits seen during activities such as walking. Walking is a task-specific activity that is at least partially controlled by spinal cord level automaticity via the presence of a complex system of spinal interneurons or central pattern generator (CPG).10-12 This generator has been proposed to act in concert with both peripheral afferent input and supraspinal control to produce functional and coordinated walking behavior.13-15 As such, evaluation of voluntary motor impairment using FM-LE may have limited ability to measure the neural determinants of walking dysfunction. In particular, control of isolated voluntary force and movement differs markedly from the cyclical patterns of walking, which rely heavily on spinal circuits as well as integration of descending motor commands and peripheral sensory inputs.
Recent advances have been made in the use of electromyographic activity (EMG) factorization procedures to identify shared patterns of activation among groups of muscles. Such approaches are consistent with the historical clinical perspective of functional muscle groupings but allow more objective determination of muscle groupings under any movement condition, including complex functional activities. Ivanenko et al16 have used a principal component analysis to identify 5 basic underlying factors that explain most of the variance of muscle EMG during gait activities that are modulated by both descending and proprioceptive signals. Using the jumping, swimming, and walking patterns of frogs, d’Avella et al17 used EMG recordings to identify a mixture of synergies that may be isolated or shared to account for all the movement variability. This control is speculated as being downstream of the processes that generate motor activation (ie, cortical inputs).18 Using microstimulation, iontophoresis, and behavioral analysis, Bizzi et al19 localized this modular organization at the level of the spinal cord in frogs and rats, and defined the ability to generate a specific pattern of motor output with a specific pattern of input into these spinal modules. Additionally, nonnegative matrix factorization (NNMF) analysis has demonstrated that these synergistic activation patterns produce previously reported stereotypical responses to postural perturbations to promote balance equilibrium.20,21 Recent work in our laboratory has used the NNMF to identify the number and composition of EMG modules (NNMF factors) accounting for lower-extremity EMG during walking in healthy adults and in adults poststroke.22
The purpose of this study is to test whether the motor impairment measured by the FM-LE is indicative of motor dysfunction during walking in adults with poststroke hemiparesis. Specifically, we hypothesize that the FM-LE and FM assessment synergy (FMS) portion will demonstrate weak correlations with biomechanical and clinical measurements of walking performance. As both the FM-LE and NNMF have been used to probe the underlying neural determinants of motor function, a secondary analysis will be to quantitatively analyze whether these 2 methods are measuring the same construct. Furthermore, we test whether the classification of stroke patients based on each of these approaches yields associations between the level of classification and deficits in walking performance, hypothesizing that NNMF classification will better stratify patients according to walking performance. NNMF is a mathematical algorithm used to investigate motor control complexity, and at this time, it is not recommended as an outcome measure to supplant the FM-LE but rather will be used as proof of concept for the importance of developing activity-specific outcome measures to assess motor control during walking.
Materials and Methods
Individuals with chronic (greater than 6 months poststroke) hemiparesis participated in a study at the Department of Veterans Affairs Medical Center in Gainesville, FL. Thirty-four individuals (15 female and 19 male), aged 60 ± 12.2 years (standard deviation), 13 with right and 21 with left hemiparesis participated in the study. Participants had a history of a single unilateral stroke, were ambulatory without contact assistance, were able to follow a multiple-step command, and did not have other medical issues interfering with their ability to walk. In addition, 17 healthy controls (3 male, 14 female, mean age 65.1 ± 10.7 years) participated in the study and walked at 0.6 m/s to match the average walking speed of the hemiparetic participants. All participants signed written informed consent approved by the University of Florida Institutional Review Board/Gainesville, FL. VA Subcommittee for Clinical Investigation.
Each participant walked for two 30-second trials at his or her self-selected speed on an instrumented treadmill at (Techmachine, Andrezieux Boutheon, France) to collect ground reaction forces (GRFs) and kinematic data. GRF data were acquired at 2000 Hz and were filtered with a low-pass, fourth-order Butterworth filter at 20 Hz forward and backward in time. The A-P GRF component (normalized by each individual’s body weight) was used in the subsequent analysis. Surface EMG (Konigsberg Instruments, Pasadena, CA) was acquired using bipolar Ag-AgCl surface electrodes (Vermed, Inc, Bellows Falls, VT) during treadmill walking and the FM-LE from 8 different muscles at 2000 Hz: tibialis anterior (TA), soleus (SOL), gastrocnemius (GAS), vastus medialis (VM), rectus femoris (RF), biceps femoris (BF), semimembranosus (SM), and gluteus medius (GM). Reference electrodes were placed over the electrically neutral patella. EMG signals were filtered with a 40-Hz high-pass filter and then a 20-Hz low-pass filter for averaging multiple steps of walking data or a 4-Hz low-pass filter to smooth one-trial data for the subsequent FM-LE analysis. Retroreflective markers were placed at 30 landmarks in addition to clusters at bilateral thighs, shanks, and feet according to a modified Helen Hayes marker set. Three-dimensional kinematics were captured using a motion analysis system (Vicon Motion Systems, Los Angeles, CA) with twelve 200-Hz cameras.
FM-LE Stratification
Each participant was stratified by severity according to the 22-point subsection of the FM-LE, which examines the ability to perform voluntary isolated movement independent from mass patterns of whole-limb coactivation (FMS) and excludes the reflex and coordination/speed parameters (severe, n = 11; moderate, n = 14; mild, n = 9). Participants were then examined with a battery of walking-specific clinical and biomechanical assessment tools. In this stratification, a FMS score of ≤15 characterized severe hemiparesis, 15 to 19 characterized moderate hemiparesis, and ≥20 determined mild hemiparesis.23 These cutoffs are based on theoretical limitations of moving within abnormal synergy patterns, combining synergy patterns, or moving at least partially outside of the patterns.
Nonnegative Matrix Factorization
For each participant, the EMG were combined into an m × t matrix (EMGo), where m indicates the number of muscles and t is the time base (t = Number of strides × 101, which corresponds to each 1% of the gait cycle from 0 to 100). A nonnegative matrix factorization algorithm was then applied to this matrix for a set of consecutive gait cycles because inherent stride-to-stride variability contains structured information that is critical to differentiating between independent factors and establishing robust factor definitions. NNMF defined the factors by populating 2 matrices:
an m × n matrix (n is the number of factors) that indicates the relative weighting of each muscle within each factor, and
an n × t matrix reflecting the activation timing profile of the factor across the gait cycle.
NNMF allows muscles to belong to more than 1 factor, but the relative activation of the muscles comprising each module (the weightings) remains fixed. The 2 matrices were multiplied to produce an m × t matrix of reconstructed EMG (EMGr), which was then compared with the original EMGo and the agreement quantified by calculating the sum of the squared errors: (EMGo - EMGr).20 NNMF systematically tries many different configurations of module weightings, choosing interim solutions that reduce the difference between the original and reconstructed EMG (our cost function) and module timing profiles until it converges on the one that minimizes the cost function (iterative optimization). Separate NNMF analyses were performed with the output constrained to 1, 2, 3, 4, and 5 factors. To determine how many factors were actually needed for each leg of each participant, we calculated the variability accounted for (VAF = 1 - [EMGo - EMGr]2/EMGo2). VAF was calculated for each muscle across the entire gait cycle and for all muscles within each of 6 phases of the gait cycle (calculated as the cumulative VAF for all muscles within each phase). Beginning with a single factor, if VAF was greater than or equal to 90% for all 14 conditions (8 muscles, 6 phases), then it was concluded that additional factors were not needed. Otherwise, the number of factors was increased until all conditions achieved 90% VAF or until adding an additional factor did not substantially increase the VAF for the muscle(s) and/or phase(s) with the lowest VAF.
Each participant was characterized as having 2, 3, or 4 factors required to explain their EMG variability.
Clinical and Biomechanical Assessment Tools
Walking speed
Self-selected walking speed overground was measured on an instrumented walkway (GAITRite, CIR Systems, Inc, Havertown, PA)
Berg balance test (BBT)
This is a 14-item test that requires an individual to perform everyday tasks of increasing difficulty such as sitting, moving from one chair to another, standing up, turning around, and picking up an item from the floor as well as the performance of more challenging tasks such as standing on 1 foot.24,25
Dynamic Gait Index
This index rates performance from 0 (poor) to 3 (excellent) on 8 different gait tasks, including gait on even surfaces, gait when changing speeds, gait and head turns in a vertical or horizontal direction, stepping over or around obstacles, and gait with pivot turns and steps.26
Paretic propulsion (Pp)
This is a quantitative measure of the coordinated output of the paretic leg, which describes the contribution of the paretic leg in propelling the center of mass forward during walking and is defined as the percentage of propulsion performed by the paretic leg.27 The percentage of propulsion (impulse derived from the positive anterior component of the GRF) generated by the paretic leg was calculated by dividing the total propulsive impulse of the paretic leg by the sum of the propulsive impulses of both legs. Statistics were run on the absolute deviation from normal (0.5).
Paretic step ratio (PSR)
This ratio is defined as the percentage of stride length performed by the paretic step.28 Statistics were run on the absolute deviation from normal (0.5). PSR was calculated from the kinematic data.
Paretic preswing (PPS)
This is a measure of the percentage of the gait cycle spent in the double-limb support phase prior to the paretic step.29 PPS was calculated from the kinematic data.
The gait cycle was divided into 6 phases for data analysis (Figure 1), and cycle events were determined by transitions of the vertical GRF. Vertical GRF transitions were defined by a customized k-means clustering program in Matlab and then verified by visual inspection. The k-means clustering analysis defines the group of common minimal points as 0 and quantifies changes away from 0 (initial contact) and to 0 (toe off) with greater sensitivity than an absolute threshold. The percentage of EMG activity in each phase was compared between each FMS severity level and for controls.
Figure 1.
Phase descriptions for the gait cycle for someone with right hemiparesis. The first double-support phase defines phase 1; phases 2 and 3 are the first and second 50% of the single-limb support; the second double-limb support (paretic preswing) is defined as phase 4; phases 5 and 6 are the first and second 50% of the swing phase.
Statistical Analysis
Group analyses were completed using a nonparametric Kruskal-Wallis H test with rank sums tests post hoc analyses. FM-LE and FMS values as well as the number of NNMF factors explaining EMG variability were correlated to the walking assessment battery using the nonparametric Spearman’s correlation coefficient. Significance for all tests was set at a < .05. All statistics were run using SPSS version 15.0 (SPSS, Inc).
Results
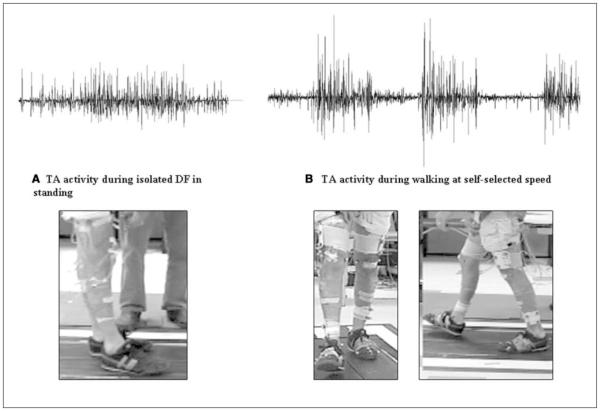
Of the 34 participants, several demonstrated higher activation of the TA during walking than during isolated dorsiflexion (DF) tasks in the supine position (n = 12, 35%), during sitting voluntary DF (n = 17, 50%), and during standing DF (n = 18, 53%). An example of this decreased TA activation during isolated tasks is seen in Figure 2, in which an individual exhibited no active DF during the isolated FM task but demonstrated active DF movement during walking accompanied by discrete bursting of the TA EMG (Figure 2). The x and y scaling for the EMG tracing was kept consistent for the purposes of visual comparison. The accompanying images illustrate the inability to dorsiflex the ankle in Figure 2A and functional DF during the walking cycle in Figure 2B.
Figure 2.
Tibialis anterior (TA) bursting patterns during right isolated dorsiflexion (DF;A) and during walking (B). Axes are identical for the 2 tracings. Note the higher amplitude and clear bursting pattern in walking (B) compared with the fairly tonic activity in isolated movements (A); in addition, note the lack of DF movement in (A) compared with functional right DF during the walking cycle in (B).
Fugl-Meyer Assessment (FMA) and EMG Activation
When stratifying according to the FMS severity, 5 of the 6 FM-LE tasks show varying degrees of differences between severity levels. In the supine extension task, TA (P = .046), MG (P = .048), BF (P = .005), SM (P = .001), and GM (P = .008) demonstrate main effects, and of these, BF (P = .004), SM (P = .002), and GM (P = .0008) show significant increases in EMG activity in the moderate group compared with the mild group. In the sitting knee flexion task, only BF demonstrated a main effect (P = .05) with significantly greater activation in the mild group compared with the moderate group (P = .037). TA demonstrated a main effect in both the sitting DF (P = .017) and standing DF (P = .013) tasks, although only the sitting DF demonstrated a significant increase in activation for the moderate group, compared with the severe group (P = .018). Standing knee flexion demonstrated a significant main effect among a majority of muscles: VM (P = .013), BF (P = .001), SM (P = .011), and GM (P = .002). Of these, the BF (P = .008), SM (P = .006), and GM (P = .001) demonstrated significantly higher activation in the mild hemiparesis group. Even though there are significant differences among muscles, there are no mass extension or mass flexion patterns noticed in the more complex tasks for the moderate and severe groups, and generally mild hemiparesis is represented by increased activation of the primary movers.
Assessing Walking EMG Patterns With FMS Severity
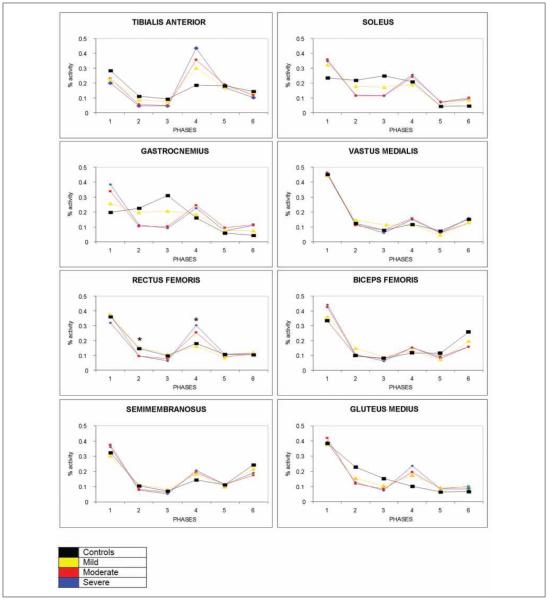
Among those with hemiparesis, only phase 2 (P = .026) and phase 4 (P = .011) of the RF demonstrate a main effect among the severity groups (Figure 3). In phase 2, the moderate and severe groups demonstrate a decrease in activation, whereas these same groups demonstrate an increase in activation in phase 4 compared with those with mild hemiparesis (RF phase 2, P = .027 and RF phase 4, P = .044).
Figure 3.
Walking electromyographic patterns with Fugl-Meyer assessment synergy (FMS) severity. Significant differences between FMS groups are only noted for rectus femoris for phases 2 and 4, demonstrating that those in differing FMS generally activate similarly during walking; differences from controls (black line) can be clearly noticed in tibialis anterior (TA), soleus, gastrocnemius (GASTROC), and gluteus medius (GLUT MEDIUS).
* Denotes significant differences in the post hoc analysis.
Observationally, large bursts are seen consistently in phases 1 and 4, representative of paretic limb loading and swing initiation, respectively. The control curve, however, is much more differentiated and shows additional bursting in the MG and GAS consistent with late stance plantarflexor activity. Additionally, the control curves demonstrate increased late swing activity (phase 6) in the BF and SM consistent with limb deceleration and preparation for initial contact.
Fugl-Meyer and Walking Performance
Of the 6 clinical and biomechanical measures of walking performance examined, walking speed, BBT, and PSR were significantly correlated with the FM-LE, whereas only the walking speed and PSR correlated significantly with the FMS (Table 1).
Table 1.
Fugl-Meyer (FM) and Walking Performance Measures
| Speed | BBT | DGI | Pp | PSR | PPS | |
|---|---|---|---|---|---|---|
| FM-total LE |
r = .588 P < .001 |
r = .369 P = .032 |
r = .116 P = .534 |
r = −.075 P = .674 |
r = −.357 P = .038 |
r = −.291 P = .126 |
| FM-synergy |
r = .456 P = .007 |
r = .325 P = .061 |
r = .058 P = .758 |
r = −.149 P = .400 |
r = −.365 P = .034 |
r = −.258 P = .177 |
Abbreviations: NNMF, nonnegative matrix factorization; BBT, Berg balance test; DGI, dynamic gait index; Pp, paretic propulsion; PSR, paretic step ratio; PPS, paretic preswing; LE, lower extremity.
Nonnegative Matrix Factorization and Walking Performance
When the number of NNMF factors required to explain their EMG variability were correlated with the battery of clinical and biomechanical walking performance variables, there was strong evidence for correlation with all variables (P < .025; Table 2).
Table 2.
NNMF Correlations With Walking Assessment Measures
| Speed | BBT | DGI | Pp Deviation | PSR Deviation | PPS | |
|---|---|---|---|---|---|---|
| NNMF Factors |
r = .451 P = .008 |
r = .504 P = .003 |
r = .545 P = .002 |
r = −.389 P = .023 |
r = −.558 P = .001 |
r = −.398 P = .020 |
Abbreviations: NNMF, nonnegative matrix factorization; BBT, Berg balance test; DGI, dynamic gait index; Pp, paretic propulsion; PSR, paretic step ratio; PPS, paretic preswing.
Discussion
When examined against a battery of clinical and biomechanical walking measures, the FM-LE and FMS both correlated significantly with self-selected walking speed and PSR, and the FM-LE was additionally correlated with the BBT. The correlation with walking speed is consistent with previous reports in the literature8 and likely reflects the general motor impairment that is present in this clinical population. Similarly, while establishing correlations between the FMA and both self-selected and fastest comfortable walking speeds, Nadeau et al8 also performed a regression analysis using the FMA (balance, FM-LE, and sensation portions entered separately), a spasticity index, isometric dynamometry scores, and spatiotemporal analysis values into a multiple regression model to examine specific contributions to the walking speed. Hip flexor strength, balance, and FMA were all significantly correlated to both self-selected and maximal walking speeds, but the multiple regression analysis indicated that only the hip flexor strength was predictive of self-selected walking speed. Hip flexor strength, sensation of the lower extremities, and plantarflexor strength were all predictive of maximal walking speed, but again, the FMA was not part of the predictive model.
This failure of the FM-LE to contribute significantly to a predictor model is true for walking speed as well as functional walking profiles such as the functional ambulation categories (FACs).9 In this study, assessments were taken longitudinally 18 times during the first year poststroke and included the following measurements: FAC, FM-LE, motricity index leg score, letter cancellation task, FM-balance, and the timed balance test. The primary outcome measure of the study was the change over time of the FAC and the contribution of other outcomes to the regression model. All the covariates listed above were significantly correlated with the FAC change score when analyzed with a bivariate regression model, with timed balance test having the strongest relationship, followed by FM balance, FMA, letter cancellation task, and motricity index. Multivariate modeling indicated that when all the above factors were combined into a single regression model, the model only predicted 18% of the change in the FAC.9
It is inconsistent with previous literature that Pp did not significantly correlate with hemiparetic severity because previous reports from our laboratory found significant correlations between Pp and Brunnstrom levels.27 However, this earlier sample had a higher level of ambulatory function (0.77 ± 0.34 m/s in the earlier sample versus 0.57 ± 0.24 m/s in the current sample). In addition, Pp was calculated in the current article by using the absolute deviation from the normal value of 0.5, whereas in the previous sample, Pp was treated as a continuous variable, and raw values were correlated with Brunnstrom levels and not raw FM scores. Another possible cause of this difference is the calculation of Pp from treadmill walking, as some investigators have argued that TM walking differs from overground walking.30 However, comparisons between treadmill walking and overground walking in healthy controls yield no significant differences in the anterior propulsive forces.31 Additionally, the instrumented treadmill used in this experiment was recently demonstrated to be valid for laboratory gait analysis, in that ground reactions; hip, knee, and ankle sagittal rotations; and torques, power, and surface EMG from 4 thigh and leg muscles were all not significantly different from the overground walking parameters, with the exception of an 8% decrease in stride length.32
When examining muscle activation patterns within the FM-LE, the clinical examination failed to distinguish hemiparetic severity consistently based on muscle activation patterns. Only supine extension and standing knee flexion demonstrated significant main effects in more than 1 muscle, and both showed group differences only in the BF, SM, and GM. Other than in these 2 tasks, EMG activity within the FM-LE is fairly consistent, regardless of severity group. Therefore, it may be inferred that 4 of the 6 tasks within the FM-LE offer practically no information regarding hemiparetic severity, at least as it relates to muscle activation patterns. The supine extension task illustrates the interesting finding of increased BF, SM, and GM activity in the most mildly hemiparetic group, indicating that these muscle groups may be active as hip extensors during the task. A limitation of the FM-LE is that there are no other extension tasks to which the supine extension results may be compared. Conversely, the BF, SM, and GM are significantly more active in the standing knee flexion task in the mild hemiparesis group, indicating increased muscle activity during a flexor phase. This flexibility of response seen in the mild group may reflect adaptability within the nervous system that is not seen in those with moderate and severe hemiparesis. Alternatively, those with more severe hemiparesis may not be able to activate the BF, SM, and GM during the tasks, but there is no evidence of mass extension and flexor patterns as the theory behind the FM-LE would assert. In analyses of voluntary single-plane motions while in a functionally significant standing position, Neckel et al33 demonstrated that while individuals poststroke produce reduced torque in 6 of the 8 motions in the paretic leg, they used similar strategies to controls in 7 of the motions. The only evidence of an abnormal synergy pattern producing the desired movement emerged with maximal hip abduction when hip flexor torque was also recorded in the stereotyped “flexor synergy” activity.33
EMG analysis of walking further illustrates the inability of the FM-LE to differentiate between hemiparetic severities because only the RF demonstrates any differences among the severity groups because the mild group differs from the moderate in phases 2 and 4. This analysis also fails to illustrate any type of mass extension or flexion strategy in the severe and moderate groups. What is seen during the walking trials is a consistent burst of activity in paretic loading and PPS. This reflects a nondifferentiated burst of activity across all measured muscles when activation is required to stabilize the body or prepare for swing initiation. Although only significant in the RF, several other muscles (TA and GM) demonstrate an increased peak in phase 4 for the severe hemiparetic group, even when activity in those muscles is not generally associated with preswing activity. The controls, on the other hand, demonstrate additional peaks, namely during phase 3 for the SOL and GAS and phase 6 for the BF and SM.
These 4 peaks seen in the control group are consistent with NNMF analysis, which indicates 4 factors that explain normal locomotion.22 These 4 factors are associated with weight acceptance (at approximately 10% of the gait cycle), propulsion (at approximately 45% of the gait cycle), ground clearance (at approximately 70% of the gait cycle), and leg deceleration during the end of swing (at approximately 95% of the gait cycle). These factors and their timing correspond very well to the peaks seen in phases 1, 3, 4, and 6 (Figure 3). As those with hemiparesis have fewer peaks of activity, this too may reflect a decrease in the number of factors required to explain the EMG activity in walking: 17 individuals with stroke required only 2 factors, 15 required 3, and only 2 required 4 factors to explain the variance in their EMG. Table 2 illustrates the degree to which classification by NNMF factors differentiates walking performance measures, which is more highly effective than by FM-LE or FMS (Table 1). The identification of a larger number of NNMF factors occurs when there is greater independence among the activation patterns of each muscle. These data strongly imply that an increase in NNMF factors is associated with increased complexity of the motor pattern and differentiation of muscle activation. Perhaps because NNMF is based on data collected while a participant is walking, its construct may more closely reflect walking performance than an assessment whose construct is based on voluntary, isolated movements as in the FM-LE.
Interparticipant differences in the complexity of the walking pattern revealed by NNMF may reflect the differences in the interaction of supraspinal, spinal, and peripheral input following stroke. Current evidence does not suggest either that human walking is controlled exclusively by the spinal cord or that the motor cortex alone is responsible for activation of muscles during walking.13 Emergence of an increased number of factors explaining EMG variability and the relationship of those factors with clinical and biomechanical measures of performance may reflect the improved organization of motor output from the central nervous system. However, this emergence of complexity of behavior may also relate to activity in the periphery such as integration of spinal neuronal circuitry and processing of afferent signals. Although additional work is necessary to delineate the role and neural mechanisms of spinal modules of motor control, it may be that these modules are integral to the coordination of multiple inputs for the control of human locomotion.
At this time, the number of modules as determined by NNMF is not appropriate to serve as a clinical measure because of the need for EMG data and detailed mathematical analyses. However, the FM-LE appears to be insufficient to capture necessary information about walking performance, and its use as an outcome measure for poststroke motor control should be limited to non-walking-related activities. The FMA’s effectiveness as a measure of upper-extremity motor control may be related to the more direct corticospinal connections to the arms and decreased reliance on patterned, spinally modulated movement.34,35 Future work developing clinical analogs to assess and monitor the presence and/or emergence of NNMF factors may greatly assist clinicians in accurately describing walking-specific motor control poststroke.
Acknowledgments
The authors would like to thank Erin Carr, BS, and Chitra Balasubramanian, PhD, PT, for their assistance with data collection and Ryan Knight, MS, for his assistance with the data analysis.
Funding
This work was funded by VA Rehabilitation R&D Grant B3983R, VA Rehabilitation R&D Center of Excellence grant F2182C (Brain Rehabilitation Research Center), and NIH R01 HD46820. This material is the result of work supported in part by the Office of Research and Development, Rehabilitation R & D Service, Department of Veterans Affairs, and the Malcom Randall VA Medical Center, Gainesville, FL.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 2.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- 3.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 4.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Hsieh CL, Dai MH, Chen CH, Lai YF. Inter-rater reliability and validity of the stroke rehabilitation assessment of movement (stream) instrument. J Rehabil Med. 2002;34:20–24. doi: 10.1080/165019702317242668. [DOI] [PubMed] [Google Scholar]
- 6.Lamontagne A, Malouin F, Richards CL. Locomotor-specific measure of spasticity of plantarflexor muscles after stroke. Arch Phys Med Rehabil. 2001;82:1696–1704. doi: 10.1053/apmr.2001.26810. [DOI] [PubMed] [Google Scholar]
- 7.Dobkin BH, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage. 2004;23:370–381. doi: 10.1016/j.neuroimage.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadeau S, Arsenault AB, Gravel D, Bourbonnais D. Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil. 1999;78:123–130. doi: 10.1097/00002060-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kollen B, van de Port I, Lindeman E, Twisk J, Kwakkel G. Predicting improvement in gait after stroke: a longitudinal prospective study. Stroke. 2005;36:2676–2680. doi: 10.1161/01.STR.0000190839.29234.50. [DOI] [PubMed] [Google Scholar]
- 10.Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- 11.Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 2004;96:1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- 12.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen JB. How we walk: central control of muscle activity during human walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- 14.Yang JF, Gorassini M. Spinal and brain control of human walking: implications for retraining of walking. Neuroscientist. 2006;12:379–389. doi: 10.1177/1073858406292151. [DOI] [PubMed] [Google Scholar]
- 15.Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev. 2005;33:54–60. [PubMed] [Google Scholar]
- 16.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- 18.Ivanenko YP, Poppele RE, Lacquaniti F. Motor control programs and walking. Neuroscientist. 2006;12:339–348. doi: 10.1177/1073858406287987. [DOI] [PubMed] [Google Scholar]
- 19.Bizzi E, Cheung VC, d’Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev. 2008;57:125–133. doi: 10.1016/j.brainresrev.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- 21.Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. 2007;98:2144–2156. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 22.Clark DJ, Subramanian S, Neptune RR, Ting LH, Kautz SA. Fewer basic activation patterns account for lower extremity EMG during walking in adults post-stroke compared to healthy controls; Poster presented at: Neural Control of Movement Annual Meeting; Naples, FL. Apr, 2008. [Google Scholar]
- 23.Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol. 2005;93:2460–2473. doi: 10.1152/jn.00963.2004. [DOI] [PubMed] [Google Scholar]
- 24.Berg KO, Maki BE, Williams JI, Holliday PJ, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73:1073–1080. [PubMed] [Google Scholar]
- 25.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 26.Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 27.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 29.De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78:1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Harris-Love ML, Macko RF, Whitall J, Forrester LW. Improved hemiparetic muscle activation in treadmill versus overground walking. Neurorehabil Neural Repair. 2004;18:154–160. doi: 10.1177/0888439004267678. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg EJ, Kautz SA, Neptune RR. Can treadmill walking be used to assess propulsion generation? J Biomech. 2008;41:1805–1808. doi: 10.1016/j.jbiomech.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tesio L, Rota V. Gait analysis on split-belt force treadmills: validation of an instrument. Am J Phys Med Rehabil. 2008;87:515–526. doi: 10.1097/PHM.0b013e31816f17e1. [DOI] [PubMed] [Google Scholar]
- 33.Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J Neuroeng Rehabil. 2006;3:17. doi: 10.1186/1743-0003-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]