Abstract
Introduction
Malignant cell transformation and tumor progression are associated with alterations in glycolysis, fatty acid synthesis, amino acid delivery and production of reactive oxygen species. With increased understanding of the role of metabolism in tumors, there has been interest in developing agents that target tumor specific metabolic pathways. Numerous promising agents targeting altered metabolic pathways are currently in Phase I – III clinical trials.
Areas covered
This paper reviews the early phase clinical trial development of these agents and provides perspective on the future direction of this emerging field. Specifically, the authors describe novel and repurposed therapies, focusing on the effects of each agent on tumor metabolism and results from relevant Phase I and II clinical trials.
Expert opinion
Metabolism modulating agents, alone and in combinations with other classes of agents, have shown efficacy in the treatment of neoplasm, which, the authors believe, will bear positive results in future studies. Because of the significant crosstalk between metabolic pathways and oncogenic signaling pathways, the authors also believe that combining metabolic modifiers with targeted agents will be an important strategy. An increased understanding of cancer metabolism, in addition to the continued study of metabolic modulators, should lead to further advances in this nascent therapeutic field in the future.
Keywords: cancer, cell metabolism, drugs, metabolism modulating agents, phase I and II
1. Introduction
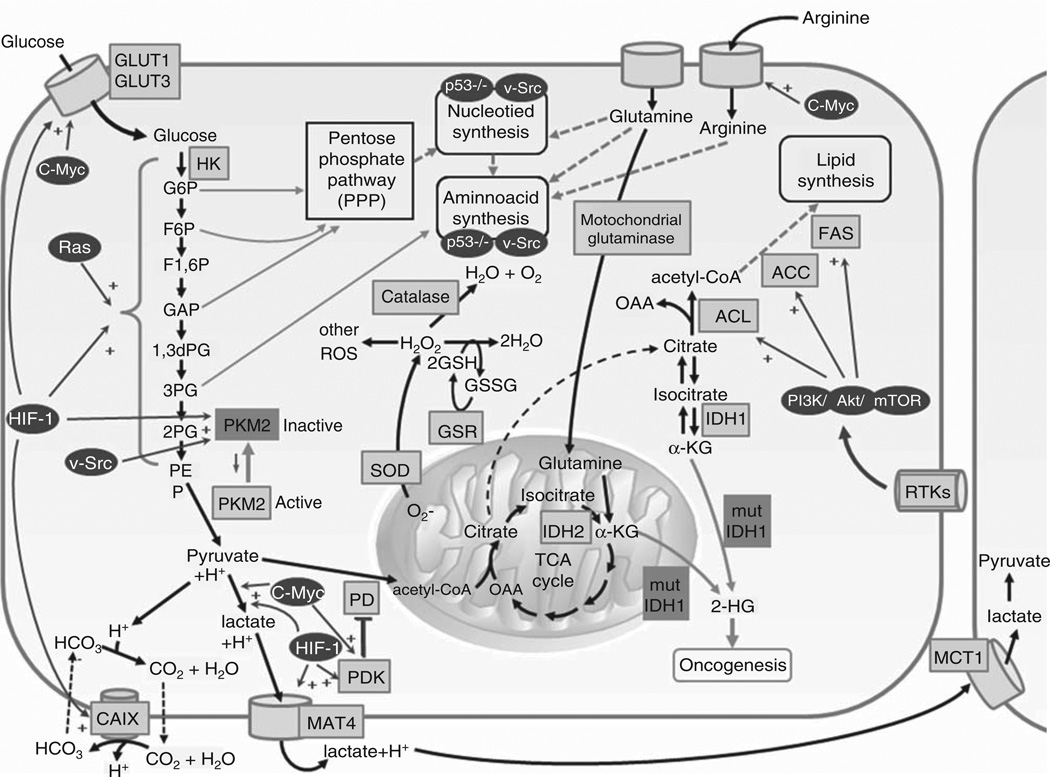
Normal cells are equipped with complex signaling networks coordinated by key control enzymes that sense environmental cues and activate metabolic pathways to provide sufficient energy for survival. During proliferation, various metabolites and enzymes act to dampen or accelerate cell metabolism. In the 1920s, Otto Warburg studied tumor metabolism and demonstrated that tumor cells predominantly produce energy via cytosolic lactate fermentation, rather than by mitochondrial oxidative phosphorylation [1,2]. It is now evident that the Warburg effect was only the tip of the iceberg with regard to metabolic changes that accompany malignant transformation. In malignant cell metabolism, changes in enzyme activity, signaling pathways and metabolite excretion allow for continued cell proliferation irrespective of the typical environmental cues. Malignant cell metabolism results from mutated oncogenes and tumor suppressor genes that modulate intracellular signaling pathways. This modulation of cell metabolism and signaling facilitates tumor growth and, in some instances, is essential for malignant transformation [3]. Metabolic derangements of cancer cells include upregulation of glycolysis, increased lactate fermentation, a shift away from mitochondrial oxidative metabolism, increased production of reactive oxygen species (ROS), altered amino acid delivery and increased fatty acid oxidation and synthesis (Figure 1) [1,4]. Increased understanding has led to the preclinical and clinical development of numerous anti-neoplastic compounds that target metabolism-related enzymes and pathways.
Figure 1. Metabolic pathways and oncogenic signaling in tumor cells.
Malignant transformation is associated with derangements of major metabolic pathways, including glycolysis, mitochondrial metabolism, amino acid metabolism, and fatty acid synthesis.
1,3 dPG: 1,3-biphosphoglyceric acid; 2-HG: 2-hydroxyglutarate; 2PG: 2-phosphoglyceric acid; 3PG: 3-phosphoglyceric acid; ACC: Acetyl-CoA carboxylase; ACL: ATP citrate lyase; CAIX: Carbonic anhydrase IX; CoA: Coenzyme A; F1,6P: Fructose 1,6-phosphate; F6P: Fructose 6-phosphate; FAS: Fatty acid synthase; G6P: Glucose 6-phosphate; GAP: Glyceraldehyde 3-phosphate; GLUT: Glucose transporter proteins; GSH: Reduced glutathione; GSR: Glutathione reductase; GSSG: Glutathi-one disulfide; HIF-1: Hypoxia-inducible factor 1; HK: Hexokinase; IDH1: Isocitrate dehydrogenase 1; IDH2: Isocitrate dehydrogenase 2; MCT1: Lactate transporter; MCT4: Monocarboxylate transporter 4; OAA: Oxaloacetic acid; PD: Pyruvate dehydrogenase; PDK: Pyruvate dehydrogenase kinase; PEP: Phosphoenolpyruvic acid; PKM2: Pyruvate kinase isozyme 2; ROS: Reactive oxygen species; SOD: Superoxide dismutase; TCA: Tricarboxylic acid cycle; α-KG: α-ketoglutarate.
In this paper, we discuss novel and repurposed agents that have been investigated in early phase clinical trials for their ability to disrupt tumor metabolism and cause regression (Table 1). We highlight the mechanisms of action, safety, tolerability and clinical efficacy of drugs. After providing an overview of these trials, we hypothesize about the future developments in the field and the role for further evaluation of many of these agents, especially in combinatorial trials. We propose that the combination of metabolism modulating agents with other types of agents will lead to increased efficacy and other benefits. As there is extensive crosstalk between metabolic and oncogenic signaling, we speculate that the most effective combinations may be those that incorporate molecularly targeted agents. We also recognize that, in the future, combinations with emerging epigenetic drugs may increase the efficacy of drugs that alter metabolism. We believe that the development of therapies targeting the unique tumoral metabolic landscape will grow to become increasingly important and, indeed, look forward to a future when these agents will significantly contribute to our armamentarium of anti-neoplastics.
Table 1.
Metabolism modulating agents evaluated in Phase I and II trials.
| Agent | Target | Developed by |
|---|---|---|
| Silybin | GLUT | Generic |
| Lonidamine/ TH-070 | HK | Threshold Pharmaceuticals |
| 2-Deoxyglucose | G6P isomerase | Generic |
| TLN-232 | PKM2 dimers | Thallion Pharmaceuticals |
| Dichloroacetate | PDK | Generic |
| (DCA) | ||
| AZD-3965 | MCT1 | AstraZeneca |
| CPI-613 | PDH | Cornerstone Pharmaceuticals |
| Mangafodipir | SOD | GE Healthcare |
| Calmangafodipir | SOD | PledPharma |
| Motexafin | TrxR | Pharmacyclics Pharmaceuticals |
| ARQ-501 | NADPH:NQO1 | Arqule Pharmaceuticals |
| STA-4783 | Cu2+ | Synta Pharmaceuticals |
| As2O3 (Trisonex) | GPx/TrxR | Cephalon, Inc. |
| 2ME | SOD | Generic |
| ENMD-1198 | SOD | EntreMed |
| ATN-224 | SOD1 | Wilson Therapeutics |
| Phenylethyl | GPx/NF-KB | Generic |
| isothiocyanate | ||
| Imexon | GSH | AmpliMed Corp. |
| Buthionine | GSH | Cayman Chemical |
| sulfoximine | ||
| Elspar | Asparagine | Merck and Company, Inc. |
| Oncaspar | Asparagine | Enzon Pharmaceuticals |
| Erwinaze | Asparagine | Eusa Pharma |
| ADI-PEG 20 | Arginine | Polaris Group |
| EGCG | FAS | Generic |
| AG-120 | IDH1 | Agios Pharmaceuticals |
| AG-221 | IDH2 | Agios Pharmaceuticals |
| cG250 (Rencarex) | CAIX | Wilex AG |
| E7070 (Indisulam) | CAIX | Eisai Inc. |
| Statins | HMGCR | Generic |
| Metformin | Multiple | Generic |
3-HMGCR: Hydroxy-3-methylglutaryl-CoA reductase; ADI: Arginine deiminase; CAIX: Carbonic anhydrase IX; EGCG: Epigallocatechin gallate; FAS: Fatty acid synthase; GLUT: glucose transporter; HK: Hexokinase; IDH: Isocitrate dehydrogenase; MCT: Monocarboxylate transporter; SOD: Superoxide dismutase.
2. Cell metabolism pathways
2.1 Glycolysis
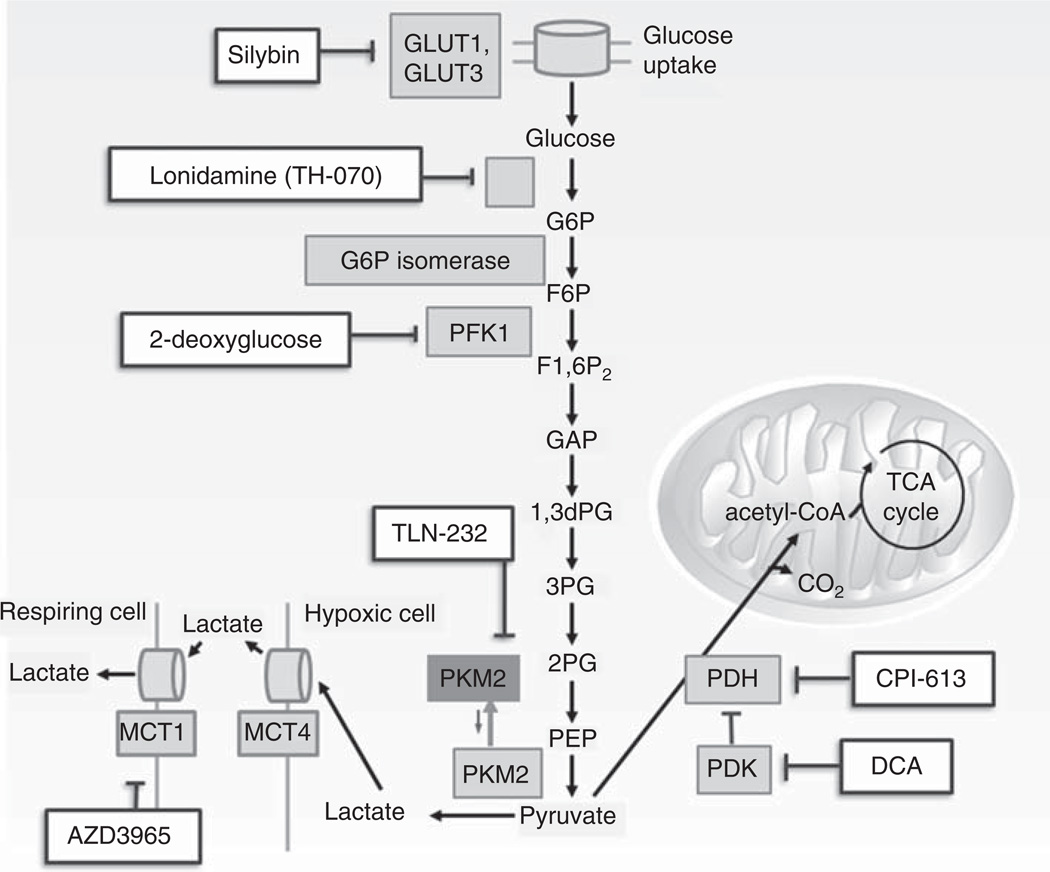
Glycolysis involves a series of enzymatic steps by which one molecule of glucose is catabolized to two molecules of pyruvate with a resultant net gain of two molecules of ATP. Cancer cells exhibit high rates of glycolysis (Figure 2). As metabolic alterations in glycolysis are common to various cancers, targeting the glycolytic pathway may have therapeutic implications for a wide range of tumor types (Table 2).
Figure 2. Agents that modulate glycolysis.
Multiple agents have shown the propensity to inhibit various steps of the glycolytic pathway. Silybin is an inhibitor of GLUT transporter proteins, londamine inhibits hexokinase, 2-deoxyglucose acts as a competitive inhibitor of glucose metabolism, AZD3965 is a MCT1 inhibitor, TLN-232 inhibits the PKM2 dimer, CPI-613 is a small molecular inhibitor of PDH and α-ketoglutarate dehydrogenase, and dichloroacetate (DCA) inhibits PDK.
CoA: Coenzyme A; G6P: Glucose-6-phosphate; HK: Hexokinase; MCT: Monocarboxylate transporter; PDH: Pyruvate dehydrogenase complex; PDK: Pyruvate dehydrogenase kinase; PFK1: Phosphofructokinase 1; PKM: Pyrvate kinase isoenzyme; TCA: Tricarboxylic acid cycle.
Table 2.
Inhibitors of glycolysis in Phase I and II trials.
| Agent | Mechanism | Phase/Trial/Number of patients | Outcome |
|---|---|---|---|
| Silybin (SIL) | Inhibitor of GLUT, EGFR, and angiogenesis; antioxidant and causes G1 arrest | 1/Advanced stage prostate cancer/13 1/Localized prostate cancer/6 1/Hepatocellular carcinoma/3 |
Well tolerated. No decrease in PSA Poor tissue penetrance Clinical benefit in 33% |
| Lonidamine (LND) | HK Inhibitor of HK and lactate efflux; reverses drug resistance and causes mitochondrial permeability | 2/Glioblastoma multiforme (+diazepam)/16 2/Ovarian (+paclitaxel, cisplatin)/10 2/NSCLC (+Cis, Dox, Vin)/31 |
No response 80% RR, tolerated 89% responders. Prolonged survival in stage IIB |
| 2-deoxyglucose (2-DG) | Inhibitor of HK phosphorylation of glucose | 1/Castrate resistant prostate cancer/12 1/Advanced solid tumors (+docetaxel)/34 1/Glioblastoma multiforme (+radiation)/6 |
Most significant DLT: QTc prolongation 35% RR, associated with hypoglycemia Reduction in radiation-induced side effects |
| TLN-232 | PKM2 inhibitor | 2/Metastatic renal cell/3 | Well tolerated, 2 SD |
| Dichloroacetate (DCA) | PDK inhibitor | 1/CNS malignancy/15 2/NSCLC and breast cancer/7 |
Well tolerated SD in 1 breast cancer patient, no response in NSCLC |
| CPI-613 | PDH inhibitor | 1 & 2/Advanced solid and hematologic malignancies (+Gem in solid tumors)/100 | 38% RR in hematologic malignancies with 100% RR in MDS; no DLTs |
GLUT: Glucose transporter; HK: Hexokinase; MDS: Myelodysplastic syndrome; SD: Stable disease.
Malignant cells have high glucose requirements. As a result, they should be more sensitive to glucose deprivation than non-malignant cells. The first rate-limiting step for glucose metabolism is transport across the plasma membrane, which is mediated by facilitative glucose transporter (GLUT) proteins (Figure 2). Although it is generally desirable to lower glucose levels in malignant cells, it should be recognized that administration of glucose may lower tumor cell pH and thus facilitate drug delivery. The GLUTs are upregulated by HIF-1α and Akt [5]. Silybin (SIL), also known as silibinin or silibin, is a GLUT inhibitor and the major active component of silymarin, a flavonoid extract of milk thistle. This agent induces G1 cell cycle arrest, inhibits EGFR and NF-κB, down-regulates survivin and suppresses angiogenesis [6]. A Phase I trial in patients with prostate cancer treated with SIL revealed that patients had no reduction in prostate-specific antigen, likely a result of the agent’s poor tumor penetrance and short half-life [7]. In a Phase I trial in three patients with advanced stage hepatocellular carcinoma (HCC), all patients quickly progressed and the study was halted [8].
After entering the cell, ATP-dependent phosphorylation of glucose results in the formation of glucose-6-phosphate (G6P). This is the first reaction of glycolysis and is catalyzed by tissue-specific isoenzymes known as hexokinases (HK) (Figure 2) [9]. Threshold Pharmaceuticals’ lonidamine (TH-070), a derivative of indazole-3-carobxylic acid, is an HK inhibitor which also binds to the adenine nucleotide translocator, causing increased mitochondrial permeability, intracellular acidification and apoptosis [10]. The lowering of intracellular pH, which can induce tumor cell death, is also proposed to result from inhibition of monocarboxylate transporter isoform 1 (MCT1) [11]. These complementary modes of action lead to cellular energy depletion and a consequent reduction in efflux of cytotoxic chemo-therapeutics in vitro [12]. Clinically, this translated into early activity signals in combination with chemotherapy, compared to lackluster single-agent activity. A Phase II trial in 35 patients with ovarian cancer demonstrated that lonidamine in combination with paclitaxel and cisplatin was associated with an 80% objective response rate (ORR) [13]. In a Phase II trial in 31 patients with NSCLC who were treated with cisplatin, epidoxorubicin and vindesine, 89% of patients had either a partial remission (PR) or stable disease (SD), and 15 patients with stage IIIB disease had an overall survival (OS) that extended beyond the median OS of 12 months [14]. After 2 negative randomized Phase III trials of lonidamine in combination with chemotherapy, further development of this agent was terminated [15,16].
2-deoxyglucose (2-DG) is a glucose analog that acts as a competitive inhibitor of G6P isomerase. After 2-DG is transported into cells, it is phosphorylated by HK to 2-DG-phosphate (2-DG-P) (Figure 2). Unlike G6P, 2-DG-P cannot be metabolized by G6P isomerase, so it accumulates and subsequently inhibits glycolysis [17–19]. Clinical trials with 2-DG include dose escalation Phase I trials in patients with castrate-resistant prostate cancer and advanced solid tumors. Single agent treatment resulted in asymptomatic QTc prolongation that limited dose escalation. 2-DG was also studied in combination with radiation in patients with glioblastoma multiforme [20,21]. In this population, treatment was well tolerated and resulted in a reduction of late radiation effects.
Further downstream, pyruvate kinase (PK) converts phosphoenolpyruvic acid to pyruvate to yield ATP. Pyruvate is then either reduced to lactate by lactate dehydrogenase (LDH) in the cytosol or enters the mitochondria to produce ATP via the tricarboxylic acid (TCA) cycle (Figure 2). Alternative splicing results in four PK isoforms: the L (PKL), R (PKR), M1 (PKM1), and M2 (PKM2). These isoforms are important targets of multiple oncogenic pathways. For example, HIF-1α upregulates the expression of the PKM gene. PKM1 predominates in most healthy adult tissues, but c-Myc activation can induce premalignant cells that favor an embryonic PKM2 isoform [1,22,23]. The PKM2 protein is normally active as a tetramer, but in malignant cells, through direct interaction with v-Src, it forms catalytically inactive dimers. The predominance of these dimers reduces the rate of phosphoenolpyruvic acid (PEP) conversion to pyruvate and results in the accumulation of glycolytic intermediates that are used for nucleotide and amino acid synthesis [24]. PKM2 dimers also act as transcriptional co-factors that promote HIF-1α binding to hypoxia-response elements that enhance Oct4-mediated transcription and promote β-catenin-mediated transactivation of cyclin D and c-Myc [1,22–24]. PKM2, therefore, is an important driver of the transition to the aerobic glycolytic phenotype and contributes significantly to malignant proliferation. Thallion Pharmaceuticals initiated a Phase II clinical trial with the PKM2 dimer-inhibiting agent TLN-232 in 10 patients with refractory metastatic renal cell carcinoma. Treatment was well tolerated and associated with SD in 2 out of the 3 evaluable patients [25]. In 2010, Thallion lost its license to develop this agent.
Pyruvate is used as a substrate in the TCA cycle; it is converted to acetyl-coenzyme A (CoA) in the mitochondria by pyruvate dehydrogenase (PDH) (Figure 2). Activated PDH kinase (PDK) phosphorylates and inactivates PDH causing cells to shift into anaerobic metabolism. In tumor cells, under hypoxia conditions, HIF-1α activates HK and PDK and increased glycolysis and enhanced lactate production results [9]. Preclinical investigation revealed that the reactivation of PDH results in the resumption of the Krebs cycle, disruption of aberrant tumor physiology and induction of apoptosis [26]. Dichloroacetate (DCA) is an inhibitor of PDK. In tumor cells, it decreases lactate and the mitochondrial membrane potential and increases ROS and mitochondria-dependent apoptosis [27,28]. A Phase I trial of single-agent DCA in 15 patients with recurrent CNS malignancy revealed that treatment was limited by the onset of peripheral neuropathies in this population [29,30]. A Phase II trial of single-agent DCA in patients with NSCLC and breast cancer was terminated early due to two deaths, one of unknown cause and the other from a fatal pulmonary embolism [31]. DCA is currently being evaluated in clinical trials in patients with head and neck cancer, glioblastoma and other solid tumors. Preclinical results indicate that DCA may synergize well with chemotherapeutic agents such as 5-FU and cisplatin through inhibition of glucose-dependent, hypoxia-induced chemoresistance [31,32].
Via a diametrically opposite effect, PDH inhibition has also been associated with potent anticancer effects in human pancreatic and non-small cell cancer xenograft models [33]. CPI-613, developed by Cornerstone Pharmaceuticals, is a small molecule inhibitor of PDH and α-ketoglutarate dehydrogenase that targets multiple points of the TCA cycle (Figure 2) [34]. In Phase I trials in patients with advanced solid tumors, single agent CPI-613 was well tolerated. Patients with relapsed/refractory hematologic malignancies treated with single agent CPI-613 had an overall ORR of 38, and 100% response was seen in patients with myelodysplastic syndrome (MDS) [35]. In a Phase I-II trial combining CPI-613 with gemcitabine in patients with advanced solid tumors study, responses occurred in patients with colon cancer [36,37]. CPI-613 has orphan drug designation from the US FDA for the treatment of MDS and several additional Phase I and II trials in patients with hematologic malignancies and advanced solid tumors are ongoing.
Accumulation of lactate within tumors is associated with poor clinical outcomes and may contribute to tumor progression [10]. MCT1 and MCT4 catalyze the bidirectional transport of lactate and hydrogen ion (H+) [38]. In tumors, accumulated lactate and H+ are either exported from the cell to maintain intracellular pH or imported for use as an energy source or amino acid synthesis. Under hypoxic conditions, HIF-1α upregulation of MCT-4 removes lactate and H plus; from the hypoxic cell. After removal of lactate and H+, MCT1 transfers lactate and H+ to neighboring respiring cells (Figure 2) [39]. AZD 3965, an inhibitor of MCT1, is being investigated in solid tumor and lymphoma preclinical models, and a Phase I single agent trial is currently underway.
2.2 Mitochondrial metabolism and control of ROS
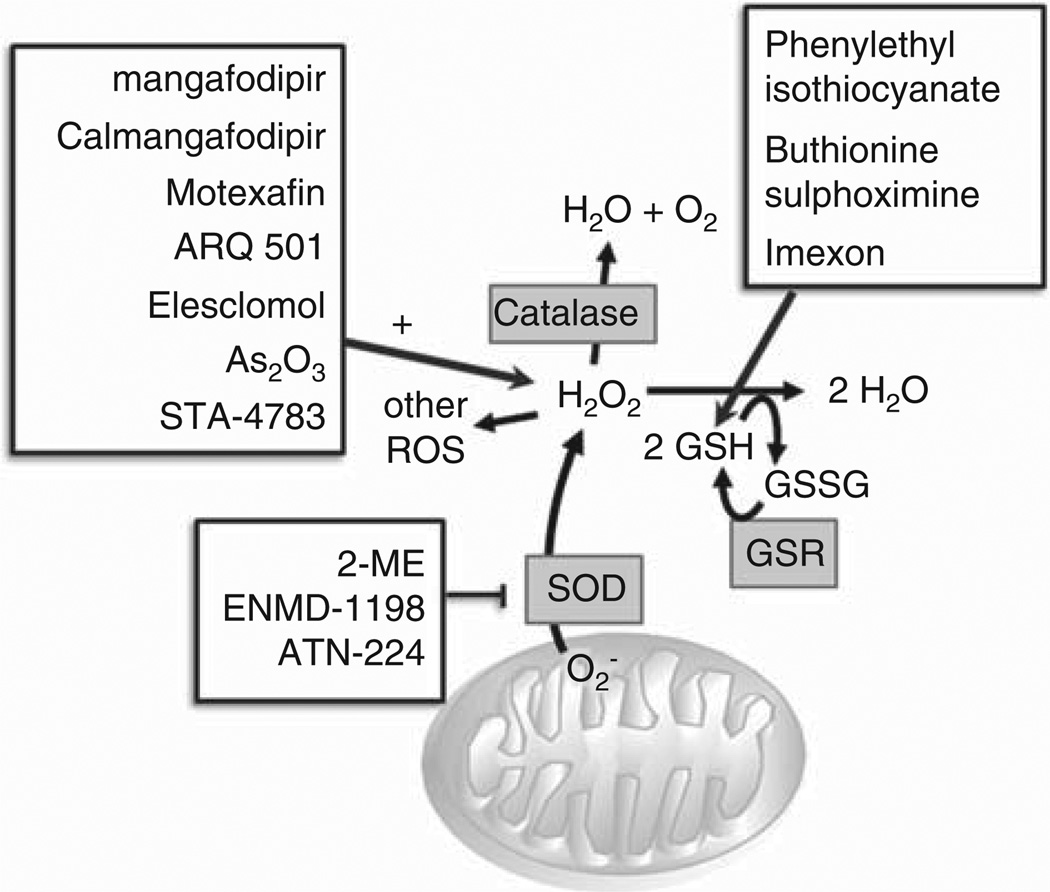
In the mitochondria, superoxide dismutase (SOD) converts oxygen (O2) from the electron transport chain to hydrogen peroxide (H2O2). Glutathione (GSH) reductase catalyzes the formation of reduced GSH. GSH then converts H2O2 to two molecules of water and catalase converts H2O2 to water and O2. Residual H2O2 yields additional ROS. A moderate amount of ROS promotes malignant cell proliferation by altering the function of growth signaling molecules such as AKT and STAT3, and transcription factors including NF-κB, AP-,1 and c-Myc [40,41]. When ROS levels exceed the toxic threshold, the antioxidant capacity of malignant cells is overwhelmed and apoptosis occurs. Metabolic agents that increase ROS production and activity therefore represent a novel and potentially efficacious antitumor therapeutic modality when used alone or in combination with conventional chemotherapeutic agents. On the other hand, certain chemotherapeutic agents like oxaliplatin and 5-FU can induce the overproduction of ROS in healthy tissues, and agents with antioxidant properties may protect patients against the adverse neurologic and hematologic effects resulting from use of these medications [42]. ROS-modulating agents and antioxidants are currently undergoing clinical development, and have been associated with variable antitumor efficacy and reduction of chemotherapy-related adverse effects (Figure 3, Table 3).
Figure 3. Agents that modulate reactive oxygen species.
Agents targeting multiple steps of mitochondrial mechanism have been evaluated in early phase clinical trials. Agents that augment production of reactive oxygen species include mangafodipir/calmangafodipir, motexafin, ARQ-501, ele-sclomol, arsenic trioxide, STA-4783, 2-ME, ENMD-1198, and ATN-224. Other agents affect mitochondrial metabolism via alternate routes, including phenylethyl isothiocyanate downregulation of glutathione, buthionine sulphoximine inhibition of GSH synthesis, and imexon depletion of GSH via thiol binding.
GSH: Reduced glutathione; GSR: Glutathione reductase; GSSG: Glutathione disulfide; H2O: Water; H2O2: Hydrogen peroxide; O2: Oxygen; ROS: Reactive oxygen species; SOD: Superoxide dismutase.
Table 3.
Phase II trials investigating reactive oxygen species (ROS) modulating agents.
| Agent | Mechanism | Trial/Number of patients | Outcome |
|---|---|---|---|
| ARQ-501 | DNA damage checkpoint activator | Head and neck cancer/59 LMS/45 Advanced pancreatic cancer/58 |
1 SD 1 PR, 3 SD 13/20 evaluable patients with ≥ SD |
| STA-4783 (Elesclomol) ATN-224 | Copper chelation Copper chelation/SOD1 inhibitor |
Metastatic melanoma (+ paclitaxel)/53 Hormone naïve prostate cancer/47 Hormone refractory prostate cancer/19 Advanced kidney cancer/15 Malignant mesothelioma/30 Resectable esophageal cancer/69 |
Doubling of median PFS Median PFS 28 weeks PD 4 SD 3 years OS for stage 1 & 2 41m, stage 3 15m 3 years OS 45% |
| Arsenic Trioxide (excluding trials in pediatric patients) | Enhancement of oxidative stress | Myelodysplastic syndrome/secondary AML (+Gemtuzumab Ozogamicin)/30 Multiple myeloma/10 Multiple myeloma (+VAD)/11 Multiple myeloma (+Vel, Mel, ascorbic acid Preparative regimen prior to autologous HSCT)/6 T-cell ALL (+IFN-α, zidovudine)/10 Metastatic urothelial carcinoma/12 Lymphoid malignancies (+ ascorbic acid)/16 Pancreatic adenocarcinoma/13 |
Overall RR 30% MDS and 25% AML 1 CR, 4 SD 4 PR Median OS 17.4 – 20.7m RR 100%, 7 CR 4 SD, median OS 6.5m Overall RR 6% Median PFS 1.6m, median OS 3.8m |
| 2ME | HIF-1α inhibitor, elevates tumor superoxide | Metastatic carcinoid tumors (+bevacizumab)/31 Ovarian cancer/peritoneal carcinomatosis/18 Prostate cancer – castrate resistant/21 Prostate cancer – hormone refractory/33 Metastatic renal cell carcinoma (+sunitinib)/17 |
68% with tumor reduction 7 SD Well tolerated, no antitumor activity Decreased PSA in 8 4 SD |
| Imexon (Amplimexon) | Increases ROS, disrupts mitochondrial membranes | Metastatic melanoma (+dacarbazine)/68 Pancreatic adenocarcinoma (+gemcitabine) |
2% PR, 30% SD Unreported |
| Motexafin | Trx inhibitor | Chronic Lymphocytic Leukemia/13 Non-small cell lung carcinoma/72 Metastatic Renal Cell Carcinoma/25 |
No responses, disease activity present 8% PR, 57% SD 8 SD, median OS 10.1m |
ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia; CR: Complete response; MDS: Myelodysplastic syndrome OS: Overall survival; PR: Partial remission; SD: Stable disease; SOD: Superoxide dismutase.
In general, single agent efficacy has been minimal whereas combination therapy has been promising, likely because the ROS-modulating agents enhance chemotherapy-induced cytotoxicity. Arqule’s ARQ 501 (β-lapachone), a quinone derivative, increases apoptosis via induction of E2F-1 and ROS production in cells overexpressing NAD(P)H:quinone oxidoreductase (NQO1). ARQ 501 has been investigated in numerous Phase I and II trials and has shown antitumor efficacy as a single agent and when added to chemotherapy. In single-agent Phase I trials, treatment with ARQ 501 was well tolerated in patients with head and neck cancer and other advanced solid tumors [43,44]. In a Phase II trial in patients with unresectable pancreatic adenocarcinoma, the combination of ARQ 501 and gemcitabine resulted in SD in 65% of patients after being on trial for 8 weeks [45]. Synta Pharmaceutical’s elesclomol/STA-4783 is a first-in-class cytotoxic agent that induces oxidative stress and mitochondrial apoptosis via copper chelation [46]. It is currently being evaluated as a single-agent in a Phase I trial in patients with relapsed/refractory acute myeloid leukemia (AML), as well as in combination trials including in prostate cancer with docetaxel and prednisone; in ovarian, fallopian tube and primary peritoneal cancers with paclitaxel; in stage IIIB/IV NSCLC with paclitaxel and carboplatin, and in soft tissue sarcomas with paclitaxel. In a randomized Phase II trial in patients with metastatic melanoma, the combination of paclitaxel and elesclomol showed a more than doubled progression free survival (PFS) compared to paclitaxel alone [47]. The recent follow-up Phase III SYMMETRY trial was a randomized, double-blinded study that evaluated elesclomol with paclitaxel versus single agent paclitaxel in patients with stage IV chemotherapy-naïve melanoma. In contrast to the Phase II trial results, this study failed to meet its primary PFS end point; the trial was terminated after an interim analysis revealed a disproportionally higher rate of deaths in the elesclomol-containing arm, particularly in patients with high levels of LDH [48]. The SOD1 inhibitor ATN 224 (tetrathiomolybdate) also acts as a copper chelator [49]. Phase I trials confirmed tolerability of single agent therapy in patients with advanced solid tumors [50]. In Phase II trials in patients with metastatic renal cancer [51], hormone-naïve or refractory prostate cancer [52,53], and resectable esophageal cancer [54], there were no objective clinical responses. Currently, trials combining ATN 224 with carboplatin/pemetrexed in patients with metastatic non-squamous non-small cell lung carcinoma, and with chemo-radiation in patients with breast cancer are underway. Arsenic trioxide (ATO) increases H2O2 and is currently in clinical trials in colorectal carcinoma, CNS malignancies (glioma, astrocytoma), multiple myeloma, urothelial carcinoma and in a variety of other hematological malignancies. The HIF-1α inhibitor 2-methoxyestradiol (2ME) elevates tumoral superoxide levels and has antitumor and anti-angiogenic properties. A Phase II trial combining 2ME and sunitinib in patients with renal cell carcinoma showed SD in 4 of the 17 patients evaluated [55]. In another Phase II trial combining 2ME and bevacizumab in patients with metastatic carcinoid, 68% of patients had evidence of tumor reduction [56]. A 2ME successor analog, ENMD-1198, is better tolerated and associated with SD in Phase I trials in patients with neuroendocrine carcinoma of the pancreas and ovarian and prostate cancers [57]. Pharmacyclics Pharmaceuticals’ motexafin gadolinium selectively accumulates in tumor tissue, where it catalyzes O2− and H2O2 formation. Motexafin has been investigated in single agent and combination trials in patients with gliomas, glioblastomas, lung cancer, chronic lymphocytic leukemia (CLL), renal cancer, non-Hodgkin’s lymphoma, ovarian cancer, head and neck cancer and multiple myeloma. In Phase III trials comparing whole brain radiation therapy with motexafin versus WBRT alone, motexafin did not improve antitumor activity or OS in patients with NSCLC [58,59].
Agents that down-regulate GSH have been clinically investigated. Phenethyl isothiocyanate (PEITC) disables the GSH antioxidant system by conjugating GSH and exporting it out of the malignant cells. In susceptible tumors, PEITC-induced ROS increase results in tumor growth suppression [60]. PEITC is currently being tested as an antitumor agent in lymphoproliferative disorders. Imexon (amplimexon) is an aziridine-derived iminopyrrolidone that depletes the GSH pool by binding thiol. It is associated with induction of apoptosis via oxidative stress, mitochondrial damage and the release of cytochrome C [61]. Single-agent and combination Phase I trials have been performed in patients with non-Hodgkin lymphoma, multiple myeloma, melanoma, breast, lung, and prostate and pancreatic cancers. Imexon alone and in combination regimens have been generally well tolerated. Phase II combinatorial trials in patients with metastatic pancreatic adenocarcinoma, breast, lung, and prostate cancers and metastatic melanoma resulted in PRs, but no complete responses (CRs). Buthionine sulphoximine inhibits GSH synthesis; it underwent Phase I trial investigation in combination with melphalan during the mid-1990s and had limited activity [62]. The combination of buthionine sulphoximine and melphalan was evaluated in pediatric patients with neuroblastoma; final results have not been reported.
ROS-modulating agents have been tested as treatment adjuncts to decrease the severity of chemotherapy related adverse events. GE Healthcare’s mangafodipir – an SOD mimetic, catalase and GSH reductase – suppresses ROS production in tumor cells. It was initially approved for use as a MRI contrast agent but was found to have protective antioxidant properties. In patients receiving FOLFOX for colon cancer, mangafodipir pretreatment decreased myelosuppression and protected against the neurotoxic effects of oxaliplatin [63,64]. The therapeutic effects of mangafodipir are dependent on an intact manganese (Mn2+) complex, an effect that is compromised by free or loosely bound zinc in the plasma. Zinc has an ~ 1000 times higher binding affinity for fodipir and can cause the release of free Mn2+. Because free Mn2+ can cause neurotoxic side effects, PledPharma’s calmangafodipir (PledOx®) was developed to mitigate the potential adverse neurological effects of mangafodipir treatment. Calmangafodipir replaces Mn2+ with Ca2+ and is a safer and more stable agent than mangafodipir. Preclinical data indicates that it is more myeloprotective and has increased antitumor effects when used in combination with oxaliplatin [64].
2.3 Amino acid delivery and malignant cell metabolism
Certain tumor types are auxotrophic for asparagine and arginine, lacking the capacity to synthesize enough of these amino acids to sustain growth. Asparaginase converts asparagine to aspartic acid and ammonia, whereas arginine deiminase (ADI) converts arginine to citrulline and ammonia. Asparaginase and ADI starve malignant cells of asparagine and arginine and cause cell death (Table 4) [65–67]. Since its initial FDA approval in 1970, Escherichia coli-derived asparaginase (Elspar) has been a standard agent for the treatment of acute lymphocytic leukemia (ALL). Variants such as pegylated asparaginase (Oncaspar or PEG-asparaginase) and Erwinia chrysanthemi-derived asparaginase (Erwinaze) have more recently been approved for patients who are hypersensitive to the bacterially derived enzyme. A multicenter Phase II with single agent L-asparaginase in ALL demonstrated ORR of 78% and is now a backbone of therapy [68]. A Phase II trial with L-asparaginase in ovarian cancer, however, was terminated early due to safety concerns [69]. ADI-PEG 20 is currently being evaluated in the Phase I – III setting in patients with lymphoma, advanced solid tumors and other hematologic malignancies. A Phase II trial was associated with SD in 9 patients with melanoma, including 4 with uveal melanoma [70]. ADI-PEG 20 with docetaxel has been evaluated in a Phase I trial in 18 patients with advanced solid tumors; in 11 evaluable patients, one had PR and 6 had SD [71]. In a randomized Phase II trial in patients with HCC, treatment with ADI-PEG 20 resulted in SD in 31% of heavily pretreated patients with advanced disease [72]. ADI-PEG 20 is currently being investigated in a Phase III trial in patients with HCC.
Table 4.
Phase I and II trials using other agents that modulate metabolism.
| Agent | Mechanism | Phase/Trial/Number of patients | Outcome |
|---|---|---|---|
| Asparaginase | Depletes asparagine | 2/Ovarian/4 | Terminated early d/t toxicity (4) |
| ADI-PEG 20 | Inhibits arginine deaminase | 1 & 2/Advanced Melanoma/31 2/Advanced solid tumors (+docetaxel)/18 2/Advanced HCC/71 |
SD in 9 patients (4 with uveal melanoma) 1 PR, 6 SD 31% SD |
| EGCG | Induction of apoptosis | 1b/Hormone-receptor negative breast cancer/40 2/Chronic lymphocytic leukemia/42 2/Advanced ovarian cancer/16 |
Well tolerated in chemopreventive setting 69% Rai stage 0 – 2 with biologic response Five women without recurrence at 18m |
| AG-221 | IDH2 inhibitor | 1: Hematologic malignancies with IDH2 mutations/22 |
6/7 evaluable pts with RR |
| cG250 (Rencarex®) | Chimeric monoclonal antibody against CAIX | 1/Advanced renal cell carcinoma/23 1 & 2/Metastatic renal cell carcinoma (+IFN-a)/31 |
Well tolerated, 74% SD at 3m 2 PR, 14 SD at 4m |
| E7070 (Indisulam) | Decreases glutathione synthase and glutathione reductase | 1/Advanced solid tumors (+Xeloda)/35 1/Advanced solid tumors (+carboplatin)/16 2/Metastatic renal cell carcinoma/30 2/Advanced NSCLC/44 |
2 PR, 17 SD Well tolerated Majority of patients with PD* 1 MR |
ADI: Arginine deiminase; CAIX: Carbonic anhydrase IX; EGCG: Epigallocatechin gallate; HCC: Hepatocellular carcinoma; IDH: Isocitrate dehydrogenase; PR: Partial remission; SD: Stable disease.
2.4 Fatty acid synthesis
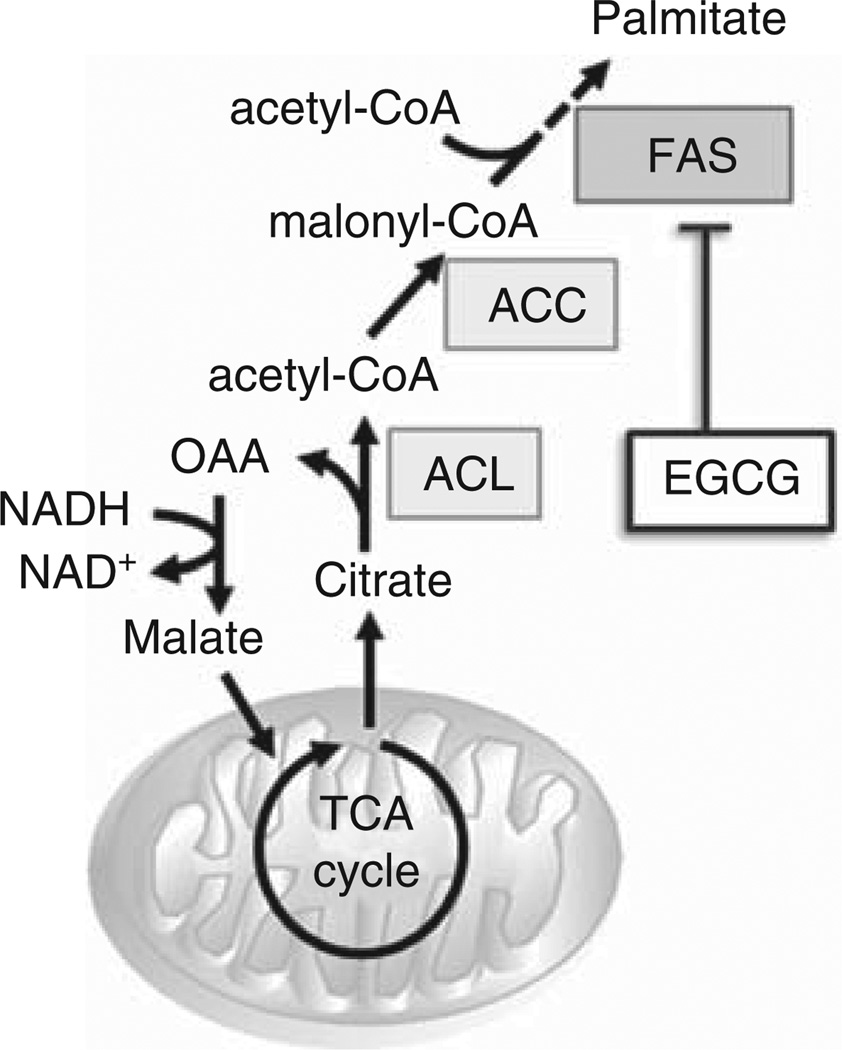
Malignant cells synthesize fatty acids for production of membranes and storage of energy [73]. Fatty acid synthesis is regulated by ATP citrate lyase (ACL), acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS). Most inhibitors of these enzymes are still in preclinical development, but the FAS inhibitor in green tea extract, epigallocatechin gallate (EGCG), has been evaluated in Phase I and II trials (Figure 4, Table 4).
Figure 4. Agents that modulate fatty acid synthesis.
Limited agents that act on fatty acid synthesis are in clinical development, but the fatty acid synthase inhibitor, epigallo-catechin gallate has been evaluated in phase 1 and 2 trials.
ACC: Acetyl-CoA carboxylase; ACL: Adenosine triphosphate citrate lyase; CoA: Coenzyme A; EGCG: Epigallocatechin gallate; FAS: Fatty acid synthase; NAD: Nicotinamide adenine dinucleotide; OAA: Oxaloacetic acid; TCA: Tricarboxylic acid cycle.
FAS converts acetyl-CoA and malonyl-CoA to palmitate. Palmitic acid is the first fatty acid synthesized and the precursor to longer fatty acids; it is important for the production of membrane phospholipids and assembly of lipid rafts. FAS inhibition triggers malignant cell apoptosis [74] and increased FAS expression has been described in breast, prostate, colon, lung, bladder, ovarian, gastric, endometrial, renal, skin and esophageal cancers; high levels of FAS confer a poor prognosis [75]. A Phase II trial evaluating EGCG in patients with advanced ovarian cancer showed no objective responses, but patients with Rai stage 0 – 2 CLL experienced clinical benefits. In the latter trial, 31% of patients had a sustained reduction of ≥ 20% in their absolute lymphocyte count and of patients with palpable adenopathy, 69% had at least a 50% reduction in the sum of the products of all lymph node areas at some time point during the 6 months of active treatment [76].
2.5 Other critical enzymes and their inhibitors
Mutations in the cytoplasmic enzyme isocitrate dehydrogenase (IDH) 1 and the mitochondrial enzyme IDH2 were originally identified in glioma, AML and myeloproliferative disorders/MDS but are increasingly found in a diverse set of other tumors such as chondrosarcoma, lymphoma, melanoma, and thyroid cancer [77–79]. Mutations in IDH augment the activity of numerous enzymes involved in the metabolism of citrate and result in the generation of a unique 2-hydroxyglutarate (2-HG) onco-metabolite [80]. 2-HG inhibits the function of TET2, a DNA demethylase enzyme, and results in hypermethylation [81,82]. In hematologic malignancies, DNA hypermethylation impairs hematopoietic differentiation and promotes leukemogenesis [83]. Additionally, excess 2-HG leads to an increased risk of developing solid tumors and may induce oncogenic progression in these malignancies [77]. Agios Pharmaceuticals has two oral IDH inhibitors in clinical development: an orally available selective IDH1 inhibitor, AG-120, and an orally available selective IDH2 inhibitor, AG-221. In a Phase I dose escalation trial investigating AG-221 in patients with IDH2 mutations and either AML or MDS, an early report demonstrated that 6 of 10 AML patients had objective responses, including 2 CRs and four other ongoing responses [84]. Three patients were not evaluable due to disease-related sepsis. Correlative analyses indicated that response was associated with a reduction of 2-HG of at least 90%.
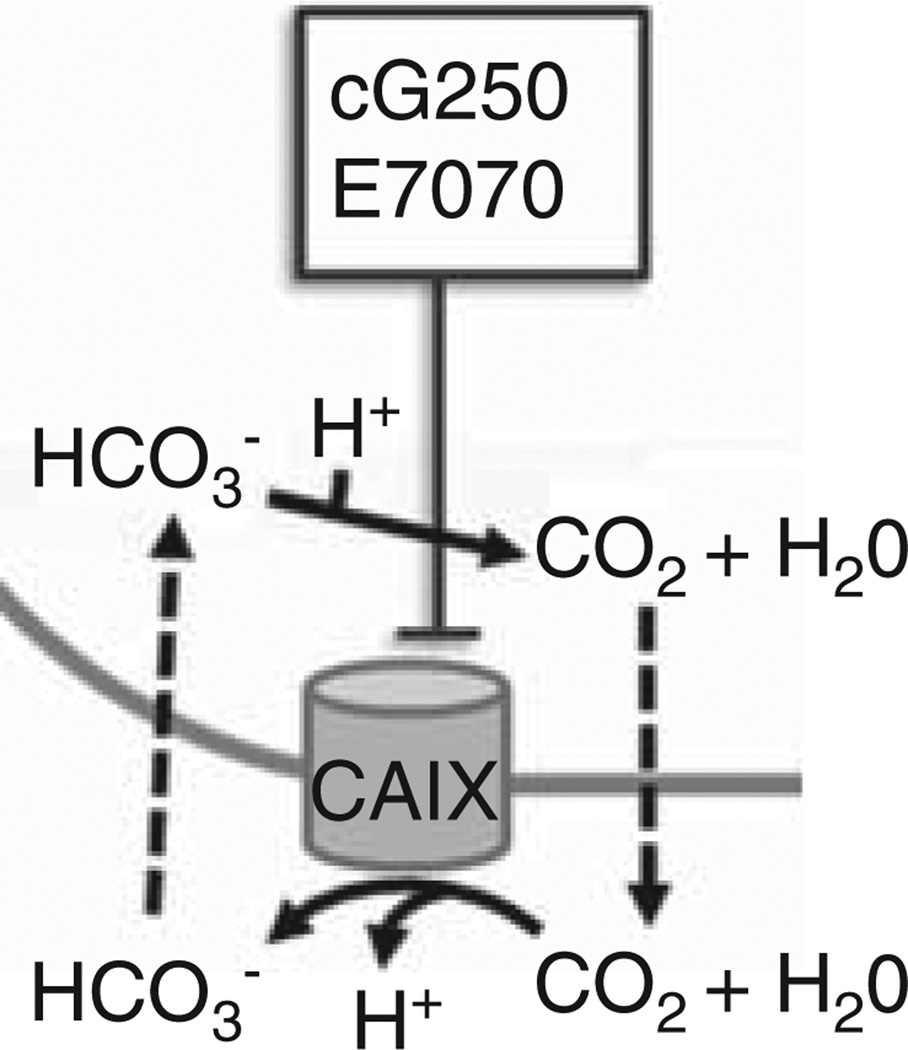
Carbonic anhydrase IX (CAIX) is present on cellular membranes where it catalyzes the hydration of CO2, removes excess protons from the cell, and acidifies the cellular microenvironment (Figure 5) [85]. Removal of intracellular protons maintains intracellular pH and promotes tumoral survival, growth and invasiveness. CAIX is upregulated in esophageal, lung, renal, colon, breast, cervical, endometrial, brain, bladder, head and neck cancers and other cancers. Wilex’s cG250 (Rencarex) is a monoclonal antibody that targets CAIX. In a Phase I/II trial evaluating the safety and efficacy of combination Rencarex and low-dose IFN-α in patients with metastatic clear cell renal carcinoma (mRCC), treatment was well tolerated and objective clinical responses were observed. Two of the 31 patients had PRs for up to 17 months, and 14 patients had evidence of SD at 4 months [86]. The preliminary results of the follow-up randomized Phase III ARISER trial that evaluated monotherapy adjuvant cG250 versus placebo in patients with localized clear cell RCC indicate that only those patients with a high CAIX score had a significantly improved disease free survival [87]. This study provides significant evidence for the use of this agent in a selected population subset. Eisai, Inc.’s indisulam/E7070, another CAIX inhibitor, continues to be tested in numerous clinical trials. In Phase I trials, the combination of indisulam and carboplatin or capecitabine was generally well tolerated [88,89]. In a Phase I trial in patients with advanced solid tumors, combination treatment with indisulam and capecitabine resulted in a PR in one patient with colon cancer and another PR in a patient with pancreatic adenocarcinoma; SD was documented in 17 patients after one cycle of treatment. However, in Phase II trials in patients with mRCC and NSCLC, single agent treatment resulted in no objective clinical responses [90,91].
Figure 5. Agents that modulate the carbonic anhydrase IX pathway.
Carbonic anhydrase IX is upregulated in numerous malignancies, and cG250, the anti-CAIX monoclonal antibody, and CAIX inhibitor E7070 have been evaluated in clinical trials.
CAIX: Carbonic anhydrase 9; CO2: Carbon dioxide; H+: Hydrogen; H2O: Water; HCO3−: Bicarbonate.
The conversion of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) to mevalonic acid by HMG-CoA reductase is an early, rate-limiting step in cholesterol synthesis. Dysregulation of this pathway is associated malignant transformation [92]. Statins have been repurposed as anti-neoplastic agents and are currently being investigated in neoadjuvant, adjuvant and metastatic settings in multiple solid tumors and hematologic malignancies. Data from three Phase II trials indicated that addition of statins to chemotherapy was not associated with additional clinical benefit in chemotherapy-naïve patients with extensive stage small cell lung carcinoma [93], locally advanced or metastatic pancreatic adenocarcinoma [94]. There is evidence, however, indicating increased response rate and prolonged progression free survival in patients with wild-type EGFR non-adenocarcinoma NSCLC when statins are used in combination with gefitinib [95]. Additionally, the SWOG0919 Phase II study investigated the combination of pravastatin to intermediate dose cytarabine and idarubicin in 36 patients with relapsed AML [96]. The trial showed that the combination resulted in a median OS of 12 months and a 75% ORR, inclusive of 20 patients with CR and 7 CR with incomplete count recovery; this promising result supports the premise that inhibition of cholesterol uptake and synthesis can sensitize AML cells to chemotherapy.
Metformin, an oral biguanide derivative, has also been repurposed as an anti-neoplastic agent. It may suppress cancer growth through the induction of cell cycle arrest and/or apoptosis, inhibition of protein synthesis, reduction of insulin in the blood, activation of the immune system, eradication of cancer stem cells and inhibition of the unfolded protein response [97,98]. This agent inhibits the mitochondrial complex and oxidative phosphorylation in malignant cells and causes these cells to be less reliant on glycolysis and more reliant on glutamine and glutaminolysis for TCA intermediates [99]. Metformin is also known to inhibit the PI3K and AKT/mTOR1 pathways [100]. There have been > 175 oncology trials that have explored metformin in either the prophylactic or therapeutic setting. Metformin has been studied primarily in large retrospective epidemiologic studies and in small noncontrolled prospective trials. A trial in endometrial cancer showed an improvement in OS in patients taking metformin versus those who were not, but there was no improvement in time to recurrence [101]. This finding led the researchers to question if the increased OS was secondary to improved all-cause mortality or if metformin actually had an anti-neoplastic effect. In addition to endometrial cancer, metformin has also been extensively investigated in patients with breast cancer and a Phase III placebo-controlled trial is currently ongoing [102]. Many other early-stage clinical trials are underway that investigate the potential of metformin to prevent an array of cancers. There are numerous epidemiological studies and animal models suggesting that augmentation of fat, fiber and vegetable intake may have important implications in cancer prevention or progression [103]. The benefit seen in metformin underpins a larger question of how changes in diet affect malignant cell growth. Changes in diet may also alter gut flora and impact immune response to malignant cells. The role of combining dietary changes with various cancer treatments is being explored.
3. Conclusion
It has become evident over the last fifty plus years that tumor metabolism differs in multiple highly significant ways from normal cellular metabolism. These alterations result from the tumoral state itself as well as from mutations and changes in enzyme expression that ultimately promotes tumorigenesis, malignant cell survival and proliferation. These metabolic derangements are affected by oncogenic signaling and metabolic abnormalities in turn affect oncogenic signaling. We propose that an increased understanding and investigation of tumor anti-metabolics will pave the way to a new frontier of antitumor therapeutics. From the data presented in this review, it is clear that metabolic inhibitors have promise in the treatment of cancer, often particularly when used in combination with chemotherapy, radiation therapy, pathway specific oncogenic-targeted agents, other anti-metabolics or epigenetic drugs. It is also clear that certain tumor types respond better to specific anti-metabolics than others; determining which agents work best in specific tumors will be increasingly important. Likewise, identification of patients whose tumors are characterized by identifiable mutations and/or enzyme overexpression will be important in determining whom to treat. Obviously, much work will be required to explore inhibition of enzymes for which there are no current modulators; similarly, much work is needed in developing agents with previously studied metabolic targets. Resistance will likely be an issue in the future as tumors alter their metabolism to survive and proliferate despite inhibition of metabolic pathways.
4. Expert opinion
A greater understanding of cancer cell metabolism has increased the clinical investigation of novel and repurposed agents that modulate metabolic targets. In defining the emerging role for these agents, we must be aware of both successes and failures in their development to date.
The majority of metabolism modulating agents have been well tolerated and do not interfere with normal cellular metabolism in a clinically meaningful manner. Clinical trials with some agents, including CPI-613, AG-221, ADI-PEG 20, and Rencarex have demonstrated single-agent efficacy. However, many agents only produced clinical benefit when combined with radiation and/or chemotherapy. ARQ-501 and 2ME showed clinical promise when used in combination regimens. ATO continues to be evaluated in hematologic malignancies following its therapeutic success with ATRA in patients with acute promyelocytic leukemia. Other agents, including 2-DG, mangafodipir and motexafin reduced the risk of adverse side effects resulting from chemotherapy and radiation. It is our opinion that several agents such as CPI-613 and AG-221 seem more promising than others based on their activities in Phase I-II trials. However, many metabolic inhibitors have transitioned into Phase III and could be deemed a success in that regard (Table 5). We believe these initial successes will prompt future research, and that this investigation will focus on defining therapeutic mechanisms, categorizing susceptible tumor subtypes and identifying ideal combinatorial strategies.
Table 5.
Selected ongoing early phase clinical trials in adult patients.
| Agent | Trial/Tumor type | Regimen (single agent unless otherwise specified) |
|---|---|---|
| 2-deoxyglucose Dichloroacetate | Pilot/Stage IV radiosensitive tumors 1/Recurrent head and neck cancer 1/Advanced malignancies 1/CNS 1/Head and neck cancer |
DCA + cisplatin + radiation |
| CPI-613 | 1/Relapsed/refractory AML 1 & 2/Advanced malignancies 1/Small cell lung cancer 1/Myelodysplastic syndrome 1/Metastatic pancreatic cancer 1/Metastatic bile duct cancer |
CPI-613 + cytarabine + mitoxantrone CPI-613 + FOLFIRINOX |
| AZD-3965 Mangafodipir ARQ-501 STA-4783 |
1/Advanced malignancies 2/Metastatic colorectal 1/Advanced solid tumors 1/Relapsed/refractory AML 2/Recurrent or persistent ovarian epithelial, fallopian, primary peritoneal |
FOLFOX +/− mangafodipir STA-4783 + paclitaxel |
| Arsenic Trioxide | 1/NSCLC 1/Myelofibrosis 1/Chronic myelogenous leukemia 1 & 2/Malignant glioma 2/Relapsed/refractory AML 1/Acute promyelocytic leukemia 2/Hepatocellular carcinoma 2/Small cell lung cancer |
ATO + icotinib ATO +/− ascorbic acid ATO + TKI ATO + temozolomide + radiation ATO + ATRA + GO TACE +/− ATO |
| ATN-224 | 1/Metastatic non-squamous NSCLC 1/Esophageal cancer 2/Breast cancer |
ATN-224 + Carboplatin/pemetrexed ATN-224 + chemoradiation |
| Imexon Elspar | 2/Relapsed follicular and aggressive lymphoma 1/Hematologic malignancies 1 & 2/Acute lymphoblastic leukemia |
|
| Oncaspar ADI-PEG 20 |
T-cell lymphoma 1/Mesothelioma, NSCLC 1/Prostate cancer, NSCLC 1/HER2-breast cancer 1/Metastatic melanoma 2/Acute myelogenous leukemia 2/Non-Hodgkin lymphoma |
Oncaspar + dexamethasone ADI-PEG 20 + pemetrexed + cisplatin ADI-PEG 20 + docetaxel ADI-PEG 20 + doxorubicin ADI-PEG 20 + cisplatin |
| EGCG | 1/Head and neck cancer 2/Amyloidosis |
EGCG + erlotinib |
| AG-120 | 1/Advanced solid tumors, glioma 1/Advanced hematologic malignancy |
|
| AG-221 E7070 |
1/Advanced hematologic malignancy 2/Relapsed AML, MDS |
E7070 + idarubicin + cytarabine |
ADI: Arginine deiminase; AML: Acute myeloid leukemia; ATO: Arsenic trioxide; EGCG: Epigallocatechin gallate; MDS: Myelodysplastic syndrome.
Despite these relative successes, many other agents have been clinically disappointing. Agents such as lonidamine and STA-4783 have successfully progressed into the Phase III trials but efficacy was not confirmed in this setting. Other agents, such as L-asparaginase in ovarian cancer, have been limited by excessive toxicity. Yet others, like SIL, failed due to poor tumor penetrance despite positive preclinical data. Inadequate preclinical models have hindered the clinical development of some metabolic inhibitors. Additionally, with the exception of 2ME in combination with sunitinib and bevacizumab, trials have not investigated combination regimens that simultaneously affect metabolic and oncogenic pathways. We believe that combining such agents will be therapeutically useful in the future because of the extensive crosstalk between oncogenic and metabolic signaling.
It will be of utmost importance to identify the specific mutations that cause the development and progression of tumors. It will be necessary to investigate targeted agents in the context of this knowledge, as is being done with IDH1 and IDH2 inhibitors. Once susceptible patient subsets are identified, successful clinical development of metabolism-directed therapies will depend on the appropriate understanding of the pharmacokinetic and pharmacodynamic properties of each agent.
We are excited about the progress with this new class of agents and look forward to a time when modulating tumor metabolism will become a major modality for treating cancer. We propose that a better understanding of metabolism and continued preclinical development of metabolic modulating therapies will propel further research. We eagerly anticipate the results of future investigations with these drugs as single agents and when used in combination with chemotherapy, radiation therapy, oncogenic-targeted therapies, other metabolic modulating agents and immune therapies. Ultimately, we believe that metabolic modulation will become one of the major successful ways of treating malignant disease; the data presented in this paper suggests that this will be possible with continued investigation.
Article highlights.
Many discoveries on alterations in tumor cell metabolism occurred after Otto Warburg described the reliance of malignant cells on aerobic glycolysis.
Several oncogenic signaling pathways alter tumor metabolism.
Tumor metabolism causes alterations in oncogenic signaling.
Metabolic modulators that alter essential malignant cell survival pathways have been developed with some success in recent years and they continue to be developed.
The success of metabolic modulating agents in cancer will depend on a better understanding of their mechanism and identification of the ideal tumor type to target.
Metabolic inhibitors should be studied both as single agents and in combination with other agents.
Acknowledgements
Timothy Schulz, PhD, Technical Resources International, Rockville, MD consulted on this project as a medical writer. DW Sborov and BM Haverkos contributed equally to this work.
Footnotes
Declaration of interest
DW Sborov and BM Haverkos are supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA165998 (PI Miguel Villalona and Steven Devine). Both are Hematology and Oncology fellows at Ohio State University. PJ Harris is an employee of the National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. •• Excellent review of crosstalk between metabolic and oncogenic signaling.
- 2. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. •• Original description of Warburg effect.
- 3.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med. 2013;45(10):e45. doi: 10.1038/emm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202(3):654–662. doi: 10.1002/jcp.20166. • Good review of the role of glucose transporter family proteins in tumor metabolism.
- 6.Dhanalakshmi S, Singh R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24(7):1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 7.Flaig TW, Glode M, Gustafson D, et al. A study of high-dose oral silybinphytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70(8):848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 8.Siegel AB, Narayan R, Rodriguez R, et al. A Phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma. Integr Cancer Ther. 2014;13(1):46–53. doi: 10.1177/1534735413490798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JW, Gao P, Liu YC, et al. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27(21):7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pelicano H, Martin D, Xu R, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. •• Comprehensive review of glycolysis in cancer and early agents that inhibit it.
- 11.Fang J, Quinones QJ, Holman TL, et al. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol. 2006;70(6):2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 12.Floridi A, Bruno T, Miccadei S, et al. Enhancement of doxorubicin content by the antitumor drug lonidamine in resistant Ehrlich ascites tumor cells through modulation of energy metabolism. Biochem Pharmacol. 1998;56(7):841–849. doi: 10.1016/s0006-2952(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 13.De Lena M, Lorusso V, Latorre A, et al. Paclitaxel cisplatin lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37(3):364–368. doi: 10.1016/s0959-8049(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 14.Portalone L, Lombardi A, Antilli A, et al. Treatment of inoperable non-small cell lung carcinoma stage IIIb and IV with cisplatin, epidoxorubicin, vindesine and lonidamine: a phase II study. Tumori. 1998;85(4):239–242. doi: 10.1177/030089169908500405. [DOI] [PubMed] [Google Scholar]
- 15.Berruti A, Bitossi R, Gorzegno G, et al. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a phase III study with a factorial design. J Clin Oncol. 2002;20(20):4150–4159. doi: 10.1200/JCO.2002.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Pacini P, Rinaldini M, Algeri R, et al. FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) versus EM (epidoxorubicin and mitomycin-C) with or without lonidamine as first-line treatment for advanced breast cancer. A multicentric randomised study. Final results. Eur J Cancer. 2000;36(8):966–975. doi: 10.1016/s0959-8049(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 17. Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol. 2009;5(5):581–585. doi: 10.2217/fon.09.44. • Review of 2-deoxy-d-glucose in cancer therapy.
- 18.Landau BR, Laszlo J, Stengle J, Burk D. Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-D-glucose. J Natl Cancer Inst. 1958;21(3):485–494. [PubMed] [Google Scholar]
- 19.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53(2):116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 20.Dwarakanath BS, Singh D, Banerji AK, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21–S26. doi: 10.4103/0973-1482.55136. [DOI] [PubMed] [Google Scholar]
- 21. Singh D, Banerji AK, Dwarakanath BS, et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181(8):507–514. doi: 10.1007/s00066-005-1320-z. • Highlights potential role of combination 2-deoxy-d-glucose with radiation.
- 22.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Liotta LA, Petricoin EF. Cancer metabolism and mass spectrometry-based proteomics. Cancer Lett 2013. doi: 10.1016/j.canlet.2013.11.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. Wong N, De Melo J, Tang D. PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol. 2013;2013:242513. doi: 10.1155/2013/242513. • Good overview of PKM2 in cancer metabolism.
- 25.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12(11):829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara S, Kawano Y, Yuki H, et al. PDK1 inhibition is a novel therapeutic target in multiple myeloma. Br J Cancer. 2013;108(1):170–178. doi: 10.1038/bjc.2012.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99(7):989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehouse S, Cooper RH, Randle PJ. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunbar E, Coats B, Shroads A, et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs. 2014;32(3):452–464. doi: 10.1007/s10637-013-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shroads AL, Langaee T, Coats BS, et al. Human polymorphisms in the glutathione transferase zeta 1/maleylacetoacetate isomerase gene influence the toxicokinetics of dichloroacetate. J Clin Pharmacol. 2012;52(6):837–849. doi: 10.1177/0091270011405664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garon EB, Christofk HR, Hosmer W, et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140(3):443–452. doi: 10.1007/s00432-014-1583-9. • Improved efficacy of a PDK inhibitor, dicloroacetate with chemotherapy.
- 32. Xuan Y, Hur H, Ham IH, et al. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321(2):219–230. doi: 10.1016/j.yexcr.2013.12.009. • Improved efficacy of dichloroacetate with chemotherapy.
- 33.Stuart SD, Schauble A, Gupta S, et al. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014;2(1):4. doi: 10.1186/2049-3002-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zachar Z, Marecek J, Maturo C, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med. 2011;89(11):1137–1148. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- 35. Pardee T, Lee K, Luddy J, et al. A phase I study of the safety, efficacy and pharmacokinetics of the first in class pyruvate dehydrogenase complex inhibitor CPI-613 in patients with advanced hematologic malignancies. Blood. 2013;122(21):A486. • Description of Phase I trial with CPI- 613 in which efficacy was shown.
- 36.Lee K, Khaira D, Rodriguez R, et al. Long-term stable disease of stage IV pancreatic neuroendocrine tumors and without significant adverse effect by CPI-613, an investigational novel anti-cancer agent. Case Stud Case Rep. 2011;1:137–145. [Google Scholar]
- 37.Senzer N, Bedell C, Maturo C, et al. CPI-613, an investigational novel anti-cancer agent, provides long-term stable disease without significant adverse effects in a patient with stage IV relapsed hepatocellular carcinoma. Case Stud Case Rep. 2012;2(2):38–45. [Google Scholar]
- 38.Dimmer KS, Friedrich B, Lang F, et al. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–227. [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6(1):127–148. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egler RA, Fernandes E, Rothermund K, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24(54):8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 41. Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10(7):709–720. doi: 10.1096/fasebj.10.7.8635688. •• Well-written manuscript about anti- oxidant and redox regulation of gene transcription.
- 42.Doroshow JH. Redox modulation of chemotherapy-induced tumor cell killing and normal tissue toxicity. J Natl Cancer Inst. 2006;98(4):223–225. doi: 10.1093/jnci/djj065. [DOI] [PubMed] [Google Scholar]
- 43.Kawecki A, Adkins D, Cunningham C, et al. A phase II study of ARQ 501 in patients with advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2007;25:16509. [Google Scholar]
- 44.Shapiro G, Supko J, Ryan D, et al. Phase I trial of ARQ 501, an activated checkpoint therapy (ACT) agent, in patients with advanced solid tumors. J Clin Oncol. 2005;23:3042. [Google Scholar]
- 45. Khong H, Dreisbach L, Kindler H, et al. A phase 2 study of ARQ 501 in combination with gemcitabine in adult patients with treatment naive, unresectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:15017. • Combination of ARQ-501 and gemcitabine resulted in stable disease.
- 46.Nagai M, Vo NH, Shin Ogawa L, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic Biol Med. 2012;52(10):2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 47.O’Day S, Gonzalez R, Lawson D, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol. 2009;27(32):5452–548. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- 48.O’Day SJ, Eggermont AM, Chiarion-Sileni V, et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J Clin Oncol. 2013;31(9):1211–1218. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 49.Khan G, Merajver S. Copper chelation in cancer therapy using tetrathiomolybdate: an evolving paradigm. Expert Opin Investig Drugs. 2009;18(4):541–548. doi: 10.1517/13543780902845622. [DOI] [PubMed] [Google Scholar]
- 50.Lowndes SA, Adams A, Timms A, et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin Cancer Res. 2008;14(22):7526–7534. doi: 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- 51.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9(5):1666–1672. 2003. [PubMed] [Google Scholar]
- 52.Henry NL, Dunn R, Merjaver S, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2007;71(3–4):168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 53.Lin J, Zahurak M, Beer TM, et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/ zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naive prostate cancer. Urol Oncol. 2013;31(5):581–588. doi: 10.1016/j.urolonc.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider BJ, Lee JS-J, Hayman JA, et al. Pre-operative chemoradiation followed by post-operative adjuvant therapy with tetrathiomolybdate, a novel copper chelator, for patients with resectable esophageal cancer. Invest New Drugs. 2013;31(2):435–442. doi: 10.1007/s10637-012-9864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruce JY, Eickhoff J, Pili R, et al. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest New Drugs. 2012;30(2):794–802. doi: 10.1007/s10637-010-9618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulke MH, Chan JA, Meyerhardt JA, et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68(2):293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Q, Gustafson D, Nallapareddy S, et al. A phase I dose-escalation, safety and pharmacokinetic study of the 2-methoxyestradiol analog ENMD-1198 administered orally to patients with advanced cancer. Invest New Drugs. 2011;29(2):340–346. doi: 10.1007/s10637-009-9383-9. • Demonstrates that ENMD-1198 was well tolerated.
- 58.Mehta MP, Shapiro WR, Phan SC, et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73(4):1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 59. Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. doi: 10.1200/JCO.2004.05.128. • Concomitant treatment with motexafin decreased the adverse effects of radiation.
- 60.Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 61. Dragovich T, Gordon M, Mendelson D, et al. Phase I trial of imexon in patients with advanced malignancy. J Clin Oncol. 2007;25(13):1779–1784. doi: 10.1200/JCO.2006.08.9672. • Article presents an example of how reactive oxygen species inhibiting agent, mangafodipir, decreases toxicity of co-administered chemotherapy.
- 62.Bailey HH, Ripple G, Tutsch KD, et al. Phase I study of continuous-infusion L-S, R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89(23):1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 63.Coriat R, Alexandre J, Nicco C, et al. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J Clin Invest. 2014;124(1):262–272. doi: 10.1172/JCI68730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlsson JOG, Kurz T, Flechsig S, et al. Superior therapeutic index of calmangafodipir in comparison to mangafodipir as a chemotherapy adjunct. Transl Oncol. 2012;5(6):492. doi: 10.1593/tlo.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bach SJ, Swaine D. The effect of arginase on the retardation of tumour growth. Br J Cancer. 1965;19:379–386. doi: 10.1038/bjc.1965.45. • Early pre-clinical work demonstrating that forced metabolism of the auxotrophic nutrient arginine retards tumor cell growth.
- 66.Kuo MT, Savaraj N, Feun LG. Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes. Oncotarget. 2010;1(4):246–251. doi: 10.18632/oncotarget.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Story MD, Voehringer DW, Stephens LC, Meyn RE. L-asparaginase kills lymphoma cells by apoptosis. Cancer Chemother Pharmacol. 1993;32(2):129–133. doi: 10.1007/BF00685615. • Pre-clinical data about L-asparginase-induced apoptosis in lymphoma cells.
- 68.Ettinger LJ, Kurtzberg J, Voute P, et al. An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer. 1995;75:1176–1176. doi: 10.1002/1097-0142(19950301)75:5<1176::aid-cncr2820750519>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 69.Hays JL, Kim G, Walker A, et al. A phase II clinical trial of polyethylene glycol-conjugated L-asparaginase in patients with advanced ovarian cancer: early closure for safety. Mol Clin Oncol. 2013;1(3):565–569. doi: 10.3892/mco.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ott PA, Carvajal RD, Pandit-Taskar N, et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs. 2013;31(2):425–434. doi: 10.1007/s10637-012-9862-2. • Example of efficacy of a metabolic modulator, arginine deiminase.
- 71.Tomlinson BK, Bomalaski JS, Diaz M, et al. Phase I trial of ADI-peg 20 plus docetaxel (DOC) in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31(Suppl) doi: 10.1158/1078-0432.CCR-14-2610. abstract 2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang T, Lu S, Chao Y, et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer. 2010;103(7):954–960. doi: 10.1038/sj.bjc.6605856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ookhtens M, Kannan R, Lyon I, Baker N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol. 1984;247(1 Pt 2):R146–R153. doi: 10.1152/ajpregu.1984.247.1.R146. [DOI] [PubMed] [Google Scholar]
- 74. Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6(4):551–562. doi: 10.2217/fon.10.11. • Good description of fatty acid synthase and its inhibitors in cancer.
- 75.Liu H, Liu JY, Wu X, Zhang JT. Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker. Int J Biochem Mol Biol. 2010;1(1):69–89. [PMC free article] [PubMed] [Google Scholar]
- 76.Shanafelt TD, Call TG, Zent CS, et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119(2):363–370. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med. 2010;16(9):387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 78. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. • Landmark article on isocitrate dehydrogenase (IDH1) mutation in glioblastoma multiforme.
- 79.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. • Seminal article about IDH1 mutation and 2-hydroxyglutarate synthesis.
- 81.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chaturvedi A, Cruz MMA, Jyotsana N, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood. 2013;122(16):2877–2887. doi: 10.1182/blood-2013-03-491571. • Discusses role of IDH as a driver in acute myeloid leukemia.
- 84. Stein E, Tallman M, Pollyea D, et al. Clinical safety and activity in a phase I trial of AG-221, a first in class, potent inhibitor of the IDH2-mutant protein, in patients with IDH2 mutant positive advanced hematologic malignancies; Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014. pp. 5–9. • Encouraging results about AG-221, an IDH2 inhibitor, in Phase I in hematologic malignancies.
- 85.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29(50):6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 86.Siebels M, Rohrmann K, Oberneder R, et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol. 2011;29(1):121–126. doi: 10.1007/s00345-010-0570-2. [DOI] [PubMed] [Google Scholar]
- 87. Belldegrun A, Kloepfer P, Fall B, et al. ARISER: a randomized double blind phase III study to evaluate adjuvant cG250 treatment versus placebo in patients with high-risk ccRCC - results and implications for adjuvant clinical trials. J Clin Oncol. 2013;31(Suppl) abstract 4507. • Provides data that a Carbonic anhydrase IX (CAIX) inhibitor, Rencarex, improves outcome in a subset of patients with high CAIX scores.
- 88.Dittrich C, Zandvliet A, Gneist M, et al. A phase I and pharmacokinetic study of indisulam in combination with carboplatin. Br J Cancer. 2007;96(4):559–566. doi: 10.1038/sj.bjc.6603606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegel-Lakhai W, Zandvliet A, Huitema A, et al. A dose-escalation study of indisulam in combination with capecitabine (Xeloda) in patients with solid tumours. Br J Cancer. 2008;98(8):1320–1326. doi: 10.1038/sj.bjc.6604300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raftopoulos H, Escudier B, Renshaw G, et al. A phase II multicenter study of the cyclin-dependent kinase inhibitor indisulam in patients with progressive inoperable and/or metastatic renal cell carcinoma (RCC) J Clin Oncol. 2004;22:4629. [Google Scholar]
- 91.Talbot DC, von Pawel J, Cattell E, et al. A randomized phase ii pharmacokinetic and pharmacodynamic study of indisulam as second-line therapy in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2007;13(6):1816–1822. doi: 10.1158/1078-0432.CCR-06-0249. [DOI] [PubMed] [Google Scholar]
- 92. Zhong C, Fan L, Yao F, et al. HMGCR is necessary for the tumorigenecity of esophageal squamous cell carcinoma and is regulated by Myc. Tumor Biol. 2014;35:4123–4129. doi: 10.1007/s13277-013-1539-8. • Demonstrates that dysregulated cholesterol synthesis can result in tumorigenesis.
- 93.Han JY, Lim KY, Yu SY, et al. A phase 2 study of irinotecan, cisplatin, and simvastatin for untreated extensive-disease small cell lung cancer. Cancer. 2011;117(10):2178–2185. doi: 10.1002/cncr.25790. [DOI] [PubMed] [Google Scholar]
- 94.Hong JY, Nam EM, Lee J, et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73(1):125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 95.Han J-Y, Lee S-H, Yoo NJ, et al. A randomized phase II. study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17(6):1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 96.Advani AS, McDonough S, Copelan E, et al. SWOG0919: a Phase 2 study of idarubicin and cytarabine in combination with pravastatin for relapsed acute myeloid leukaemia. Br J Haematol. 2014 doi: 10.1111/bjh.13035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karnevi E, Said K, Andersson R, Rosendahl AH. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer. 2013;13:235. doi: 10.1186/1471-2407-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2012;29(2):1314–1327. doi: 10.1007/s12032-011-9846-7. [DOI] [PubMed] [Google Scholar]
- 99.Bost F, Sahra IB, Le Marchand-Brustel Y, Tanti JF. Metformin and cancer therapy. Curr Opin Oncol. 2012;24(1):103–108. doi: 10.1097/CCO.0b013e32834d8155. [DOI] [PubMed] [Google Scholar]
- 100. Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–156. doi: 10.1038/nrendo.2013.256. •• Elegant review of the role of metformin in cancer therapy and diabetes mellitus.
- 101. Ko EM, Walter P, Jackson A, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014;132(2):438–442. doi: 10.1016/j.ygyno.2013.11.021. • Nice example of metformin’s anti-neoplastic activity.
- 102.Thompson AM. Molecular pathways: preclinical models and clinical trials with metformin in breast cancer. Clin Cancer Res. 2014;20(10):2508–2515. doi: 10.1158/1078-0432.CCR-13-0354. [DOI] [PubMed] [Google Scholar]
- 103.Aragόn F, Perdigόn G, de LeBlanc A. Modification in the diet can induce beneficial effects against breast cancer. World J Clin Oncol. 2014;5(3):455. doi: 10.5306/wjco.v5.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]