Abstract
The development of alcoholic liver disease (ALD) can be attributed to many factors that cause damage to the liver and alter its functions. Data collected over the last 30 years strongly suggests that an immune component may be involved in the onset of this disease. This is best evidenced by the detection of circulating autoantibodies, infiltration of immune cells in the liver, and the detection of hepatic aldehyde modified proteins in patients with ALD. Experimentally, there are numerous immune responses that occur when proteins are modified with the metabolites of ethanol. These products are formed in response to the high oxidative state of the liver during ethanol metabolism, causing the release of many inflammatory processes and potential of necrosis or apoptosis of liver cells. Should cellular proteins become modified with these reactive alcohol metabolites and be recognized by the immune system, then immune responses may be initiated. Therefore, it was the purpose of this article to shed some insight into how the immune system is involved in the development and/or progression of ALD.
Keywords: Alcoholic liver disease, Liver endothelial cells, Aldehyde adducts, Oxidative stress, Immune system, Cytokines, Metabolism
INTRODUCTION
It has become commonly accepted that immune mechanisms are partially responsible for the onset and/or progression of alcoholic liver disease (ALD). This is demonstrated by alcohol abuse increasing the immune deficiency in numerous diseases including: pneumonia, tuberculosis, human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis B virus (HBV), and many other less common infections[1]. This observation is often overlooked because of the many other complications of alcohol abuse such as: malnutrition, ingestion of high saturated fats, vitamin deficiency, drug abuse, and smoking[2]. The immune system has been suggested as playing a role in ALD because of the many clinical manifestations that have been observed and are thought to contribute to the damage of liver tissue[3,4]. However, the combination of immune function, metabolism, genetics, nutrition, and environmental factors most likely together play a role in the development of ALD. This review will begin to digest some of the potential hypothesis as to how the immune system, in combination with other factors contribute to the onset or progression of alcoholic liver disease.
LIVER TOXICITY DUE TO ETHANOL AND ITS METABOLITES
General byproducts
After ethanol is ingested, it is absorbed in the gut and transported to the liver by the bloodstream where it is metabolized by either the alcohol dehydrogenase or cytochrome-P450 pathways. The first and most important pathway is alcohol dehydrogenase, which is isolated in the mitochondria and oxidizes ethanol to acetaldehyde. Acetaldehyde is further oxidized to acetate by acetaldehyde dehydrogenase. These reactions help form NADH and NAD, which alter the redox state of the cell, causing harmful effects on lipid and carbohydrate metabolism when the normal ratio is altered[5]. Elevated levels of NADH inhibits fatty acid metabolism leading to the possible formation of acute fatty liver[6] or they may play a role in the formation of scar tissue in the liver (i.e. fibrosis)[7]. In the second method of ethanol metabolism, CYP2E1 (an isoenzyme of the cytochrome-P450 system) is involved. However, it is very limited at low doses, but can be induced by continuous exposure to higher levels of ethanol when the need arises[8]. As a general rule, alcoholics tend to have higher levels of this enzyme than non alcoholics[9].
Studies have found that CYP2E1 can metabolize certain fatty acids to exacerbate the CYP2E1 pathway causing a build-up of fatty infiltrate into the liver[10]. During CYP2E1 breakdown of alcohol, there is a release of highly reactive oxygen species (ROS). These ROS molecules damage the liver by altering the degradation of fat molecules, resulting in oxidative stress and free radical build up[11]. Therefore, both ethanol metabolism pathways contribute to the build-up of fats and oxidative stress in the liver, providing a source for highly reactive molecules capable of modifying or altering proteins in the liver.
Metabolite adducts
In the livers of chronic alcoholics, a number of metabolites are produced that could potentially become reactive to form stable protein adducts. Previous findings have demonstrated that acetaldehyde (AA) protein adducts form during the metabolism of ethanol to acetaldehyde[12-15]. These acetaldehyde adducts are immunogenic and have been found in the serum of both alcohol fed rats[16] and alcoholic patients[17]. Researchers have found that these AA-adducts interact with specific amino acids, particularly lysine, during their formation[18,19], as lysine residues on proteins tend to be highly reactive when protonated, and the level of modification depends on the concentration of acetaldehyde.
One AA-adduct that was investigated for a number of years is the N-ethyl lysine adduct that can be formed when AA is incubated under reducing conditions. However, the development of monoclonal and affinity purified polyclonal antibodies to this adduct have shown that it is not present in the tissue of rats chronically consuming ethanol or human alcoholics. This now makes sense, since the levels of reduction necessary to form this product do not occur in the liver during alcohol metabolism (which is an oxidative mechanism)[20,21].
As mentioned above, the metabolism of ethanol occurs through two pathways, both of which are oxidative and induce oxidative stress. Lipid peroxidation metabolizes fats to form malondialdehdye (MDA) and 4-hydroxy-2-nonenal (HNE), which can react with proteins to generate still other adducts[19,22]. Both of these adducts have been suggested to be detected in patients with alcoholic liver disease, with their concentrations possibly correlating with the severity of the disease[23,24].
The increased concentration of acetaldehyde levels and the oxidation of the fats in the liver due to chronic ethanol intake, increase the oxidative state of the liver, providing a key component in the formation of another adduct that “molds” these two observations. Recent studies have shown that both malondialdehyde (from lipid peroxidation) and acetaldehyde (from ethanol metabolism) react with lysines on proteins in a synergistic manner to form hybrid adducts different from either of these two aldehydes alone[25]. This adduct has been called Malondialdehye-Acetaldehyde adduct (MAA) and has been shown to form two different components on the lysine residue. The first is a relatively stable compound formed on lysine with two molecules of MDA and 1 molecule of AA. This 2:1 adduct is extremely stable and fluorescent due to its characteristic ring structure. The second compound is formed on lysine with one molecule of MDA and one molecule of AA. This 1:1 adduct is very unstable and most likely serves at the precursor to the 2:1 adduct[26]. The MAA-adduct has been found in both rats[27] and human subjects[28] chronically consuming alcohol and in human studies, it has been shown that increased levels of adducts in the serum correlated with severity of liver disease[28].
Immune response to MAA-adducts
The MAA-adduct has been the focus of numerous studies to determine its role in the onset of alcoholic liver disease. One thing that is clear is that these MAA-protein adducts, when injected into animals (in the absence of adjuvant), produce strong antibodies to the adduct, carrier protein, and the protein carrier conjugate[21]. Studies performed in our laboratory have demonstrated that MAA-adducts initiate the immune system to respond. These responses are achieved by binding to scavenger receptors, up-regulating adhesion molecules, inducing pro-inflammatory cytokines, producing antibody and T-cell responses, and increasing the pro-fibrotic response.
Clearance of MAA-adducts
Recent evidence has shown that MAA-adducts bind to cells expressing scavenger receptors on their surface[29,30]. Chronic ethanol feeding studies with rats has shown that ethanol impairs the receptor(s) on liver endothelial cells impairing their ability to clear acetaldehyde and MAA adducts[31-33]. The inability to clear these molecules out of the circulation leaves them available to other cells of the immune system. These immune cells bind modified adducts and induce inflammatory responses.
MAA-adducts upregulate adhesion molecules
In alcoholic liver disease, there is an increase in the infiltration of immune cells, which accounts for the necrosis of hepatocytes[34]. The recruitment of these cells occurs by the increase in adhesion molecule expression on liver sinusoidal endothelial cell (SECs) surface[35]. This action occurs when expression of selectins on SECs mediate loose attachment of leukocytes to the vessel walls. Chemoattractants cause the activation of integrins on leukocytes and these integrins bind to intercellular adhesion molecules (ICAMs) on the surfaces wall of the SECs. The leukocytes then respond to the amount of chemoattractant and diapedese through the SECs cell junction[36]. It has been shown that metabolites of alcohol can increase the expression of these adhesion molecules on the surface of SECs[37-41].
The ability of these adducts to increase chemoattractants (TNF-Alpha, MCP-1, and MIP-2) and adhesion molecules (ICAM, p-selectin, and L-selectin) provide evidence for the recruitment of immune cells into the liver. Damage to the liver results from the movement of these cells into the liver parenchyma subsequently, causing an increase in cell death.
MAA-adducts induce pro-inflammatory cytokines
Aldehyde adducts have also been shown to induce the release of pro-inflammatory cytokines and chemokines in kupffer, endothelial, and stellate cells[42-45]. These adducts most likely bind to scavenger receptors located on these cells and signal the release of cytokines. Since these adducts are not cleared efficiently, they are able to be re-circulated and induce pro-inflammatory responses. Also, T-cells and antibody can be elicited to exacerbate this response.
It has been determined that levels of the cytokines TNF-alpha, IL-1 and IL-6 are elevated in alcohol liver disease patients[46-48]. One possible mechanism for the increased levels of TNF-alpha is that increased gut permeability due the ethanol (leaky gut), causes the translocation of lipopolysaccharide (LPS) from the lumen into the blood stream[49]. LPS generated from bacterial cell walls has been shown to stimulate macrophages to release TNF-alpha and other cytokines[50-53]. Data generated from our laboratory has shown that LPS (in extremely small amounts) in conjunction with MAA-modified proteins actually increases the release of TNF-alpha, MCP-1, and MIP-1 by rat liver kupffer and endothelial cells[42]. One could postulate that both the metabolites of ethanol (adduct formation on proteins) and the increased levels of LPS in the blood stream (gut derived) provide the necessary components for endothelial and kupffer cells to begin releasing pro-inflammatory cytokines. Stellate cells have also been shown to increase the release of these pro-inflammatory cytokines in response to aldehyde adducts[45,54,55].
Increase in antibody and T-cell responses to MAA-adducts
Immune responses to MAA-adducts have been shown to be induced in the absence of adjuvants[19,21]. This response may represent an important mechanism by which T and B-cells respond to soluble proteins. It has been shown that with as little as 25 micrograms of MAA-modified protein, a tremendous antibody response is demonstrated; both to the MAA-adduct and the protein carrier conjugate[21].
Involvements of T-cells in these responses are shown by the strong expansion of T-cells to the MAA-adduct in in vitro studies[56]. These responses were determined to be to the adduct and protein carrier conjugate. It was also determined that scavenger receptors were involved in the uptake of the MAA-adduct and presentation to T-cells[30]. Recent studies in our laboratory have shown that MAA-modified self proteins can become immunogenic, potentially modifying liver proteins, and increasing the risk of specific organ damage.
Pro-fibrotic response to MAA-adducts
Hepatic fibrosis is the start of the wound-healing process resulting from the injury to the liver caused by years of alcohol consumption. This process can be reversed should ethanol consumption be eliminated. However, if ethanol consumption continues, fibrosis will occur, followed by scarring and finally the development of cirrhosis[57,58]. Fibrosis is the build up of excessive depositions of extracellular matrix (ECM) proteins, which consists of collagen and fibronectin[59]. These ECM proteins may cause the characteristic scar tissue formed in the liver after an injury has occurred. Byproducts of ethanol metabolism have been shown to increase the release of these products. Hepatic stellate cells up-regulate collagen genes in response to stimulation with acetaldehyde[60,61]. Also of interest is the finding that SECs secrete the isoform (EIIIA) of fibronectin in response to MAA-adducts, which are capable of causing stellate cells to release collagen[62]. Activation of the stellate cells is the key component in the fibrogenic process, providing the main production of fibrillar collagen. The build up of modified protein adducts causes disability of effective clearance, thereby increasing the activation of SECs and stellate cells, resulting in a fibrogenic response. The only way to intervene in the continual response form fibrosis to cirrhosis is to remove the ethanol from the system. Some anti-fibrotic drugs have been experimentally tested, yet there are still problems concerning delivery and concentration[63-65].
IMMUNE FUNCTION AND ALCOHOL
Innate immune response
Ethanol affects many functions of the innate immune system. The cell types involved in this early response include: macrophage, neutrophils, and natural killer cells. One of the most active cells, the macrophage, is designed to respond to bacterial cell wall antigens by releasing cytokines, and engulfing foreign agents. In alcoholic liver disease, kupffer cells in the liver are activated by lipopolysaccaride (LPS) caused from a breakdown in the intestinal wall permeability. This phenomenon has been called “leaky gut” and occurs when alcohol increases gut permeability, causing bacteria from the intestinal tract to escape into the blood stream[66,67]. When LPS is present, it activates kupffer cells to release TNF-alpha and superoxides that result in an inflammatory response. Recent studies in our laboratory have shown that adducts and very small amounts of LPS can stimulate kupffer cells and SECs to release these pro-inflammatory cytokines[42]. Once these cytokines are initiated, inflammation and necrosis occurs to hepatocytes and other cell types of the liver.
Neutrophils are the cell type that is predominantly recruited to the liver in response to the increase in cytokine concentrations. When these cells infiltrate into the site of infection, they phagocytose antigens and release proteolytic enzymes capable of destroying cell walls. They also release more chemoattractants to expedite the inflammatory process[68]. In alcoholic liver disease, these cells play a role in the propagation of the disease by infiltrating and cleaning up dead or dying cells. In fact, it has been shown that IL-8, a known neutrophil chemoattractant, is elevated in patients with alcoholic liver damage[69,70]. These cell types can be seen microscopically infiltrating the liver in patients with alcoholic hepatitis[1].
Adaptive immune system
The adaptive immune system consists of cells types designed to induce memory to specific antigens. It is characterized by the presentation of antigen to T-cells and clonal expansion of these cells to increase specificity to proteins, further increasing disposal of foreign agents. More importantly, there is a tolerance to self antigens, protecting the organism from self elimination[71]. When a breakdown in the adaptive immune response occurs, autoimmune diseases are sometimes initiated.
T-cells and NK T-cells have been implicated in the development of alcoholic liver disease by the increased numbers found in human livers following ethanol injury[72]. Studies have demonstrated that co-culturing lymphocytes (from alcoholic cirrhotic patients) with ethanol increased the expression of adhesion molecules and TNF-alpha as compared to control patients[38]. In this same study, the control patient lymphocytes when cultured with ethanol had a suppressive effect on the release of these molecules. The T-cells in the liver help drive the inflammatory process by releasing more cytokines including: TNF-alpha and INF-gamma. Another study implicating lymphocytes in ALD was done by transferring T cells from ethanol-fed rats to control recipient rats. In this experiment, damage to the naive livers was shown using reactive T-cells from alcohol-fed animals[73]. Cytotoxic T cells can be generated in response to acetaldehyde-modified spleen cells[74], giving aldehyde-modified proteins a role in the activation of these cells. Additionally, we have shown that aldehyde-modified proteins at high levels (non-physiological) can cause cell death and apoptosis to these cells[75]. Therefore, the build up of these adducts in the liver could potentially increase the level of cell death/apoptosis, increase cytokine production in all immune cell types, and cause increased inflammation in the liver leading to cirrhosis.
Alcoholic liver disease has often been associated with circulating antibodies and lymphocytes specific to hepatic antigens[76,77]. Circulating antibodies specific to acetaldehyde adducts and hydroxyethyl-free radicals have also been found to correlate with ALD[78,79]. Malondialdehyde-acetaldehyde (MAA-adducts) have been found to be significantly increased in patients with ALD, which correlated with the severity of liver damage[28]. Work done in animals has shown that MAA-adducted proteins are immunogenic without the use of adjuvants[21]. Antibodies directed against these proteins were specific for both the MAA-adduct and the carrier protein. When hen egg lysozyme (HEL) was use as the carrier antigen, an antibody response to the carrier protein and not the adduct was observed[56]. This provides a possible mechanism of autoantibody production wherein metabolites of ethanol modify hepatic self antigens and induce an autoimmune response against the liver.
In order to induce a autoimmune response, the right set of circumstance must be present to override the fail-safe mechanism(s) put in place by immune cells of the organism. In the adaptive immune response, this means that tolerance must be broken. How is tolerance broken in alcoholic liver disease? When metabolites of alcohol are present in the liver (as described above), the immune response is tremendously increased in the liver. Toxicity due to the chemical breakdown of these metabolites and the inflammation of immune cells increase the number of hepatocyte damage. Spilling of cellular material into the liver is cleaned up by macrophage and neutrophils. Self proteins from hepatocytes could become modified with acetaldehyde, malondialdehdye, or both. These self proteins could bind to and be taken up by macrophage, endothelial cells, or dendritic cells and presented to T-cells. If this occurs, reactive cytotoxic T-cells or the production of antibody could aid in damaging the liver. Experimental evidence to support this hypothesis has been demonstrated in animal models[80,81].
Previous studies with maleylated mouse serum albumin (MSA) have shown the breaking of tolerance by inducing an antibody response to the carrier protein (MSA)[82]. These proteins were found to bind to scavenger receptors on the surface of macrophage and presented to T-cells from the spleen. In fact, many different aldehyde-modified proteins have been shown to bind to scavenger receptors[29,83-85]. Studies performed in our laboratory have demonstrated similar findings when MSA was modified with acetaldehyde and malondialdehdye[21]. These MAA-modified self proteins appear to bind scavenger receptors, are processed and presented in the cell, and increase the proliferative response in T-cells[30]. Further proof for these observations is the increase in the co-stimulatory molecules B7-1 and B7-2 by splenocytes stimulated with MAA-modified proteins[86]. These co-stimulatory molecules are the key component in autoimmune regulation and the breaking of tolerance[87]. When the T-cell binds an antigen presenting cell, the T-cell receptor binds to the matching peptide presented in the groove of the MHC molecule. If co-stimulatory molecules are ligated, then the cell is signaled to respond and the T-cells begin to proliferate. Specificity is to the peptide in the groove, and if this is a self protein, the immune system sees these proteins (liver specific) as a foreign invader and eliminates the threat. This threat is eliminated with cyototoxic T-cells destroying the cell with their powerful enzymes[71]. If these proteins happen to be presented to multiple B-cells and crosslinking of receptors occurs, antibody production specific for the self-protein is initiated with the help of T-helper cells. Antibodies would then bind to the self proteins and be taken up by Fc receptors located on NK cells and attacking the liver cells[71]. See Figure 1 for a description of this hypothesis.
Figure 1.
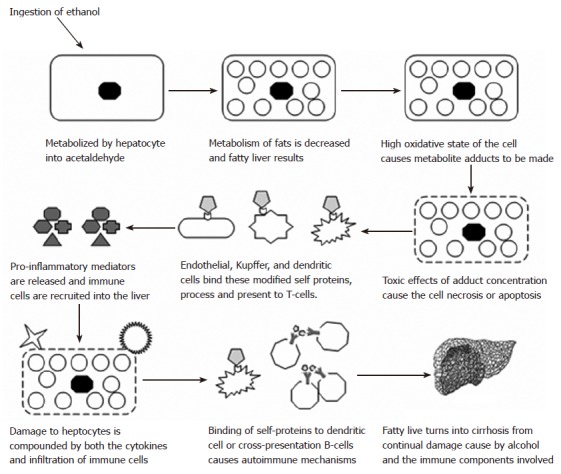
The proposed involvement of immune mechanisms in the development and/or progression of alcoholic liver disease. A schematic depicting the involvement of the immune system in the progress of fatty liver to end stage cirrhosis.
INTERACTION OF DIET, ETHANOL, AND IMMUNE FUNCTION
Diet and ethanol intake
Alcoholics have always been considered to be malnourished due to the exchange of food calories for alcohol, leaving a reduction in valuable nutrients. One explanation for this is that increased alcohol intake interferes with absorption of these nutrients from the intestine, making them unavailable to the body[8]. Thus, a decrease in body fat is the case for a number of individual alcoholics. However, metabolic syndrome in individuals has become a risk factor with the increase in high fat diets[88]. Metabolic syndrome is a chronic inflammatory condition that promotes insulin resistance and fat accumulation in the liver[89]. This disease, known as fatty liver, is quickly becoming an epidemic in our society as a result of increased dietary fat intake[90]. Taken into account the chronic inflammatory condition in alcoholic liver disease alone, one could assume that the two diseases together would greatly increase the severity of the disease. To support this claim experimentally, investigators have shown in a rat model that incorporates an ethanol diet with polyunsaturated fats, there is an increase in the severity of liver damage[91].
As mentioned above, alcohol metabolism increases the accumulation of fats in the liver. This is caused by suppression in lipid oxidation as a result of the increased production of acetate produced from the metabolism of ethanol[92]. Fatty liver can also occur when levels of NADH are increased. This enzyme is increased when alcohol is broken down by alcohol dehydrogenase and accounts for a number of metabolic conditions including: hyperlipemia, hypoglycemia, hyperlactacidemia, hyperuricmeia, and gout attacks[8]. In looking at the increased inflammatory state and oxidative stress of the liver under these conditions, one could speculate that lipoproteins and other proteins are modified by the metabolites of ethanol.
Lipoproteins are oxidized under certain conditions. These lipoproteins are under investigation for contributing to coronary heart disease[93]. However, there is some indication that these modified lipoproteins may play a role in damage to the liver. They have been shown to bind to scavenger receptors on stellate cells and stimulate extracellular matrix proteins[94]. Aldehyde-modified proteins are similar in nature to these modified LDL proteins, in that they bind to some of the same receptors[95]. Interestingly, in other studies, MAA-adducted proteins were found in atherosclerotic aortas, implicating a similar connection between MAA-modified proteins and oxidized LDL[96]. These data indicate that modified proteins may not be limited to the liver and may reach the blood stream and travel throughout the body. Since these modified proteins bind to scavenger receptors on endothelial cells, it seems likely that excess presence of these adducts could be harmful.
Malondialdehyde, one of the breakdown products of lipid metabolism, has been implicated in autoimmune disease[97]. Anti-MDA adducts have been found in patients with systemic lupus erythematosus (SLE) and correlated with antibody markers specific for this disease. MDA-adducts have also been found to correlate with atherosclerosis and many other diseases by their ability to form lipofuscin[98,99]. MDA-adducts in our laboratory has been found in atherosclerotic plaques using immunohistochemical techniques[96].
These adducts formed from the metabolism of fats or alcohol and have been shown experimentally in human subjects to have many harmful effects. The fact that they can be toxic to cells at high levels increases the cell death or apoptotic numbers in the organ[75,100,101]. This increases the amount of pro-inflammatory cytokines in the organ and calls immune cells in to clean up the debris. If aldehyde adducts bind to the cellular debris, and this material is picked up by an antigen-presenting cell, the risk for an increased immune response is prevalent. If these molecules happen to be presented to an activated dendritic cell, the immune response is generated that is specific for the self protein.
Taken altogether, the observation that many individuals are overweight or already have fatty liver without the consumption of alcohol[102], there is an increased risk of liver damage when alcohol is consumed in large quantities. Metabolism of fats and ethanol together constitute a fine balance between clearance and build up of reactive metabolites. Acetaldehyde from alcohol and malondialdehyde from lipids increase in number until the product is eliminated from the body. If and when individuals continue to consume either ethanol, fats, or both in large quantities, they run a risk of adduct formation. As discussed above, adduct formation can lead to a number of diseases including: autoimmunity, atherosclerosis, alcoholic liver disease, non-alcoholic liver disease, and many others.
Management or treatment options
In looking at treatment options for alcohol abuse and obesity, the fastest way to resolve these diseases is to eliminate the source. Alcoholic steatosis can be reversed if withdrawal from ethanol is accomplished. In fact, abstinence from ethanol can improve cirrhosis and/or fibrosis within only several weeks if eliminated from the diet[103]. As for obesity, any loss in weight has large implications in improving disease state of any kind. However, individuals will continue to consume fats and ethanol so treatment using other methods could help in the quest for improved disease state.
Nutrition of patients with alcoholic liver disease has been a key component, as many of these individuals are malnourished. This puts pressure on the immune system in its ability to clear infections and aid in liver regeneration[103]. Increasing proteins, vitamins, essential amino acids, and decreasing saturated fat intake can aid in the recovery of many liver disease patients[91,104]. However, diet change is hard for individuals so administration of certain vitamins has been somewhat successful. These include injections of vitamin B1, supplements of vitamins B2 and B6, and increasing folic acid levels[8].
When nutritional changes are not working for these patients, various compounds have had some success. These include: steroids, anti-TNF, S-adenosylmethionine, betaine, antibiotics, and possibly statins. Corticosteroids have been able to reduce the inflammatory response in numerous diseases. In alcoholic liver disease, steroids have been shown to reduce cytokine production, suppress acetaldehyde adducts, and inhibit the production of collagen[105]. Studies have demonstrated that TNF-alpha levels are greatly increased in the liver following chronic alcohol consumption[106]. This cytokine is toxic to cells and is the major source of inflammation in the liver. Treating with anti-TNF antibodies immobilizes the effects of TNF-alpha on hepatocytes and other cells in the liver[107].
Treatment with anti-oxidants has been somewhat successful in the quest for alcoholic liver disease therapy. S-adenosylmethionine (SAM) and betaine have been shown to restore glutathione levels, which increase the ability of the liver to metabolize fats[108-110]. SAM and betaine work by correcting alterations in methionine metabolic pathways, restoring methylation reactions in the oxidative liver. Use of antibiotics for treatment of liver cirrhosis in individuals with bacterial infections is also beneficial[111,112]. Bacterial infections in these patients are due in part to the increase in gut permeability, where by alcohol cause the release of normal flora into the blood stream. Lipopolysaccaride from the cell walls of these bacteria increase levels of cytokines greatly enhancing toxicity to cells in the liver[48]. By decreasing the level of bacteria in the system, activation of the immune system by these cytokines can be reduced.
Statins are commonly used to lower harmful cholesterol in the blood stream[113]. These drugs are mainly used to prevent atherosclerosis and heart disease. However, recent evidence for preventing other diseases has become available. For example, statins have been postulated to have an anti-inflammatory and immunomodulatory role in protecting the body against endotoxin from bacteria[114]. Evidence has also shown that these drugs may have implications in the treatment or prevention of metabolic syndrome[115]. The cholesterol lowering and anti-inflammatory properties of these drugs might be helpful in the future to lower the fatty liver associated with alcoholic liver disease. Studies will need to be performed to determine the efficacy of statins.
There is some suggestion that ethanol in moderation might have some beneficial effects to certain individuals. Decreased risk of coronary heart disease has been associated with individuals who drink as little as one to six alcoholic beverages per week[116]. Experts think that small doses of alcohol may increase the level of good cholesterol (HDL) in the blood stream regardless of the beverage consumed[117]. However, the overall benefit of alcohol does not outweigh the risk of the toxic effects of this drug. Like all things in life, moderation is the key to living a healthy lifestyle.
CONCLUSION
While there are a number of contributing factors resulting in the development and/or progression of alcoholic liver disease, the immune system appears to be a major player. Immune function in alcoholics has shown circulating autoantibodies, hypergammaglobulinemia[3,118], antibodies to metabolite adducts[24,28,79], and the infiltration of immune cells into the liver[34,37,72]. One other factor to support the immune system is seen in alcoholic patients who have received a liver transplant. If these individuals start consuming ethanol, hepatic fibrosis or cirrhosis occurs much more quickly than the years it took to develop prior to transplant[119]. This quick memory response to the liver suggests that the adaptive immune system is involved.
Other factors that contribute to the development of ALD include: metabolism, toxicity, genetics, and nutritional factors. The metabolism of excess ethanol causes many harmful effects that could contribute to the formation of metabolite adducts. If these adducts form while cells are dying from the toxic effects of ethanol, self-proteins could become modified, providing the necessary mechanism for autoimmune disease. If you figure in the susceptibility of certain individuals and their genetic make up, the risk for liver disease becomes higher. Also factored into to this is the observation that many alcoholics are malnourished, consuming many of their calories from alcohol[8]. It has been shown that the immune system works better when a healthy balanced diet is followed[120]. One other common issue is the consumption of high levels of fatty foods in the diets of most individuals. Alcohol metabolism already contributes to the build-up of fats in the liver (fatty liver); compound that with the addition of more fats in the diet and the potential exists for a double-edged sword of damage and oxidative stress.
The fact remains that alcohol consumption has many harmful effects on the body. However, getting people to stop drinking completely is an unrealistic expectation. The need for understanding the mechanisms of alcoholic liver disease remains necessary for determining better therapy and intervention strategies.
ACKNOWLEDGMENTS
Dr. Dean J Tuma, Karen C Easterling, Carlos D Hunter, Bartlett C Hamilton III, Amy L DeVeney, Kristin M Lenczowski, Jennifer C Thiele, and Chris D Peters whom work in the Experimental Immunology Laboratory.
Footnotes
S- Editor Ma N L- Editor Alpini GD E-Editor Li JL
References
- 1.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 2.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 3.Bailey RJ, Krasner N, Eddleston AL, Williams R, Tee DE, Doniach D, Kennedy LA, Batchelor JR. Histocompatibility antigens, autoantibodies, and immunoglobulins in alcoholic liver disease. Br Med J. 1976;2:727–729. doi: 10.1136/bmj.2.6038.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetterman RK, Sorrell MF. Immunologic aspects of alcoholic liver disease. Gastroenterology. 1981;81:616–624. [PubMed] [Google Scholar]
- 5.Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280–286. doi: 10.1136/pmj.76.895.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalant H. Research on alcohol metabolism: a historical perspective. Keio J Med. 1991;40:113–117. doi: 10.2302/kjm.40.113. [DOI] [PubMed] [Google Scholar]
- 7.Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–765. [PubMed] [Google Scholar]
- 8.Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220–231. [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumi M, Lasker JM, Shimizu M, Rosman AS, Lieber CS. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10:437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- 10.Koop DR, Casazza JP. Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem. 1985;260:13607–13612. [PubMed] [Google Scholar]
- 11.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoerner M, Behrens UJ, Worner T, Lieber CS. Humoral immune response to acetaldehyde adducts in alcoholic patients. Res Commun Chem Pathol Pharmacol. 1986;54:3–12. [PubMed] [Google Scholar]
- 13.Lin RC, Smith RS, Lumeng L. Detection of a protein-acetaldehyde adduct in the liver of rats fed alcohol chronically. J Clin Invest. 1988;81:615–619. doi: 10.1172/JCI113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niemelä O, Parkkila S, Ylä-Herttuala S, Villanueva J, Ruebner B, Halsted CH. Sequential acetaldehyde production, lipid peroxidation, and fibrogenesis in micropig model of alcohol-induced liver disease. Hepatology. 1995;22:1208–1214. [PubMed] [Google Scholar]
- 15.Freeman TL, Tuma DJ, Thiele GM, Klassen LW, Worrall S, Niemelä O, Parkkila S, Emery PW, Preedy VR. Recent advances in alcohol-induced adduct formation. Alcohol Clin Exp Res. 2005;29:1310–1316. doi: 10.1097/01.alc.0000171484.52201.52. [DOI] [PubMed] [Google Scholar]
- 16.Worrall S, De Jersey J, Shanley BC, Wilce PA. Ethanol induces the production of antibodies to acetaldehyde-modified epitopes in rats. Alcohol Alcohol. 1989;24:217–223. [PubMed] [Google Scholar]
- 17.Lin RC, Lumeng L, Shahidi S, Kelly T, Pound DC. Protein-acetaldehyde adducts in serum of alcoholic patients. Alcohol Clin Exp Res. 1990;14:438–443. doi: 10.1111/j.1530-0277.1990.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 18.Tuma DJ, Newman MR, Donohue TM, Sorrell MF. Covalent binding of acetaldehyde to proteins: participation of lysine residues. Alcohol Clin Exp Res. 1987;11:579–584. doi: 10.1111/j.1530-0277.1987.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 19.Worrall S, Thiele GM. Protein modification in ethanol toxicity. Adverse Drug React Toxicol Rev. 2001;20:133–159. [PubMed] [Google Scholar]
- 20.Thiele GM, Wegter KM, Sorrell MF, Tuma DJ, McDonald TL, Klassen LW. Specificity of N-ethyl lysine of a monoclonal antibody to acetaldehyde-modified proteins prepared under reducing conditions. Biochem Pharmacol. 1994;48:183–189. doi: 10.1016/0006-2952(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 21.Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22:1731–1739. [PubMed] [Google Scholar]
- 22.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Stewart SF, Vidali M, Day CP, Albano E, Jones DE. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39:197–203. doi: 10.1002/hep.20021. [DOI] [PubMed] [Google Scholar]
- 24.Viitala K, Makkonen K, Israel Y, Lehtimäki T, Jaakkola O, Koivula T, Blake JE, Niemelä O. Autoimmune responses against oxidant stress and acetaldehyde-derived epitopes in human alcohol consumers. Alcohol Clin Exp Res. 2000;24:1103–1109. [PubMed] [Google Scholar]
- 25.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 26.Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32:303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology. 1998;115:686–692. doi: 10.1016/s0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 28.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Aricó S, Sartori M, Bellomo G, Klassen LW, Thiele GM, et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31:878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 29.Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68:1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- 30.Willis MS, Thiele GM, Tuma DJ, Klassen LW. T cell proliferative responses to malondialdehyde-acetaldehyde haptenated protein are scavenger receptor mediated. Int Immunopharmacol. 2003;3:1381–1399. doi: 10.1016/S1567-5769(03)00136-X. [DOI] [PubMed] [Google Scholar]
- 31.Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Chronic ethanol consumption impairs receptor-mediated endocytosis of MAA-modified albumin by liver endothelial cells. Biochem Pharmacol. 2003;66:1045–1054. doi: 10.1016/s0006-2952(03)00416-7. [DOI] [PubMed] [Google Scholar]
- 32.Thiele GM, Miller JA, Klassen LW, Tuma DJ. Chronic ethanol consumption impairs receptor-mediated endocytosis of formaldehyde-treated albumin by isolated rat liver endothelial cells. Hepatology. 1999;29:1511–1517. doi: 10.1002/hep.510290517. [DOI] [PubMed] [Google Scholar]
- 33.Thiele GM, Miller JA, Klassen LW, Tuma DJ. Long-term ethanol administration alters the degradation of acetaldehyde adducts by liver endothelial cells. Hepatology. 1996;24:643–648. doi: 10.1002/hep.510240329. [DOI] [PubMed] [Google Scholar]
- 34.Haydon G, Lalor PF, Hubscher SG, Adams DH. Lymphocyte recruitment to the liver in alcoholic liver disease. Alcohol. 2002;27:29–36. doi: 10.1016/s0741-8329(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 35.Adams DH. Leucocyte adhesion molecules and alcoholic liver disease. Alcohol Alcohol. 1994;29:249–260. [PubMed] [Google Scholar]
- 36.Adams DH. Lymphocyte-endothelial cell interactions in hepatic inflammation. Hepatogastroenterology. 1996;43:32–43. [PubMed] [Google Scholar]
- 37.Sacanella E, Estruch R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict Biol. 2003;8:371–378. doi: 10.1080/13556210310001656376. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Perez JL, Diez-Ruiz A, Luna-Casado L, Soto-Mas JA, Wachter H, Fuchs D, Gutierrez-Gea F. T-cell activation, expression of adhesion molecules and response to ethanol in alcoholic cirrhosis. Immunol Lett. 1996;50:179–183. doi: 10.1016/0165-2478(96)02541-2. [DOI] [PubMed] [Google Scholar]
- 39.Mándi Y, Nagy I, Krenács L, Ocsovszky I, Nagy Z. Relevance of ICAM-1 to alcoholic liver cirrhosis. Pathobiology. 1996;64:46–52. doi: 10.1159/000164005. [DOI] [PubMed] [Google Scholar]
- 40.Bautista AP. Chronic alcohol intoxication enhances the expression of CD18 adhesion molecules on rat neutrophils and release of a chemotactic factor by Kupffer cells. Alcohol Clin Exp Res. 1995;19:285–290. doi: 10.1111/j.1530-0277.1995.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 41.Miller JA, Klassen LW, Duryee MJ, Tuma DJ, Thiele GM. Increased adhesion molecule and TNF-alpha expression by liver endothelial cells after exposure to MAA-modified proteins. Hepatology. 1997;26:808. [Google Scholar]
- 42.Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Liz G, Flores-Hernández J, Arias-Montaño JA, Reyes-Esparza JA, Rodríguez-Fragoso L. Modulation of urokinase-type plasminogen activator by transforming growth factor beta1 in acetaldehyde-activated hepatic stellate cells. Pharmacology. 2005;73:23–30. doi: 10.1159/000081071. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura Y, Yokoyama H, Higuchi S, Hara S, Kato S, Ishii H. Acetaldehyde accumulation suppresses Kupffer cell release of TNF-Alpha and modifies acute hepatic inflammation in rats. J Gastroenterol. 2004;39:140–147. doi: 10.1007/s00535-003-1278-5. [DOI] [PubMed] [Google Scholar]
- 45.Kharbanda KK, Todero SL, Shubert KA, Sorrell MF, Tuma DJ. Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol. 2001;25:123–128. doi: 10.1016/s0741-8329(01)00174-4. [DOI] [PubMed] [Google Scholar]
- 46.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- 47.Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221–225. [PMC free article] [PubMed] [Google Scholar]
- 48.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 49.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 50.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayub S, Verma J, Das N. Effect of endosulfan and malathion on lipid peroxidation, nitrite and TNF-alpha release by rat peritoneal macrophages. Int Immunopharmacol. 2003;3:1819–1828. doi: 10.1016/j.intimp.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 53.Niemelä O, Parkkila S, Pasanen M, Iimuro Y, Bradford B, Thurman RG. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res. 1998;22:2118–2124. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 54.George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39:756–764. doi: 10.1016/s0168-8278(03)00376-3. [DOI] [PubMed] [Google Scholar]
- 55.Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683–693. doi: 10.1042/BJ20020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willis MS, Klassen LW, Tuma DJ, Sorrell MF, Thiele GM. Adduction of soluble proteins with malondialdehyde-acetaldehyde (MAA) induces antibody production and enhances T-cell proliferation. Alcohol Clin Exp Res. 2002;26:94–106. [PubMed] [Google Scholar]
- 57.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert Rev Mol Med. 2003;5:1–23. doi: 10.1017/S1462399403005684. [DOI] [PubMed] [Google Scholar]
- 59.Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res. 2005;29:102S–109S. doi: 10.1097/01.alc.0000189275.97419.58. [DOI] [PubMed] [Google Scholar]
- 60.Nieto N, Dominguez-Rosales JA, Fontana L, Salazar A, Armendariz-Borunda J, Greenwel P, Rojkind M. Rat hepatic stellate cells contribute to the acute-phase response with increased expression of alpha1(I) and alpha1(IV) collagens, tissue inhibitor of metalloproteinase-1, and matrix-metalloproteinase-2 messenger RNAs. Hepatology. 2001;33:597–607. doi: 10.1053/jhep.2001.22520. [DOI] [PubMed] [Google Scholar]
- 61.Ma X, Svegliati-Baroni G, Poniachik J, Baraona E, Lieber CS. Collagen synthesis by liver stellate cells is released from its normal feedback regulation by acetaldehyde-induced modification of the carboxyl-terminal propeptide of procollagen. Alcohol Clin Exp Res. 1997;21:1204–1211. [PubMed] [Google Scholar]
- 62.Thiele GM, Duryee MJ, Freeman TL, Sorrell MF, Willis MS, Tuma DJ, Klassen LW. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593–1600. doi: 10.1016/j.bcp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Beljaars L, Meijer DK, Poelstra K. Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front Biosci. 2002;7:e214–e222. doi: 10.2741/A917. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 65.Bennett RG, Dalton SR, Mahan KJ, Gentry-Nielsen MJ, Hamel FG, Tuma DJ. Relaxin receptors in hepatic stellate cells and cirrhotic liver. Biochem Pharmacol. 2007;73:1033–1040. doi: 10.1016/j.bcp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 67.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 68.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–G314. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 69.Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology. 1993;18:576–580. [PubMed] [Google Scholar]
- 70.Sheron N, Bird G, Koskinas J, Portmann B, Ceska M, Lindley I, Williams R. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology. 1993;18:41–46. [PubMed] [Google Scholar]
- 71.Parker GA, Picut CA. Liver immunobiology. Toxicol Pathol. 2005;33:52–62. doi: 10.1080/01926230590522365. [DOI] [PubMed] [Google Scholar]
- 72.Sakai Y, Izumi N, Marumo F, Sato C. Quantitative immunohistochemical analysis of lymphocyte subsets in alcoholic liver disease. J Gastroenterol Hepatol. 1993;8:39–43. doi: 10.1111/j.1440-1746.1993.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 73.Cao Q, Batey R, Pang G, Clancy R. Altered T-lymphocyte responsiveness to polyclonal cell activators is responsible for liver cell necrosis in alcohol-fed rats. Alcohol Clin Exp Res. 1998;22:723–729. [PubMed] [Google Scholar]
- 74.Terabayashi H, Kolber MA. The generation of cytotoxic T lymphocytes against acetaldehyde-modified syngeneic cells. Alcohol Clin Exp Res. 1990;14:893–899. doi: 10.1111/j.1530-0277.1990.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 75.Willis MS, Klassen LW, Tuma DJ, Thiele GM. Malondialdehyde-acetaldehyde-haptenated protein induces cell death by induction of necrosis and apoptosis in immune cells. Int Immunopharmacol. 2002;2:519–535. doi: 10.1016/s1567-5769(01)00195-3. [DOI] [PubMed] [Google Scholar]
- 76.Paronetto F. Immunologic reactions in alcoholic liver disease. Semin Liver Dis. 1993;13:183–195. doi: 10.1055/s-2007-1007348. [DOI] [PubMed] [Google Scholar]
- 77.Klassen LW, Tuma D, Sorrell MF. Immune mechanisms of alcohol-induced liver disease. Hepatology. 1995;22:355–357. [PubMed] [Google Scholar]
- 78.Clot P, Bellomo G, Tabone M, Aricò S, Albano E. Detection of antibodies against proteins modified by hydroxyethyl free radicals in patients with alcoholic cirrhosis. Gastroenterology. 1995;108:201–207. doi: 10.1016/0016-5085(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 79.Worrall S, de Jersey J, Nicholls R, Wilce P. Acetaldehyde/protein interactions: are they involved in the pathogenesis of alcoholic liver disease? Dig Dis. 1993;11:265–277. doi: 10.1159/000171418. [DOI] [PubMed] [Google Scholar]
- 80.Lohse AW. Experimental models of autoimmune hepatitis. Semin Liver Dis. 1991;11:241–247. doi: 10.1055/s-2008-1040442. [DOI] [PubMed] [Google Scholar]
- 81.Thiele GM, Freeman TL, Willis MS, Hunter CD, Tuma DJ, Klassen LW. Identification of liver cytosolic proteins associated with malondialdehyde-acetaldehyde (MAA) adducted liver cytosol induced autoimmune hepatitis. Hepatology. 2003;38:488A. [Google Scholar]
- 82.Abraham R, Choudhury A, Basu SK, Bal V, Rath S. Disruption of T cell tolerance by directing a self antigen to macrophage-specific scavenger receptors. J Immunol. 1997;158:4029–4035. [PubMed] [Google Scholar]
- 83.Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283–292. doi: 10.1007/s00726-003-0029-5. [DOI] [PubMed] [Google Scholar]
- 84.Dhaliwal BS, Steinbrecher UP. Scavenger receptors and oxidized low density lipoproteins. Clin Chim Acta. 1999;286:191–205. doi: 10.1016/s0009-8981(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 85.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 86.Thiele GM, Klassen LW, Tuma DJ, Willis MS. The increased binding of MAA (malondialdehyde-acetaldehyde) adducted protein to scavenger receptors and the up-regulation of B7 may play a role in immunogenicity. Hepatology. 1999;30:328A. [Google Scholar]
- 87.Hasler P, Zouali M. Immune receptor signaling, aging, and autoimmunity. Cell Immunol. 2005;233:102–108. doi: 10.1016/j.cellimm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 88.Diehl AM. Hepatic complications of obesity. Gastroenterol Clin North Am. 2005;34:45–61. doi: 10.1016/j.gtc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, Kral JG. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513–1517. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 90.Machado M, Cortez-Pinto H. Non-alcoholic steatohepatitis and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2006;9:637–642. doi: 10.1097/01.mco.0000241677.40170.17. [DOI] [PubMed] [Google Scholar]
- 91.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 92.Suter PM. Is alcohol consumption a risk factor for weight gain and obesity? Crit Rev Clin Lab Sci. 2005;42:197–227. doi: 10.1080/10408360590913542. [DOI] [PubMed] [Google Scholar]
- 93.Holvoet P. Oxidized LDL and coronary heart disease. Acta Cardiol. 2004;59:479–484. doi: 10.2143/AC.59.5.2005219. [DOI] [PubMed] [Google Scholar]
- 94.Schneiderhan W, Schmid-Kotsas A, Zhao J, Grünert A, Nüssler A, Weidenbach H, Menke A, Schmid RM, Adler G, Bachem MG. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology. 2001;34:729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- 95.Willis MS, Klassen LW, Carlson DL, Brouse CF, Thiele GM. Malondialdehyde-acetaldehyde haptenated protein binds macrophage scavenger receptor(s) and induces lysosomal damage. Int Immunopharmacol. 2004;4:885–899. doi: 10.1016/j.intimp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Hill GE, Miller JA, Baxter BT, Klassen LW, Duryee MJ, Tuma DJ, Thiele GM. Association of malondialdehyde-acetaldehyde (MAA) adducted proteins with atherosclerotic-induced vascular inflammatory injury. Atherosclerosis. 1998;141:107–116. doi: 10.1016/s0021-9150(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 97.Khan MF, Wu X, Ansari GA. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol Appl Pharmacol. 2001;170:88–92. doi: 10.1006/taap.2000.9086. [DOI] [PubMed] [Google Scholar]
- 98.Itakura K, Uchida K. Evidence that malondialdehyde-derived aminoenimine is not a fluorescent age pigment. Chem Res Toxicol. 2001;14:473–475. doi: 10.1021/tx010055u. [DOI] [PubMed] [Google Scholar]
- 99.Chowdhury PK, Halder M, Choudhury PK, Kraus GA, Desai MJ, Armstrong DW, Casey TA, Rasmussen MA, Petrich JW. Generation of fluorescent adducts of malondialdehyde and amino acids: toward an understanding of lipofuscin. Photochem Photobiol. 2004;79:21–25. [PubMed] [Google Scholar]
- 100.Goldin RD, Wickramasinghe SN. Hepatotoxicity of ethanol in mice. Br J Exp Pathol. 1987;68:815–824. [PMC free article] [PubMed] [Google Scholar]
- 101.Wickramasinghe SN, Gardner B, Barden G. Circulating cytotoxic protein generated after ethanol consumption: identification and mechanism of reaction with cells. Lancet. 1987;2:122–126. doi: 10.1016/s0140-6736(87)92330-0. [DOI] [PubMed] [Google Scholar]
- 102.Sachdev MS, Riely CA, Madan AK. Nonalcoholic fatty liver disease of obesity. Obes Surg. 2006;16:1412–1419. doi: 10.1381/096089206778870012. [DOI] [PubMed] [Google Scholar]
- 103.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 104.Bergheim I, Parlesak A, Dierks C, Bode JC, Bode C. Nutritional deficiencies in German middle-class male alcohol consumers: relation to dietary intake and severity of liver disease. Eur J Clin Nutr. 2003;57:431–438. doi: 10.1038/sj.ejcn.1601557. [DOI] [PubMed] [Google Scholar]
- 105.Zhang FK, Zhang JY, Jia JD. Treatment of patients with alcoholic liver disease. Hepatobiliary Pancreat Dis Int. 2005;4:12–17. [PubMed] [Google Scholar]
- 106.Fernandez-Checa JC, Colell A, Mari M, García-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29:151S–157S. [PubMed] [Google Scholar]
- 107.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, et al. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419–425. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 108.Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, et al. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G857–G867. doi: 10.1152/ajpgi.00044.2006. [DOI] [PubMed] [Google Scholar]
- 109.Kharbanda KK, Rogers DD, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519–524. doi: 10.1093/jn/135.3.519. [DOI] [PubMed] [Google Scholar]
- 110.Barak AJ, Beckenhauer HC, Tuma DJ. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol. 1994;11:501–503. doi: 10.1016/0741-8329(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 111.Almeida D, Paraná R. Current aspects of antibiotic prophylaxis for upper gastrointestinal bleeding in cirrhosis patients. Braz J Infect Dis. 2002;6:266–268. doi: 10.1590/s1413-86702002000500009. [DOI] [PubMed] [Google Scholar]
- 112.Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 113.Cuddy PG. Lipid lowerers. RN. 2006;69:27–32; quiz 33. [PubMed] [Google Scholar]
- 114.Kruger PS. Statins: the next anti-endotoxin. Crit Care Resusc. 2006;8:223–226. [PubMed] [Google Scholar]
- 115.Daskalopoulou SS, Mikhailidis DP, Elisaf M. Prevention and treatment of the metabolic syndrome. Angiology. 2004;55:589–612. doi: 10.1177/00033197040550i601. [DOI] [PubMed] [Google Scholar]
- 116.Eidelman RS, Vignola P, Hennekens CH. Alcohol consumption and coronary heart disease: a causal and protective factor. Semin Vasc Med. 2002;2:253–256. doi: 10.1055/s-2002-35393. [DOI] [PubMed] [Google Scholar]
- 117.Sesso HD. Alcohol and cardiovascular health: recent findings. Am J Cardiovasc Drugs. 2001;1:167–172. doi: 10.2165/00129784-200101030-00002. [DOI] [PubMed] [Google Scholar]
- 118.Iturriaga H, Pereda T, Estévez A, Ugarte G. Serum immunoglobulin A changes in alcoholic patients. Ann Clin Res. 1977;9:39–43. [PubMed] [Google Scholar]
- 119.Bonet H, Manez R, Kramer D, Wright HI, Gavaler JS, Baddour N, Van Thiel DH. Liver transplantation for alcoholic liver disease: survival of patients transplanted with alcoholic hepatitis plus cirrhosis as compared with those with cirrhosis alone. Alcohol Clin Exp Res. 1993;17:1102–1106. doi: 10.1111/j.1530-0277.1993.tb05671.x. [DOI] [PubMed] [Google Scholar]
- 120.Plat J, Mensink RP. Food components and immune function. Curr Opin Lipidol. 2005;16:31–37. doi: 10.1097/00041433-200502000-00007. [DOI] [PubMed] [Google Scholar]


