Abstract
AIM: To investigate the expression profile of IL-8 in inflammatory and malignant colorectal diseases to evaluate its potential role in the regulation of colorectal cancer (CRC) and the development of colorectal liver metastases (CRLM).
METHODS: IL-8 expression was assessed by quantitative real-time PCR (Q-RT-PCR) and the enzyme-linked immunosorbent assay (ELISA) in resected specimens from patients with ulcerative colitis (UC, n = 6) colorectal adenomas (CRA, n = 8), different stages of colorectal cancer (n = 48) as well as synchronous and metachronous CRLM along with their corresponding primary colorectal tumors (n = 16).
RESULTS: IL-8 mRNA and protein expression was significantly up-regulated in all pathological colorectal entities investigated compared with the corresponding neighboring tissues. However, in the CRC specimens IL-8 revealed a significantly more pronounced overexpression in relation to the CRA and UC tissues with an average 30-fold IL-8 protein up-regulation in the CRC specimens in comparison to the CRA tissues. Moreover, IL-8 expression revealed a close correlation with tumor grading. Most interestingly, IL-8 up-regulation was most enhanced in synchronous and metachronous CRLM, if compared with the corresponding primary CRC tissues. Herein, an up to 80-fold IL-8 overexpression in individual metachronous metastases compared to normal tumor neighbor tissues was found.
CONCLUSION: Our results strongly suggest an association between IL-8 expression, induction and progression of colorectal carcinoma and the development of colorectal liver metastases.
Keywords: Interleukin-8, Gene expression profiling, RNA and protein expression, Colorectal cancer, Colorectal liver metastases
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers, with nearly one million estimated new patients per year world wide in 2006. Despite continuous improvements in diagnosis and therapy, approximately 60% of colon cancer patients develop metastases. While there is a good survival rate for patients diagnosed with localized disease, the CRC prognosis worsens dramatically with advancing stages and distant metastases and only 5% of patients diagnosed with distant metastases survive 5 years[1,2]. Colorectal specimens and cell lines derived from them have been shown to express a variety of chemokines, including the multifunctional cytokine Interleukin (IL)-8, a member of the CXC chemokine superfamily of structurally and functionally related inflammatory cytokines that stimulate the migration of distinct subsets of cells[3,4]. IL-8 and other anti-inflammatory cytokines have been shown to act as potent chemoattractants for leucocytes, such as neutrophils and natural killer cells, thus contributing to healing processes but also stimulating inflammatory diseases such as rheumatoid arthritis, sepsis and inflammatory bowel disease[5-7]. Subsequent studies revealed that IL-8 also promotes the movement of cells of different lineages, such as fibroblasts and keratinocytes[8,9]. In recent years, IL-8 was reported to induce the migration of tumor cells and its expression was correlated with tumor growth, angiogenesis and metastatic potential in various human carcinomas and animal models[10-16].
Yet, up to now, the pathophysiological role of IL-8 in tumor development and metastasis is not fully understood and still a matter of debate. For example, various recent studies point out that IL-8 expression correlates with disease progression in human melanoma[17-19]. On the other hand, IL-8 expression in melanoma is only associated with early malignancy, whereas metastatic melanoma as well as dysplastic naevi specimens showed little or no IL-8 up-regulation as shown by immunohistochemical staining[20]. Although constitutive IL-8 expression was determined in melanoma cells from surgical specimens, no correlation was found between the degree of cytokine expression and the clinical tumor stage[21]. Accordingly, there is no distinct relationship between IL-8 expression and metastatic potential in various cell lines representing different clinical stages[22,23] and some studies have even reported on antitumoral IL-8 effects[24].
However, increasing data suggest that constitutive expression of IL-8 in colon carcinoma cell lines is associated with the metastatic potential and that IL-8 might act as an autocrine/paracrine growth factor in colon cancer progression and metastasis[25,26]. Still it remains unclear, whether IL-8 expression is related to cancer progression and the metastatic potential in colorectal carcinoma tissues. Therefore, the purpose of this study was to examine the expression profile of IL-8 in inflammatory as well as non-malignant and malignant colorectal diseases and colorectal liver metastases (CRLM) to evaluate its role in the regulation of colorectal cancer (CRC) and the development of CRLM.
MATERIALS AND METHODS
Patients
Surgical specimens and corresponding normal tissue from the same samples were collected from patients with ulcerative colitis (UC, n = 6), colorectal adenomas (CRA, n = 8), colorectal carcinomas of different cancer stages (n = 48) and synchronous or metachronous CRLM (n = 16) who underwent surgical resection at our department between 2003 and 2006. Informed consent for tissue procurement was obtained from all patients. The study was approved by the ethics commission of the Ärztekammer of the Saarland. The clinical variables were obtained prospectively from the clinical and pathological records and are in accordance with the UICC/TNM classification[27] (Tables 1 and 2).
Table 1.
Clinical characteristics of patients with colorectal carcinomas and colorectal liver metastases
| Factor | CRC (n = 48) | CRLM (n = 161) |
| Localization of primary tumor | ||
| Colon | 22 | 6 |
| Rectum | 26 | 8 |
| Gender | ||
| Male | 29 | 7 |
| Female | 19 | 7 |
| Age, yr2 | 63.7 (47-78) | 60.3 (41-76) |
| Hepatitis (A, B or C) | ||
| Positive | 6 | 2 |
| Negative | 42 | 12 |
| Liver cirrhosis | ||
| Positive | 1 | 0 |
| Negative | 47 | 14 |
| Adipositas | ||
| Positive | 12 | 4 |
| Negative | 36 | 10 |
| Largest tumor diameter (cm)2 | 4.6 (1.2-9.1) | 4.2 (1.5-5.5) |
| TNM stage of primary tumor | ||
| I | 8 | 1 |
| II | 15 | 2 |
| III | 15 | 10 |
| IV | 10 | 1 |
| Grading | ||
| I | 0 | 0 |
| II | 21 | 4 |
| III | 27 | 10 |
| Lymphatic permeation | ||
| Positive | 27 | 10 |
| Negative | 21 | 4 |
| Vascular invasion | ||
| Positive | 6 | 5 |
| Negative | 42 | 9 |
| Chemotherapy before operation | 0 | 2 |
| Radiotherapy before operation | 0 | 2 |
TNM: Tumor-node-metastasis; CRC: Colorectal carcinoma; CRLM: Colorectal liver metastases.
16 CRLM originating from 14 CC patients;
Median with range in parentheses.
Table 2.
Clinical characteristics of patients with ulcerative colitis and colorectal adenomas
| Factor | UC (n = 6) | CRA (n = 8) |
| Localization of biopsy | ||
| Colon | 6 | 6 |
| Rectum | 0 | 2 |
| Gender | ||
| Male | 4 | 5 |
| Female | 2 | 3 |
| Age (yr)1 | 51.7 (23-78) | 65.3 (41-75) |
| Adipositas | ||
| Positive | 0 | 5 |
| Negative | 6 | 3 |
UC: Ulcerative colitis; CRA: Colorectal adenoma.
Median with range in parentheses.
Tissue preparation
Tissue samples were collected immediately after resection, snap frozen in liquid nitrogen and then stored at -80°C until they were processed under nucleic acid sterile conditions for RNA and protein extraction. Tumor samples were taken from vital areas of histopathologically confirmed (M.W.) adenocarcinomas and liver metastases, respectively. As corresponding normal tissue we used adjacent unaffected mucosa, 2-3 cm distal to the resection margin from the same resected adenocarcinoma or liver specimen, respectively. All tissues obtained were reviewed by a minimum of two experienced pathologists (M.W. et al) and examined for the presence of tumor cells. As minimum criteria for usefulness for our studies we only chose tumor tissues in which tumor cells occupied a major component (> 80%) of the tumor sample.
Single-strand cDNA synthesis
Total RNA was isolated using RNeasy columns from Qiagen (Hilden, Germany) according to the manufacturer’s instructions. RNA integrity was confirmed spectrophotometrically and by electrophoresis on 1 g/L agarose gels. For cDNA synthesis 5 μg of each patient total RNA sample were reverse-transcribed in a final reaction volume of 50 μL containing 1 × TaqMan RT buffer, 2.5 μmol/L random hexamers, 500 μmol/L each dNTP, 5.5 mmol/L MgCl2, 0.4 nakt/L RNase inhibitor, and 1.25 nakt/L Multiscribe RT. All RT-PCR reagents were purchased from Applied Biosystems (Foster City, CA). The reaction conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
Real-time PCR
All Q-RT PCR assays containing the primer and probe mix were purchased from Applied Biosystems, (Applied Biosystems, Foster City, CA) and utilized according to the manufacturer’s instructions. PCR reactions were carried out using 10 μL 2 × Taqman PCR Universal Master Mix No AmpErase® UNG and 1 μL gene assay (Applied Biosystems, Foster City, CA), 8 μL Rnase-free water and 1 μL cDNA template (50 mg/L). The theoretical basis of the qRT assays is described in detail elsewhere[28]. All reactions were run in duplicates along with no template controls and an additional reaction in which reverse transcriptase was omitted to to assure absence of genomic DNA contamination in each RNA sample. For the signal detection, ABI Prism 7900 sequence detector was programmed to an initial step of 10 min at 95°C, followed by 40 thermal cycles of 15 s at 95°C and 10 min at 60°C and the log-linear phase of amplification was monitored to obtain CT values for each RNA sample.
Gene expression of IL-8 was analyzed in relation to the levels of the slope matched housekeeping gene Cyclophilin C (CycC)[29]. Since reporting of data obtained from raw CT values falsely represent the variations, we converted the individual CT values to the linear form as follows:
Fold difference = 2-(mean CT pathological tissue - mean CT calibrator) = 2-delta CT
Hence, the normal tissue became the 1 × sample, and all other quantities were expressed as an n-fold difference relative to this tissue.
Isolation of total protein
Protein lysates from frozen tissues were extracted with the radioimmunoprecipitation (RIPA) cell lysis and extraction buffer from Pierce (Pierce, Rockford, USA). Total protein content was assessed by using the Pierce BCA protein assay reagent kit (Pierce, Rockford, USA).
Enzyme-linked immunosorbant assay
IL-8 tissue concentrations in the lysates were determined by sandwich-type Enzyme-linked immunosorbant assay (ELISA) according to the manufacturer’s protocol: R&D systems (R&D Systems Inc. Minneapolis, Minnesota, USA) for the quantification of IL-8. The absorbance was read at 450 nm.
Laser Capture Microdissection
Laser microbeam microdissection (LMM) was employed for obtaining pure tumor cell and pure normal cell samples for subsequent genetic analysis. LMM was performed on three samples for each tissue type for IL-8. Histochemical staining was used on cryo sections before microdissection. Specimen preparation, microdissection and catapulting were performed following a laser pressure catapulting protocol according to the manufacturer’s instructions (P.A.L.M. Microlaser Technologies, Bernried, Germany). RNA was extracted using the P.A.L.M. RNA extraction kit and for reverse transcription the invitrogen reverse transcription kit (Invitrogen Life Technologies, Karlsruhe, Germany) was applied. Subsequently quantitative PCR analysis was performed.
Statistical analysis
IL-8 expression profiles in the different groups are presented as mean and standard error of the mean (SEM). Statistical calculations were done with the MedCalc software package (MedCalc software, Mariakerke, Belgium)[30]. Where appropriate, either the parametric t-tests or the Wilcoxon’s rank sum test were applied to test for group differences of continuous variables. P ≤ 0.05 was deemed significant.
RESULTS
IL-8 expression in colorectal carcinomas related to tumor stage
Recently, we demonstrated the involvement of the murine macrophage inflammatory protein (MIP)-2, a functional murine analogue of the human IL8, in angiogenesis and tumor growth[16]. Here, we analysed IL-8 expression in human tissue specimens of various colorectal diseases and cancer stages using Q-RT-PCR and ELISA. Our tissue specimens descended from 6 patients with UC, 8 patients with CRA and 48 patients with CRC of various cancer stages with different metastatic potentials (Tables 1 and 2). Adjacent, disease and tumor free tissue samples of the UC, CRA and CRC patients served as control groups, respectively.
Q-RT-PCR analysis revealed significant up-regulation of IL-8 mRNA expression in all pathological tissue specimens analysed, if compared with the unaffected corresponding UC-, CRA- and CRC neighbor tissues, as presented in Figure 1A. In UC- and CRA tissue specimens IL-8 mRNA expression was on average 11- and 10-fold up-regulated (P < 0.05, respectively). Yet, in the different T- stages of the CRC specimens IL-8 expression was more than 25-fold up-regulated (P < 0.05). The results shown in Figure 1A represent the pooled mean expression levels and the SEMs from 6 UC-, 8 CRA-, 8 T1-, 15 T2-, 15 T3- and 10 T4 stage CRC patients, respectively. Since averaging out the CT values or ratios could mask significant differences between individual paired samples, we also analysed the differences between gene expression from matched normal/cancer samples. The results obtained from this level of analysis corresponded widely with the results presented in Figure 1A thus confirming the correlation between the degree of IL-8 expression and advanced tumor stage. Sections of tumor and normal cells have been microdissected in three specimens of each tissue type, followed by subsequent RT-PCR gene expression analysis to ensure that the expression data corresponded well with the results presented in Figure 1A.
Figure 1.
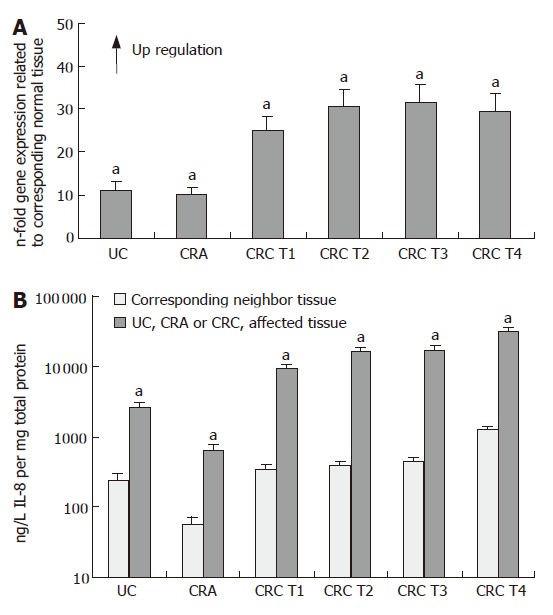
IL-8 expression in ulcerative colitis (UC), colorectal adenoma (CRA) and colorectal carcinomas (CRC). A: Q-RT-PCR; B: ELISA (mean ± SE, aP < 0.05, n = 6, 8, 8, 15, 15 and 10, respectively).
In consistence with our results obtained at the RNA level, IL-8 protein expression, as assessed by ELISA, revealed also significant up-regulation of IL-8 protein expression in all pathological tissue specimens in comparison to the unaffected corresponding UC-, CRA- and CRC mucosa tissues, respectively (Figure 1B). Analysis of IL-8 tissue protein concentrations showed a similar profile with an average 12- and 11-fold overexpression in the UC- and CRA tissue specimens in relation to normal mucosa tissues (P < 0.05), and on average 30-fold higher IL-8 expression in the different T- stages of the CRC specimens, if compared with the respective tumor neighboring mucosa tissues (P < 0.05). Moreover, absolute expression on the protein level revealed a distinct increase in IL-8 expression levels in tumor- and tumor-free neighbor tissues of CRC patients with T-stage 4 in comparison to CRC patients with T-stages 1-3 (P < 0.05). However, most notably, we observed that IL-8 protein concentrations were significantly higher expressed in all CRC specimens compared to non-malignant and inflammatory tissue samples (P < 0.05, Figure 1B), showing on average 30-fold IL-8 protein up-regulation in the CRC specimens in comparison to the CRA tissues. For example, comparative analysis of the absolute protein levels between the CRC specimens and the CRA tissues revealed a significantly higher IL-8 expression of almost 30 000 ng/L IL-8 in the CRC tissues of tumor- stage 4 compared to approximately 600 ng/L IL-8 in the CRA tissues (P < 0.05, Figure 1B).
IL-8 expression in colorectal liver metastases
In the next set of experiments, we analysed IL-8 mRNA and protein expression in 16 CRLM of synchronous and metachronous origin and the corresponding colorectal cancer tissues where the metastases originated from (Table 1). Healthy tumor neighbor tissues of the CRLM and corresponding CRC tissues served again as control groups. On the RNA level we found on average a 52- fold increase in IL-8 expression in the synchronous metastases compared with the corresponding tumor neighbor tissues and a 62- fold increase in IL-8 expression in the metachronous metastases (P < 0.05, respectively, Figure 2A). These findings were well in line with the results obtained on the protein level, which showed a 47- fold increase in IL-8 expression in synchronous metastases and a 60- fold increase in metachronous metastases (P < 0.05, respectively) with up to 80-fold IL-8 overexpression in individual metastases, when compared to the corresponding tumor-free hepatic neighbor tissues, respectively (Figure 2B). In our survey, neither gender nor the specific tumor localization (colon versus rectum) influenced IL-8 expression profiles in our CRC patient series. However, CRC patients with synchronous or metachronous metastases beyond the age of 60 expressed significantly more IL-8 than CRC patients younger than 60 years (P < 0.05). Most interestingly, the highest IL-8 overexpression was detected in synchronous and metachronous colorectal metastases, if compared to their corresponding primary CRC tissues (P < 0.05, respectively Figure 2B). For example, comparative analysis of the absolute protein quantities between the CRLM specimens and their corresponding CRC tissues revealed an IL-8 expression of almost 50 000 ng/L in the CRLM tissues of metachronous metastases compared to approximately 18 000 ng/L in the corresponding CRC primary tumor tissues (P < 0.05, Figure 2B).
Figure 2.
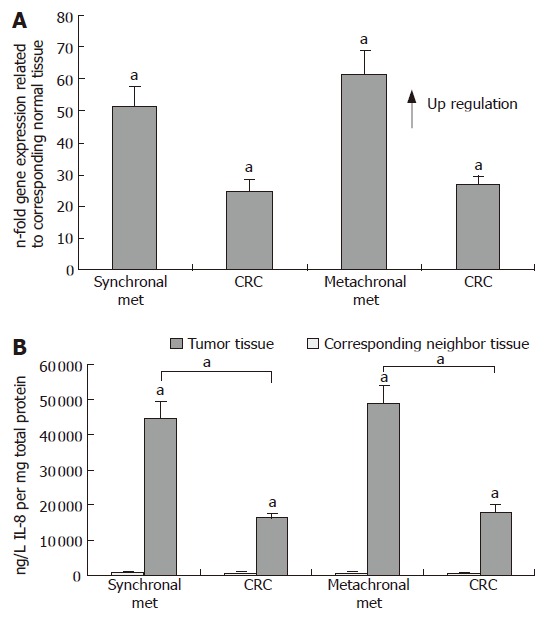
IL-8 expression in colorectal liver metastases of synchronal (Synchronal Met) and metachronal (Metachronal Met) origin and corresponding colorectal carcinomas (CRC). A: Q-RT-PCR; B: ELISA (mean ± SE, aP < 0.05, n = 9 and 7, respectively).
DISCUSSION
In this study, we present an unprecedented complete and comparative analysis of the IL-8 RNA and protein expression profile of various inflammatory, non-malignant and malignant histopathlogical colorectal entities comprising ulcerative colitis and colorectal adenomas, both representing known premalignant conditions often preceding the development of colorectal malignancies. We further included colorectal carcinomas of different tumor stages as well as synchronous and metachronous colorectal liver metastases along with their related primary colorectal tumors.
The major findings of this study demonstrate that the magnitude of IL-8 expression in surgical CRC tissue specimens correlates with colorectal cancer progression and the development of synchronous and metachronous liver metastases. Moreover, to our knowledge we could demonstrate for the first time that irrespective of the tumor stage IL-8 is significantly higher expressed in CRC tissues compared to inflammatory colorectal conditions and adenomas of the colon/rectum. Since such conditions often constitute prevalent pre-existing disease states in the etiopathogenesis of colorectal cancer, our results strongly suggest a close association of IL-8 up-regulation and the development of CRC. In line with this conclusion is the concept that inflammation is a critical component of tumor progression. Chronic inflammation can activate cell actions that increase the induction of irreversible DNA damage and the risk of cancer and it is now becoming clear that tumor cells have co-opted some of the signalling molecules of the innate immune system, such as chemokines and their receptors for invasion, migration and metastasis[31]. In addition, we demonstrated significant IL-8 overexpression in CRLM in comparison to the related primary colorectal tumors, indicating a correlation between IL-8 expression and the malignant status of colorectal cancer cells. Thus, our results not only suggest an association between IL-8 expression and the induction and progression of colorectal carcinoma, but also clearly point to a correlation between IL-8 expression and the development of CRLM. As our results are based on expression data, they will need further evaluation. Therefore, the next step in our investigations will comprise the functional analysis in cell culture assays. However, even at this point, the clinical significance of our findings has meaningful consequences. Examining the IL-8 expression profile in CRC patients may potentially help to assess more precisely the course of the cancerous condition. In this respect it is conceivable to monitor the IL-8 expression level in CRC patients that show no diagnosable symptoms of CRLM at the time of presentation, but may still carry a high risk for developing such metastases. A significantly up-regulated level of IL-8 might thus be a useful tool to evaluate the prognosis of patients with CRC with an important impact on future treatment strategies. Thus, patients with a higher risk of developing CRLM may receive a different treatment from patients with a lower risk of developing CRLM.
Our findings are supported by previous studies[32], exploiting the potential clinical significance of serum IL-8 measurements in untreated CRC patients in comparison to healthy volunteers. Circulating IL-8 serum concentrations revealed a significant correlation with the clinical stage of CRC, bowel wall invasion and liver metastasis[32,33]. Our results are also well in line with the outcome of recent surveys which compared the expression of a number of different chemokines and growth factors in a panel of arbitrarily chosen CRC specimens with normal mucosa by use of ELISA. The authors also found significant IL-8 over-expression in the malignant tissues that correlated with tumor size, depth of infiltration, Dukes stage and shorter overall survival times[34,35]. Accordingly, Li and colleagues[25] studied IL-8 expression in vitro in human colon carcinoma cells with different metastatic potentials and found an association between constitutive IL-8 expression and aggressiveness of human colon carcinoma cells, which was assigned to autocrine growth and angiogenic properties of IL-8 in the regulation of multiple biological activities in endothelial cells[36,37]. In animal studies inhibition of IL-8 reduced tumorigenesis of human non-small cell lung cancer in SCID mice[38], and our group[16,39] reported that MIP-2 dose-dependently promotes angiogenesis as well as hepatic engraftment and tumor growth of colon cancer cells. In other tumor types a direct role of IL-8 was demonstrated, e.g. by enhancing proliferation and invasive potential of metastatic melanoma cells[19]. Although several authors investigated possible pathways[40,41], the precise molecular mechanisms underlying the IL-8 mediated regulation of tumor cells still remain obscure. Recently, IL-8 was reported to promote cell proliferation and migration through metalloproteinase-cleavage in human colon carcinoma cells[42], and other studies indicated that IL-8 mediated tumor progression may be regulated by tumor-associated stress factors[43].
In spite of the increasing number of studies that outlined an important role and potential mechanisms of IL-8 contributing to cancer progression in various cancer types through its regulative, autocrine and paracrine functions, its interference with respect to the development of malignancies, metastasis and angiogenesis still remains controversial. For example, Kassim and colleagues demonstrated an association between IL-8 expression and a dismal prognosis in epithelial ovarian cancer patients[44], while other studies demonstrated that IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration[45]. Accordingly, in a model of colon cancer in rats, IL-8 was found to have a highly reproducible antitumoral effect that was associated with a systemic activation of T lymphocytes[24,46]. Likewise, various metastatic tumor cell lines were shown to produce IL-8 constitutively[19,25], while other cell lines like some metastatic prostate tumor cell lines do not express IL-8 at detectable levels[22]. Finally, in some studies no correlation between IL-8 secretion and tumor growth kinetics or metastatic potential was found at all[47].
Despite still ongoing controversies regarding the biological relevance of IL-8 in cancer biology, our clinical and experimental findings strongly support a multifunctional role for IL-8 in tumor induction, progression and metastasis. Taken together, our data outline a significant correlation between IL-8 expression and the induction and progression of CRC as well as the development of CRLM. The predominance of high IL-8 expression levels in surgical colorectal carcinoma specimens might thus be a useful indicator of poor prognosis. In addition to its prognostic significance we therefore propose IL-8 as a putative target for the development of novel treatment concepts in the therapy of CRC. Herein, neutralization of IL-8 or inhibition of its production and activity might be a future basic treatment strategy in the management of CRC.
ACKNOWLEDGMENTS
C Weber and B Kruse for excellent technical assistance.
COMMENTS
Background
In recent years, an increasing number of studies suggested an involvement of IL-8 in the migration of tumor cells and correlated its expression with tumor growth, angiogenesis and metastatic potential in various human carcinomas and animal models. Yet, up to now, the pathophysiological role of IL-8 in tumor development and metastasis is not fully understood and discussed controversially. Moreover, it remains unclear, whether IL-8 expression is related to cancer progression and the metastatic potential in colorectal carcinoma tissues.
Research frontiers
The research hotspot is to elucidate a potential pathophysiological role of IL-8 in CRC development and metastatic spread and to clarify the precise molecular mechanisms underlying the IL-8 mediated regulation of colorectal carcinoma (CRC) cells.
Innovations and breakthroughs
In recent years, IL-8 expression was reported to be associated with colon cancer cell proliferation and the metastatic potential in colon carcinoma cell lines. Moreover, IL-8 was suggested to act as an autocrine/paracrine growth factor in colon cancer progression. In order to further evaluate the role of IL-8 in the regulation of CRC and the development of colorectal liver metastases (CRLM), we examined the expression profile of IL-8 in ulcerative colitis (UC) and colorectal adenoma (CRA) as conditions often preceding the development of colorectal malignancies, and moreover, in CRC tissues of different tumor stages as well as in synchronous and metachronous CRLM along with their related primary colorectal tumors. We demonstrated a significant correlation between IL-8 expression and the induction and progression of CRC as well as the development of CRLM.
Applications
Our findings suggest that monitoring the IL-8 expression level in CRC patients may potentially help to assess the course of the cancerous condition and the prognosis of patients with respect to the development of CRLM. Thus, we propose IL-8 as a useful indicator of poor prognosis and a putative target for the development of drugs in the therapy of CRC.
Terminology
UC is a disease that causes ulcers in the lining of the rectum and colon. It is one of a group of diseases called inflammatory bowel disease. CRA means a benign epithelial neoplasm of the colon or rectum, which extends from the ileocacal junction to the sigmoid colon. CRC develops as a locally invasive cancer in the colon and rectum often evolving from polyps with a very high incidence of metastatic spread.
Peer review
This is very nice work looking at IL-8 and the correlation with induction, progression and metastatic potential colorectal cancer. The scientific and innovative contents as well as readability can reflect the advanced levels of the clinical and basic researches in gastroenterology.
Footnotes
S- Editor Ma N L- Editor Alpini GD E- Editor Lu W
References
- 1.McLeod HL, McKay JA, Collie-Duguid ES, Cassidy J. Therapeutic opportunities from tumour biology in metastatic colon cancer. Eur J Cancer. 2000;36:1706–1712. doi: 10.1016/s0959-8049(00)00150-7. [DOI] [PubMed] [Google Scholar]
- 2.Booth RA. Minimally invasive biomarkers for detection and staging of colorectal cancer. Cancer Lett. 2007;249:87–96. doi: 10.1016/j.canlet.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clin Sci (Lond) 1999;97:385–390. [PubMed] [Google Scholar]
- 4.Eckmann L, Jung HC, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima K, Baldwin ET, Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- 6.Sebok K, Woodside D, al-Aoukaty A, Ho AD, Gluck S, Maghazachi AA. IL-8 induces the locomotion of human IL-2-activated natural killer cells. Involvement of a guanine nucleotide binding (Go) protein. J Immunol. 1993;150:1524–1534. [PubMed] [Google Scholar]
- 7.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 8.Dunlevy JR, Couchman JR. Interleukin-8 induces motile behavior and loss of focal adhesions in primary fibroblasts. J Cell Sci. 1995;108(Pt1):311–321. doi: 10.1242/jcs.108.1.311. [DOI] [PubMed] [Google Scholar]
- 9.Michel G, Kemény L, Peter RU, Beetz A, Ried C, Arenberger P, Ruzicka T. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992;305:241–243. doi: 10.1016/0014-5793(92)80677-9. [DOI] [PubMed] [Google Scholar]
- 10.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–3721. [PubMed] [Google Scholar]
- 11.Singh RK, Varney ML, Bucana CD, Johansson SL. Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res. 1999;9:383–387. doi: 10.1097/00008390-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- 13.Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. 2004;91:1970–1976. doi: 10.1038/sj.bjc.6602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitadai Y, Haruma K, Mukaida N, Ohmoto Y, Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ, et al. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin Cancer Res. 2000;6:2735–2740. [PubMed] [Google Scholar]
- 15.Masood R, Cai J, Tulpule A, Zheng T, Hamilton A, Sharma S, Espina BM, Smith DL, Gill PS. Interleukin 8 is an autocrine growth factor and a surrogate marker for Kaposi's sarcoma. Clin Cancer Res. 2001;7:2693–2702. [PubMed] [Google Scholar]
- 16.Kollmar O, Scheuer C, Menger MD, Schilling MK. Macrophage inflammatory protein-2 promotes angiogenesis, cell migration, and tumor growth in hepatic metastasis. Ann Surg Oncol. 2006;13:263–275. doi: 10.1245/ASO.2006.03.096. [DOI] [PubMed] [Google Scholar]
- 17.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 18.Nürnberg W, Tobias D, Otto F, Henz BM, Schadendorf D. Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol. 1999;189:546–551. doi: 10.1002/(SICI)1096-9896(199912)189:4<546::AID-PATH487>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125:209–216. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 20.Hensley C, Spitzler S, McAlpine BE, Lynn M, Ansel JC, Solomon AR, Armstrong CA. In vivo human melanoma cytokine production: inverse correlation of GM-CSF production with tumor depth. Exp Dermatol. 1998;7:335–341. doi: 10.1111/j.1600-0625.1998.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 21.Ciotti P, Rainero ML, Nicolò G, Spina B, Garrè C, Casabona F, Santi PL, Bianchi-Scarrà G. Cytokine expression in human primary and metastatic melanoma cells: analysis in fresh bioptic specimens. Melanoma Res. 1995;5:41–47. doi: 10.1097/00008390-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Balbay MD, Pettaway CA, Kuniyasu H, Inoue K, Ramirez E, Li E, Fidler IJ, Dinney CP. Highly metastatic human prostate cancer growing within the prostate of athymic mice overexpresses vascular endothelial growth factor. Clin Cancer Res. 1999;5:783–789. [PubMed] [Google Scholar]
- 23.Scheibenbogen C, Möhler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5:179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lejeune P, Reisser D, Onier N, Lagadec P, Lindley I, Jeannin JF. Interleukin-8 has antitumor effects in the rat which are not associated with polymorphonuclear leukocyte cytotoxicity. Cancer Immunol Immunother. 1994;38:167–170. doi: 10.1007/BF01525637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 26.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 27.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 29.Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, Brittner B, Ludwig B, Schilling M. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101–109. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 31.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, Michalski W, Chechlinska M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biol. 2005;26:186–194. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- 33.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 34.Baier PK, Eggstein S, Wolff-Vorbeck G, Baumgartner U, Hopt UT. Chemokines in human colorectal carcinoma. Anticancer Res. 2005;25:3581–3584. [PubMed] [Google Scholar]
- 35.Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37:166–172. doi: 10.1159/000085964. [DOI] [PubMed] [Google Scholar]
- 36.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 37.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 38.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollmar O, Junker B, Rupertus K, Menger MD, Schilling MK. Studies on MIP-2 and CXCR2 expression in a mouse model of extrahepatic colorectal metastasis. Eur J Surg Oncol. 2007;33:803–811. doi: 10.1016/j.ejso.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- 41.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 42.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Shi Q, Xiong Q, Le X, Xie K. Regulation of interleukin-8 expression by tumor-associated stress factors. J Interferon Cytokine Res. 2001;21:553–566. doi: 10.1089/10799900152547812. [DOI] [PubMed] [Google Scholar]
- 44.Kassim SK, El-Salahy EM, Fayed ST, Helal SA, Helal T, Azzam Eel-D, Khalifa A. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem. 2004;37:363–369. doi: 10.1016/j.clinbiochem.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Lee LF, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K, Ting JP. IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol. 2000;164:2769–2775. doi: 10.4049/jimmunol.164.5.2769. [DOI] [PubMed] [Google Scholar]
- 46.Reisser D, Lejeune P, Lagadec P, Onier N, Dasilva C, Lindley I, Jeannin JF. Interleukin-8 antitumour effect is associated with a local infiltration but not with a systemic activation of T lymphocytes. Anticancer Res. 1994;14:977–979. [PubMed] [Google Scholar]
- 47.Schadendorf D, Fichtner I, Makki A, Alijagic S, Küpper M, Mrowietz U, Henz BM. Metastatic potential of human melanoma cells in nude mice--characterisation of phenotype, cytokine secretion and tumour-associated antigens. Br J Cancer. 1996;74:194–199. doi: 10.1038/bjc.1996.337. [DOI] [PMC free article] [PubMed] [Google Scholar]


