Abstract
AIM: To assess the trends in the incidence of inflammatory bowel disease (IBD) over 23 years in the same area and to identify genetic factors related to incidence evolution.
METHODS: Patients with IBD arising from North-western Greece were systematically recorded through the 1983-2005 period. Trends in disease incidence and genetic patterns related to CARD15 variants were documented and correlated.
RESULTS: A total of 447 patients with IBD were recorded (23.5% Crohn’s disease, 72.7% Ulcerative colitis and 3.8% indeterminate colitis). Mean annual incidence rates of CD and UC were 0.9/100 000 (95% CI 0.1-1.7) and 2.7/100 000 (95% CI 1.7-4.1) inhabitants, respectively. There was a statistically significant increase of CD incidence (P < 0.01) during the study period, in contrast to the UC incidence. There were no statistical differences in CARD15 variants over the study period.
CONCLUSION: The incidence of CD in North-western Greece has risen disproportionately to that of UC in the 21st century. This is not related to alterations of genetic background though.
Keywords: Inflammatory bowel disease, Crohn’s disease, Epidemiology, CARD15
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses two distinct clinicopathological entities, Crohn’s disease (CD) and ulcerative colitis (UC), possessing different etiological and epidemiological correlations. Both are considered of unknown aetiology, and various endogenous and exogenous factors have been etiologically implicated, leading to the concept of a multifactorial pathogenetic process involving interplay of environmental risk factors and immunologically triggered alterations[1]. Environmental factors have for long been incriminated in the pathogenesis of both forms of IBD, usually based on data of geographic or temporal variation of disease incidence. A global north-south variation in the incidence of IBD has been reported[2]. In Europe, the EC-IBD population based study showed a higher incident of UC (11.8/100 000 vs 8.7/100 000) and CD (7.0/100 000 and 3.9/100 000, respectively) in Northern compared to Southern Europe[3]. Such variations do exist in the overall prevalence of each form, with variable data on the incidence trends of CD in recent years[4-6], but an invariable increase in CD incidence in Southern European regions[7,8]. Evaluation of the factors triggering this evolving incidence may allow for identifying major endogenous or exogenous factors related to the pathogenesis of the disease.
In North-western Greece, an area traditionally categorized to the poorest of Europe, previous studies have shown rarity of CD and common appearance of UC during the previous decades[9,10]. This study assesses current evolution of incidence of CD and correlates these trends with genetic (CARD15 mutations) factors.
MATERIALS AND METHODS
Study area
The study area, North-western Greece, represents a population of 506 142 inhabitants according to the National Census of 2001, including four Districts situated on the mainland (Districts of Ioannina, Arta, Preveza and Thesprotia) and two Districts situated in islands (Districts of Corfu and Lefkada). Urban residents represented about 40% of the total population, residing mainly in the District capitals.
Case identification and diagnostic criteria
Cases have been recorded in the frame of a systematic recording system for IBD, using multiple sources of retrieval, developed in this defined area of North-western Greece between January 1983 and December 2005. The health care system in our area includes both a National Health Service and a private sector. All gastroenterologists are members of North-western Greece Gastroenterology Group and were updated about the methods and aims of this study both through newsletters and meetings. The system records cases from the following sources: (1) in- and outpatients referred to the Hepato-gastroenterology Unit of the Ioannina University Hospital; (2) patients referred to outpatient Gastroenterology clinics in Districts hospitals and (3) patients referred to the private gastroenterologists practicing inside the area. A review of collected data was performed in the University Hospital of Ioannina, by two investigators (ME and EVT).Diagnosis of CD and UC was based on typical clinical, radiological, endoscopic and histological criteria and the diagnosis of intermediate colitis (IC) was only set when a distinct diagnosis of UC or CD could not be established. CD was classified according to the Vienna System[11] and UC according to Lennard-Jones[12]. Perianal disease was defined by the presence of perianal abscesses, fistulae or ulcers, but not by the presence of skin tags. A final diagnosis of CD, UC, or ulcerative proctitis (UP) though was made by two expert gastroenterologists and recorded as definite, probable, or possible following criteria previously published[9]. Phenotypic details were obtained on 2 occasions by retrospective case-note review between January and December 2005 by 2 investigators (ME and EVT). Duration of follow-up was defined as the interval between diagnosis and latest case-note review. Details regarding other demographic characteristics were further supplemented by a patient interview or completed postal questionnaire. During the last year blood was collected from all CD patients. An incidence case was defined as any IBD patient, diagnosed during the study period, resident in the study area for at least one year before the diagnosis. All patients first diagnosed during the study period 1983-2005 were included in the study. All patients included in the study were evaluated at least twice during the study period.
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Genotyping
Three NOD2/CARD15 variants previously identified as being independently associated with Crohn’s disease, R702W, G908R, L1007fsinsC[13-15] were genotyped. Each individual reaction contained primers to amplify a nonpolymorphic genomic control sequence. A single thermocycling program was used for all reactions. The products were electrophorerized on 1% agarose gels with ethidium bromide and viewed under ultraviolet light. An image was recorded digitally. Healthy controls match for age and gender with CD patients were randomly selected from Blood Donors in University Hospital of Ioannina and District Hospital of Corfu.
Statistical analysis
Incidence rates were calculated as the number of cases per 100 000 inhabitants, and 95% Confidence Intervals were estimated using the normal distribution. Population data were based on databases of the National Statistical Service (National Censuses 1981, 1991, and 2001). The significance of time trends of incidence rates was tested using the χ2-trends in proportion test. Frequency and susceptibilities of NOD2/CARD15 variants among CD patients, UC patients and controls was compared with the chi-square test. Odds ratios (OR) were calculated with the chi-squared distribution test and 95% confidence intervals (95% CI). The Fisher exact test was used for comparing differences between allele frequencies in patients and controls. The P values obtained were two-tailed and statistical significance was assumed below 0.01. Inference was aided by GraphPad InStat (version 3.00; GraphPad Software).
RESULTS
During the study period, a total of 447 IBD cases were recorded in the study area. One hundred and five patients were diagnosed with CD (23.5%), 325 with UC (72.7%), and 17 with Indeterminate Colitis (3.8%). The majority of the patients were derived from the regions of Ioannina and Corfu, accounting for 92% of the total CD cases and 78% of the total UC cases.
The main demographic and clinical characteristics of CD and UC patients are presented in Table 1.
Table 1.
Demographic characteristics of IBD patients diagnosed during the period 1983-2005, in northwest Greece
| Crohn’s disease | Ulcerative colitis | |
| Total numbers of patients | 105 | 325 |
| Region of origin | ||
| Ioannina | 59 | 140 |
| Arta | 5 | 10 |
| Preveza | 2 | 36 |
| Thesprotia | 5 | 21 |
| Corfu | 33 | 113 |
| Lefkada | 1 | 5 |
| Male: female (ratio) | 66:39 (1.69) | 208:117 (1.77) |
| Age at diagnosis (yr) (mean ± SD) | 33.2 ± 12.9 | 45.7 ± 16.1 |
| (range) | (18-70) | (18-93) |
| Men | 33.4 ± 12.9 | 47.3 ± 15.1 |
| Women | 33.1 ± 13.2 | 42.9 ± 17.3 |
| Follow up (mo) (mean ± SD) | 92.9 ± 72.3 | 119.6 ± 73.8 |
| (range) | (4-264) | (5-276) |
A familial history of IBD was present in 11 patients (2.5%). Terminal ileal localization of the disease was present in 35 (33.3%), ileocolonic in 28 (26.7%) and colonic in 42 (40%) of the CD patients; stenotic disease was recorded in 23 (21.9%), fistulating in 25 (23.8%) and perianal disease in 26 (24.8%) CD patients. Of the UC patients, 71 (21.8%) had pancolitis, 99 (30.5%) had proctitis, and 155 (47.7%) had left-sided UC.
Mean annual crude incidence rates of CD and UC were 0.9 and 2.9/100 000 inhabitants, respectively. Age-adjusted incidence rates were 0.9/100 000 (95% CI 0.1-1.7) and 2.7 (95% CI 1.7-4.1), respectively. The age-adjusted mean annual incidence rate of IC was 0.2/100 000 (95% CI 0.1-0.4).
The incidence of CD was higher in men than in women. The highest incidence rate was found in the age group 18-44 years for men and women. UC incidence was higher in men also. The highest incidence rate was found in the age group 45-64 for both sexes.
There was a statistically significant increase of CD incidence (P < 0.01) during the study period, while the incidence of UC increased slightly and not in a statistically significant manner (Figure 1). As a consequence, the ratio of CD to UC practically doubled, surpassing 1 in the district of Ioannina, the largest population district and centre of the study, in the year 2002, and remaining above 1 for the following years (Table 2).
Figure 1.
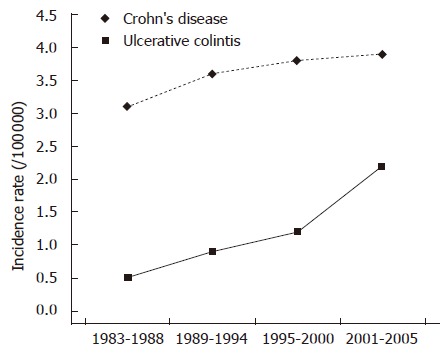
Trends in standardised incidence of Crohn’s disease (CD) and Ulcerative colitis (UC) (Northwestern Greece 1983-2005).
Table 2.
Evolution of genetic, demographic and clinical correlations of Crohn’s disease through the study period
| Period | Any CARD15 mutation | M:F ratio | Mean age (yr) | CD:UC ratio, cumulative | CD:UC ratio, b region of Ioannina | CD:UC ratio, region of Corfu | Perianal |
| Pre-1994 | 46.10% | 1.4 | 30.4 | 0.23 | 0.26 | 0.2 | 13.80% |
| 1995-1999 | 61.90% | 3.4 | 35.4 | 0.32 | 0.46 | 0.3 | 27.20% |
| 2000-2005 | 46.90% | 1.55 | 33.1 | 0.61 | > 1 | 0.5 | 31.30% |
| Total | 51% | 1.83 | 32.8 | 0.36 | 0.38 | 0.32 | 25.50% |
When demographic and clinical parameters were evaluated in correlation with the increase in CD incidence, age, gender, and regional patient distribution did not exhibit any statistically significant trends. There were also no differences in the percentages of patients with terminal ileal or colonic localization, and stenotic or fistulating disease during the pre-1995 period, compared to the 1995-1999 and the 2000-2005 periods. A statistically significant increase in the percentage of patients with perianal disease was observed in the latter two periods compared to the pre-1995 period (Table 2).
The association of CD with mutations in the three casual CARD15 variants is shown in Table 3.
Table 3.
CARD15 variant allele frequencies
|
Genotype |
Mutant allele frequency (%) | P; OR (95% CI) | |||
| Samples | -/- | -/+ | +/+ | ||
| R702W | |||||
| CD | 85 | 8 | 3 | 7.29 | 0.015; 3.85 (1.24-11.93) |
| UC | 171 | 9 | 0 | 2.5 | 0.78; 1.26 (0.38-4.13) |
| Control | 96 | 4 | 0 | 2 | |
| G908R | |||||
| CD | 83 | 13 | 0 | 6.77 | 0.70; 0.83 (0.39-1.78) |
| UC | 156 | 24 | 0 | 6.66 | 0.61; 0.82 (0.42-1.58) |
| Control | 84 | 16 | 0 | 8 | |
| Leu1007fsincC | |||||
| CD | 52 | 36 | 8 | 27.08 | < 0.0001; 7.06 (3.46-14.37) |
| UC | 166 | 9 | 5 | 5.27 | 1; 1.06 (0.48-2.32) |
| Control | 90 | 10 | 0 | 5 | |
-/-, homozygous wild-type; -/+, heterozygous; +/+, homozygous mutant. CD: Crohn’s disease; UC: Ulcerative colitis; OR: Odds Ratio; CI: Confidence Interval. The CARD15 gene variants determined in 96 CD patients (from 102 living CD patients out of 105, from our cohort 6 refused genetic test), 180 out of 300 living UC patients and 100 randomly chosen healthy blood donors.
There were no statistically significant differences on any mutations over time (Table 2).
DISCUSSION
North-western Greece is comprised of the mainland district of Epirus and the islands Corfu and Lefkada. Due to geographical reasons and relatively underdeveloped transport facilities to the rest of Greece the region can be considered secluded, and thus an ideal frame for epidemiological analysis of certain diseases. We have shown in the past that UC was a common entity in this region, while CD on the contrary was all too rare, with the CD:UC ratio in the 1980 s and early 1990 s being near 0.1[9,10]. Herein, the results of this 23-year period study, clearly show a rapid and sustained increase in CD incidence from 2000 on in North-western Greece, an increase that overturned the CD:UC ratio in the largest province of the study, the region of Ioannina, by 2002.
These findings are in accordance with formerly published studies showing an increasing trend for CD[16-22]. In these studies the increasing trend was often related to increasing age at diagnosis and could thus be related to improvements in average survival and surveillance (global increase[20,21] or a higher proportion of older patients[5,18,22]).
This is not the case in our study in which the majority of CD patients are young, similar to other studies[6,19] and the average patient age has not altered significantly over the years. Furthermore this increase could not be attributed to enhanced surveillance and patient reporting; characteristically, the increase in incidence is predominantly expressed in the region of Ioannina, which, hosting the study centre for the past 25 years, guarantees total patient reporting and absence of bias. Moreover, a similar increase was not observed for UC during this period. This rising incidence suggests that unknown triggering factors continue to work in North-western Greece. To explain this increase in CD incidence throughout the period 1983-2005 we hypothesized that certain etiologic factors exist, and we evaluated the potential role of genetic susceptibility changing slowly over time in this incidence trend.
Although there are differences in ethnic, racial and geographic distribution[23], the genetic association of CD with CARD15 is undoubtedly replicated widely at present[24,25]. There were no significant differences in the prevalence of CARD15 variants during the study period in our study, indicating an absence of alterations in the genetic background. The prevalence of the three CARD15 variants is similar with that found in cohorts in central Europe and much higher than in northern Europe[25]. Yet these results are different from two other Greek studies: the first, based on the island of Crete outlined the rarity of SNP 13[26], an inconsistency that may be attributed to the fact that Crete is an isolated geographic region where this mutation does not seem to predispose to the disease, or to the relatively small size of the examined sample. The relatively higher frequencies in all three SNPs in the second study may be attributed to patient selection (hospitalized patients in a period of time, high proportion of ileocolonic disease)[27]. Our study, being population based, may be more representative of prevalence of CARD15 variants in Greece.
One could speculate that the increased incidence of CD recorded in recent years may actually reflect increase in detection, but not incidence of colonic disease, more easily recognized through increased endoscopy implementation. Although the total number of endoscopies performed naturally increased over time, there was no difference in endoscopy success rates, furthermore significant trends in disease localization over time were not recorded, apart from the already mentioned perianal CD (data not shown).
A potential criticism of the recruitment methods used in this study would be that ascertainment might have been incomplete, missing patients with CD or those with mild ulcerative proctitis who might have never required gastroenterologic consultation or hospital admission. However in the Greek population, during the time period of this study, there was an almost universal admission policy for investigation of IBD. Although North-western Greece has a relatively small population and isolated geographic area, this has actually been a great advantage and has resulted in a comprehensive study of the entire population, excluding any case leaks to other centers or existence of not notified cases followed-up by general practitioners.
In conclusion, we have shown that a significant increase in CD incidence in a secluded geographical region of Greece where CD was previously rare is not related to any alterations in the genetic profile or typical demographical factors. The study being population based outlines the need for further investigation of the interplay of environmental factors and disease.
ACKNOWLEDGMENTS
The authors thank the patients that participated in this study. The authors acknowledge the contribution of Dr M Gazouli for advice and technical support in genetic analysis, Kleopatra Garallea, Gioula Georgitsi, Lamprini Gouma, Alexandra Kioulou, for organization and collection of blood specimens and Drs E Zervou and M Tzilianos for helping to collect samples from healthy blood donors. We thank all gastroenterologists of North-western Greece Gastroenterology Group and Medical personnel of 1st Department of Internal Medicine University Hospital of Ioannina.
COMMENTS
Background
The incidence of Crohn’s disease and ulcerative colitis varies greatly between specific geographic areas, and is supposed to be related to exogenous factors such as affluence and dietary habits, as well as endogenous factors such as genetic predisposition. North-western Greece was characterized by a low incidence of Crohn’s disease (CD) although Ulcerative Colitis (UC) was not uncommon according to a previous study undertaken in the early 1990s.
Innovations and breakthroughs
The present study is one of the few to evaluate genetic predisposition to Crohn’s disease and ulcerative colitis through time in a given area. The presence of absence of relation to incidence evolution allows for evaluation of the etiological role of genetic predisposition in disease.
Applications
Similar studies, when applied in larger areas, may be hampered by planning difficulties though.
Terminology
NOD2/CARD15 mutations are mutations of a specific genetic locus that have been shown to interfere with altered immune response and induce increased predisposition to the development of Crohn’s disease.
Peer review
The author examined the relation between the change in the annual incidence rates of IBD patients and CARD15 variants in North-western Greece. Although the incidence of CD has significantly increased during the study period, there was no statisitical differences in NOD2/CARD15 variants among the Groups. The data is interesting and certainly provides the new evidences in this research field.
Footnotes
Supported by General Secretariat for Research and Technology, Greece and the European Union, PENED03ED770
S- Editor Zhu LH L- Editor Rampone B E- Editor Liu Y
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munkholm P, Langholz E, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of Crohn's disease in the county of Copenhagen, 1962-87: a sixfold increase in incidence. Scand J Gastroenterol. 1992;27:609–614. doi: 10.3109/00365529209000127. [DOI] [PubMed] [Google Scholar]
- 5.Lapidus A, Bernell O, Hellers G, Persson PG, Löfberg R. Incidence of Crohn's disease in Stockholm County 1955-1989. Gut. 1997;41:480–486. doi: 10.1136/gut.41.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinié F, Gower-Rousseau C, Yzet T, Merle V, Grandbastien B, Marti R, Lerebours E, Dupas JL, Colombel JF, Salomez JL, et al. Opposite evolution in incidence of Crohn's disease and ulcerative colitis in Northern France (1988-1999) Gut. 2004;53:843–848. doi: 10.1136/gut.2003.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maté-Jimenez J, Muñoz S, Vicent D, Pajares JM. Incidence and prevalence of ulcerative colitis and Crohn's disease in urban and rural areas of Spain from 1981 to 1988. J Clin Gastroenterol. 1994;18:27–31. doi: 10.1097/00004836-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Trallori G, Palli D, Saieva C, Bardazzi G, Bonanomi AG, d'Albasio G, Galli M, Vannozzi G, Milla M, Tarantino O, et al. A population-based study of inflammatory bowel disease in Florence over 15 years (1978-1992) Scand J Gastroenterol. 1996;31:892–899. doi: 10.3109/00365529609051998. [DOI] [PubMed] [Google Scholar]
- 9.Tsianos EV, Masalas CN, Merkouropoulos M, Dalekos GN, Logan RF. Incidence of inflammatory bowel disease in north west Greece: rarity of Crohn's disease in an area where ulcerative colitis is common. Gut. 1994;35:369–372. doi: 10.1136/gut.35.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsianos EV, Katsanos KH, Christodoulou D, Dimoliatis I, Kogevinas A, Logan RF. Continuing low incidence of Crohn's disease in Northwest Greece. Dig Liver Dis. 2003;35:99–103. doi: 10.1016/s1590-8658(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 11.Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16-19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 13.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 14.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 15.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 16.Thomas GA, Millar-Jones D, Rhodes J, Roberts GM, Williams GT, Mayberry JF. Incidence of Crohn's disease in Cardiff over 60 years: 1986-1990 an update. Eur J Gastroenterol Hepatol. 1995;7:401–405. [PubMed] [Google Scholar]
- 17.Moum B, Vatn MH, Ekbom A, Aadland E, Fausa O, Lygren I, Stray N, Sauar J, Schulz T. Incidence of Crohn's disease in four counties in southeastern Norway, 1990-93. A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996;31:355–361. doi: 10.3109/00365529609006410. [DOI] [PubMed] [Google Scholar]
- 18.Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 19.Björnsson S, Jóhannsson JH. Inflammatory bowel disease in Iceland, 1990-1994: a prospective, nationwide, epidemiological study. Eur J Gastroenterol Hepatol. 2000;12:31–38. doi: 10.1097/00042737-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lee FI. Nguyen-van-Tam JS. Prospective study of incidence of Crohn's disease in northwest England: no increase since the late 1970's. Eur J Gastroenterol Hepatol. 1994;6:27–31. [Google Scholar]
- 21.Timmer A, Breuer-Katschinski B, Goebell H. Time trends in the incidence and disease location of Crohn's disease 1980-1995: a prospective analysis in an urban population in Germany. Inflamm Bowel Dis. 1999;5:79–84. doi: 10.1097/00054725-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kyle J. Crohn's disease in the northeastern and northern Isles of Scotland: an epidemiological review. Gastroenterology. 1992;103:392–399. doi: 10.1016/0016-5085(92)90826-k. [DOI] [PubMed] [Google Scholar]
- 23.Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JP. Differential effects of NOD2 variants on Crohn's disease risk and phenotype in diverse populations: a metaanalysis. Am J Gastroenterol. 2004;99:2393–2404. doi: 10.1111/j.1572-0241.2004.40304.x. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire S, Rutgeerts P. Current status of genetics research in inflammatory bowel disease. Genes Immun. 2005;6:637–645. doi: 10.1038/sj.gene.6364257. [DOI] [PubMed] [Google Scholar]
- 25.Gaya DR, Russell RK, Nimmo ER, Satsangi J. New genes in inflammatory bowel disease: lessons for complex diseases? Lancet. 2006;367:1271–1284. doi: 10.1016/S0140-6736(06)68345-1. [DOI] [PubMed] [Google Scholar]
- 26.Roussomoustakaki M, Koutroubakis I, Vardas EM, Dimoulios P, Kouroumalis EA, Baritaki S, Koutsoudakis G, Krambovitis E. NOD2 insertion mutation in a Cretan Crohn's disease population. Gastroenterology. 2003;124:272–273; author reply 272-273;. doi: 10.1053/gast.2003.50036. [DOI] [PubMed] [Google Scholar]
- 27.Gazouli M, Zacharatos P, Mantzaris GJ, Barbatis C, Ikonomopoulos I, Archimandritis AJ, Lukas JC, Papalambros E, Gorgoulis V. Association of NOD2/CARD15 variants with Crohn's disease in a Greek population. Eur J Gastroenterol Hepatol. 2004;16:1177–1182. doi: 10.1097/00042737-200411000-00016. [DOI] [PubMed] [Google Scholar]


