Abstract
Uncomplicated reflux disease comprises the non-erosive reflux disease (NERD) and erosive reflux disease (ERD). The objectives of treatment are the adequate control of symptoms with restoration of quality of life, healing of lesions and prevention of relapse. Treatment of NERD consists in the administration of proton pump inhibitors (PPI) for 2-4 wk, although patients with NERD show an overall poorer response to PPI treatment than patients with ERD owing to the fact that patients with NERD do not form a pathophysiologically homogenous group. For long-term management on-demand treatment with a PPI is probably the best option. In patients with ERD, therapy with a standard dose PPI for 4-8 wk is always recommended. Long-term treatment of ERD is applied either intermittently or as continuous maintenance treatment with an attempt to reduce the daily dosage of the PPI (step-down principle). In selected patients requiring long-term PPI treatment, antireflux surgery is an alternative option. In patients with troublesome reflux symptoms and without alarming features empirical PPI therapy is another option for initial management. Therapy should be withdrawn after initial success. In the case of relapse, the long-term care depends on a careful risk assessment and the response to PPI therapy.
Keywords: Erosive reflux disease, Non-erosive reflux disease, Proton pump inhibitor, Uninvestigated reflux disease
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common condition affecting approximately 10-20% of the adult population of industrialized countries[1]. In the great majority of patients with GERD, the disease does not lead to complications, instead presents with often severe symptoms. Some 60% of patients in primary care with troublesome reflux symptoms have no endoscopically recognizable lesions of the esophageal mucosa, 35% have erosive esophagitis (75% of which are mild, corresponding to Los Angeles A/B, and 25% severe, corresponding to Los Angeles C/D). In about 5% of the patients, complications, such as stricture, ulcer and in particular Barrett’s esophagus or even adenocarcinoma, must be expected (Figure 1)[2]. Epidemiological data support the hypothesis that GERD is not a spectrum disease with occasional reflux symptoms but no lesions at the one end, and severe complications at the other, but can instead be classified into three distinct categories-nonerosive reflux disease (NERD)-erosive reflux disease (ERD), and Barrett’s esophagus-in each of which the respective patient remains, that is, progression of the disease over time is, overall, very rare[3]. This category model of GERD is supported by the latest data from a large prospective European study (ProGERD) involving more than 6 000 patients with NERD and ERD: the rate of progression (to severe esophagitis or Barretts’) for patients with NERD and mild erosive esophagitis (Los Angeles A/B) was less than 1% per year (Labenz et al unpublished data).
Figure 1.
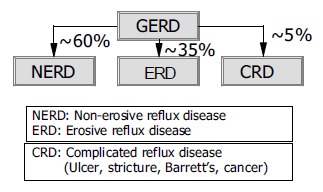
GERD-a categorical disease with three distinct entities[2,3].
INITIAL MANAGEMENT OF GERD
Uncomplicated reflux disease comprises the non-erosive form, that is, symptoms that impact negatively on the patient’s quality of life, but which are not associated with endoscopic evidence of mucosal breaks in the esophagus, and erosive reflux esophagitis of varying degrees of severity, e.g. grades A-D in the Los Angeles classification[4].
Contrary to commonly held beliefs, symptom evaluation is the most important assessment for the initial phase of GERD management[5], although an evidence-based analysis of symptoms is hardly possible[6]. Characteristic symptoms are heartburn and acid regurgitation[6]. However, these symptoms are predictive of GERD in only 70% of the patients, even in cases with an unequivocal history[7]. It must be emphasized that there is no diagnostic gold standard for GERD: endoscopy has a sensitivity of only 30-40%, microscopic features such as dilated intracellular spaces and regenerative changes in the absence of endoscopically visible mucosal breaks of the squamous epithelium in the distal esophagus are currently not sufficiently validated, and pH-monitoring is far from being a diagnostic gold standard, since 30-60% of patients with NERD, as well as 10-20% of those with ERD, have normal results of 24-h pH-monitoring, and intra-individual comparisons have also shown that pH-metry is subject to appreciable fluctuations[4,8]. Moreover, there is consistent observation that there is virtually no correlation between the severity of endoscopic findings and symptom severity (Figure 2)[9]. All these aspects have an important impact on the clinical management of GERD which is distinct in the initial phase and long-term care.
Figure 2.
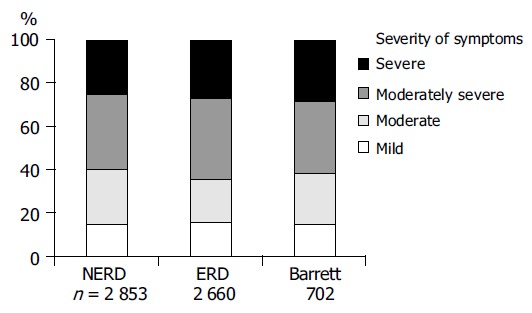
No correlation between endoscopic findings and symptom severity in patients with GERD[9].
Basic goals of treatment are:
- to provide complete, or at least sufficient, control of symptoms,
- to maintain symptomatic remission,
- to heal underlying esophagitis and maintain endoscopic remission, and
- to treat or, ideally, prevent complications.
Adequate control of symptoms is considered to have been achieved when mild reflux symptoms occur at most once a week-more frequent or more pronounced complaints are not accepted as satisfactory by the patient[10]. Initially, a symptom-based diagnosis is established, and an individual risk assessment made (Figure 3). If such alarming symptoms such as dysphagia, unintended weight loss and/or signs of bleeding are present, an endoscopic examination is mandatory, with further management dictated by the endoscopic findings. Other indications for endoscopy at this point in time may include, for example, a family history of upper gastrointestinal tract malignancies, a long prior history of severe complaints, age over 50 years, use of NSAIDs, and a positive Helicobacter pylori status[11]. Otherwise, empirical therapy can be offered (Figure 3). Withholding endoscopy in the initial phase is, of course, associated with the theoretical risk that serious complications of GERD or other significant pathologies in the upper gastrointestinal tract mimicking the symptoms of reflux disease may be overlooked or recognized too late. On the other hand, given the facts that GERD is extremely common, and complications are generally rare, endoscopic evaluation of all patients with GERD is hardly justifiable, especially since an endoscopy-based management strategy has not been subjected to appropriate evaluation. In a cross-sectional study from Finland the detection rate of serious complications of GERD did not differ between regions with low and those with high referral to endoscopy[12]. Considering that most patients have mild GERD and that the disease is not progressive over time, restricted use of endoscopy does not appear to put the patients at risk. However, the economic impact of different diagnostic strategies on expense remains to be established, at least in countries with low endoscopy costs[13]. A recent study in 742 patients with uncomplicated GERD showed no correlation between endoscopic findings and subsequent therapeutic decisions[14]. Further arguments for a primarily symptom-driven strategy are that the ultimate benchmark for the clinical efficacy of treatment of GERD is patient satisfaction and that accurate determination of esophagitis requires the withholding of therapy before endoscopy, which in many cases is not possible[15].
Figure 3.
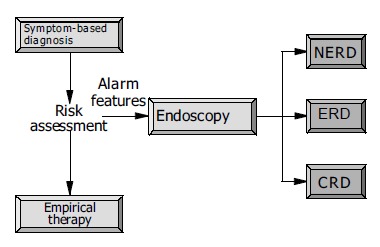
Initial management of patients with symptoms suggestive of GERD.
For the sake of simplicity, the following sections first discuss the initial and long-term treatment of patients with NERD and ERD (endoscopy-based approach), and then consider the management of uninvestigated GERD, which is doubtless the more common treatment that is applied in the clinical setting.
TREATMENT OF NERD
While NERD is the most common manifestation of reflux disease, patients with this entity do not form a pathophysiologically homogeneous group. A differentiation can be made between patients with unequivocally pathological acid reflux, patients with an acid-sensitive (hypersensitive) esophagus, which means that more than 50% of the symptomatic episodes were associated with acid reflux (positive symptom index), and those with symptoms that are independent of acid-reflux events (functional heartburn)[8,16]. This latter category explains the observation that patients with NERD did not respond as well to acid suppressants as patients with erosive esophagitis do[17,18]. Possible pathophysiological causes of functional heartburn include non-acid reflux (liquid, gas, mixed), minute changes in the esophageal acidity above a pH of 4, motility disorders such as sustained contractions of the longitudinal musculature, visceral hypersensitivity, and emotional and psychological abnormalities[19].
Initial therapy of NERD
Initially, patients should receive a proton pump inhibitor (PPI) for 2-4 wk (Figure 4). The effect of other substances such as H2blockers or prokinetic drugs is hardly better than that of placebo[20]. In a large, placebo-controlled study, a dose-response relationship was established for omeprazole: omeprazole 20 mg proved to be more effective than omeprazole 10 mg[21]. In a further controlled study, lansoprazole 30 mg was no more effective than lansoprazole 15 mg[22]. The S-isomer of omeprazole, esomeprazole, was investigated in two large, double-blind, multicenter studies involving patients with NERD[23]. Esomeprazole at a dose of 20 and 40 mg per day proved more effective than placebo, but a dose-response effect could not be shown. Three further randomized, double-blind, multicenter studies involving a total of more than 2 600 patients with NERD treated for 4 wk with omeprazole 20 mg, and esomeprazole 20 or 40 mg revealed comparable success rates (resolution of symptoms in 60-70% of the patients)[24]. Assessment of the response to treatment in studies like these are greatly influenced by the target criterion (e.g. complete elimination of symptoms, satisfactory symptom control), so that the studies can hardly be compared. From the above remarks it may be concluded that appropriately dosed PPI treatment can achieve a satisfactory initial response in some two-thirds of the patients. If initial treatment with 4 wk of PPI fails to elicit adequate symptom control (Figure 4), increasing the PPI dose (e.g. standard dose PPI twice daily) is recommended, since studies have shown that patients with acid-sensitive esophagus respond better to a high PPI dose[25-27]. In non-responders to appropriate PPI treatment, it is recommended that esophageal pH-monitoring be performed during PPI therapy and, if symptomatic acid reflux can be excluded, to discontinue PPI therapy and initiate a trial with a low-dose tricyclic antidepressant at bedtime[28]. Potential therapeutic options for the future might be serotonin reuptake inhibitors, kappa agonists and substances with an impact on transient sphincter relaxation such as baclofen.
Figure 4.
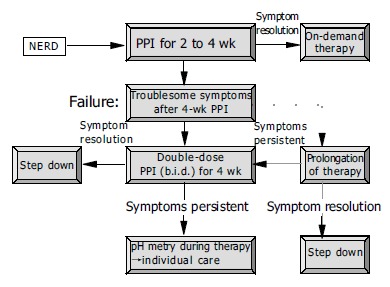
Initial therapy and long-term care of patients with NERD.
Long-term care of patients with NERD
If initial treatment is successful, medication should be discontinued, since 25% (or more) of the patients may remain in remission over prolonged periods of time[29], and this clinical entity does not appear to necessitate measures aimed at preventing complications. In the event of a relapse, indicating the need for long-term management, a number of different options are available: continuous maintenance therapy starting with a PPI and subsequent attempts to step down to lower dosages of the PPI or even less potent drugs (Figure 5)[5], intermittent courses of treatment for 2-4 wk with initially successful PPI[30], and patient-controlled on-demand therapy with a PPI[11]. On-demand therapy means that the patient himself determines both the start and the end of treatment. Medication should be discontinued when the symptoms have been eliminated. This last option in particular, has met with great interest in recent years on account of its potential economic advantages[31,32]. In a first large randomized, controlled study lasting 6 mo, Lind et al[33] were able to show that more than 80% of the patients were satisfactorily treated with an on-demand strategy employing omeprazole 20 mg. In this study, omeprazole 20 mg proved more effective than omeprazole 10 mg. The convincing efficacy of this new treatment option was then confirmed with esomeprazole 20 mg[34,35]. All these studies also showed that roughly one-half of these patients were satisfactorily treatable with placebo medication and the use of antacids as required. In a recently presented randomized, open international multicenter study involving 598 patients, on-demand treatment with esomeprazole 20 mg was compared with continuous treatment with esomeprazole 20 mg o.d. in patients with NERD[36]. The vast majority of patients in both treatment groups were satisfied with the regimen, and medication consumption was considerably lower in the on-demand therapy arm (average consumption: 0.41 vs 0.91 tablets per day). However, the final endoscopic examination revealed mild erosive esophagitis (Los Angeles A: n = 14; Los Angeles B: n = 1) in 5% of the patients receiving on-demand treatment, while none of the patients on continuous treatment had this finding. From the viewpoint of a clinician, this observation occasions no major concern, since alternation between the categories NERD and mild ERD (corresponding to Los Angeles A and B) often occurs during the spontaneous course of the disease. In another large scale study, 622 patients with NERD were randomized to esomeprazole 20 mg on demand or lansoprazole 15 mg o.d. for 6 mo after an initial successful treatment with esomeprazole[37]. Based on the target criterion “willingness to continue” on-demand treatment was superior to continuous treatment (93% vs 88%;P = 0.02). Patients on continuous treatment complained more often about heartburn and adverse events, the main reasons for “unwillingness to continue”. Other PPIs (lansoprazole, pantoprazole, rabeprazole) have also proven their superiority to placebo in on-demand treatment in individual studies[11]. Head-to-head comparisons between various PPIs are, however, lacking, so that a comparative assessment is not possible.
Figure 5.
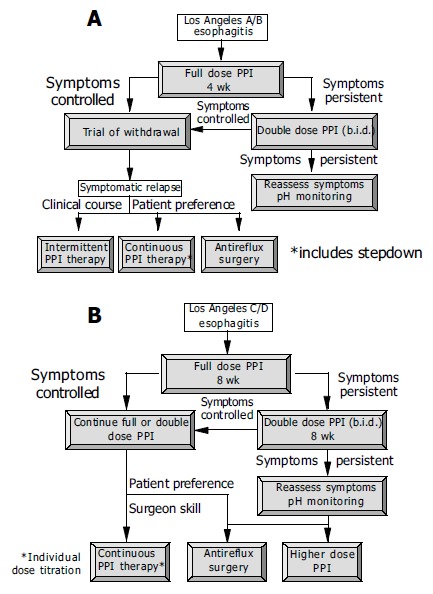
Management of patients with mild to moderate erosive esophagitis (A) and moderate to severe esophagitis (B)[4,5].
TREATMENT OF ERD
Erosive reflux esophagitis can be found in about 30-40% of GERD patients[2]. Endoscopically, reflux esophagitis is categorized into various degrees of severity. In recent years, and especially in therapeutic studies, the Los Angeles classification in particular has been applied[38,39]. This distinguishes the four degrees of severity A-D (A: mucosal breaks of less than 5 mm on the top of folds; B: mucosal breaks >5 mm in extent on mucosal folds; C: circumferential spreading of mucosal breaks involving less than 75% of the circumference; D: mucosal breaks involving more than 75% of the circumference). The gradings A and B correspond to mild to moderate esophagitis and C and D to moderate to severe esophagitis. One fourth of patients with erosive esophagitis are categorized in grade C or D[40-42].
Numerous controlled studies have investigated the efficacy of a variety of medications in the healing of esophagitis and the elimination of symptoms. In a meta-analysis, Chiba et al[43] showed that PPIs (omeprazole, lansoprazole, pantoprazole) heal the esophagitis within 8 wk in 83.6% of the patients, with a symptom-resolution rate of 77.4%. All other medications (H2-receptor antagonists, cisapride, sucralfate) were appreciably less effective. A placebo-related healing rate of 28.2% documents the fluctuating nature of the course of reflux disease in some patients, with spontaneous remissions and exacerbations. When using highly potent PPIs, elimination of symptoms after 8 wk is predictive for healing of the esophagitis[41,42].
Initial therapy of ERD
In patients with erosive reflux esophagitis, treatment with a standard dose of a PPI is always recommended (Figure 5)[4]. Mild cases (Los Angeles grade A/B) usually heal within 4 wk, while severe cases (Los Angeles C/D) often require longer treatment-eight or, in some cases, more weeks. Resolution of symptoms in those responding to therapy is achieved appreciably more quickly (median time to sustained symptom resolution 5-10 d). The major predictive factor for the healing rate is the severity of the erosive esophagitis, but also of significance are concomitant Barrett’s metaplasia in the lower esophagus, which reduces the healing rate, and infection with H pylori, which enhances the efficacy of the PPI[44,45].
Is there any clinically relevant difference between the PPIs available in the market? Racemic PPIs (omeprazole, lansoprazole, pantoprazole, rabeprazole) differ in such pharmacokinetic characteristics as bioavailability and the rapidity with which an effect occurs. This, however, is irrelevant for the healing of esophagitis at 4 and 8 wk[46,47], although the substances do differ in terms of the time required to eliminate symptoms. In a large randomized, controlled study involving more than 3 500 patients with erosive esophagitis, lansoprazole 30 mg o.d. relieved heartburn significantly faster than did omeprazole 20 mg o.d.[48]. There is a linear relationship between the degree of acid suppression measured by the time per day that gastric pH is higher than 4, and the healing kinetics of esophagitis[49]. With regard to the healing rates of reflux esophagitis after 4 and 8 wk, no differences are to be seen between the standard doses of the racemic PPIs (omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, rabeprazole 20 mg)[46,47], nor did doubling the individual dose (e.g. lansoprazole 60 mg, pantoprazole 80 mg) increases efficacy. Of significance for the healing rates and symptom elimination, however, is cytochrome 2C19 polymorphism. Thus, it has been recently shown that the response to treatment with lansoprazole 30 mg o.d. is poorer in extensive metabolizers than in intermediate and poor metabolisers[50].
Cross-over pH-monitoring studies in healthy volunteers and patients with GERD have shown that esomeprazole is more effective than corresponding doses of the racemic PPIs omeprazole, lansoprazole, pantoprazole and rabeprazole[51,52]. In large controlled studies, significantly higher healing rates were achieved with esomeprazole at a dose of 40 mg o.d. than with omeprazole 20 mg o.d., lansoprazole 30 mg o.d., and pantoprazole 40 mg o.d.[40-42,53,54]. The therapeutic advantage of esomeprazole over the other PPIs increased with increasing severity of the esophagitis as defined by the Los Angeles classification[55]. These studies also showed a significant superiority of esomeprazole in terms of the time to sustained symptom (heartburn) resolution. Small non-inferiority studies claiming equivalence between different PPIs did not have the statistical power to detect a difference of the magnitude that has been consistently established by the large scale studies mentioned above[56-58].
In the event of inadequate efficacy (insufficient control of symptoms or healing of the esophagitis), doubling the individual dose of PPI does not reliably improve clinical efficacy, but switching to another PPI, or shortening the interval between doses (e.g. twice daily) might increase the response to treatment[59]. In recent years there has been intense discussion on the clinical relevance of nocturnal acid breakthrough (NABT) in difficult-to-treat cases[60,61]. NABT is defined as a decrease in gastric pH to <4 for more than 1 h during the course of the night. The clinical relevance of this phenomenon has not yet been established. H2-receptor antagonists given at bedtime can prevent this acid breakthrough, but when administered over a longer period of time, they rapidly lose this effect[62]. Treatment with combinations of PPI and prokinetic drugs is of unproven value.
Long-term care of patients with ERD
After responding well to initial treatment, ERD shows a tendency to relapse. Up to 90% of the patients will relapse already within the next 6 mo[29]. Patients with mild esophagitis (Los Angeles A/B) often have a longer relapse-free interval than patients with severe esophagitis (Los Angeles C/D), who frequently suffer a relapse within days of discontinuing successfully the initial treatment[63,64]. In the light of these observations, it is recommended that, in patients with mild esophagitis, therapy should first be discontinued and the further course of the disease kept under surveillance, while in severe esophagitis, initial successful therapy should be followed, a priori, by maintenance treatment (Figure 5). Established options for long-term management are intermittent treatment for some weeks and continuous maintenance treatment with an attempt to reduce the daily dosage of the PPI (step-down principle)[4,30]. On-demand therapy has, to date, been investigated in only two studies in patients with erosive esophagitis[65,66]. Satisfactory control of symptoms was achieved in the vast majority of patients, but continuous therapy proved to be superior with respect to maintenance of remission of erosive esophagitis, so that an evidenced-based recommendation is currently not possible. However, since GERD is usually not progressive, attempts to realize on-demand treatment does not appear to harm the patients[3].
For the prevention of relapse in patients with healed esophagitis, PPIs are clearly superior to H2-receptor antagonists, prokinetic drugs and combinations of these medications[67-69]. The yield between a standard dose of a PPI and one-half of this dose is, in individual studies, often small, although significant, and even probably clinically relevant differences have occasionally been observed[67]. On the basis of a cost effectiveness analysis using a Markov model designed to simulate the economic and clinical outcomes of GERD in relation to the cost per symptom-free patient years gained and the cost per QALY gained treatment with a standard dose of a PPI appears to be superior despite the higher drug costs[69]. Nevertheless, in view of the overall high response rates, an initial attempt with half the standard dose of a PPI is recommended in patients with mild erosive esophagitis, while patients with more severe disease should be kept on the dose of PPI required to induce remission (Figure 5). If this approach proves successful, a dose reduction, or a changeover to a less potent drug can be attempted[70,71]. If the reduced dose proves unsuccessful, the dose must be increased appropriately. Occasionally, a higher-than-standard dose may be necessary to maintain remission[72].
With long-term therapy also, differences are found between the isomeric PPI esomeprazole, and the racemic PPIs lansoprazole or pantoprazole. In large double-blind randomized studies in patients with healed esophagitis, esomeprazole 20 mg o.d. applied over 6 mo was significantly more effective than lansoprazole 15 mg o.d. (therapeutic gain 8-9%), or pantoprazole 20 mg o.d. (therapeutic gain 12%)[73-75]. As in the case of acute treatment, this superiority was more pronounced with increasing disease severity. Apart from the severity of the baseline esophagitis, concomitant Barrett’s esophagus (poorer results) and H pylori infections (better results) also have a role as predictors of treatment outcome[76].
Eradication of Helicobacter pylori in patients with GERD
Whether a concomitant H pylori infection in patients with GERD should be treated or not is still under discussion[77,78]. Since H pylori is probably not involved in the pathogenesis of GERD, it cannot be expected that its eradication can heal this condition[79]nor, according to the data of Moayyedi et al[45] is an aggravation of the spontaneous course of GERD to be expected. H pylori does, however, have an impact on the pH-elevating effect of PPIs, which leads to higher healing rates and faster elimination of symptoms in patients with reflux esophagitis. PPI treatment in H pylori-infected patients leads to an aggravation of corpus gastritis, possibly also accompanied by an accelerated development of atrophy, while at the same time, antral gastritis is improved. The resulting gastritis type (corpus dominant) is found more frequently in patients with gastric cancer, and is therefore termed as “risk gastritis” or “gastritis of the cancer phenotype”. Whether long-term PPI treatment in patients with H pylori gastritis actually does increase the gastric cancer risk is unclear. It does, however, appear certain that PPI treatment for over more than 10 years is also safe in patients infected with H pylori[72]. Whether this also applies to treatment for over 20, 30 or more years is not known at present. On the basis of these considerations, some authors advocate the eradication of H pylori before initiating long-term PPI treatment[80].
Antireflux surgery
In selected patients requiring long-term PPI treatment, a possible alternative option is antireflux surgery, which, however, is no more effective than tailored PPI therapy, and also carries a significant complication risk[81-83]. To date, no advantages of surgery in terms of economics have been unequivocally demonstrated[83,84]. The best candidates for fundoplication are probably those with esophagitis documented by endoscopy, a need for continuous PPI therapy, abnormal pH monitoring studies, normal esophageal motility studies, and at least partial symptom relief with PPI therapy[85]. Further arguments for surgery are high-volume reflux and young age. Relevant concomitant diseases, in contrast, tend to militate in favor of sticking with a conservative approach. A “treatment-refractory” GERD patient should certainly not be automatically referred for antireflux surgery.
Endoscopic antireflux procedures
In recent years, a number of different methods for endoscopic endoluminal treatment of GERD have been investigated (endoscopic gastroplication with differing suturing techniques, application of radiofrequency energy to the lower esophageal sphincter, endoscopic submucosal or intramuscular injection of inert materials). To date, the efficacy of endoluminal therapy for GERD is not supported by a high level of evidence[86]. Only a single fully published controlled study (radio-frequency energy delivery vs sham procedure) that documented a benefit in terms of symptom relief, but no effect on acid reflux, has been reported[87]. Overall, too few data are currently available on efficacy and safety, so that the use of these methods outside of controlled studies cannot be recommended. In particular, controlled studies comparing endoscopic antireflux procedures with the established options of treatment would be desirable.
UNINVESTIGATED GERD
In patients with troublesome reflux symptoms but no alarm symptoms (e.g. dysphagia, unintended loss of weight, signs of bleeding), empirical PPI therapy is another option for initial management.
The goals of empirical therapy are: (1) to succeed with initial therapy; (2) to determine need for ongoing therapy; (3) to maintain satisfactory symptom control; (4) to minimize risks from esophagitis and other consequences of abnormal reflux.
These aims should be achieved at the lowest possible cost and with minimal risks[88]. Initial therapy should via rapid relief of symptoms confirm the symptom based diagnosis, reassure the patient as to the benign and treatable nature of the reflux disease, and if present cure the esophagitis. For many years, patients with GERD received step-up therapy beginning with weakly effective substances, such as antacids and H2-receptor antagonists, and increasing the intensity of the treatment if the effect was inadequate. With this strategy, the above-mentioned aims of empirical treatment cannot be achieved. For this reason, initiation of treatment with a PPI at a standard dose applied for 4 wk is favored (step-in approach) (Figure 6). However, few scientific data are available on this approach. In a four-arm controlled double-blind study involving 593 patients and conducted over 20 wk, the patients initially received lansoprazole 30 mg o.d. or ranitidine 150 mg b.i.d. over a period of 8 wk, followed by either continuation of this medication, or a step-down from lansoprazole to ranitidine or a step-up from ranitidine to lansoprazole[89]. The most effective strategy was step-in with a PPI and continuation with this medication. These results were confirmed in another study comparing omeprazole with ranitidine[90].
Figure 6.
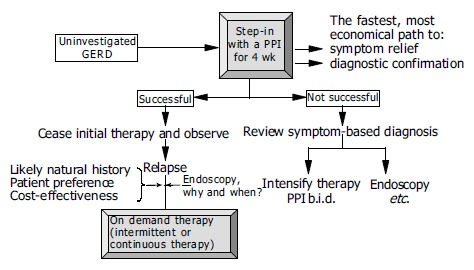
Proposal for the empirical management of patients with uninvestigated GERD[88].
Therapy should be withdrawn after initial success. In the case of a relapse, the long-term care depends on a careful assessment of the risk and the response to PPI therapy. Potential strategies are on-demand therapy or intermittent treatment. In a controlled three-arm study involving 1 357 patients with uninvestigated GERD, Meineche-Schmidt et al[91] compared on-demand therapy with esomeprazole 20 mg and GP-controlled intermittent strategy with esomeprazole 40 mg o.d. for 2 or 4 wk applied over 6 mo. The direct medical costs were similar in all three arms, but the total costs were substantially higher in patients treated with a GP-controlled intermittent strategy. If continuous maintenance therapy is needed to preserve remission, or if an initial positive response is rapidly followed by relapse, an endoscopic evaluation to exclude/detect severe erosive esophagitis or complicated reflux disease is recommended. If initial treatment is not successful, and if the clinical data militate against a severe form of GERD, the PPI dose can be increased (standard dose twice daily) or a changeover to a more potent substance implemented[59]; otherwise, in this clinical situation, too, endoscopy should be performed[88]. It is not clear whether patients who respond to initial treatment with a PPI and are then well controlled with on-demand therapy need to be submitted to endoscopy at all. Earlier calls for “once in a life-time” endoscopy for every patient with reflux disease are no longer considered mandatory. Moreover, the timing of endoscopy is critical: endoscopy off therapy is required to correctly assess the severity of esophagitis, which is important for the choice of further management, and endoscopy on therapy is needed to assess Barrett’s esophagus which is important with regard to cancer risk and the planning of surveillance.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Quigley EM. Non-erosive reflux disease: part of the spectrum of gastro-oesophageal reflux disease, a component of functional dyspepsia, or both? Eur J Gastroenterol Hepatol. 2001;13 Suppl 1:S13–S18. [PubMed] [Google Scholar]
- 3.Fass R, Ofman JJ. Gastroesophageal reflux disease--should we adopt a new conceptual framework? Am J Gastroenterol. 2002;97:1901–1909. doi: 10.1111/j.1572-0241.2002.05912.x. [DOI] [PubMed] [Google Scholar]
- 4.Dent J, Brun J, Fendrick AM, Fennerty MB, Janssens J, Kahrilas PJ, Lauritsen K, Reynolds JC, Shaw N, Talley NJ. An evi-dence-based appraisal of reflux disease management – the Genval Workshop Report. Gut. 1999;44(Suppl 2):S1–S16. doi: 10.1136/gut.44.2008.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent J. Management of reflux disease. Gut. 2002;50 Suppl 4:iv67–iv71. doi: 10.1136/gut.50.suppl_4.iv67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent J, Armstrong D, Delaney B, Moayyedi P, Talley NJ, Vakil N. Symptom evaluation in reflux disease: workshop background, processes, terminology, recommendations, and discussion outputs. Gut. 2004;53 Suppl 4:iv1–i24. doi: 10.1136/gut.2003.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klauser AG, Schindlbeck NE, Müller-Lissner SA. Symptoms in gastro-oesophageal reflux disease. Lancet. 1990;335:205–208. doi: 10.1016/0140-6736(90)90287-f. [DOI] [PubMed] [Google Scholar]
- 8.Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537–545. doi: 10.1046/j.1365-2036.2003.01423.x. [DOI] [PubMed] [Google Scholar]
- 9.Kulig M, Nocon M, Vieth M, Leodolter A, Jaspersen D, Labenz J, Meyer-Sabellek W, Stolte M, Lind T, Malfertheiner P, et al. Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol. 2004;57:580–589. doi: 10.1016/j.jclinepi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Junghard O, Carlsson R, Lind T. Sufficient control of heartburn in endoscopy-negative gastro-oesophageal reflux disease trials. Scand J Gastroenterol. 2003;38:1197–1199. doi: 10.1080/00365520310004920. [DOI] [PubMed] [Google Scholar]
- 11.Bytzer P, Blum AL. Personal view: rationale and proposed algorithms for symptom-based proton pump inhibitor therapy for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:389–398. doi: 10.1111/j.1365-2036.2004.02093.x. [DOI] [PubMed] [Google Scholar]
- 12.Mäntynen T, Färkkilä M, Kunnamo I, Mecklin JP, Juhola M, Voutilainen M. The impact of upper GI endoscopy referral volume on the diagnosis of gastroesophageal reflux disease and its complications: a 1-year cross-sectional study in a referral area with 260,000 inhabitants. Am J Gastroenterol. 2002;97:2524–2529. doi: 10.1111/j.1572-0241.2002.06034.x. [DOI] [PubMed] [Google Scholar]
- 13.Koop H. Gastroesophageal reflux disease and Barrett's esophagus. Endoscopy. 2004;36:103–109. doi: 10.1055/s-2004-814177. [DOI] [PubMed] [Google Scholar]
- 14.Blustein PK, Beck PL, Meddings JB, Van Rosendaal GM, Bailey RJ, Lalor E, Thomson AB, Verhoef MJ, Sutherland LR. The utility of endoscopy in the management of patients with gastroesophageal reflux symptoms. Am J Gastroenterol. 1998;93:2508–2512. doi: 10.1111/j.1572-0241.1998.00594.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones MP. Acid suppression in gastro-oesophageal reflux disease: Why? How? How much and when? Postgrad Med J. 2002;78:465–468. doi: 10.1136/pmj.78.922.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fass R, Tougas G. Functional heartburn: the stimulus, the pain, and the brain. Gut. 2002;51:885–892. doi: 10.1136/gut.51.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 18.Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965–973. doi: 10.3109/00365529709011211. [DOI] [PubMed] [Google Scholar]
- 19.Tack J, Fass R. Review article: approaches to endoscopic-negative reflux disease: part of the GERD spectrum or a unique acid-related disorder? Aliment Pharmacol Ther. 2004;19 Suppl 1:28–34. doi: 10.1111/j.0953-0673.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- 20.Lauritsen K. Management of endoscopy-negative reflux disease: progress with short-term treatment. Aliment Pharmacol Ther. 1997;1(Suppl 2):87–92. [Google Scholar]
- 21.Lind T, Havelund T, Carlsson R, Anker-Hansen O, Glise H, Hernqvist H, Junghard O, Lauritsen K, Lundell L, Pedersen SA, et al. Heartburn without oesophagitis: efficacy of omeprazole therapy and features determining therapeutic response. Scand J Gastroenterol. 1997;32:974–979. doi: 10.3109/00365529709011212. [DOI] [PubMed] [Google Scholar]
- 22.Richter JE, Kovacs TOG, Greski-Rose PA, Huang B, Fisher R. Lansoprazole in the treatment of heartburn in patients with-out erosive oesophagitis. Aliment Pharmacol Ther. 1999;13:795–804. doi: 10.1046/j.1365-2036.1999.00558.x. [DOI] [PubMed] [Google Scholar]
- 23.Katz PO, Castell DO, Levine D. Esomeprazole resolves chronic heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther. 2003;18:875–883. doi: 10.1046/j.1365-2036.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong D, Talley NJ, Lauritsen K, Moum B, Lind T, Tunturi-Hihnala H, Venables T, Green J, Bigard MA, Mössner J, et al. The role of acid suppression in patients with endoscopy-negative reflux disease: the effect of treatment with esomeprazole or omeprazole. Aliment Pharmacol Ther. 2004;20:413–421. doi: 10.1111/j.1365-2036.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 25.Bate CM, Riley SA, Chapman RW, Durnin AT, Taylor MD. Evaluation of omeprazole as a cost-effective diagnostic test for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1999;13:59–66. doi: 10.1046/j.1365-2036.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 26.Fass R, Ofman JJ, Gralnek IM, Johnson C, Camargo E, Sampliner RE, Fennerty MB. Clinical and economic assessment of the omeprazole test in patients with symptoms suggestive of gastroesophageal reflux disease. Arch Intern Med. 1999;159:2161–2168. doi: 10.1001/archinte.159.18.2161. [DOI] [PubMed] [Google Scholar]
- 27.Watson RG, Tham TC, Johnston BT, McDougall NI. Double blind cross-over placebo controlled study of omeprazole in the treatment of patients with reflux symptoms and physiological levels of acid reflux--the "sensitive oesophagus". Gut. 1997;40:587–590. doi: 10.1136/gut.40.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahrilas PJ. Refractory heartburn. Gastroenterology. 2003;124:1941–1945. doi: 10.1016/s0016-5085(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalvag A, et al. Gastro-oesophageal reflux disease (GORD) in primary care – an international study of different treatment strategies with omeprazole. Eur J Gastroenterol Hepatol. 1998;10:119–124. [PubMed] [Google Scholar]
- 30.Bardhan KD, Müller-Lissner S, Bigard MA, Bianchi Porro G, Ponce J, Hosie J, Scott M, Weir DG, Gillon KR, Peacock RA, et al. Symptomatic gastro-oesophageal reflux disease: double blind controlled study of intermittent treatment with omeprazole or ranitidine. The European Study Group. BMJ. 1999;318:502–507. doi: 10.1136/bmj.318.7182.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerson LB, Robbins AS, Garber A, Hornberger J, Triadafilopoulos G. A cost-effectiveness analysis of prescribing strategies in the management of gastroesophageal reflux disease. Am J Gastroenterol. 2000;95:395–407. doi: 10.1111/j.1572-0241.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 32.Wahlqvist P, Junghard O, Higgins A, Green J. Cost effective-ness of proton pump inhibitors in gastro-oesophageal reflux disease without oesophagitis: comparison of on-demand esomeprazole with continuous omeprazole strategies. Pharmacoeconomics. 2002;20:267–277. doi: 10.2165/00019053-200220040-00005. [DOI] [PubMed] [Google Scholar]
- 33.Lind T, Havelund T, Lundell L, Glise H, Lauritsen K, Pedersen SA, Anker-Hansen O, Stubber�d A, Eriksson G, Carlsson R, et al. On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis – a placebo-controlled randomized trial. Ali-ment Pharmacol Ther. 1999;13:907–914. doi: 10.1046/j.1365-2036.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 34.Talley NJ, Lauritsen K, Tunturi-Hihnala H, Lind T, Moum B, Bang C, Schulz T, Omland TM, Delle M, Junghard O. Esomeprazole 20 mg maintains symptom control in endos-copy-negative gastro-oseophageal reflux disease: a controlled trial of on-demand therapy for 6 mo. Aliment Pharmacol Ther. 2001;15:347–354. doi: 10.1046/j.1365-2036.2001.00943.x. [DOI] [PubMed] [Google Scholar]
- 35.Talley NJ, Venables TL, Green JR, Armstrong D, OKane KP, Giaffer M, Bardhan KD, Carlsson RG, Chen S, Hasselgren GS. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 mo. Eur J Gastroenterol Hepatol. 2002;14:857–863. doi: 10.1097/00042737-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Bayerdörffer E, Sipponen P, Bigard M, Weiss W, Mearin F, Rodrigo L, Dominguez-Munoz J, Grundling H, Nauclér E, Svedberg L, et al. Esomeprazole 20 mg continous versus on demand treatment of patients with endoscopy-negative reflux disease (ENRD) Gut. 2004;53(Suppl 4):A106. [Google Scholar]
- 37.Tsai HH, Chapman R, Shepherd A, McKeith D, Anderson M, Vearer D, Duggan S, Rosen JP. Esomeprazole 20 mg on-demand is more acceptable to patients than continuous lansoprazole 15 mg in the long-term maintenance of endoscopy-negative gastro-oesophageal reflux patients: the COMMAND Study. Aliment Pharmacol Ther. 2004;20:657–665. doi: 10.1111/j.1365-2036.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 39.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche P, Johnson F, Hongo M, Richter JE, Spechler SJ, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richter JE, Kahrilas PJ, Johanson J, Maton P, Breiter JR, Hwang C, Marino V, Hamelin B, Levine JG. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–665. doi: 10.1111/j.1572-0241.2001.3600_b.x. [DOI] [PubMed] [Google Scholar]
- 41.Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, Skammer W, Levine JG. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575–583. doi: 10.1111/j.1572-0241.2002.05532.x. [DOI] [PubMed] [Google Scholar]
- 42.Labenz J, Armstrong D, Lauritsen K, Katelaris P, Schmidt S, Schütze K, Wallner G, Juergens H, Preiksaitis H, Keeling N, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther. 2005;21:739–746. doi: 10.1111/j.1365-2036.2005.02368.x. [DOI] [PubMed] [Google Scholar]
- 43.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 44.Malfertheiner P, Lind T, Willich S, Vieth M, Jaspersen D, Labenz J, Meyer-Sabellek W, Junghard O, Stolte M. Prognostic influence of Barrett's oesophagus and Helicobacter pylori infection on healing of erosive gastro-oesophageal reflux disease (GORD) and symptom resolution in non-erosive GORD: report from the ProGORD study. Gut. 2005;54:746–751. doi: 10.1136/gut.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holtmann G, Cain C, Malfertheiner P. Gastric Helicobacter pylori infection accelerates healing of reflux esophagitis during treatment with the proton pump inhibitor pantoprazole. Gastroenterology. 1999;117:11–16. doi: 10.1016/s0016-5085(99)70544-5. [DOI] [PubMed] [Google Scholar]
- 46.Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther. 2001;15:1729–1736. doi: 10.1046/j.1365-2036.2001.01128.x. [DOI] [PubMed] [Google Scholar]
- 47.Vakil N, Fennerty MB. Direct comparative trials of the efficacy of proton pump inhibitors in the management of gastro-oesophageal reflux disease and peptic ulcer disease. Aliment Pharmacol Ther. 2003;18:559–568. doi: 10.1046/j.1365-2036.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 48.Richter JE, Kahrilas PJ, Sontag SJ, Kovacs TO, Huang B, Pencyla JL. Comparing lansoprazole and omeprazole in onset of heartburn relief: results of a randomized, controlled trial in erosive esophagitis patients. Am J Gastroenterol. 2001;96:3089–3098. doi: 10.1111/j.1572-0241.2001.05263.x. [DOI] [PubMed] [Google Scholar]
- 49.Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51 Suppl 1:59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 50.Kawamura M, Ohara S, Koike T, Iijima K, Suzuki J, Kayaba S, Noguchi K, Hamada S, Noguchi M, Shimosegawa T. The effects of lansoprazole on erosive reflux oesophagitis are influenced by CYP2C19 polymorphism. Aliment Pharmacol Ther. 2003;17:965–973. doi: 10.1046/j.1365-2036.2003.01539.x. [DOI] [PubMed] [Google Scholar]
- 51.Miner P, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–2620. doi: 10.1111/j.1572-0241.2003.08783.x. [DOI] [PubMed] [Google Scholar]
- 52.Röhss K, Wilder-Smith C, Nauclér E, Jansson L. Esomeprazole 20mg provides more effective intragastric Acid control than maintenance-dose rabeprazole, lansoprazole or pantoprazole in healthy volunteers. Clin Drug Investig. 2004;24:1–7. doi: 10.2165/00044011-200424010-00001. [DOI] [PubMed] [Google Scholar]
- 53.Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D´Amico D, Hamelin B, Joelsson B. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. Aliment Pharmacol Ther. 2000;14:1249–1258. doi: 10.1046/j.1365-2036.2000.00856.x. [DOI] [PubMed] [Google Scholar]
- 54.Fennerty MB, Johanson J, Hwang C, Hoyle P, Sostek M. Esomeprazole 40 mg versus lansoprazole 30 mg in healing and symptom relief in patients with moderate to severe erosive oesophagitis (Los Angeles C & D) Gut. 2004;53(Suppl 4):A111–112. [Google Scholar]
- 55.Labenz J, Armstrong D, Katelaris P, Schmidt S, Nauclér E, Eklund S. Analysis of healing associated with 4 weeks' esomperazole 40 mg treatment relative to lansoprazole 30 mg and pantoprazole 40 mg in patients with all grades of erosive esophagitis. Gut. 2004;53(Suppl 4):A105. [Google Scholar]
- 56.Gillessen A, Beil W, Modlin IM, Gatz G, Hole U. 40 mg pantoprazole and 40 mg esomeprazole are equivalent in the healing of esophageal lesions and relief from gastroesophageal reflux disease-related symptoms. J Clin Gastroenterol. 2004;38:332–340. doi: 10.1097/00004836-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Scholten T, Gatz G, Hole U. Once-daily pantoprazole 40 mg and esomeprazole 40 mg have equivalent overall efficacy in relieving GERD-related symptoms. Aliment Pharmacol Ther. 2003;18:587–594. doi: 10.1046/j.1365-2036.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- 58.Tinmouth JM, Steele LS, Tomlinson G, Glazier RH. Are claims of equivalency in digestive diseases trials supported by the evidence? Gastroenterology. 2004;126:1700–1710. doi: 10.1053/j.gastro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Fass R, Thomas S, Traxler B, Sostek M. Patient reported out-come of heartburn improvement: doubling the proton pump inhibitor (PPI) dose in patients who failed standard dose PPI versus switching to a different PPI. Gastroenterology. 2004;126:A37. [Google Scholar]
- 60.Hatlebakk JG, Katz PO, Kuo B, Castell DO. Nocturnal gastric acidity and acid breakthrough on different regimens of omeprazole 40 mg daily. Aliment Pharmacol Ther. 1998;12:1235–1240. doi: 10.1046/j.1365-2036.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 61.Ours TM, Fackler WK, Richter JE, Vaezi MF. Nocturnal acid breakthrough: clinical significance and correlation with esophageal acid exposure. Am J Gastroenterol. 2003;98:545–550. doi: 10.1111/j.1572-0241.2003.07304.x. [DOI] [PubMed] [Google Scholar]
- 62.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625–632. doi: 10.1053/gast.2002.31876. [DOI] [PubMed] [Google Scholar]
- 63.Vakil NB, Shaker R, Johnson DA, Kovacs T, Baerg RD, Hwang C, D'Amico D, Hamelin B. The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6-mo, randomized, double-blind, placebo-controlled study of efficacy and safety. Aliment Pharmacol Ther. 2001;15:927–935. doi: 10.1046/j.1365-2036.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 64.Johnson DA, Benjamin SB, Vakil NB, Goldstein JL, Lamet M, Whipple J, Damico D, Hamelin B. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol. 2001;96:27–34. doi: 10.1111/j.1572-0241.2001.03443.x. [DOI] [PubMed] [Google Scholar]
- 65.Johnsson F, Moum B, Vilien M, Grove O, Simren M, Thoring M. On-demand treatment in patients with oesophagitis and reflux symptoms: comparison of lansoprazole and omeprazole. Scand J Gastroenterol. 2002;37:642–647. doi: 10.1080/00365520212499. [DOI] [PubMed] [Google Scholar]
- 66.Sjöstedt S, Befrits R, Sylvan A, Carling L, Harthon C, Modin S, Stubberöd A, Toth E, Lind T. On demand versus continu-ous treatment with esomeprazole (ESO) 20 mg once daily in subjects with healed erosive esophagitis (EE) after initial heal-ing with ESO 40 mg once daily. An open, randomised, Swed-ish multicenter study. Gut. 2004;53(Suppl 4):A68. [Google Scholar]
- 67.Richter JE, Fraga P, Mack M, Sabesin SM, Bochenek W. Prevention of erosive oesophagitis relapse with pantoprazole. Aliment Pharmacol Ther. 2004;20:567–575. doi: 10.1111/j.1365-2036.2004.02121.x. [DOI] [PubMed] [Google Scholar]
- 68.Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, Di Mario F, Battaglia G, Mela GS, Pilotto A. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106–1110. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 69.You JH, Lee AC, Wong SC, Chan FK. Low-dose or standard-dose proton pump inhibitors for maintenance therapy of gastro-oesophageal reflux disease: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2003;17:785–792. doi: 10.1046/j.1365-2036.2003.01526.x. [DOI] [PubMed] [Google Scholar]
- 70.Inadomi JM, Jamal R, Murata GH, Hoffman RM, Lavezo LA, Vigil JM, Swanson KM, Sonnenberg A. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;121:1095–1100. doi: 10.1053/gast.2001.28649. [DOI] [PubMed] [Google Scholar]
- 71.Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol. 2003;98:1940–1944. doi: 10.1111/j.1572-0241.2003.07665.x. [DOI] [PubMed] [Google Scholar]
- 72.Klinkenberg-Knol EC, Nelis F, Dent J, Snel P, Mitchell B, Prichard P, Lloyd D, Havu N, Frame MH, Roman J, et al. Long-term omeprazole treatment in resistant gastroesoph-ageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 73.Lauritsen K, Devière J, Bigard MA, Bayerdörffer E, Mózsik G, Murray F, Kristjánsdóttir S, Savarino V, Vetvik K, De Freitas D, et al. Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed reflux oesophagitis: Metropole study results. Aliment Pharmacol Ther. 2003;17:333–341. doi: 10.1046/j.1365-2036.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 74.DeVault KR, Liu S, Hoyle P, Sostek M. Esomeprazole 20 mg versus lansoprazole 15 mg for maintenance of healing of ero-sive esophagitis. Am J Gastroenterol. 2004;99(Suppl):S6–S7. [Google Scholar]
- 75.Labenz J, Armstrong D, Katelaris P, Schmidt S, Adler J, Eklund S. A comparison of esomeprazole and pantoprazole for maintenance treatment of healed erosive esophagitis. Gut. 2004;53(Suppl 4):A108. [Google Scholar]
- 76.Labenz J, Armstrong D, Katelaris P, Schmidt S, Eklund S. The effect of Helicobacter pylori status on maintenance therapy for healed erosive esophagitis with esomeprazole 20 mg or pantoprazole 20 mg. Helicobacter. 2004;9:544. [Google Scholar]
- 77.Labenz J. Protagonist: Should we eradicate Helicobacter pylori before long term antireflux therapy? Gut. 2001;49:614–616. doi: 10.1136/gut.49.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freston JW. Antagonist: Should we eradicate Helicobacter pylori before long term antireflux therapy? Gut. 2001;49:616–617. doi: 10.1136/gut.49.5.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moayyedi P, Bardhan C, Young L, Dixon MF, Brown L, Axon AT. Helicobacter pylori eradication does not exacerbate reflux symptoms in gastroesophageal reflux disease. Gastroenterology. 2001;121:1120–1126. doi: 10.1053/gast.2001.29332. [DOI] [PubMed] [Google Scholar]
- 80.Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 81.Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Liedman B, Hatlebakk JG, Julkonen R, Levander K, Carlsson J, Lamm M, et al. Continued (5-year) followup of a randomized clinical study comparing antireflux surgery and omeprazole in gastroesophageal reflux disease. J Am Coll Surg. 2001;192:172–179; discussion 172-179;. doi: 10.1016/s1072-7515(00)00797-3. [DOI] [PubMed] [Google Scholar]
- 82.Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Thor K, Lamm M, Blomqvist A, Hatlebakk JG, Janatuinen E, Levander K, et al. Long-term management of gastro-oesophageal reflux disease with omeprazole or open antireflux surgery: results of a prospective, randomized clinical trial. The Nordic GORD Study Group. Eur J Gastroenterol Hepatol. 2000;12:879–887. doi: 10.1097/00042737-200012080-00007. [DOI] [PubMed] [Google Scholar]
- 83.Arguedas MR, Heudebert GR, Klapow JC, Centor RM, Eloubeidi MA, Wilcox CM, Spechler SJ. Re-examination of the cost-effectiveness of surgical versus medical therapy in patients with gastroesophageal reflux disease: the value of long-term data collection. Am J Gastroenterol. 2004;99:1023–1028. doi: 10.1111/j.1572-0241.2004.30891.x. [DOI] [PubMed] [Google Scholar]
- 84.Myrvold HE, Lundell L, Miettinen P, Pedersen SA, Liedman B, Hatlebakk J, Julkunen R, Levander K, Lamm M, Mattson C, et al. The cost of long term therapy for gastro-oesophageal reflux disease: a randomised trial comparing omeprazole and open antireflux surgery. Gut. 2001;49:488–494. doi: 10.1136/gut.49.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freston JW, Triadafilopoulos G. Review article: approaches to the long-term management of adults with GERD-proton pump inhibitor therapy, laparoscopic fundoplication or endoscopic therapy? Aliment Pharmacol Ther. 2004;19 Suppl 1:35–42. doi: 10.1111/j.0953-0673.2004.01837.x. [DOI] [PubMed] [Google Scholar]
- 86.Arts J, Tack J, Galmiche JP. Endoscopic antireflux procedures. Gut. 2004;53:1207–1214. doi: 10.1136/gut.2003.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corley DA, Katz P, Wo J, Stefan A, Patti M, Rothstein R, Edmundowicz S, Kline M, Mason R, Wolfe MM. Improve-ment of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlled trial. Gastroenterology. 2003;125:668–676. doi: 10.1016/s0016-5085(03)01052-7. [DOI] [PubMed] [Google Scholar]
- 88.Dent J, Talley NJ. Overview: initial and long-term management of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17 Suppl 1:53–57. doi: 10.1046/j.1365-2036.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 89.Howden CW, Henning JM, Huang B, Lukasik N, Freston JW. Management of heartburn in a large, randomized, community-based study: comparison of four therapeutic strategies. Am J Gastroenterol. 2001;96:1704–1710. doi: 10.1111/j.1572-0241.2001.03861.x. [DOI] [PubMed] [Google Scholar]
- 90.Armstrong D, Barkun AN, Chiba N, Veldhuyzen van Zanten S, Thomson ABR, Smyth S, Chakraborty B, Sinclair P. ‘Start high’- A better acid suppression strategy for heartburn-dominant uninvestigated dyspepsia (DU) in primary care practice (PCP) – the CADET-HR study. Gastroenterology. 2002;122:A472. [Google Scholar]
- 91.Meineche-Schmidt V, Juhl HH, Østergaard JE, Luckow A, Hvenegaard A. Costs and efficacy of three different esomeprazole treatment strategies for long-term management of gastro-oesophageal reflux symptoms in primary care. Aliment Pharmacol Ther. 2004;19:907–915. doi: 10.1111/j.1365-2036.2004.01916.x. [DOI] [PubMed] [Google Scholar]


