Abstract
AIM: To investigate the possible mechanism for HBV X gene to induce apoptosis of hepatocyte HL-7702 cells.
METHODS: HBV X gene eukaryon expression vector pcDNA3-X was established and transfected into HL-7702 cells by lipid-mediated transfection, including transient and stable transfection. Positive clones were screened by incubating in the selective medium with 600 mg/mL G418 and named HL-7702/HBV-encoded X protein (HBx) cells. The expressions of Fas/FasL, Bax/Bcl-2, and c-myc mRNA were measured by semi-quantitative RT-PCR in HL-7702/HBx and control group, respectively.
RESULTS: RT-PCR analysis confirmed that HBV X gene was transfected into HL-7702 cells successfully. By semi-quantitative RT-PCR analysis, Bax and c-myc mRNA levels in HL-7702/HBx cells of transient transfection were significantly higher than those in control, FasL and c-myc mRNA levels in HL-7702/HBx cells of stable transfection were significantly higher than those in control, whereas the Bcl-2 mRNA levels in HL-7702/HBx cells of transient and stable transfection were significantly lower than those in control.
CONCLUSION: HBV X gene may promote the apoptosis of hepatocytes by regulating the expressions of Fas/FasL, Bax/Bcl-2, and c-myc gene in a dose-dependent manner.
Keywords: Hepatitis B virus, X gene, Apoptosis, Gene expression
INTRODUCTION
HBV X gene is the smallest open reading frame of HBV, codes for a 16.5-ku protein (X protein, HBx) consisted of 154 amino acids. Previous studies showed that HBV X gene and HBx modulate apoptosis of hepatocytes and play an important role in HBV-associated liver disease[1]. HBV X gene could inhibit apoptosis of hepatocytes in several ways and contribute to the generation of hepatocellular carcinoma (HCC). It was reported that HBx displays a pro-apoptotic function and induces apoptosis of liver cells[2-5]. But the relationship between this function of HBx and HBV-associated liver disease remains obscure. So far no molecular mechanism or target for HBx-mediated apoptosis has been clearly elucidated. In this study, we transfected X gene into hepatocyte line HL-7702 by transient and stable transfection. Thus, cell lines that expressed different levels of HBx were established. To explore the possible mechanism for HBx to modulate apoptosis, we investigated the effect of HBx on expressions of apoptosis-associated genes in hepatocytes. The expressions of Fas/FasL, Bax/Bcl-2, and c-myc mRNA were measured by semi-quantitative RT-PCR in HL-7702/HBx and control group, respectively.
MATERIALS AND METHODS
Materials
PcDNA3 expression vector and HBV X gene eukaryon expression vector pcDNA3-X were previously constructed and stored[6]. The normal hepatic cell line HL-7702 was provided by Cell Bank of Chinese Academy of Sciences. Reverse transcription system, DNA purification system, G418, and TransFastTM transfection reagent were obtained from Promega Biotech. Total RNA isolation kit was purchased from Jingmei Biotech Company. PCR primers were synthesized by Shanghai Biotechnology Company. RPMI-1640 was bought from Gibco BRL Company.
Transfection and expression of HBV X gene in HL-7702
Cell culture and DNA transfection HL-7702 cells were cultured in RPMI-1640 supplemented with 20% FBS. The cells in logarithmic growth were separately transfected with pcDNA3-X and pcDNA3 plasmids using lipid-mediated transfection technique according to the protocol for transient or stable transfection of adherent cells. About 2 mL transfection mixture containing 5 mg plasmid DNA, 15 mL TransFastTM reagent and RPMI-1640 was added to a 25-mm2 culture plate. At 48 h post-transfection, all cells were trypsinized. Cells for transient transfection were transferred to a 25-mm2 culture plate and cultured in RPMI-1640 supplemented with 20% FBS for 72 h. Cells used for stable transfection were cultured in the selective medium with 600 mg/mL G418 for 2 wk. Then, drug-resistant individual clones were isolated and transferred to a 96-well plate for further amplification in the presence of selective medium. Cells that transiently transfected with pcDNA3-X and pcDNA3 were named as HL-7702/HBxT and HL-7702/pcDNA3T, respectively. Cells that stably transfected with pcDNA3-X and pcDNA3 were named as HL-7702/HBxS and HL-7702/pcDNA3S, respectively. HL-7702 was used as control.
Determination of X gene expression by RT-PCR analysis Total RNA was extracted separately from the cells of each group with a RNA isolation kit, and RT-PCR was carried out. b-Actin served as an internal control, 2 mL of RT product was used as template, X gene and b-actin were amplified together. The sequence of b-actin primers was 5-'GGCATCGTGATGGACTCCG-3' and 5'-GCTGGAAGGTGGACAGCGA-3'. The sequence of X gene primers was: 5'-ATGCAAGCTTATGGCTGCT-AGGCTGTACTG-3' and 5'-TGCGAATTCTTAGGCA-GAGGTGAAAAAGTTG-3'. The expected amplification fragment length of b-actin and X gene was 607 and 467 bp, respectively. PCR was carried out as follows: pre-denaturation at 95 °C for 5 min, 32 amplification cycles (denaturation at 94 °C for 35 s, annealing at 65 °C for 35 s, and extension at 72 °C for 1 min), and a final extension at 72 °C for 7 min.
Effect of HBV X gene on apoptosis-associated gene mRNA expression in hepatocytes by transient and stable transfection
RT-PCR for Fas/FasL, Bax/Bcl-2, and c-myc Total RNA was extracted from HL-7702/HBx, HL-7702/PcDNA3, and HL-7702 according to the RNA isolation kit instructions. The content and purity of total RNA were determined by spectrophotography. RNA (260/280 was between 1.8 and 2.0) was further used for reverse transcription reaction, which was carried out according to the reverse transcription kit instructions. Two microgrammes of total RNA was used in each reverse transcription reaction and the final volume was 20 mL. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control and amplified together with target genes. PCR was performed in 50 mL reaction volume containing 5 mL 10×PCR buffer, 5 mL 2 mmol/L MgCl2, 1 mL 10 mmol/L dNTP, 1 mL 20 pmol/mL target gene sense and anti-sense primers, 0.4 mL 12.5 pmol/mL GAPDH primer pair, 2 mL RT product, 1.5 U Taq DNA polymerase. The specific sets of primers and the target gene amplification conditions are shown in Table 1. All initial denaturations were at 94 °C for 5 min. Finally an additional extension step at 72 °Cfor 7 min was done.
Table 1.
Primer sequences for PCR and amplification conditions for each target gene
| Primer | Sequence | Amplification conditions | Product (base) |
| Fas | 5-TCAGTACGGAGTTGGGGAAG-3’ | Denaturation at 94 °C for 45 s, | 207 |
| 5-CAGGCCTTCCAAGTTCTGAG-3 | annealing at 63 °C for 30 s and synthesizing | ||
| at 72 °C for 1 min for 35 cycles | |||
| FasL | 5-GATGATGGAGGGGAAGATGA-3 | Denaturation at 94 °C for 45 s, | 203 |
| 5-TGGAAAGAATCCCAAAGTGC-3’ | annealing at 58 °C for 30 s and synthesizing | ||
| at 72 °C for 1 min for 30 cycles | |||
| Bax | 5-TTTGCTTCAGGGTTTCATCC-3’ | Denaturation at 94 °C for 45 s, | 246 |
| 5-CAGTTGAAGTTGCCGTCAGA-3’ | annealing at 58 °C for 30 s and synthesizing | ||
| at 72 °C for 1 min for 30 cycles | |||
| Bcl-2 | 5-CGACGACTTCTCCCGCCGCTACCGC-3’ | Denaturation at 94 °C for 45 s, | 318 |
| 5-CCGCATGCTGGGGCCGTACAGTTCC-3’ | annealing at 67 °C for 30 s and synthesizing | ||
| at 72 °C for 1 min for 30 cycles | |||
| c-myc | 5-TTCGGGTAGTGGAAAACCAG-3’ | Denaturation at 94 °C for 45 s, | 203 |
| 5-CAGCAGCTCGAATTTCTTCC-3’ | annealing at 58 °C for 30 s and synthesizing | ||
| at 72 °C for 1 min for 28 cycles | |||
| GAPDH | 5-ACCACAGTCCATGCCATCAC-3’ | Changed according to different target genes | 452 |
| 5-TCCACCACCCTGTTGCTGTA-3’ |
Result determination The PCR products were electrop-horesed on 2% agarose gel and visualized by ethidium bromide staining. Bioimaging system was used to detect the densities of bands of the PCR products. The ratio of target gene density to GAPDH density was respectively used to represent the relative expression level of Fas/FasL, Bax/Bcl-2, and c-myc mRNA. The semi-quantitative detection was analyzed five times.
Statistical analysis
All data were expressed as mean±SE. The significance for the difference between the groups was assessed with SPSS 10.0 by one-way ANOVA. P<0.05 was considered statistically significant.
RESULTS
Expression of HBV X gene mRNA in HL-7702 cells
HBV X gene mRNA was detected in HL-7702/HBxT and HL-7702/HBxS cells by RT-PCR. The expected band was found between 400 and 500 bp in both kinds of transfected cells (Figure 1). The relative level of HBV X gene mRNA in HL-7702/HBxT was higher than that in HL-7702/HBxS by about 2.5-folds (0.815±0.013 vs 0.308±0.021), indicating that HBV X gene mRNA could be expressed in HL-7702 after transient or stable transfection, and cell lines that expressed different levels of HBx were established.
Figure 1.
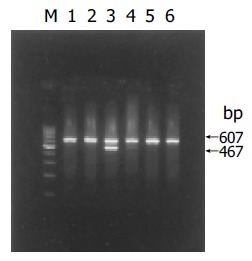
(PDF) RT-PCR analysis of HBV X gene mRNA expression in HL-7702. M: 100-bp DNA ladder; lane 1: HL-7702T; lane 2: HL-7702/pcDNA3T; lane 3: HL-7702/HBxT; lane 4: HL-7702/HBxS; lane 5: HL-7702/pcDNA3S; lane 6: HL-7702S.
Effects of HBV X gene on apoptosis-associated genes mRNA expression in HL-7702 by transient transfection
Fas/FasL mRNA As shown in Figures 2A, B, and 3A, both Fas mRNA and FasL mRNA were expressed in HL-7702 of each group. Both of their expression levels had no significant differences among the three groups, indicating that HBx had no effect on Fas and FasL expression in normal hepatocytes though it was expressed at a relatively high level after transient transfection.
Figure 2.
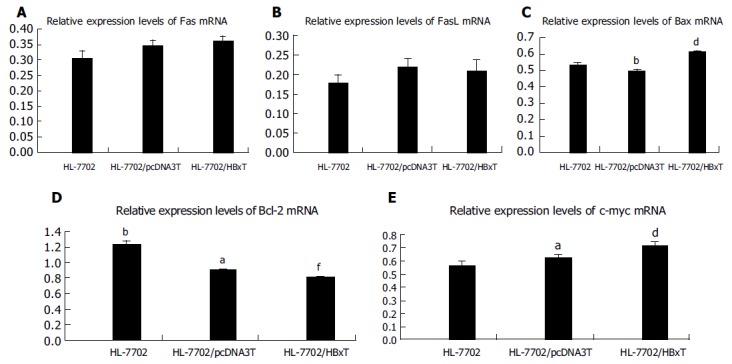
(PDF) Relative expression levels of apoptosis-associated gene mRNA in HL-7702 of different transiently transfected groups assessed by RT-PCR. A: Relative Fas mRNA expression levels; B: relative FasL mRNA expression levels; C: relative Bax mRNA expression levels; D: relative Bcl-2 mRNA expression levels; E: relative c-myc mRNA expression levels. aP<0.05 vs HL-7702/HBxT, bP<0.01 vs HL-7702/HBxT, dP<0.01 vs HL-7702, fP<0.01 vs HL-7702/pcDNA3T, P>0.05 between random two groups.
Bcl-2/Bax mRNA Both Bax and Bcl-2 mRNA were expressed in HL-7702 after transient transfection (Figures 2C, D, and 3B). As it could be seen from Figures 2C, 3B, and C, the expression level of Bax mRNA in HL-7702/HBxT was higher than that in HL-7702 or in HL-7702/pcDNA3T (0.6140.014 vs 0.5360.009 or 0.4940.015, P<0.01). The expression level of Bcl-2 mRNA in HL-7702/HBxT was lower than that in HL-7702 or in HL-7702/pcDNA3T (0.811±0.010 vs 1.243±0.033, P<0.01) (0.811±0.010 vs0.901±0.014, P<0.05). There was also a significant difference in Bcl-2 mRNA expression level between the two control groups (0.901±0.014 vs 1.243±0.033, P<0.01; Figures 2D, 3B, and D). The data indicated that HBx could induce the pro-apoptotic factor Bax expression in normal hepatocytes and inhibit the anti-apoptotic factor Bcl-2 expression.
c-myc mRNA c-myc mRNA was expressed in both HL-7702 and HL-7702/pcDNA3, and there was no significant difference between them. The expression level of c-myc mRNA was significantly higher in HL-7702/HBxT, suggesting that HBx was able to promote the c-myc expression in normal hepatocytes (0.719±0.020 vs 0.569±0.026, P<0.01, 0.719±0.020 vs 0.630±0.012,P<0.05; Figures 2E and 3C).
Figure 3.
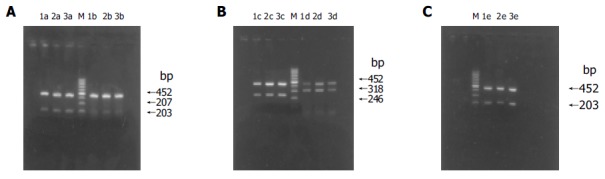
(PDF) RT-PCR results of apoptosis-associated gene mRNA expression in HL-7702 of different transiently transfected groups. A: RT-PCR results of Fas/FasL mRNA expression; B: RT-PCR results of Bax/Bcl-2 mRNA expression; C: RT-PCR results of c-myc mRNA expression; M: 100-bp DNA ladder; lane 1: HL-7702; lane 2: HL-7702/pcDNA3T; lane 3: HL-7702/HBxT; a: Fas; b: FasL; c: Bax; d: Bcl-2; e: c-myc.
Effects of HBV X gene on apoptosis-associated gene mRNA expression in HL-7702 by stable transfection
Fas/FasL mRNA The expression levels of Fas mRNA had no significant difference among the three groups (Figures 4A and 5A). There was no significant difference in FasL mRNA expression between HL-7702 and HBV X gene expressing stable cell line HL-7702/HBxS. The expression of FasL in HL-7702/HBxS was significantly higher than that in HL-7702/pcDNA3S (0.261±0.043vs 0.217±0.012, P<0.01), suggesting that HBx at a low level could improve the FasL expression, which was different from the effect of HBx at a relatively higher level as shown in the former part (Figures 4B, 5A, and B).
Figure 4.
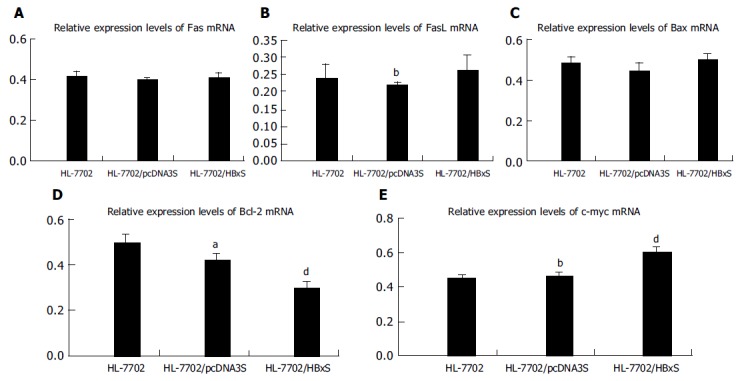
(PDF) Relative expression levels of apoptosis-associated gene mRNA in HL-7702 of different stably transfected groups assessed by RT-PCR. A: Relative Fas mRNA expression levels; B: relative FasL mRNA expression levels; C: relative Bax mRNA expression levels; D: relative Bcl-2 mRNA expression levels; E: relative c-myc mRNA expression levels. aP<0.05 vs HL-7702/HBxS; bP<0.01 vs HL-7702/HBxS, dP<0.01 vs HL-7702, P>0.05 between random two groups.
Figure 5.
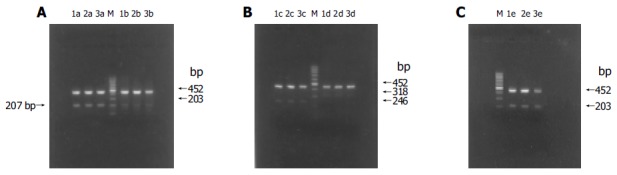
(PDF) RT-PCR results of apoptosis-associated gene mRNA expression in HL-7702 of different stably transfected groups. A: RT-PCR results of Fas/FasL mRNA expression; B: RT-PCR results of Bax/Bcl-2 mRNA expression; C: RT-PCR results of c-myc mRNA expression; M: 100-bp DNA ladder; lane 1: HL-7702; lane 2: HL-7702/pcDNA3S; lane 3: HL-7702/HBxS; a: Fas; b: FasL; c: Bax; d: Bcl-2; e: c-myc.
Bax/Bcl-2 mRNA HBV X gene stably expressed in HL-7702/HBxS seemed to have no effect on Bax mRNA expression (Figures 4C, 5B, and C). The expression level of Bcl-2 mRNA had no significant difference between the two control groups, HL-7702 and HL-7702/pcDNA3S, but it was lower in HL-7702/HBxS (0.300±0.028 vs 0.498±0.035, P<0.01; 0.300±0.028 vs 0.420±0.032,P<0.05; Figures 4D, 5B, and D), suggesting that HBx could downregulate Bcl-2 expression in normal hepatocytes.
c-myc mRNA Similar to the effect of HBV X gene transient transfection, c-myc was expressed in both HL-7702 and HL-7702/pcDNA3S, and there was no significant difference between them. The expression level of c-myc mRNA was significantly higher in HL-7702/HBxS, suggesting that even a low level of HBx was able to upregulate the c-myc expression in normal hepatocytes (0.603±0.035 vs 0.449±0.023 or 0.461±0.022, P<0.01; Figures 4E and 5C).
DISCUSSION
HBV infection is a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma and accounts for one million deaths annually. HBx is a multifunctional protein that is implicated in the pathogenesis of HBV-associated liver disease by regulating gene transcription, causing cell proliferation, suppressing DNA repair and inducing cell death[7]. The role of HBx in carcinogenesis and its transactivation function have been well documented[8]. The role of HBx in liver cell proliferation and apoptosis is still controversial. A number of studies have revealed that HBx exert dual activity on cell apoptosis[9,10].
On the one hand, HBx has an anti-apoptosis function in different ways and plays an important role in the development of HCC. HBx can activate and upregulate transcription factor NF-kB[11]. It induces Fas-ligand in human hepatoma cells, which contributed to the apoptosis of T cells, thus the hepatoma cells might escape from immune surveillance[12]. There is evidence that HBx blocks apoptosis via downregulating expression of Bid in human hepatocellular carcinoma[13]. The distal C-terminal domain of HBx, independent of its transactivation activity, is complexed with p53 in the cytoplasm and partially prevents its nuclear entry and ability to induce apoptosis[14]. On the other hand, HBx induces or sensitizes cells to apoptotic killing by pro-apoptotic stimuli[2-5]. The possible mechanisms may include upregulating the expression of Fas and Fas-ligand in hepatocytes[15,16], inducing cell death by causing loss of mitochondrial membrane potential and mitochondrial aggregation at the nuclear periphery[17,18], antagonizing the anti-apoptosis function of the normal level of p53[19], abrogating the apoptosis-inhibitory function of c-FLIP and enhancing the death-inducing signal[20], inducing TNF-a expression and sensitizing liver cells to TNF-a-mediated apoptosis even below the threshold concentration[20-22], affecting the apoptosis control function of Bcl-2 family members by interaction with mitochondria[23]. The above data suggest that the possible mechanism for HBx to regulate cell apoptosis is a "network" implicated with multiple factors and various pathways, and the exact mechanism remains obscure.
Fas and FasL are regarded as the most important factors that switch on the Fas/FasL-mediated apoptosis. The Bcl-2 family proteins play an important role in modulating cell survival and apoptosis. Bcl-2 and Bax act as an anti-apoptotic factor and a pro-apoptotic factor respectively. The proportion of apoptosis-inducing forces and apoptosis-inhibiting forces determines the cells response to death-inducing signals and their final fate[24-26]. C-myc, usually acting as a proto-oncogene and exerting a dual activity on cell proliferation and apoptosis, is also involved in apoptosis regulation. We have previously demonstrated that the apoptosis rate in HBx-expressing cells by transient or stable transfection is much higher than that in control groups, suggesting that there is a quantity-effect relationship between HBx expression and apoptosis rate, and that HBx can induce apoptosis in normal hepatocytes, during which the expression level of HBV X gene may play a vital role. This study aimed to investigate the possible mechanism for HBV X gene to induce apoptosis of hepatocytes. Thereby, we established cell models that expressed different levels of HBV X gene by transfecting HBV X gene into HL-7702 through transient and stable transfection, and studied the effect of HBx on the mRNA expression of apoptosis-associated genes.
We found that HBx at a relatively higher level could induce the pro-apoptotic factor Bax expression in normal hepatocytes and inhibit the anti-apoptotic factor Bcl-2 expression, though it had no effect on Fas and FasL expression. However, HBx at a low level could improve FasL expression, while it had no effect on Bax. The data show that HBx may induce the apoptosis of HL-7702 by reinforcing the death signal of Fas-FasL pathway and increasing the proportion of pro-apoptotic forces and anti-apoptotic forces in Bcl-2 family members. The dose of HBx may decide which is the chief pathway. The c-myc expression in HL-7702 significantly increased after transient or stable transfection with X gene expression vector, which is consistent with previous reports[9,27]. C-myc protein could sensitize cells to apoptotic killing in certain conditions, such as during exposure to TNF-a or other factors[28-30]. Thus, c-myc may also act as an important medium in HBx-mediated apoptosis. Su et al[30] found that the combination of HBx and c-myc increases the sensitivity of WT 3T3 cells to TNF-a killing by about 10-fold. They also demonstrated that cell killing by HBx plus TNF-a is suppressed by activation of NF-kB, but can be overridden by a high level of c-myc protein.
Our results show that different levels of HBV X gene expression have different effects on apoptosis-associated gene expression. Wang et al[31] studied the effects of regulatory endoplasmic HBx expression on hepatic cell apoptosis and found that low HBx expression cannot induce apoptosis. Thereby, the effect of HBx on apoptosis may be dose dependent. We hypothesize that there may be a threshold concentration for HBx to induce apoptosis, only that beyond the threshold can exert a pro-apoptosis function. Further studies are needed to confirm it.
In conclusion, HBx may induce apoptosis in part via regulating the expression of apoptosis-associated genes. HBV X gene expression may play a vital role, although the underlying mechanism may be complicated and crisscrossing.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Supported by the Science and Technology Fund of Fujian Province, No. 99-Z-162
References
- 1.Birrer RB, Birrer D, Klavins JV. Hepatocellular carcinoma and hepatitis virus. Ann Clin Lab Sci. 2003;33:39–54. [PubMed] [Google Scholar]
- 2.Terradillos O, Pollicino T, Lecoeur H, Tripodi M, Gougeon ML, Tiollais P, Buendia MA. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115–2123. doi: 10.1038/sj.onc.1202432. [DOI] [PubMed] [Google Scholar]
- 3.Pollicino T, Terradillos O, Lecoeur H, Gougeon ML, Buendia MA. Pro-apoptotic effect of the hepatitis B virus X gene. Biomed Pharmacother. 1998;52:363–368. doi: 10.1016/s0753-3322(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 4.Chirillo P, Pagano S, Natoli G, Puri PL, Burgio VL, Balsano C, Levrero M. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc Natl Acad Sci USA. 1997;94:8162–8167. doi: 10.1073/pnas.94.15.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shintani Y, Yotsuyanagi H, Moriya K, Fujie H, Tsutsumi T, Kanegae Y, Kimura S, Saito I, Koike K. Induction of apoptosis after switch-on of the hepatitis B virus X gene mediated by the Cre/loxP recombination system. J Gen Virol. 1999;80(Pt 12):3257–3265. doi: 10.1099/0022-1317-80-12-3257. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Tang NH, Zhang SJ, Chen ZX, Wang XZ. Construction of hepatitis B virus X gene expression vector in eucaryotic cells and its transfection in HL-7702 cells. Shijie Huaren Xiaohua Zazhi. 2004;12:614–617. [Google Scholar]
- 7.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 8.Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, Kim DK, Yu DY, Seong JK, Lee MO. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003;278:39076–39084. doi: 10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- 9.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Tarn C, Wang WH, Chen S, Hullinger RL, Andrisani OM. Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus nontransforming hepatocyte (AML12) cell lines. J Biol Chem. 2002;277:8730–8740. doi: 10.1074/jbc.M108025200. [DOI] [PubMed] [Google Scholar]
- 11.Su F, Schneider RJ. Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol. 1996;70:4558–4566. doi: 10.1128/jvi.70.7.4558-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin EC, Shin JS, Park JH, Kim H, Kim SJ. Expression of fas ligand in human hepatoma cell lines: role of hepatitis-B virus X (HBX) in induction of Fas ligand. Int J Cancer. 1999;82:587–591. doi: 10.1002/(sici)1097-0215(19990812)82:4<587::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen GG, Lai PB, Chan PK, Chak EC, Yip JH, Ho RL, Leung BC, Lau WY. Decreased expression of Bid in human hepatocellular carcinoma is related to hepatitis B virus X protein. Eur J Cancer. 2001;37:1695–1702. doi: 10.1016/s0959-8049(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 14.Elmore LW, Hancock AR, Chang SF, Wang XW, Chang S, Callahan CP, Geller DA, Will H, Harris CC. Hepatitis B virus X protein and p53 tumor suppressor interactions in the modulation of apoptosis. Proc Natl Acad Sci USA. 1997;94:14707–14712. doi: 10.1073/pnas.94.26.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo YG, Lee MO. Hepatitis B virus X protein induces expression of Fas ligand gene through enhancing transcriptional activity of early growth response factor. J Biol Chem. 2004;279:36242–36249. doi: 10.1074/jbc.M401290200. [DOI] [PubMed] [Google Scholar]
- 16.Sejima T, Miyagawa I. The evaluation of Fas/Fas ligand system in renal cell carcinoma--the effect of preoperative interferon-alpha therapy. Nihon Hinyokika Gakkai Zasshi. 1999;90:826–832. doi: 10.5980/jpnjurol1989.90.826. [DOI] [PubMed] [Google Scholar]
- 17.Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071–22078. doi: 10.1074/jbc.M301606200. [DOI] [PubMed] [Google Scholar]
- 18.Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965–6973. doi: 10.1038/sj.onc.1203188. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Lee H, Yun Y. X-gene product of hepatitis B virus induces apoptosis in liver cells. J Biol Chem. 1998;273:381–385. doi: 10.1074/jbc.273.1.381. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Seong BL. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara-Pezzi E, Majano PL, Gómez-Gonzalo M, García-Monzón C, Moreno-Otero R, Levrero M, López-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor alpha gene expression in hepatocytes. Hepatology. 1998;28:1013–1021. doi: 10.1002/hep.510280416. [DOI] [PubMed] [Google Scholar]
- 22.Yi YS, Park SG, Byeon SM, Kwon YG, Jung G. Hepatitis B virus X protein induces TNF-alpha expression via down-regulation of selenoprotein P in human hepatoma cell line, HepG2. Biochim Biophys Acta. 2003;1638:249–256. doi: 10.1016/s0925-4439(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 23.Terradillos O, de La Coste A, Pollicino T, Neuveut C, Sitterlin D, Lecoeur H, Gougeon ML, Kahn A, Buendia MA. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene. 2002;21:377–386. doi: 10.1038/sj.onc.1205110. [DOI] [PubMed] [Google Scholar]
- 24.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 25.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 26.Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 27.Balsano C, Avantaggiati ML, Natoli G, De Marzio E, Will H, Perricaudet M, Levrero M. Full-length and truncated versions of the hepatitis B virus (HBV) X protein (pX) transactivate the cmyc protooncogene at the transcriptional level. Biochem Biophys Res Commun. 1991;176:985–992. doi: 10.1016/0006-291x(91)90379-l. [DOI] [PubMed] [Google Scholar]
- 28.Juin P, Hueber AO, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juin P, Hunt A, Littlewood T, Griffiths B, Swigart LB, Korsmeyer S, Evan G. c-Myc functionally cooperates with Bax to induce apoptosis. Mol Cell Biol. 2002;22:6158–6169. doi: 10.1128/MCB.22.17.6158-6169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su F, Theodosis CN, Schneider RJ. Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein. J Virol. 2001;75:215–225. doi: 10.1128/JVI.75.1.215-225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HP, Chen XP, He SQ, Ding L. Effects of regulatory endoplasmic HBX expression on hepatic cell apoptosis. Zhonghua GanZangBing ZaZhi. 2003;11:440. [PubMed] [Google Scholar]


