Abstract
AIM: To investigate the relationship between Helicobacter pylori (H pylori) infection, microsatellite instability and the expressions of the p53 in gastritis, intestinal metaplasia and gastric adenocarcinoma and to elucidate the mechanism of gastric carcinogenesis relating to H pylori infection.
METHODS: One hundred and eight endoscopic biopsies and gastric adenocarcinoma were available for the study including 33 cases of normal, 45 cases of gastritis, 30 cases of intestinal metaplasia, and 46 cases of gastric adenocarcinoma. Peripheral blood samples of these patients were also collected. H pylori infection and p53 expressions were detected by means of streptavidin-peroxidase (SP) immunohistochemical method. Microsatellite loci were studied by PCR-SSCP-CE using the markers BAT-26, D17S261, D3S1283, D2S123, and D3S1611. MSI was defined as the peak shift in the DNA of the gastric tissue compared with that of the peripheral blood samples. Based on the number of mutated MSI markers, specimens were charac-terized as high MSI (MSI-H) if they manifested instability at two or more markers, low MSI (MSI-L) if unstable at only one marker, and microsatellite stable (MSS) if they showed no instability at any marker.
RESULTS: H pylori infection was detected in the samples of gastritis, intestinal metaplasia, and gastric adenocarcinoma and the infection frequencies were 84.4%, 76.7%, and 65.2%, respectively, whereas no H pylori infection was detected in the samples of normal control. There was a significant difference in the infection rates between gastritis and carcinoma samples (P = 0.035). No MSI was detected in gastritis samples, one MSI-H and two MSI-L were detected among the 30 intestinal metaplasia samples, and 12 MSI-H and 3 MSI-L were detected in the 46 gastric carcinomas. In those gastric carcinomas, the MSI-H frequency in H pylori-positive group was significantly higher than that in H pylori-negative group. No p53 expression was detected in the normal and gastritis samples from dyspeptic patients. P53-positive immunohistochemical staining was detected in 13.3% of intestinal metaplasia samples and in 43.5% of gastric carcinoma samples. The levels of p53 in H pylori-positive samples were higher than those in the negative group when the carcinoma samples were subdivided into H pylori-positive and -negative groups (P = 0.013). Eight samples were detected with positive p53 expression out of the 11 MSI-H carcinomas with H pylori infection and no p53 expression could be seen in the H pylori-negative samples.
CONCLUSION: H pylori affect the p53 pattern in gastric mucosa when MMR system fails to work. Mutations of the p53 gene seem to be an early event in gastric carcinogenesis.
Keywords: Dyspepsia, H pylori, Gastric cancer, MSI, p53
INTRODUCTION
Gastric cancer is one of the most common forms of malignant tumors in adults and is the leading cause of death from carcinomas in China. A close association between Helicobacter pylori (H pylori) and gastric cancer has been found[1,2], mainly on the basis of epidemiological data. Although H pylori has been classified as a type I carcinogen for gastric cancer by the International Agency for Research on Cancer (IARC), the exact pathway has remained indistinct[3,4]. It has been known that some gastric carcinomas are characterized by microsatellite instability resulting from defect of mismatch repair. Mismatch repair genes, as house keeping genes, have a central role in maintaining genomic stability by repairing DNA replication errors and inhibiting recombination between non-identical sequences. Loss of MMR genes causes destabilization of the genome and results in high mutation rates, which predisposes human to diverse cancers including gastric carcinoma[5-7]. The p53 protein is a transcriptional factor that arrests the cell cycle in the G1 phase when DNA is damaged by inducing the expression of the p21 protein, an inhibitor of Cdk kinase and PCNA[8,9]. Thus, damaged DNA cannot replicate, allowing time for the repair system to act[8]. If this system fails, p53 induces apoptosis by transacti-vation of the bax gene[10]. Both mismatch repair and suppressor are two main pathways involved in the tumorigenesis of gastric carcinoma. In this study, we examine microsatellite instability and p53 protein accumulation in patients with H pylori-infected gastric mucosa and in patients with gastric adenocarcinoma to elucidate whether any relationship exists between these genetic alterations and H pylori infection.
MATERIALS AND METHODS
Patients
One hundred and eight dyspeptic patients (65 men and 43 women; median age, 46 years; range, 20-73 years) undergoing upper endoscopy, and 46 consecutive patients (29 men and 17 women; median age, 56 years; range, 32-69 years) who underwent surgical excision for gastric adenocarcinoma at the hospitals of Dalian area entered the study. Peripheral blood samples of these patients were also collected. The ethical approval for this study was granted by the Local Research Ethics Committee. Endoscopic biopsies were removed with standard gastric biopsy forceps and then cut in half with sterile scalpel blades. Half the biopsy sample was fixed in 10% buffered neutral formalin and embedded in paraffin and serial sections (4-mm thick), while the other half was stored at -80 °C. Hematoxylin-eosin (HE) staining was used for the histopathological diagnosis. Among the 108 endoscopic biopsies, 33 samples were normal, 45 samples were gastritis and 30 samples were intestinal metaplasia.
DNA extraction
DNA was extracted from the frozen gastric tissues and peripheral leukocytes using a regular phenol-chloroform method and stored at -20 °C until use.
Microsatellite analysis
Microsatellite instability was studied using five markers (Table 1), PCR was performed in 12.5 mL of reaction mixture containing 1.5 mmol/L MgCl2, 200 mmol/L each dNTP, 0.5 Unit ampli Taq polymerase (TaKaRa Biotech., Dalian, China), 0.5 mmol/L of each primer, and 50 ng genomic DNA. The reaction was carried out in a thermal cycler (Perkin-Elmer Model 2700, CA, USA) at 94 °C for 30 s, 58-60 °C for 30 s, and 72 °C for 30 s, for 35 cycles with an initial denaturation step of 94 °C for 5 min and a final extension step of 72 °C for 5 min. 0.5 mL each PCR product was mixed with 1 mL GeneScan 500 size standard and 12 mL water, and heated at 95 °Cfor 10 min, then immediately put into ice water and kept for 5 min. Microsatellite was analyzed by an ABI PRISM 310 (Perkin-Elmer, ABI Prism) with 6% SLPA and 8 mol/L urea as sieving medium under constant voltage 15 kV at 60 °C. Single-stranded microsatellite fragments were detected by LIF and the data were collected and analyzed by GeneScan. MSI was defined as the peak shift in the DNA of the gastric tissue compared with that of the peripheral blood samples. Based on the number of mutated MSI markers, specimens were characterized as high MSI (MSI-H) if they manifested instability at two or more markers, low MSI (MSI-L) if unstable at only one marker, and microsatellite stable (MSS) if they showed no instability at any marker.
Table 1.
Primers of microsatellite markers
| Markers | Primers | Tm (°C) |
| BAT-26 | 5-FAM- TGACTACTTTTGACTTCAGCC | 58 |
| 5-AACCATTCAACATTTTTAACCC | ||
| D17S261 | 5-HEX-AGGGATACTATTCAGCCCGAGGTG | 60 |
| 5-ACTGCCACTCCTTGCCCCATTC | ||
| D3S1283 | 5-TET-GGCAGTACCACCTGTAGAAATG | 60 |
| 5-GAGTAACAGAGGCATCGTGTATTC | ||
| D2S123 | 5-FAM-AAACAGGATGCCTGCCTTTA | 60 |
| 5'-GGACTTTCCACCTATGGGAC | ||
| D3S1611 | 5-HEX-CCCCAAGGCTGCACTT | 60 |
| 5-AGCTGAGACTACAGGCATTTG |
Immunohistochemical staining
Immunostaining was performed using the streptavidin-peroxidase (SP) method as previously described by Lan et al Negative control sections were processed in the same manner, replacing the primary antibody with buffered saline. A total of 300 cells were counted in random fields from representative areas and the immunoreactive cells were assessed and expressed as percentages. Samples with p53 staining in more than 10% were considered positive (the nuclei, staining brown-yellow). However, the H pyloriimmunostaining was assessed positive as long as the brown-black dotish were stained on the surface of mucosa.
Statistical analysis
The c2 test and the Fisher's exact probability test were used to compute the frequencies by SPSS 12.0 for Windows. P<0.05 was considered to be statistically significant.
RESULTS
H pylori status
Sixty-one of the 108 (56.5%) dyspeptic patients and 30 of the 46 (65.2%) gastric cancer patients showed H pylori infection. None of the normal gastric mucosa was infected with H pylori. The infection rates of gastritis, intestinal metaplasia and tumor samples were largely more than the normal. c2 tests also revealed a significant difference in the infection rates between gastritis and carcinoma samples (P = 0.035, Table 2).
Table 2.
H pylori infection in the normal, gastritis, intestinal metaplasia, and tumor samples
| Tissue type | Number of samples | Infection number of H pylori | Infection rates (%) |
| Normal | 33 | 0 | 0 |
| Gastritis | 45 | 38 | 84.4 |
| Intestinal metaplasia | 30 | 23 | 76.7 |
| Carcinoma | 46 | 30 | 65.2 |
Microsatellite analysis
In the 33 normal and 45 gastritis samples, no microsatellite status shift was detected. One MSI-H and two MSI-L were detected among the 30 samples of intestinal metaplasia, whereas in the 46 gastric carcinomas, 12 MSI-H and 3 MSI-L were detected. The MSI status was significantly higher in H pylori positive samples of carcinomas than that in H pylori negative samples of carcinomas (Table 3 and Figure 1).
Table 3.
MSI frequency according to H pylori status
| MSI frequency | H pylori positive (30) | H pylori negative (16) | P1 |
| MSI-H (12) | 36.7% (11/30) | 6.3% (1/16) | 0.035 |
| MSI-L (3) | 6.7% (2/30) | 6.25 % (1/16) | 1.000 |
1H pylori positive vs H pylori negative.
Figure 1.
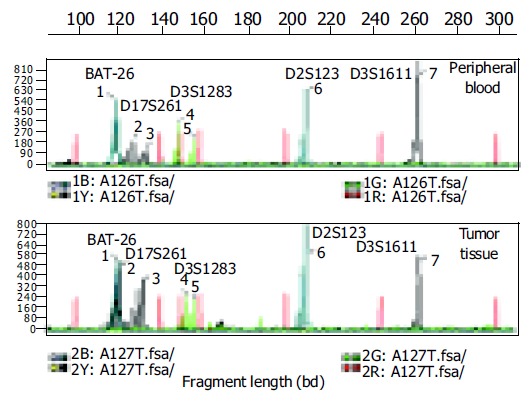
(PDF) Electropherograms of five microsatellite loci in the peripheral blood sample and tumor tissue of one gastric cancer patient. Red peaks: interval standard peaks.
p53 overexpression
No p53 expression was detected in the normal and gastritis samples from dyspeptic patients. Four of the 30 intestinal metaplasia samples showed p53-positive immunohistochemical staining, and in the 46 patients with gastric cancer, 20 (43%) p53-positive samples were identified. Eight manifested p53 positivity out of the 11 MSI-H carcinomas with H pylori infection and no p53expression could be seen in the H pylori-negative group. When the carcinoma samples were subdivided into H pylori-positive and -negative groups, immunohistochemical staining revealed that the levels of p53 in H pylori-positive samples were higher than those in the negative samples (P = 0.013, Tables 4 and 5, Figures 2A and B).
Table 4.
P53 expression with regard to H pylori status in carcinomas
| H pylori status | P53 positive (20) | P |
| H pylori positive (30) | 17 | 0.0131 |
| H pylori negative (16) | 3 |
1H pylori positive vs H pylori negative.
Table 5.
Length of representative fragments of five microsatellite loci in the normal tissue and peripheral blood sample of the gastric cancer patient
| BAT-26 |
D17S261 |
D3S1283 |
D2S123 | D3S1611 | |||
| Peak no. | 1# (bp) | 2# (bp) | 3# (bp) | 4# (bp) | 5# (bp) | 6# (bp) | 7# (bp) |
| Normal | 119.15 | 127.52 | 133.78 | 149.12 | 147.25 | 209.42 | 262.76 |
| Tumor | 120.30 | 121.63 | 131.45 | 143.23 | 147.36 | 209.30 | 262.85 |
Figure 2.
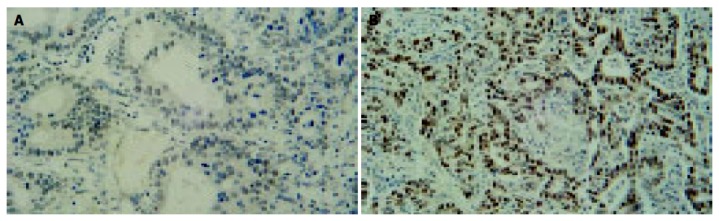
p53 in the normal and gastric carcinoma samples. A: p53-Negative staining in normal gastric glands from a dyspeptic patient, B: gastric carcinoma showing nuclear p53 immunoreactivity.
DISCUSSION
Both genetic and environmental factors are crucial in gastric cancer development and progression. H pylori infection has been documented as an important risk factor for gastric cancer[12]. It is fully agreed that the bacterium is effectively able to induce chronic mucosal injury with increased mucosal proliferation that could facilitate malignant transformation[13-15]. In this study, we detected a high infection rate of H pylori in both dyspeptic samples and gastric adenocarcinoma samples, which is consistent with the documents[16,17]. The reason why infection rate was significantly higher in gastritis samples than that in carcinoma samples is probable that H pylori density is lower in atrophic gastritis mucosa and very low in the intestinal metaplasia and in patients with gastric cancer, the degree of atrophic gastritis and intestinal metaplasia is higher than in patients without cancer. Another reason may be that different strains contribute differently to the occurrence of gastritis or gastric carcinoma, and it is not adequate to compare H pylori infection only by histology.
Epithelial cell proliferation is not carcinogenic in itself. It is likely that H pylori promote neoplastic transformation in combination with additional factors. In this study, we detected microsatellite instability and p53 protein expression in accordance with the development from gastric gastritis, intestinal metaplasia to gastric cancer with regard to H pylori infection status.
Microsatellites are short sequences of tandem repeats dispersed throughout the mammalian genome. Repeat units range from 1 to 4 bp in length, and the entire sequence of a typical repeat tract is less than 100 bp long[18,19]. Microsatellite instability is characterized by the insertion or deletion of one or more repeat units, which is caused by a failure of the DNA-MMR system to repair errors that occur during the replication of DNA[20]. MSI has been regarded as one of the most important indication of MMR defect. In this study, we detected five microsatellite loci sensitive to gastric cancer and found MSI-H in 26% carcinoma samples. The frequency of MSI-H in H pylori-positive group was significantly higher than that in H pylori-negative group. Among gastritis and intestinal metaplasia samples, the MSI-H frequency was 0% and 3% respectively although with higher H pylori infection. MSI existed in intestinal metaplasia samples although the frequency was much lower than that in carcinoma samples. That is to say, MMR defect happened before the malignant transformation of gastric mucous membrane cells. These results together indicated that H pyloriinfection induce tumorigenesis of gastric carcinoma when MMR system of gastric mucosa fails to work functionally.
It has been proved that wild type p53 protein can induce cell apoptosis but the intracellular accumulation of mutant p53 protein can inhibit cell apoptosis and promote cell transf-ormation and proliferation, resulting in carcinogenesis. The overexpression of p53protein is generally mutant forms, for the half-life of wild-type p53 is very short and p53 protein expression is usually negative in normal tissues. In our present study, p53 expressions were found in 0% of the control, 0% of gastritis samples, 13.3% of intestinal metaplasia samples and 43% gastric carcinomas samples. The detection of p53 expression in intestinal metaplasia indicates that p53mutation can be an early event in the pathogenesis of gastric cancer. When the carcinomas samples were subdivided into H pylori-positive and -negative, and the positive rates of their p53 expression compared, we found a higher expression rate in H pylori-positive group than that in H pylori-negative group. The carcinoma samples from MSI study were also analyzed to determine whether there was any relationship with p53 protein accumulation. No significant difference was shown to exist between samples in terms of their MSI status and p53 expression. Nevertheless, MSI was found in 11 with H pylori infection, 8 manifested p53 positive, this suggests that H pylori infection may play a role by inducing p53 gene mutations in those MSI or MMR defect gastric mucosa, but only in certain individuals.
In conclusion, the association between H pylori infection, MSI and p53 mutations observed in intestinal metaplasia and gastric carcinoma samples leads us to hypothesize that H pylori affect p53 pattern in gastric mucosa when MMR system fails to work. Mutations of the p53 gene seem to be an early event in gastric carcinogenesis.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 2.Meining A, Bayerdörffer E, Stolte M. [Extent, topography and symptoms of Helicobacter pylori gastrtitis. Phenotyping for accurate diagnosis and therapy?] Pathologe. 2001;22:13–18. doi: 10.1007/s002920000423. [DOI] [PubMed] [Google Scholar]
- 3.Fujioka T, Honda S, Tokieda M. Helicobacter pylori infection and gastric carcinoma in animal models. J Gastroenterol Hepatol. 2000;15 Suppl:D55–D59. doi: 10.1046/j.1440-1746.2000.02139.x. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for research on Cancer. Schistosomes, Liver flukes and Helicobacter pylori. Evaluation of carcinogenic risks to humans. IARC Monograph Evaluating Carcinogenic Risks to Humans. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob S, Praz F. DNA mismatch repair defects: role in colorectal carcinogenesis. Biochimie. 2002;84:27–47. doi: 10.1016/s0300-9084(01)01362-1. [DOI] [PubMed] [Google Scholar]
- 6.Coleman WB, Tsongalis GJ. The role of genomic instability in human carcinogenesis. Anticancer Res. 1999;19:4645–4664. [PubMed] [Google Scholar]
- 7.Duval A, Hamelin R. Genetic instability in human mismatch repair deficient cancers. Ann Genet. 2002;45:71–75. doi: 10.1016/s0003-3995(02)01115-2. [DOI] [PubMed] [Google Scholar]
- 8.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 9.Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 10.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 13.Parente F, Caselli M, Bianchi Porro G. Gastric apoptosis and Helicobacter pylori infection: an intricate matter. Scand J Gastroenterol. 2001;36:113–115. doi: 10.1080/003655201750065834. [DOI] [PubMed] [Google Scholar]
- 14.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 15.Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562–565. [PubMed] [Google Scholar]
- 16.Berloco P, Russo F, Cariola F, Gentile M, Giorgio P, Caruso ML, Valentini AM, Di Matteo G, Di Leo A. Low presence of p53 abnormalities in H pylori-infected gastric mucosa and in gastric adenocarcinoma. J Gastroenterol. 2003;38:28–36. doi: 10.1007/s005350300003. [DOI] [PubMed] [Google Scholar]
- 17.Wang XW, Tseng A, Ellis NA, Spillare EA, Linke SP, Robles AI, Seker H, Yang Q, Hu P, Beresten S, et al. Functional interaction of p53 and BLM DNA helicase in apoptosis. J Biol Chem. 2001;276:32948–32955. doi: 10.1074/jbc.M103298200. [DOI] [PubMed] [Google Scholar]
- 18.Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 19.Bowcock A, Osborne-Lawrence S, Barnes R, Chakravarti A, Washington S, Dunn C. Microsatellite polymorphism linkage map of human chromosome 13q. Genomics. 1993;15:376–386. doi: 10.1006/geno.1993.1071. [DOI] [PubMed] [Google Scholar]
- 20.Harfe BD, Jinks-Robertson S. DNA mismatch repair and genetic instability. Annu Rev Genet. 2000;34:359–399. doi: 10.1146/annurev.genet.34.1.359. [DOI] [PubMed] [Google Scholar]


