Significance
Bioinformatics and virus-induced gene silencing (VIGS)-guided gene discovery combined with biochemical enzyme assays show that tabersonine 3-oxygenase (T3O) and tabersonine 3-reductase (T3R) are required to form 3-hydroxy-16-methoxy-2,3-dihydrotabersonine, an intermediate in the formation of anticancer drug precursor vindoline from tabersonine. In the absence of T3R, tabersonine is converted by T3O to a series of byproducts that can no longer be used by T3R, suggesting a concerted reaction mechanism. Engineering the seven-gene pathway in yeast demonstrated a prototype platform of high potential for industrial production of the anticancer drug precursor vindoline.
Keywords: monoterpenoid indole alkaloid, tabersonine 3-oxygenase, tabersonine 3-reductase, virus-induced gene silencing, tabersonine-to-vindoline metabolic engineering
Abstract
Antitumor substances related to vinblastine and vincristine are exclusively found in the Catharanthus roseus (Madagascar periwinkle), a member of the Apocynaceae plant family, and continue to be extensively used in cancer chemotherapy. Although in high demand, these valuable compounds only accumulate in trace amounts in C. roseus leaves. Vinblastine and vincristine are condensed from the monoterpenoid indole alkaloid (MIA) precursors catharanthine and vindoline. Although catharanthine biosynthesis remains poorly characterized, the biosynthesis of vindoline from the MIA precursor tabersonine is well understood at the molecular and biochemical levels. This study uses virus-induced gene silencing (VIGS) to identify a cytochrome P450 [CYP71D1V2; tabersonine 3-oxygenase (T3O)] and an alcohol dehydrogenase [ADHL1; tabersonine 3-reductase (T3R)] as candidate genes involved in the conversion of tabersonine or 16-methoxytabersonine to 3-hydroxy-2,3-dihydrotabersonine or 3-hydroxy-16-methoxy-2,3-dihydrotabersonine, which are intermediates in the vindorosine and vindoline pathways, respectively. Biochemical assays with recombinant enzymes confirm that product formation is only possible by the coupled action of T3O and T3R, as the reaction product of T3O is an epoxide that is not used as a substrate by T3R. The T3O and T3R transcripts were identified in a C. roseus database representing genes preferentially expressed in leaf epidermis and suggest that the subsequent reaction products are transported from the leaf epidermis to specialized leaf mesophyll idioblast and laticifer cells to complete the biosynthesis of these MIAs. With these two genes, the complete seven-gene pathway was engineered in yeast to produce vindoline from tabersonine.
Monoterpenoid indole alkaloids (MIAs), composed of a secoiridoid moiety and an indole moiety, constitute an important class of alkaloids with very diverse structures. MIAs are well known for their complicated biosynthetic routes, represented by the model plant Catharanthus roseus (Madagascar periwinkle), in which biosynthetic pathways spread among multiple cell types, leading to a complex pool of different structures (1–7). Many MIAs and iridoids have been demonstrated for their ecological and pharmaceutical functions, including the bisindole alkaloid anticancer drugs vinblastine and vincristine from C. roseus (1). Vinblastine and vincristine are condensed from two MIA moieties, catharanthine and vindoline. Their complicated structures make the total chemical synthesis difficult and costly. Despite their medicinal importance, these drugs are still harvested from cultivated periwinkles. The elucidation of the biosynthesis of catharanthine and vindoline becomes critical: to either increase their production in planta or to engineer these pathways in microorganisms to allow industrial production of medicinally relevant compounds. The identification of relevant MIA pathway genes in this model plant is also facilitating the discovery of pathways in a number of other species that accumulate other MIAs of medicinal value.
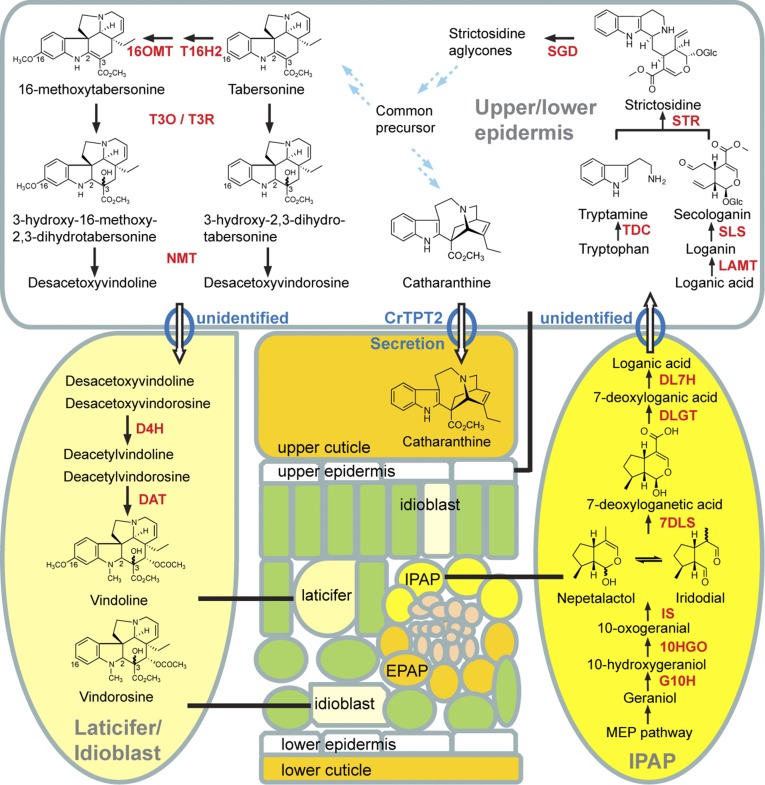
Recently, the entire nine-step biosynthetic pathway from monoterpene precursor geranyl diphosphate leading to the formation of the iridoidal MIA precursor secologanin has been fully elucidated in C. roseus (8–13). Biochemically specialized leaf internal phloem associated parenchyma (IPAP) cells convert geranyl diphosphate through multiple oxidation and cyclization steps to yield loganic acid that is transported to leaf epidermis, where it is converted to secologanin (4). Cells in the leaf epidermis express strictosidine synthase (STR) to form strictosidine from this iridoid and tryptamine (14). Subsequent cleavage of strictosidine by strictosidine β-glucosidase (SGD) (15) yields labile aglycone precursors required for the assembly of Corynanthe, iboga, and aspidosperma skeletons represented by MIAs such as ajmalicine, catharanthine, and vindoline that accumulate in C. roseus. Although the pathways leading to the formation of catharanthine or tabersonine remain to be discovered, it is known that tabersonine is converted to vindoline via hydroxylation and O-methylation to give rise to 16-methoxytabersonine (16, 17), followed by an unknown C-3-oxidation and C-2/C-3-reduction step, N-methylation (18, 19), C-4-hydroxylation, and C-4-O-acetylation (3, 20, 21) (Fig. S1). Small amounts of tabersonine can also be converted to vindorosine (16-desmethoxyvindoline) when the first two steps in the vindoline pathway are bypassed (Fig. S1). Recent discoveries have also shown that catharanthine accumulates entirely in the wax exudates on the leaf surface (6), mediated by a leaf epidermis-localized plasma membrane-associated member of the pleiotropic drug-resistance family of ATP-binding cassette transporters (7) compared with vindoline, which is found within specialized internal leaf cells that preferentially express the terminal pathway reactions (3, 6). Enzymes required for the first two steps in the pathway, tabersonine 16-hydroxylase 2 (T16H2) and 16-hydroxytabersonine O-methyltransferase (16OMT), are localized in leaf epidermis (16, 17). In comparison, the last two steps catalyzed by desacetoxyvindoline-4-hydroxylase (D4H) and deacetylvindoline-4-O-acetyltransferase (DAT) are localized in two biochemically specialized leaf mesophyll cells known as idioblasts and laticifers (3). To determine the localization of the remaining steps, sucrose gradient density centrifugation showed that 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT) activity may be associated with chloroplasts (19), suggesting its preferential expression in chloroplast-containing mesophyll cells. In addition, the intermediate 16-methoxytabersonine mainly exists in leaf body. These evidences suggest that the majority of the pathway, with the exception of the first two steps, occurs in leaf mesophyll cells.
The present study identified two enzymes, tabersonine 3-oxygenase (T3O) and tabersonine 3-reductase (T3R), that convert 16-methoxytabersonine to 3-hydroxy-16-methoxy-2,3-dihydrotabersonine through bioinformatics-guided virus-induced gene silencing (VIGS) and biochemical studies. Expression studies also suggested that T3O, T3R, and NMT are localized in leaf epidermis, leaving D4H and DAT the only enzymes in leaf mesophyll. Assembly of the complete seven-gene pathway in Saccharomyces cerevisiae (Baker’s yeast) demonstrated the prototype microbial platform for industrial vindoline production from tabersonine.
Results
Bioinformatics-Assisted Gene Discovery.
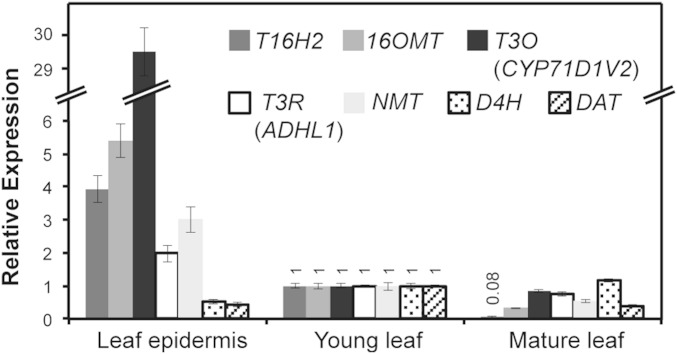
Despite the biochemical localization of NMT in leaf mesophyll (19), NMT transcripts were found in a C. roseus leaf epidermal EST database (4). To reevaluate the pathway localization, quantitative reverse transcription PCR (qRT-PCR) analyses were conducted. Consistent with in situ localization and biochemical studies (3, 16, 17), expression of early vindoline biosynthesis genes T16H2 and 16OMT is enriched (four- to fivefold) in leaf epidermis compared with that in whole leaf tissues, whereas the last two genes, D4H and DAT, are expressed with a ratio of 0.5 or less in the leaf epidermis compared to the whole leaf (Fig. 1). However, qRT-PCR suggested that NMT expression is threefold higher in leaf epidermis over whole leaf tissues. These results suggest that only the last two steps in vindoline biosynthesis may occur in specialized leaf mesophyll cells, whereas the conversion of tabersonine to desacetoxyvindoline takes place in the leaf epidermis (Fig. S1).
Fig. 1.
Expression ratios of 7 C. roseus genes that convert tabersonine to vindoline in leaf epidermis compared with those in whole leaves. The transcript abundance of selected genes in RNA extracts from young leaf epidermis and whole mature leaves was compared with those from whole young leaves. Young leaf refers to LP-1 (youngest leaf pair on a vegetative meristem), whereas manure leaf refers to LP-4 (fourth leaf pair from the youngest leaf pair). Internal control 60S ribosomal RNA was used for data normalization. Error bars indicate the SD from three technical replicates.
Candidate genes that encode “hydratase” activity for converting 16-methoxytabersonine to 3-hydroxy-16-methoxy-2,3-dihydrotabersonine were selected from an EST database enriched in transcripts preferentially expressed in C. roseus leaf epidermis (4). This database, shown to be enriched in transcripts for numerous genes involved in triterpene, very long chain fatty acid, flavonoid, and MIA biosynthesis, has been successfully used to identify candidate genes for loganic acid O-methyltransferase (LAMT) (4) and for the ATP-binding cassette transporter responsible for secretion of the catharanthine to the leaf surface (7). The database also shows that six known biochemically characterized genes involved in MIA biosynthesis [tryptophan decarboxylase (TDC), STR, SGD, T16H2, 16OMT, and NMT] are mostly well represented, together with the late iridoid pathway genes LAMT and secologanin synthase (SLS), as well as selected leaf epidermal markers for triterpene biosynthesis, amyrin synthase (AS) and amyrin oxidase (AO) (Table S1). In contrast, G10H and 10HGO, which initiate biosynthesis of iridoids in specialized leaf mesophyll IPAP cells, as well as D4H and DAT, which complete the biosynthesis of vindoline in specialized leaf mesophyll idioblast or laticifer cells, are poorly or not represented (Table S1). The realization that NMT, which catalyzed the third to last step in vindoline biosynthesis, is likely expressed in the leaf epidermis raised the possibility that the “hydratase” might also be preferentially expressed in these cells. For this proposed reaction, the C-3-oxidation might involve a cytochrome P450, whereas C-2/C-3 reduction might be simultaneously catalyzed by this CYP or by another reductase. As a result, a cytochrome p450 gene (CYP71D1V2, GenBank: KP122967; Table S1, represented eight times) and an alcohol dehydrogenase-type gene (ADHL1, GenBank: KP122966; Table S1, represented 59 times) were identified as likely candidate genes. CYP71D1V2 and ADHL1 are well represented compared with other known leaf epidermal MIA pathway transcripts (Table S1), and they have less than 65% amino acid sequence identity to genes from the leaf transcriptomes of three other MIA producing-members of the Apocynaceae family (Vinca minor, Amsonia hubrichtii, and Tabernaemontana elegans; PhytoMetaSyn Project, www.phytometasyn.ca) that do not make vindoline. In addition, as CYP71D1V2 and ADHL1 are preferentially enriched 30- and twofold in leaf epidermis compared with whole leaf tissues (Fig. 1), consistent with the patterns of epidermal enrichment of T16H2, 16OMT, and NMT, both were selected for further characterization by VIGS. The CYP71D1V2 has been described as inactive in converting amyrin to ursolic acid (22), whereas ADHL1 has not been previously described.
VIGS of Candidate Genes.
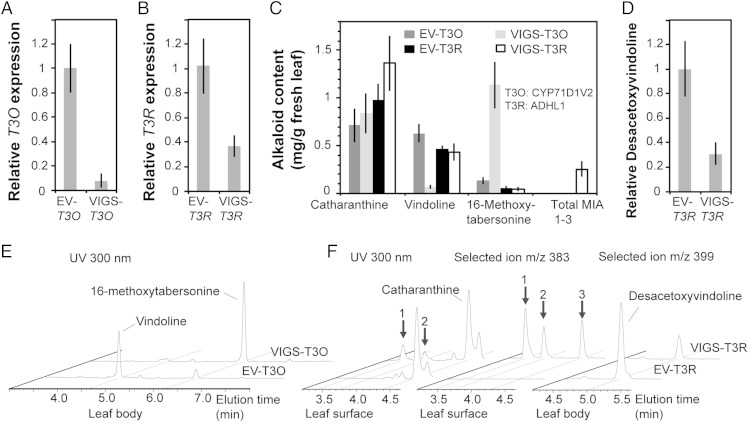
VIGS of CYP71D1V2 and ADHL1 in C. roseus leaves was monitored by comparative metabolite profiling of MIAs secreted to the leaf surface (such as catharanthine) to those found within the leaf body (such as vindoline and 16-methoxytabersonine) (6, 7). qRT-PCR analyses showed that plants could be silenced by an average of 92% for CYP71D1V2 and by 63% for ADHL1 compared with the empty vector (EV) controls (Fig. 2 A and B). The 89% decline in vindoline and 850% increase of 16-methoxytabersonine (m/z = 367) levels observed in CYP71D1V2-silenced plants compared with the EV controls (Fig. 2 C and E) suggest that 16-methoxytabersonine is a likely substrate of CYP71D1V2. Although the milder silencing of ADHL1 did not reduce vindoline levels (Fig. 2C), desacetoxyvindoline, the N-methylated MIA produced immediately after the 3-hydroxylation step, was reduced accordingly by 69% (Fig. 2 D and F). The leaf surface extracts of VIGS-ADHL1 plants also accumulated three new unidentified isomeric MIAs (m/z = 383) that were not detected in the EV controls (Fig. 2F).
Fig. 2.
Silencing T3O (CYP71D1V2) or T3R (ADHL1) by VIGS in C. roseus leaf. Relative expression of T3O (A) and T3R (B) in C. roseus first leaf pair in comparison with the EV control plants. Internal control 60S ribosomal RNA was used for data normalization. Alkaloid contents in respective plants (C). Relative content of desacetoxyvindoline in VIGS-T3R plants (D). Biological replicates: EV-T3O, n = 5; VIGS-T3O, n = 5; EV-T3R, n = 5; and VIGS-T3R, n = 4. Error bar indicates SD. Total MIA 1–3: 3-oxidized isomers of 16-methoxytabersonine found in F (Middle). Representative LC-MS chromatograms monitored at 300 nm MIAs extracted from leaf body treated with VIGS-T3O compared with EV control (E). Representative LC-MS chromatograms of leaf surface or leaf body treated with VIGS-T3R compared with EV control (F). Isomers of 3-oxidized 16-methoxytabersonine were identified by measuring absorbance at 300 nm (Left) or by selected ion m/z= 383 (Middle). The content of desacetoxyvindoline was measured by selected ion m/z= 399 (Right). The 3-oxidized isomers of 16-methoxytabersonine could also be detected in in vitro enzyme assays with T3O-expressing yeast microsome in Fig. 3A and Fig. S2 B and F.
In Vitro Biochemical Studies.
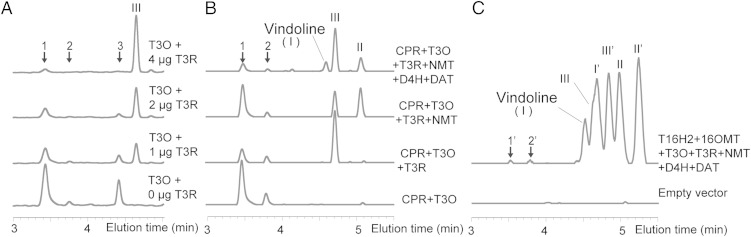
The CYP71D1V2 was expressed in yeast (Fig. S2A) along with the C. roseus cytochrome P450 reductase (CPR) (9). Only microsomal membranes containing recombinant proteins, NADPH, and O2 converted 16-methoxytabersonine (Fig. 3A and Fig. S2 B and F), 16-hydroxytabersonine (Fig. S2 C and G), or tabersonine (Fig. S2 D and H) to three isomeric MIAs [eluting with different retention times but with the same masses m/z 383 (MIA 1–3), 369 (MIA 1″-3″), and 353 (MIA 1′- 3′); Fig. S2 F–H and Fig. S3]. The mass increase of 16 m/z obtained for each MIA suggested that CYP71D1V2 catalyzed a monooxygenation reaction instead of the mass increase of 18 m/z that would be expected to generate 2,3-dihydro-3-hydroxylated MIAs. In comparison, 2,3-dihydrotabersonine was not a substrate of CYP71D1V2 (Fig. S2E), suggesting monooxygenation only occurs across the 2,3-double bond. When the living yeast expressing CYP71D1V2 was incubated with 16-methoxytabersonine, 16-hydroxytabersonine, or tabersonine, only MIA 1 and 2 (Fig. S4A), 1″ and 2″ (Fig. S4B), or 1′ and 2′ (Fig. S4C) could be isolated, suggesting MIA 3 (Fig. S2F), 3″ (Fig. S2G), or 3′ (Fig. S2H) occurring in microsomal enzyme assays is not stable within live cells. Further analyses of tabersonine-derived MIA 1′ by NMR suggested it to be 2,3-epoxytabersonine (Fig. S5). This structure was further supported by the sodium borohydride reduction of MIA 1′ to the respective diol that had the expected mass of 327 m/z, as measured by LC-MS. Together, these analyses also suggest that MIA 2′ may be the stereoisomer of MIA 1′.
Fig. 3.
T3O (CYP71D1V2) and T3R (ADHL1) are required for biosynthesis of 16-methoxy-3-hydroxy-2,3-dihydrotabersonine and for biosynthesis of vindoline in yeast cultures expressing the seven-gene pathway from tabersonine to vindoline. Recombinant T3R catalyzes the synthesis of 3-hydroxy-16-methoxy-2,3-dihydrotabersonine when coupled with recombinant T3O, with 16-methoxytabersnine as the substrates (A). Biotransformation products isolated by feeding the yeast expressing various combinations of vindoline pathway genes with 16-methoxytabersonine (B). Vindoline formation by feeding tabersonine to the yeast strain B containing the seven-gene pathway (C). The products were revealed by LC-MS at 300 nm (A and B) or total ion scan (C). I, vindoline; I′, vindorosine; II, desacetoxyvindoline; II′, desacetoxyvindorosine; III, 3-hydroxy-16-methoxy-2,3-dihydrotabersonine; III′, 3-hydroxy-2,3-dihydrotabersonine. 1, 2, and 3: T3O products derived from 16-methoxytabersonine; 1′2′: T3O products derived from tabersonine.
The three isomeric MIAs (1–3, m/z = 383) identified in CYP71D1V2 expressing yeast microsomal enzyme assays with 16-methoxytabersonine (Fig. 3A and Fig. S2 B and F) showed identical LC-MS profiles with the three MIAs (m/z = 383) found on leaf surfaces of VIGS-ADHL1 plants (Fig. 2F). These results suggested that ADHL1 might catalyze a subsequent reduction of the epoxides to form NMT substrate (Fig. S1). Although recombinant ADHL1 purified from Escherichia coli (Fig. S6D) was not active with either MIA 1 or 2 generated from CYP71D1V2-expressing yeast incubated with 16-methoxytabersonine (Fig. S6A, Left) or tabersonine (Fig. S6A, Right), ADHL1 assays containing all the three isomeric MIAs produced in CYP71D1V2-expressing yeast microsomal enzyme assays with 16-methoxytabersonine (Fig. S6B, Left) or tabersonine (Fig. S6B, Right) yielded small amounts of respective 2,3-dihydro-3-hydroxylated MIAs with a slight decline in the levels of MIA 3 or 3′. When CYP71D1V2-containing microsomes were combined with different levels of recombinant ADHL1 in the same reaction, the formation of the three isomeric MIAs derived from 16-methoxytabersonine (Fig. 3A and Fig. S6C, Left) or tabersonine (Fig. S6C, Right) declined with increasing levels of ADHL1 protein in favor of formation of large amounts of the respective 2,3-dihydro-3-hydroxylated MIAs. Substrate saturation kinetics was conducted with CYP71D1V2-containing microsomes combined with ADHL1 to give an apparent Km for tabersonine of 3.1 μM (Fig. S7). On the basis of the results, CYP71D1V2 and ADHL1 were renamed as T3O and T3R, respectively.
Assembly of Tabersonine-to-Vindoline Pathway in Yeast.
To confirm that the products of the T3O and T3R reactions are true intermediates for the biosynthesis of vindoline and vindorosine, the tabersonine-to-vindoline pathway was assembled in yeast. Yeast strains coexpressing CPR, T3O, and T3R (Fig. S8A) converted 16-methoxytabersonine (Fig. 3B and Fig. S8B, Left) or tabersonine (Fig. S8B, Right) mostly into 2,3-dihydro-3-hydroxylated MIAs, whereas additional expression of NMT allowed the detection of desacetoxyvindoline or desacetoxyvindorosine, and additional expression of D4H and DAT (strain A) triggered the formation of vindoline (Fig. 3B and Fig. S8B, Left) or vindorosine (Fig. S8B, Right). When yeast expressing all seven genes for the vindoline pathway (addition of T16H2/16OMT and omission of CPR, strain B) was fed with tabersonine, vindoline and vindorosine were clearly formed together with several other pathway intermediates (Fig. 3C).
Under tested conditions, yeast strain A was able to convert 16-methoxytabersonine at 37 mg⋅L−1 12 h−1 or 8 mg⋅g dry weight (dwt)−1 12 h−1 rate and to produce vindoline at 2.7 mg⋅L−1 12 h−1 or 0.6 mg⋅g dwt−1 12 h−1 rate. Yeast strain B was able to convert tabersonine at 17 mg⋅L−1 12 h−1 or 3.7 mg⋅g dwt−1 12 h−1 and to produce vindoline at 1.1 mg⋅L−1 12 h−1 or 0.24 mg⋅g dwt−1 12 h−1. In comparison, vindoline content in C. roseus total leaves is 2 mg⋅g dwt−1 or less (23). In addition, more than 95% of the produced vindoline and other intermediates are secreted by yeast to the medium (Fig. S9) and are easily extracted with ethyl acetate. Therefore, the yeast system could be a powerful platform for industrial production of vindoline from tabersonine.
Discussion
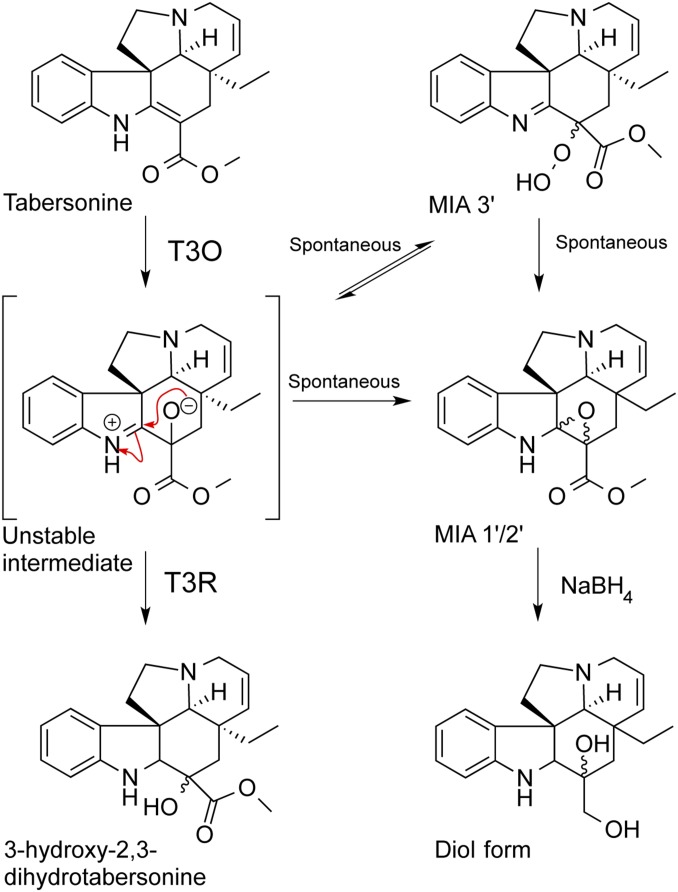
The identification of T3O and T3R completes the molecular and biochemical characterization of the only remaining unknown reactions in the tabersonine-to-vindoline pathway. It was shown that saturation of T3O with T3R eliminated the accumulation of epoxide byproduct in favor of 2,3-dihydro-3-hydroxylated MIAs and that a proposed reactive intermediate (Fig. 4) required for the T3R reaction may spontaneously be converted into unusable epoxide derivatives in its absence. The formation of the 2,3-epoxide significantly alters the UV absorption from the longer wavelengths typical of the reaction substrates to those typical of an indolenine moiety, whereas the UV absorption of MIA 3 and 3′ resembles the profile of 3-hydroxylated MIA end products (Fig. S2). We therefore speculate that MIA 3 or 3′ may be the peroxide of the ring-opened 2,3-epoxide (Fig. 4), but it appears as 2,3-epoxide isomers when analyzed by mass spectrometry because of the unstable nature of peroxide bond. Although the exact mechanism for this reaction remains to be discovered, it is likely that T3O and T3R catalyze the 3-hydroxylation step in a concerted manner in planta.
Fig. 4.
Conversion of tabersonine to 3-hydroxy-2,3-dihydrotabersonine by the combined action of T3O and T3R enzymes. In the absence of T3R, the proposed intermediate is spontaneously converted to byproduct MIAs 1′-3′. Reduction of MIA 1′ with sodium borohydride yields the expected diol.
A related study (24) that appeared while this report was in review showed that a vincamine-type MIA, rather than the 2,3-epoxide, was detected when a T3O-expressing yeast strain was cultured in the presence of 16-methoxytabsersonine. This led to the suggestion by these authors that the 2,3-epoxide can undergo rearrangement to yield the vincamine–eburnamine backbone in the absence of T3H and suggesting a biosynthetic relationship between the aspidosperma- and eburnamine-type MIAs. The present study shows, in contrast, that the 2,3-epoxide is clearly detected within VIGS-T3R-silenced plants as well as in T3O-expressing cultured yeast and in microsomal assays when tabersonine, 16-hydroxytabersonine, or 16-methoxytabersonine were supplied as MIA substrates. The proposed rearrangement in ref. 24 is likely to be chemical and well-known to occur, depending on the acidity of the growth medium or alkaloid extraction conditions, and occurs when the aspidosperma nucleus is in the indolenine oxidation state (25–27).
Previous biochemical localization studies involving organelle isolation by sucrose gradient centrifugation followed by fractionation suggested that NMT activity was associated with Catharanthus chloroplasts (19). In addition, the phylogenetic relationship of the NMT gene to nuclear-encoded but chloroplast-associated tocopherol C-methyltransferases (18) further implied that the NMT and possibly “hydratase” reactions might be associated leaf mesophyll chloroplasts. The preferential enrichment of T16H2, 16OMT, NMT, T3O, and T3R transcripts in leaf epidermis compared with whole leaf tissues (Fig. 1) and the exclusive and restricted detection of T3O-derived MIA products (Fig. 3A) to leaf surfaces of VIGS-T3R plants (Fig. 2F) implies that both reactions take place in the epidermis of Catharanthus leaves. In contrast to the leaf surface accumulation of T3O-derived MIA products (Fig. 2F), 16-methoxytabersonine was only detected in the leaf body (Fig. 2E). This raises questions about the different possible biological mechanisms that might account for the results obtained. This study further suggests that the MIA transported to idioblasts or laticifers for elaboration by D4H and DAT into vindoline is desacetoxyvindoline (Fig. 5). Taking into account this new information, it is possible that NMT is associated with plastids in the leaf epidermis, but further studies are required to determine this.
Fig. 5.
Cellular compartmentation of iridoid and MIA biosynthesis. 7DLS, 7-deoxyloganetic acid synthase; 10HGO, 10-hydroxygeraniol oxidoreductase; DL7H, 7-deoxyloganic acid 7-hydroxylase; DLGT, 7-deoxyloganetic acid glucosyltransferase; G10H, geraniol 10-hydroxylase; IS, iridoid synthase; SGD, strictosidine β-glucosidase; SLS, secologanin synthase; STR, strictosidine synthase; TDC, tryptophan decarboxylase.
As the model plant most studied for MIA biosynthesis, C. roseus revealed a fascinating picture of cell-type specific compartmentation of different parts of the vindoline pathway that would require movement of different biosynthetic intermediates for its assembly (Fig. 5). Remarkably, specialized IPAP cells preferentially express the plastid-specific methylerythritol phosphate pathway to supply geraniol for elaboration into loganic acid via seven key biochemical reactions. Loganic acid must then be transported by an uncharacterized process to the leaf epidermis, where it is converted to secologanin (3, 4, 8–13). Leaf epidermal cells preferentially express tryptophan decarboxylase together with all MIA pathway genes leading to biosynthesis of catharanthine, desacetoxyvindorosine, and desacetoxyvindoline. At this point, catharanthine is secreted via CrTPT2-mediated transport (6, 7) and accumulated on the leaf surface, and desacetoxyvindorosine and desacetoxyvindoline are transported by an unidentified process to idioblasts or laticifers for a two-step elaboration to form vindorosine and vindoline. The completion of the tabersonine-to-vindoline pathway from this and other studies (5, 16–18, 20, 21) highlights the complex nature of plant secondary metabolism.
The assembly of the seven-gene tabersonine-to-vindoline pathway in yeast for vindoline production demonstrates the feasibility of microbial MIA production. The discrepancy between the tabersonine consumption and the final vindoline yield in yeast showed great potential for further production optimization. The results suggested that T16H, NMT, and D4H activities might be bottlenecks that limit flux, as N-desmethy- and N-methyl intermediates mainly accumulated in strain B (Fig. 3C). Further gene expression optimization on these three enzymes may significantly increase vindoline yield to achieve an industrial strain superior to the plant. In the future, completion of strictosidine-to-tabersonine/catharanthine branch pathways may lead to the total synthesis of plant-derived MIA in controlled microbial systems.
Materials and Methods
VIGS.
Seeds of C. roseus cv. Little Delicata were sowed and grown in a greenhouse at 28 °C, 16/8 h photoperiod. Four-week-old seedlings with two to three pairs of true leaves were used for VIGS as described (28), with modifications. Cells of an overnight culture of Agrobacterium tumefaciens carrying pTRV1 (tobacco rattle virus) or various pTRV2 constructs were resuspended in infection buffer (10 mM Mes at pH 5.6, 10 mM MgCl2, 200 μM acetosyringone) to an OD600 1.5, and incubated at 28 °C for 2 h. The suspension of pTRV1 and pTRV2 strains were mixed in equal volume just before the infection. The stem of the seedling was poked with a sterile toothpick just underneath the apical meristem, and 150 μL infection mixture was flooded over the wound. After infection, the plants were transferred to a greenhouse at 20 °C, 16/8 h photoperiod. VIGS-phytoene desaturase was used to indicate the silencing effect (9).
Leaf Alkaloid Extraction.
The first leaf pair (LP-1) were collected and split in half along the vertical axis 25–30 d after infection. One half of the LP-1 were frozen in liquid nitrogen for RNA extraction, and the other half were weighed (10–30 mg) and submerged in 20 times chloroform (wt/vol) for 1 min to generate leaf surface extraction (6). The leaf material was thereafter transferred to 20 times methanol (wt/vol) for extraction on ice for 1 h to generate the leaf body extraction.
Yeast Biotransformation Assays.
A single yeast [BY47471 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YPL154c::kanMX4) (29)] colony carrying various constructs was inoculated in 2 mL synthetic complementary (SC) medium with appropriate amino acids dropped out, supplemented with 2% (wt/vol) glucose overnight at 30 °C. Cells were harvested and inoculated in SC medium with 2% (wt/vol) galactose and induced at 30 °C for 24 h. The cells were harvested and suspended in 1 mL biotransformation buffer (10 mM Tris⋅HCl at pH 7.5, 1 mM EDTA) with 20 μM substrates and incubated at 30 °C for 24 h. For tabersonine conversion quantification, 225 μM tabersonine was used. Basified buffer was extracted with ethyl acetate, dried, and redissolved in methanol for LC-MS analysis. For large-scale product purification, tabersonine (50 mg) was fed to 2 L yeast expressing T3O and CPR for 12 h. The extracted product was harvested from thin-layer chromatograph sheets (Silica gel 60 F254) with solvent toluene: ethyl acetate: methanol (15:4:1, vol/vol). About 8 mg MIA 1′ was subjected to further NMR and reduction analysis.
In Vitro Assay.
The assay (100 μL) contains 50 mM Hepes at pH 7.5, 1 mM NADPH, 25 μM substrate (tabersonine, 16-hydroxytabersonine 16-methoxytabersonine, 2,3-dihydrotabersonine, or reaction products of T3O), 50 μg microsomal protein, and/or various amount of recombinant T3R. The reaction took place at 30 °C for 1 h. Ethyl acetate extracted products were redissolved in methanol for LC-MS analysis. The kinetics assays (100 μL) contain 50 mM Hepes at pH 7.5, 0.25 mM NADPH, 25 μg microsomal proteins (T3O), 2 μg purified recombinant T3R, and various amount of tabersonine. The reaction took place at 30 °C, and the velocity was calculated as the consumption of NADPH measured at OD340.
Supplementary Material
Acknowledgments
We thank Dr. Dae-Kyun Ro from the University of Calgary for providing the yeast strain and expression vectors. This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (to V.D.L.), Canada Research Chairs (to V.D.L.), and the Canada Foundation for Innovation and the Ontario Ministry of Research and Innovation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KP122967 and KP122966).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501821112/-/DCSupplemental.
References
- 1.De Luca V, Salim V, Thamm A, Masada SA, Yu F. Making iridoids/secoiridoids and monoterpenoid indole alkaloids: Progress on pathway elucidation. Curr Opin Plant Biol. 2014;19:35–42. doi: 10.1016/j.pbi.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 2.De Luca V, Salim V, Atsumi SM, Yu F. Mining the biodiversity of plants: A revolution in the making. Science. 2012;336(6089):1658–1661. doi: 10.1126/science.1217410. [DOI] [PubMed] [Google Scholar]
- 3.St-Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11(5):887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murata J, Roepke J, Gordon H, De Luca V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell. 2008;20(3):524–542. doi: 10.1105/tpc.107.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata J, De Luca V. Localization of tabersonine 16-hydroxylase and 16-OH tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J. 2005;44(4):581–594. doi: 10.1111/j.1365-313X.2005.02557.x. [DOI] [PubMed] [Google Scholar]
- 6.Roepke J, et al. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci USA. 2010;107(34):15287–15292. doi: 10.1073/pnas.0911451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu F, De Luca V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci USA. 2013;110(39):15830–15835. doi: 10.1073/pnas.1307504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geu-Flores F, et al. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature. 2012;492(7427):138–142. doi: 10.1038/nature11692. [DOI] [PubMed] [Google Scholar]
- 9.Salim V, Yu F, Altarejos J, De Luca V. Virus-induced gene silencing identifies Catharanthus roseus 7-deoxyloganic acid-7-hydroxylase, a step in iridoid and monoterpene indole alkaloid biosynthesis. Plant J. 2013;76(5):754–765. doi: 10.1111/tpj.12330. [DOI] [PubMed] [Google Scholar]
- 10.Asada K, et al. A 7-deoxyloganetic acid glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar periwinkle. Plant Cell. 2013;25(10):4123–4134. doi: 10.1105/tpc.113.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salim V, Wiens B, Masada-Atsumi S, Yu F, De Luca V. 7-deoxyloganetic acid synthase catalyzes a key 3 step oxidation to form 7-deoxyloganetic acid in Catharanthus roseus iridoid biosynthesis. Phytochemistry. 2014;101:23–31. doi: 10.1016/j.phytochem.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen K, et al. The seco-iridoid pathway from Catharanthus roseus. Nat Commun. 2014;5:3606. doi: 10.1038/ncomms4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irmler S, et al. Indole alkaloid biosynthesis in Catharanthus roseus: New enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J. 2000;24(6):797–804. doi: 10.1046/j.1365-313x.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 14.Treimer JF, Zenk MH. Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation. Eur J Biochem. 1979;101(1):225–233. doi: 10.1111/j.1432-1033.1979.tb04235.x. [DOI] [PubMed] [Google Scholar]
- 15.Geerlings A, Ibañez MM, Memelink J, van Der Heijden R, Verpoorte R. Molecular cloning and analysis of strictosidine beta-D-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem. 2000;275(5):3051–3056. doi: 10.1074/jbc.275.5.3051. [DOI] [PubMed] [Google Scholar]
- 16.Levac D, Murata J, Kim WS, De Luca V. Application of carborundum abrasion for investigating the leaf epidermis: Molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J. 2008;53(2):225–236. doi: 10.1111/j.1365-313X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- 17.Besseau S, et al. A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol. 2013;163(4):1792–1803. doi: 10.1104/pp.113.222828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liscombe DK, Usera AR, O’Connor SE. Homolog of tocopherol C methyltransferases catalyzes N methylation in anticancer alkaloid biosynthesis. Proc Natl Acad Sci USA. 2010;107(44):18793–18798. doi: 10.1073/pnas.1009003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deluca V, Balsevich J, Tyler RT, Kurz WG. Characterization of a novel N-methyltransferase (NMT) from Catharanthus roseus plants : Detection of NMT and other enzymes of the indole alkaloid biosynthetic pathway in different cell suspension culture systems. Plant Cell Rep. 1987;6(6):458–461. doi: 10.1007/BF00272782. [DOI] [PubMed] [Google Scholar]
- 20.St-Pierre B, Laflamme P, Alarco AM, De Luca V. The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 1998;14(6):703–713. doi: 10.1046/j.1365-313x.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Flota F, De Carolis E, Alarco AM, De Luca V. Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol Biol. 1997;34(6):935–948. doi: 10.1023/a:1005894001516. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, et al. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta. 2012;236(5):1571–1581. doi: 10.1007/s00425-012-1712-0. [DOI] [PubMed] [Google Scholar]
- 23.Chung I-M, et al. Screening 64 cultivars Catharanthus roseus for the production of vindoline, catharanthine, and serpentine. Biotechnol Prog. 2011;27(4):937–943. doi: 10.1002/btpr.557. [DOI] [PubMed] [Google Scholar]
- 24.Kellner F, et al. Discovery of a P450-catalyzed step in vindoline biosynthesis: A link between the aspidosperma and eburnamine alkaloids. Chem Commun (Camb) March 19, 2015 doi: 10.1039/C5CC01309G. [DOI] [PubMed] [Google Scholar]
- 25.Wenkert E. Biosynthesis of indole alkaloids. The aspidosperma and Iboga bases. J Am Chem Soc. 1962;84(1):98–102. [Google Scholar]
- 26.Wenkert E, Wickberg B. General methods of synthesis of indole alkaloids. IV. A synthesis of dl-eburnamonine. J Am Chem Soc. 1965;87:1580–1589. doi: 10.1021/ja01085a029. [DOI] [PubMed] [Google Scholar]
- 27.Barton JED, Harley-Mason J. Total synthesis of Hunteria and Aspidosperma alkaloids from a common intermediate. Chem Commun (London) 1965;10:197–198. doi: 10.1016/s0040-4039(01)89153-x. [DOI] [PubMed] [Google Scholar]
- 28.Liscombe DK, O’Connor SE. A virus-induced gene silencing approach to understanding alkaloid metabolism in Catharanthus roseus. Phytochemistry. 2011;72(16):1969–1977. doi: 10.1016/j.phytochem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parr CL, Keates RAB, Bryksa BC, Ogawa M, Yada RY. The structure and function of Saccharomyces cerevisiae proteinase A. Yeast. 2007;24(6):467–480. doi: 10.1002/yea.1485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.