Significance
β-Arrestins are unique proteins that have multiple cellular functions such as G protein-coupled receptor signal desensitization, protein trafficking and signaling molecule scaffolding. Treatment of Parkinson’s disease (PD) motor symptoms by l-3,4-dihydroxyphenylalanine (l-DOPA) has been hampered by abnormal involuntary movements or dyskinetic side effects. The cause of these dyskinesias has been attributed to receptor supersensitivity and uncontrolled neuronal excitability. Here we demonstrate in multiple preclinical models of l-DOPA–induced dyskinesias and PD that expression levels of β-arrestin2 can alter manifestation of these dyskinesias by reducing receptor supersensitivity while maintaining the therapeutic effect of l-DOPA. Thus novel drugs that increase β-arrestin–dependent function at dopamine receptors may be useful in ameliorating PD motor symptoms without inducing dyskinesias.
Keywords: l-DOPA, dyskinesia, beta-arrestin, dopamine receptors, biased signaling
Abstract
Parkinson’s disease (PD) is characterized by severe locomotor deficits and is commonly treated with the dopamine (DA) precursor l-3,4-dihydroxyphenylalanine (l-DOPA), but its prolonged use causes dyskinesias referred to as l-DOPA–induced dyskinesias (LIDs). Recent studies in animal models of PD have suggested that dyskinesias are associated with the overactivation of G protein-mediated signaling through DA receptors. β-Arrestins desensitize G protein signaling at DA receptors (D1R and D2R) in addition to activating their own G protein-independent signaling events, which have been shown to mediate locomotion. Therefore, targeting β-arrestins in PD l-DOPA therapy might prove to be a desirable approach. Here we show in a bilateral DA-depletion mouse model of Parkinson’s symptoms that genetic deletion of β-arrestin2 significantly limits the beneficial locomotor effects while markedly enhancing the dyskinesia-like effects of acute or chronic l-DOPA treatment. Viral rescue or overexpression of β-arrestin2 in knockout or control mice either reverses or protects against LIDs and its key biochemical markers. In other more conventional animal models of DA neuron loss and PD, such as 6-hydroxydopamine–treated mice or rats and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine–treated nonhuman primates, β-arrestin2 overexpression significantly reduced dyskinesias while maintaining the therapeutic effect of l-DOPA. Considerable efforts are being spent in the pharmaceutical industry to identify therapeutic approaches to block LIDs in patients with PD. Our results point to a potential therapeutic approach, whereby development of either a genetic or pharmacological intervention to enhance β-arrestin2- or limit G protein-dependent D1/D2R signaling could represent a more mechanistically informed strategy.
Dopamine (DA) is a major catecholamine neurotransmitter that is released by midbrain DA neurons. DA activates G protein-coupled receptors (GPCRs), which belong to the dopamine D1 (D1R and D5R) or D2 (D2R, D3R, and D4R) class of DA receptors that are known to signal via G protein-dependent mechanisms (1, 2). However, recent studies have shown that in addition to G protein-mediated signaling, many GPCRs, including DA receptors, signal through beta-arrestin 1 and 2 (βarr1 and βarr2)-dependent mechanisms (3, 4). The two isoforms of β-arrestin, β-arrestin1 (βarr1) and β-arrestin2 (βarr2) are widely coexpressed in the brain (5, 6). β-Arrestins were originally appreciated for their ability to desensitize (i.e., turn off) GPCR signaling in a GPCR kinase (GRK)-dependent manner (7, 8). Binding of β-arrestin to the GPCR sterically hinders G protein binding and in most instances initiates receptor endocytosis via interactions with adaptor protein complex-2 (AP-2) and clathrin (9–11). It is now appreciated that β-arrestins regulate physiology and behaviors independently of G protein signaling through their ability to scaffold multiple intracellular signaling molecules such as kinases and phosphatases (3, 4, 12, 13). Studies from our laboratory have shown that through both dopamine D1 and D2 receptors, βarr2-mediated signaling plays a major role in DA-dependent locomotion (14–16).
Parkinson’s disease (PD) is a neurodegenerative disorder caused by the progressive loss of DA neurons projecting to the striatum that is characterized by severe locomotor deficits and is commonly treated with the DA precursor l-3,4-dihydroxyphenylalanine (l-DOPA) or D2 receptor agonists. Although l-DOPA treatment ameliorates the locomotor deficits, prolonged l-DOPA use causes dyskinesias, termed as l-DOPA–induced dyskinesias (LIDs) in humans or abnormal involuntary movements (AIMs) in animal models. Despite these drawbacks, l-DOPA is still the mainstay of PD treatment for its unsurpassed antiparkinsonian efficacy. Studies in animal models of PD suggest that dyskinesias are associated with enhanced G protein-mediated signaling at dopamine receptors (17–23) potentially leading to changes in gene expression and uncontrolled neuronal excitability (21, 24–28). Therefore, PD therapy strategies that moderate G protein signaling and neuronal excitability while maintaining normal movement may be an ideal way to eliminate DA receptor-associated dyskinesias. To moderate this uncontrolled signaling or neuronal excitability, several approaches have been explored such as reducing D1R surface expression (29, 30), dampening overactive intracellular signaling (20, 23, 31, 32), and inhibiting A2A (33, 34), mGluR5 (35–37) or NMDA receptors (24, 25, 38–40). Although these targets have clinical potential, several drugs to these targets have either failed clinical trials or have the potential to affect other key CNS physiological processes. As a novel approach, targeting βarr2 function in the DA system might be desirable because through its desensitization of G protein signaling, it can reduce dyskinesias and simultaneously through its signaling ability facilitate locomotion, without potentially affecting other neurotransmitter systems.
In the current study we provide evidence supporting the hypothesis that up-regulating βarr2 expression ameliorates LIDs but enhances the therapeutic effects of l-DOPA. We use four different animal models of PD and LIDs to support this notion: (i) a nonconventional mouse model of acute PD symptoms: the bilateral DA-deficient dopamine transporter (DAT)-KO (DDD) mouse (41, 42), (ii) a unilateral 6-hydroxydopamine (6-OHDA)-lesioned mouse model, (iii) a unilateral 6-OHDA–lesioned rat model, and (iv) a bilateral 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned nonhuman primate (NHP) model of PD. Using these various animal models we show that deletion of βarr2 enhances LIDs and reduces forward locomotion but overexpression of βarr2 in the striatum reduces LIDs and enhances the therapeutic effects of l-DOPA.
Results
Using genetically engineered mice, we have previously shown that βarr2 but not βarr1 plays a major role in DA-dependent locomotion (6, 14–16). Other studies have shown that β-arrestins and GRK6 are overexpressed in mouse and monkey models of PD (43, 44), and that lentiviral-mediated overexpression of GRK6 in rodent and monkey models of PD reduces LIDs (30). GRK6, which lies upstream of β-arrestins in the GPCR signaling cascade, presumably promotes β-arrestin–dependent signaling (45–47). Therefore, we reasoned that βarr2 might play a critical role in the mechanism of l-DOPA therapy in PD.
To examine this question, we used a simple but robust bilateral model of acute and reversible PD symptoms, which can be used to reliably assess behaviors and perform genetic and pharmacological manipulations. We have previously shown that the DAT-KO mice rely solely on de novo synthesis to maintain ambient levels of DA and that treatment with the irreversible inhibitor of tyrosine hydroxylase, alpha-methyl-p-tyrosine (AMPT), produces an acute, complete, and prolonged depletion of presynaptic DA bilaterally (41, 42). This DDD mouse shows characteristic PD phenotypes that can be acutely and completely reversed by l-DOPA.
As shown in Fig. 1A, when injected with AMPT [250 mg/kg, intraperitoneally (i.p.)], DAT-KO (DDD/WT) or DAT/βarr2 double KO (DDD/βarr2KO) mice become akinetic. After 1 h in this akinetic state, treatment with l-DOPA/benserazide (LD/Benz) [50/12.5 mg/kg, subcutaneously (s.c.)] induces a robust locomotor response in the DDD/WT mice, essentially returning them to their characteristic hyperactive levels (48). However, in the DDD/βarr2KO mice, a drastically reduced and shortened locomotor response is observed (Fig. 1A, **P < 0.01), which is surprisingly replaced by an increase in vertical beam breaks over this period of 2 h of acute l-DOPA exposure (Fig. 1B, ***P < 0.001). The reduction in the locomotor response in the DDD/βarr2KO mice can be attributed to genetic deletion of βarr2 and not to changes in DA levels or metabolism (Fig. S1A). We observed that unlike normal rearing behavior, the DDD/βarr2KO mice were persistently in a vertical position that was accompanied by rapid limb movements and tongue protrusions/licking (Movie S1). Vertical activity has been suggested to reflect dyskinesias in rodents because pharmacological agents that prevent dyskinesias in humans selectively inhibit vertical activity induced by l-DOPA in rodents (49). Interestingly, in the Pitx3/aphakia genetic mouse model of bilateral DA neuron degeneration, chronic l-DOPA treatment results in vertical-like activity that was termed “three-paw dyskinesia” by the authors (50) and is phenotypically similar to the behavior observed in the l-DOPA–treated DDD/βarr2KO mice. These data suggest that deletion of βarr2 renders the DDD mice acutely susceptible to the dyskinetic effects of l-DOPA. Interestingly, when acute administration of l-DOPA (Fig. 1 A and B) is substituted by a more clinically relevant chronic l-DOPA treatment strategy (daily 125 mg/kg, i.p. AMPT; an hour later l-DOPA/benserazide, 25/12.5 mg/kg, s.c.) for 17 d, the DDD/WT mice display a modest gradual decrease in forward locomotion (Fig. 1C, blue) but a gradual increase in persistent vertical activity/three-paw dyskinesia (Fig. 1D, red, **P < 0.01) that mimics the acutely injected DDD/βarr2KO mice (Fig. 1B). We observe the same phenomenon in the DDD/WT mice after an acute high dose of l-DOPA (100 mg/kg, Fig. S1 B and C).
Fig. 1.
Acute and chronic l-DOPA–induced locomotion and dyskinesias in the DDD model of PD symptoms. For acute experiments all mice were injected with AMPT (250 mg/kg, i.p.) for 60 min (DDD) followed by saline (SAL) or l-DOPA/benserazide (LD/Benz, 50/12.5 mg/kg, s.c.) and monitored for 120 min. Upon acute l-DOPA injection, (A) DAT-KO/βarr2WT (DDD/WT) mice show a robust locomotor response but DAT-KO/βarr2KO (DDD/βarr2KO) mice show a significant blunted locomotor response (**P < 0.01). (B) DDD/WT mice show low vertical activity, whereas DDD/βarr2KO mice display significantly enhanced vertical activity upon acute l-DOPA treatment (***P < 0.001). For chronic experiments all mice were injected with AMPT (125 mg/kg, i.p.) for 60 min (DDD) followed by saline (SAL) or l-DOPA/benserazide (LD/Benz, 25/12.5 mg/kg, s.c.) for 120 min daily for 17 d and cumulative total distance or vertical activity for each day was recorded. (C) DDD mice show a gradual decrease in the l-DOPA locomotor response as assessed by total distance traveled but D shows gradual increased vertical activity upon l-DOPA treatment. n = 8–10 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with saline (SAL); #P < 0.05 compared with day 1. (E) Representative blots showing ERK and S6 phosphorylation 30 min after l-DOPA exposure on last day. (F) Quantification of ERK and S6 phosphorylation normalized to total protein levels shows increased ERK and S6 phosphorylation, n = 6–8 per group per treatment. *P < 0.05 compared with saline group.
Previous studies in mice have shown that signaling cascades such as extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways might be important in mediating LIDs (20, 23). Accordingly, we assessed phosphorylation status of ERK 1/2 (T202/Y204) and S6 ribosomal protein (S235/236), two biomarkers previously correlated with increased occurrence of LIDs. Upon completion of the chronic l-DOPA behavioral study, mice were killed 30 min after saline or l-DOPA injection on the last day and Western blot analysis was performed on striatal lysates in the DDD mice. As shown in Fig. 1 E and F, a significant increase in ERK and S6 phosphorylation was observed after chronic l-DOPA injection compared with saline-injected mice (*P < 0.05), thereby providing biochemical validation to the persistent vertical activity in the DDD model as a correlate for dyskinesias.
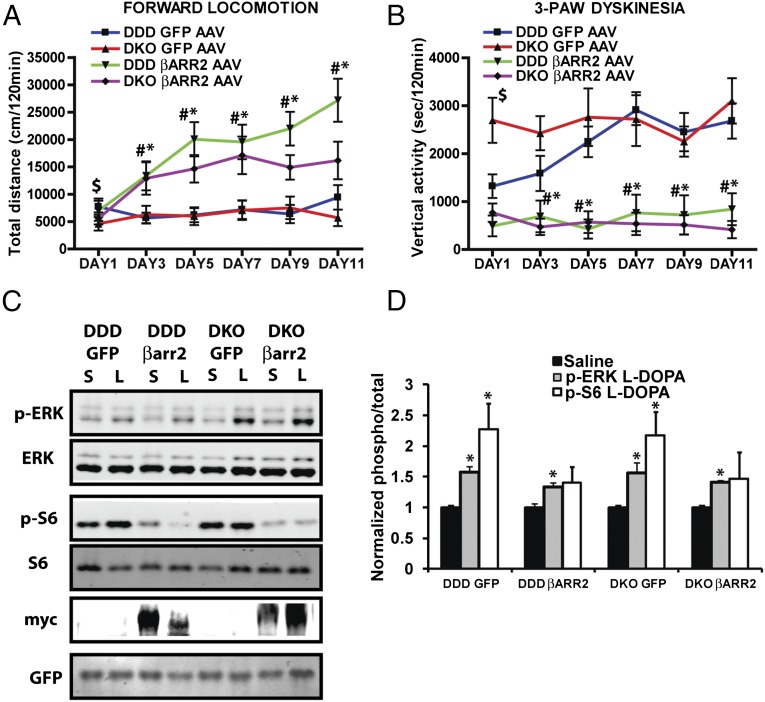
We next explored whether restoring or overexpressing βarr2 would prevent or eliminate the manifestation of dyskinesias in the DDD mice. Striata of DDD/WT (DDD) and DDD/βarr2KO (DKO) mice were infected with adeno-associated viruses (AAVs) encoding either green fluorescent protein (GFP) (control: GFP AAV) or βarr2 (βarr2 AAV) (Fig. S1D) followed by a chronic l-DOPA administration regimen as described in Fig. 1 and Materials and Methods. Chronic l-DOPA treatment does not increase forward locomotion (Fig. 2A) but markedly exacerbates the development of persistent vertical activity/three-paw dyskinesia in GFP AAV-injected DDD/βarr2KO mice (Fig. 2B DKO GFP AAV, red line, $P < 0.05) compared with GFP AAV-injected DDD/WT control mice (Fig. 2B DDD GFP AAV, blue line), which has a delayed onset of dyskinesia similar to Fig. 1C. AAV-mediated restoration of βarr2 expression in the DDD/βarr2KO (DKO) mice markedly enhances l-DOPA–mediated locomotion (Fig. 2A, purple line, #P < 0.05) while completely eliminating the dyskinetic behavior (Fig. 2B, DKO βarr2 AAV, purple line, #P < 0.05). Consistent with the beneficial effect of βarr2 signaling, overexpression of βarr2 in DDD/WT mice not only provided resistance to l-DOPA–induced dyskinesia (Fig. 2B, DDD βarr2 AAV, green line, *P < 0.05) but also markedly enhanced forward locomotion in control DDD/WT mice (Fig. 2A, DDD βarr2 AAV, green line, *P < 0.05). Together these results suggest that deletion of βarr2 in the DDD model increases dyskinesias while reducing forward locomotion, whereas overexpression or restoration of βarr2 decreases dyskinesias and at the same time enhances forward locomotion.
Fig. 2.
βarr2 expression modifies l-DOPA–induced locomotion and dyskinesias in a DDD model of PD symptoms. All mice were injected with AMPT (125 mg/kg i.p.) for 60 min (DDD) followed by saline or l-DOPA/benserazide (25/12.5 mg/kg, s.c.) and monitored for 120 min, daily for 12 d. Overexpression or restoring βarr2 enhances (A) forward locomotion but decreases (B) vertical activity in DDD/WT (DDD βarr2 AAV, green) or DKO (DKO βarr2 AAV, purple) mice. However, control mice (overexpression of GFP) show (A) reduced forward locomotion but (B) increased vertical activity/three-paw dyskinesia in DDD/WT (DDD GFP AAV, blue) or DKO (DKO GFP AAV, red) mice. n = 9–11 mice per group. *P < 0.05 compare DDD βarr2 to DDD GFP, #P < 0.05 compare DKO βarr2 to DKOGFP, $P < 0.05 compare DDDGFP to DKOGFP. (C) Representative blots showing ERK and S6 phosphorylation 30 min after saline (S) or l-DOPA (L) exposure on last day. (D) Quantification of ERK and S6 phosphorylation normalized to total protein levels show increased ERK and S6 phosphorylation. *P < 0.05 compared with saline treatment for each group.
Additionally, we observe a significant increase in ERK and S6 phosphorylation after chronic l-DOPA injection in only the GFP-injected mice (Fig. 2 C and D, *P < 0.05) but a reduced S6 phosphorylation in the βarr2 overexpressed mice, suggesting that in addition to behavioral outcomes, overexpression of βarr2 normalizes certain biochemical markers of LIDs.
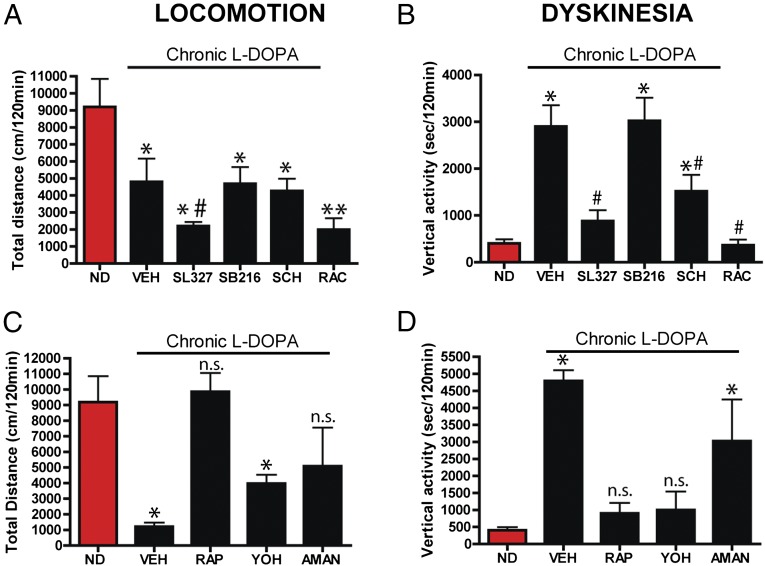
Previous evidence has shown that pharmacological inhibition of GSK3β, MEK/ERK, or mTOR signaling pathways inhibits DA-dependent locomotor behaviors in mice (23, 51, 52). Compared with acute l-DOPA–treated nondyskinetic (ND, red bar) DDD mice, chronic l-DOPA–treated dyskinetic DDD mice (vehicle, VEH) have reduced forward locomotion (Fig. 3A, *P < 0.05) but enhanced dyskinesia (Fig. 3B, *P < 0.05). Compared with dyskinetic DDD mice (VEH) acute administration of the MEK inhibitor SL327 but not the GSK3 inhibitor SB216763 inhibited remaining forward locomotor activity (Fig. 3A, #P < 0.05) and dyskinesia (Fig. 3B, #P < 0.05). However, administration of the mTOR pathway inhibitor rapamcyin restored forward locomotion and reduced dyskinesias (Fig. 3 C and D). We also tested antidyskinetic drugs that target other neurotransmitter systems such as amantadine (glutamate) and yohimbine (noradrenergic). Both amantadine and yohimbine partially rescued forward locomotion (Fig. 4C), whereas amantadine partially inhibited dyskinesia and yohimbine had a more pronounced inhibition of dyskinesia (Fig. 4D) in the chronically l-DOPA–treated dyskinetic DDD mice. We have previously shown that βarr2 promotes DA-dependent signaling in either a D1R- or D2R-dependent manner (15, 16). As shown in Fig. 3 A and B, injection with either the D1R antagonist SCH23390 or D2R antagonist raclopride along with l-DOPA inhibited both forward locomotion and dyskinesia, consistent with the classical observations of the engagement of both receptor subtypes for general DA-mediated behaviors (53–55).
Fig. 3.
Pharmacological characterization of l-DOPA behaviors in the DDD model of PD symptoms. DAT-KO mice were acutely injected with AMPT (125 mg/kg i.p.) for 60 min (DDD) followed by acute l-DOPA/benserazide (25/12.5 mg/kg, s.c.) such that they were nondyskinetic (ND, red bars) and total distance traveled and vertical activity was recorded for 120 min. The chronic group was injected with AMPT (125 mg/kg i.p.) for 60 min (DDD) followed by saline or l-DOPA/benserazide (25/12.5 mg/kg, s.c.) and monitored for 120 min daily for 14 d. (A and C) Total distance traveled, i.e., locomotion. (B and D) Vertical activity, i.e., dyskinesia recorded on the 14th day. (A and B) On the last day 15 min before l-DOPA injection, DDD mice were treated with either vehicle (VEH), SL327 (100 mg/kg i.p.), SB216273 (SB216, 10 mg/kg i.p.), SCH23390 (SCH, 0.2 mg/kg, s.c.) and raclopride (RAC, 2 mg/kg, i.p.) or (C and D) rapamycin (RAP, 5 mg/kg i.p.), yohimbine (YOH, 10 mg/kg i.p.), and amantadine (AMAN, 90 min before l-DOPA, 40 mg/kg s.c.). n = 8–10 mice per group. *P < 0.05 **P < 0.01 compared with ND DDD mice for each group. #P < 0.05 compare SL327 to VEH. ns, not significant compared with ND DDD mice.
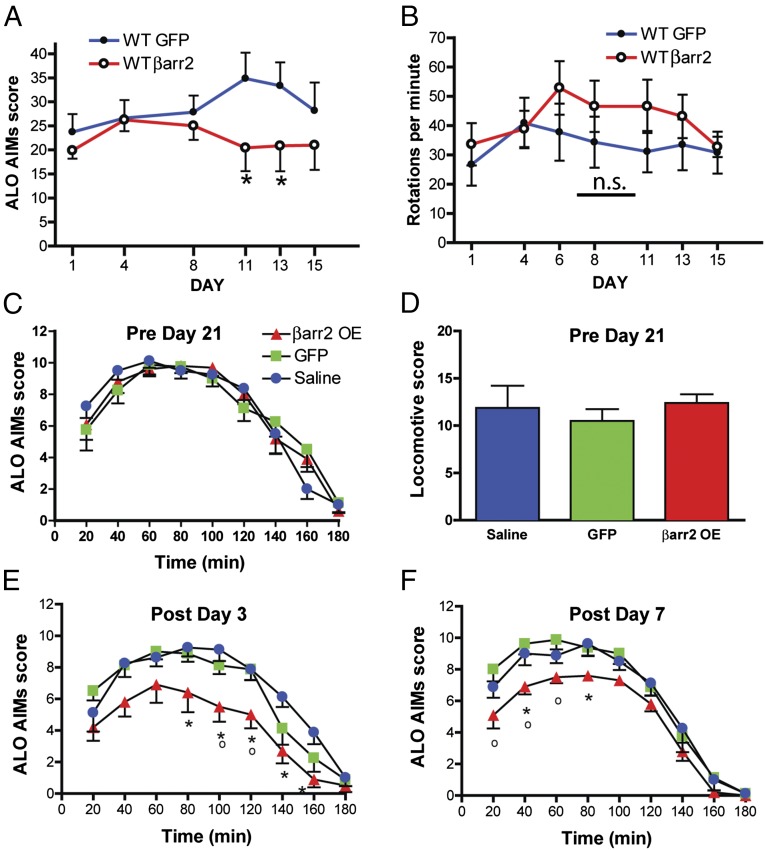
Fig. 4.
βarr2 overexpression in 6-OHDA rodent PD models. (A and B) C57BL/6J mice were unilaterally injected with 6-OHDA and infected unilaterally in the striatum with either a GFP virus or a βarr2 virus and then chronically treated with l-DOPA/benserazide (20/12.5 mg/kg s.c.) once daily for 14 d. ALO AIMs (A) and rotations (B) were recorded. n = 8 mice per group. *P < 0.05 compare GFP injected mice to βarr2 virus-injected mice. (C and D) Rats were unilaterally injected with 6-OHDA and then chronically treated with l-DOPA/benserazide (6/15 mg/kg i.p.) once daily for 21 d. ALO AIMs (C) and rotations (D) were recorded. Following chronic l-DOPA, the rats were infected in the striatum with either a GFP virus or a βarr2 virus and chronically treated with l-DOPA and ALO AIMs were recorded on day 3 (E) and day 7 (F). *P < 0.05 compare GFP or saline-injected rats to βarr2 virus-injected rats for each group. °P < 0.05 βarr2OE compared with GFP injected rats. ns, not significant.
Whereas the DDD mouse model represents a convenient pharmacological model of bilateral DA depletion that produces acute PD symptoms, it is unconventional as a PD model, because there is no neurodegeneration/DA neuron loss and the dyskinesias are atypical (vertical activity/three paw). To further confirm our observations in the DDD mice, we examined the effects of βarr2 overexpression on l-DOPA–induced dyskinesias in three additional conventional models of DA neuron loss and PD symptoms.
The unilateral 6-OHDA rodent lesion method is one of the most widely used neurodegeneration models in PD research. To assess the effect of βarr2 overexpression on dyskinesias, we first used the 6-OHDA–lesioned mouse model for which a thoroughly validated manual dyskinesia scoring protocol is widely used and can report dyskinetic manifestations of genetic or pharmacological manipulations (56–58). These dyskinesias are termed “abnormal involuntary movements” (AIMs) in rodents. The unilateral 6-OHDA lesion and viral infections were done on the same day as described in Materials and Methods. C57BL6/J wild-type mice were unilaterally injected with 6-OHDA and infected with either GFP (control) or βarr2 AAV and were allowed to recover for 3 wk to allow for the effects of 6-OHDA and viral expression (Fig. S1 E and F). The GFP or βarr2 AAV-injected/lesioned mice were then subjected to a chronic daily l-DOPA (20 mg/kg, s.c.) administration regimen for 2 wk and AIMs were analyzed as described in Materials and Methods. Similar to the DDD mice, GFP AAV-injected 6-OHDA–lesioned mice displayed a time-dependent increase in l-DOPA–induced axial, limb, orolingual (ALO) AIMs score (Fig. 4A, closed circles). However, injection of βarr2 AAV led to a significant decrease in AIM score on days 11 and 13 (Fig. 4A, open circles, *P < 0.05). Interestingly, there were no significant differences observed in locomotive AIMs as assessed by contralateral rotations (Fig. 4B, P = 0.36).
To further confirm these observations, we performed 6-OHDA lesioning in rats (Fig. S2 A and B). Dyskinesias are observed in patients with PD after a few years of l-DOPA treatment. To mimic conditions of already established dyskinesias, we initially primed rats for 21 d with l-DOPA (PRE) so that they developed AIMs and then assessed the effect of βarr2 overexpression (POST). Before viral injection, all groups of rats were treated with l-DOPA for 21 d, wherein they developed ALO and locomotive AIMs to the same extent (Fig. 4 C and D). After viral infection (Fig. S2 C and D) and 3 wk of recovery, rats were then again subjected to a chronic l-DOPA regimen. βarr2 overexpression significantly decreased ALO AIMs (Fig. 4 E and F and Fig. S3 B and D) but not locomotive AIMs (Fig. S3 A and C) upon postviral chronic l-DOPA injection. These data further confirmed that βarr2 overexpression has a beneficial inhibitory effect on l-DOPA–induced dyskinesias.
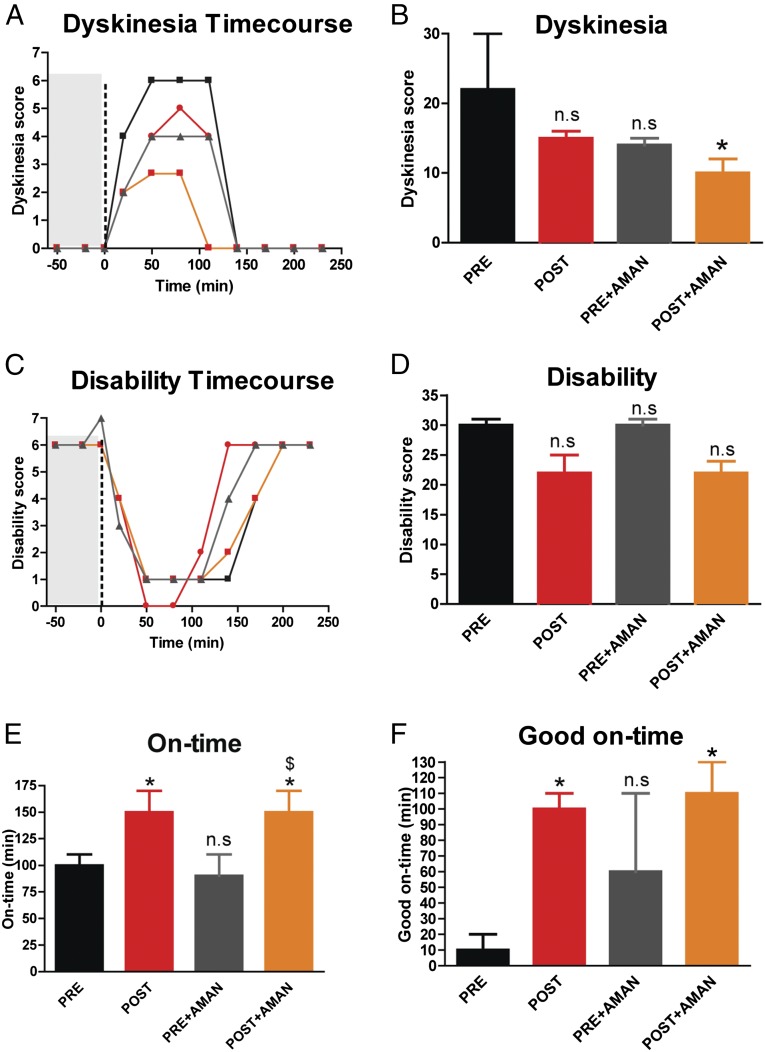
To provide an even more compelling validation of our observations, we performed experiments to test the potential effects of βarr2 in the nonhuman primate (NHP) MPTP model of PD. The NHP MPTP model of PD is considered the most clinically relevant model for dyskinesia studies because it very closely mimics the LIDs observed in human patients. Experimental details are described in Materials and Methods. Dyskinetic MPTP-lesioned macaque monkeys received stereotaxic administration of βarr2 AAV in their putamen. Behavior of the animals was investigated prior (PRE, black bars/curves, Fig. 5) and after the surgery (i.e., after βarr2 overexpression, POST, red bars/curves, Fig. 5 and Fig. S4A for transfection volume). βarr2 overexpression decreased the dyskinesia score (Fig. 5 A and B) and the disability score, i.e., further improving parkinsonism (severity of akinesia/bradykinesia) (Fig. 5 C and D) compared with PRE. Interestingly, at the same time, βarr2 overexpression significantly increased (*P < 0.05) both the “on-time” (time of absence of akinesia/bradykinesia) (Fig. 5E) and the “good on-time” (time of absence of akinesia/bradykinesia, without troublesome dyskinesia) (Fig. 5F) of l-DOPA treatment. These data suggest that βarr2 overexpression not only reduces dyskinesia but importantly increases the duration of the beneficial therapeutic effect of l-DOPA. Interestingly, βarr2 overexpression (POST, red bars) is similar to the treatment of dyskinetic NHPs with the clinically used antidyskinetic agent amantadine before surgery (PRE + AMAN, gray bars), in reducing the dyskinesia score (Fig. 5 A and B) but is significantly better at improving the disability score (Fig. 5 C and D) and the good on-time (Fig. 5 E and F). Additionally, βarr2 overexpression has a significant additive effect when combined with amantadine (POST + AMAN, orange bars) in reducing the dyskinesia score (Fig. 5 A and B) after surgery. These data suggest that unlike amantadine, βarr2 overexpression reduces dyskinesia in addition to enhancing the beneficial therapeutic effect, i.e., on-time of l-DOPA (comparing PRE and POST datasets) in the MPTP NHP and βarr2 overexpression-induced dyskinesia improvement is mechanistically different from the one of amantadine, leaving open the potential for combined treatment.
Fig. 5.
βarr2 overexpression in a MPTP macaque PD model. Macaques were injected with MPTP and chronically injected with l-DOPA (PRE) followed by l-DOPA supplemented with amantadine (PRE + AMAN). The same macaques were injected with a βarr2 virus and chronically injected with l-DOPA (POST) followed by l-DOPA supplemented with AMAN (POST + AMAN). For each condition, (A) dyskinesia time course, (B) total dyskinesia, (C) disabilty time course, (D) total disability, (E) on-time, and (F) good on-time were recorded. n = 3 monkeys. *P < 0.05 compare all groups to PRE. $P < 0.05 compare POST + AMAN to PRE + AMAN. ns, not significant compared with PRE. A and C show scores recorded for 60 min (gray area) before l-DOPA administration (vertical dashed black line at t = 0 min).
Discussion
The mainstay of PD motor symptom therapy since the 1960s is the DA precursor l-DOPA (59, 60) and will likely remain so until better therapies are developed. A rational approach from the prospect of novel pharmacotherapies should first identify and then exploit the biology that separates l-DOPA–induced therapeutic effects from l-DOPA–induced dyskinesia.
DA neurons progressively degenerate in PD, thus depriving postsynaptic DA GPCRs from their endogenous ligand, rendering them supersensitive. This sensitized state is thought to underlie, at least in part, the etiology of dyskinesias, owing to uncontrolled downstream signaling and neuronal activity. Therapeutic strategies that target this supersensitivity without compromising the beneficial effects would be ideal. Enhancing β-arrestin2 function seems to be an appropriate approach because β-arrestins are known to desensitize receptor signaling and at the same time activate signaling pathways known to mediate locomotion.
Several animal models of PD exist to study the etiology and mechanistic implications of PD and these models have been used to test therapeutic strategies for PD. Here we present evidence wherein in the bilateral acute DDD mouse model of PD symptoms, genetic manipulation of βarr2 affords a separation of the biology of the therapeutic and dyskinesia effect of l-DOPA. The DDD model, which has been shown to have reproducible, nonvariable, bilateral DA depletion, is also amenable to bilateral genetic manipulations and importantly can be subjected to automated robust behavioral screens to test therapeutic efficacy. Using the DDD model, we show that deletion of βarr2 (DDD/βarr2KO) increases vertical beam brakes but reduces forward locomotion upon acute l-DOPA exposure. Interestingly, a clinically relevant chronic l-DOPA exposure regimen in the DDD/WT mice phenocopies the acute l-DOPA–treated DDD/Barr2KO mice i.e., increased vertical beam brakes. The vertical activity measured by the automated activity monitors is the result of a change in the axis of the mouse from a horizontal to a vertical plane, thereby mimicking axial AIMs (Movie S1). Additionally, these mice also display tongue protrusions and continuous rapid limb movements (three-paw dyskinesia) mimicking orolingual and limb AIMs, respectively, that occur simultaneously with the axial AIMs. Thus, we argue that persistent vertical beam breaks represent dyskinesia and the pattern of dyskinesia observed in the DDD mice is similar to other unilateral rodent PD models of dyskinesias but is a consequence of bilateral DA depletion. Similar dyskinetic patterns are observed in other bilateral DA depletion/neurodegeneration models (49, 50) and need to be characterized further. In addition to behavioral changes observed in the DDD mice, we also observe changes in biochemical markers of AIMs such as the ERK and mTOR (phospho-S6) pathways upon chronic l-DOPA administration.
Overexpression of βarr2 in the striatum of DDD or DDD/βarr2KO mice prevents or rescues both the behavioral and biochemical phenotypes, thereby reducing dyskinesias and increasing forward locomotion and normalizing biochemical markers of LIDs—ERK and S6 phosphorylation. Similar to the DDD model, we observe that in conventional rodent or macaque neurodegeneration PD models, βarr2 overexpression reduced dyskinesia scores when they were either l-DOPA primed or nonprimed. In the macaque model, βarr2 overexpression was better than amantadine, a common clinical antidyskinetic agent (38, 39), in reducing dyskinesias and simultaneously enhancing the therapeutic effect of l-DOPA. These data not only validate the use of the DDD model and persistent vertical activity recording as a correlate for dyskinesias but also validate β-arrestin2–dependent functions as a valid target for dyskinesias in l-DOPA therapy in PD. Furthermore, these data suggest that whereas the absence of βarr2 reduces the beneficial locomotor response of l-DOPA, it enhances the manifestation of l-DOPA–induced dyskinesias. We presume that this happens because of a lack of desensitization by βarr2 and a shift of the system to predominantly G protein-dependent signaling. Conversely, overexpression of βarr2 increases desensitization of G protein signaling and at the same time enhances βarr2-dependent signaling thereby promoting locomotion. Therefore, moderating G protein signaling in PD l-DOPA therapy may be an effective way to eliminate associated dyskinesias either through directly blocking G protein signaling or increasing activation of βarr2 either genetically or pharmacologically at DA receptors, thus enhancing the desirable effects such as initiation of movement and locomotion that are beneficial to patients with PD.
Consensus exists on the primary role of D1Rs for the genesis of dyskinesias (27, 61), which is a hyperkinetic manifestation. Highlighting the pivotal role played by D1R in pathophysiology of dyskinesia should not neglect the role, although minor, of D2R in the same phenomenon. However, simultaneous action of D1 and D2 receptors are required for the therapeutic effect of l-DOPA, as both indirect and direct pathway are required for a proper goal-directed locomotion (62–64). Consistent with this notion, studies with GRK6 overexpression on l-DOPA behaviors also suggest that a balance between D1Rs and D2Rs is important for antiparkinsonian effects (30). Therefore, we propose that in addition to focusing on particular receptor subtypes, we should also focus on selective activated signaling cascades beyond their stimulation. For instance, we confirm here that pharmacological inhibition of D1R and MEK/ERK signaling inhibits l-DOPA–induced behaviors, whereas inhibition of mTOR signaling with rapamycin inhibits only AIMs and restores forward locomotion, consistent with previous findings (23). Furthermore, pharmacological inhibition of GSK3 does not alter l-DOPA behaviors, suggesting that D2R/βarr2 signaling pathways, which activate GSK3β, may not play a critical role.
The discovery of the dichotomous signaling nature of GPCRs (G protein vs. β-arrestin) has led to the development and application of the concept of biased signaling or functional selectivity. Evidence from the literature has shown that individual signaling pathways through the same receptor have the ability to mediate distinct physiological responses. Moreover, targeting these specific signaling pathways affects specific physiological outcomes without affecting others. Thus, leveraging functional selectivity to exploit this bimodal signaling might prove to be beneficial for PD l-DOPA therapy. In the future, either generating allosteric G protein-biased antagonists at D1Rs as a supplement to l-DOPA or generating β-arrestin–biased agonists at D1 and/or D2 receptors might mimic the actions of l-DOPA without inducing dyskinesias.
Materials and Methods
Animals.
Mouse.
All mouse studies were performed according to NIH guidelines for animal care and use and were approved through the Duke University Animal Care and Use Committee. All mice were housed at a maximum of five per cage, provided with food and water ad libitum, and tested at 10–20 wk of age. Mice were age and sex matched and all experiments were performed in drug-naïve animals. DAT-KO and DAT/β-arrestin2 double knockout mice have been described before (14, 48). C57BL6/J wild-type mice and βarr2KO mice (65) used for 6-OHDA experiments have been described before.
Rat and monkey.
Experiments were performed in accordance with the European Union directive of September 22, 2010 (2010/63/EU) on the protection of animals used for scientific purposes and were approved by the Institutional Animal Care and Use Committee (IACUC) of Bordeaux (CE50) under the license numbers 5012099-A (rats) and 50120102-A (monkeys). Monkey experiments were performed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility following acceptance of study design by the Institute of Lab Animal Science (Chinese Academy of Science, Beijing) IACUC.
Mouse Experimental Schedule.
DDD mice.
For behavioral analyses DAT-KO mice were injected with α-methyl-p-tyrosine (AMPT, 250 mg/kg, i.p. for acute and 125 mg/kg, i.p. for chronic experiments) to induce acute DA-depletion (DDD) and parkinsonian symptoms such as akinesia. Following AMPT injection for 1 h, DDD mice were injected with l-DOPA (25 mg/kg, s.c.) and benserazide (12.5 mg/kg, s.c.) and activity was recorded in an Accuscan activity monitor (Accuscan Instruments) as described previously (16, 66). Total distance traveled (forward locomotion) and vertical activity (dyskinesia) were measured at 5-min intervals and data were analyzed in 5-min increments for 120 min. For chronic experiments, daily injections of AMPT (125 mg/kg, i.p.) and l-DOPA/benserazide were administered and activity was recorded. For viral rescue or overexpression experiments, virus was injected 2 wk before chronic l-DOPA experiments. A total of 0.5 μL of concentrated viruses (AAV10-mCerulean-βarr2 or AAV10-GFP) were bilaterally injected at the following two coordinates from bregma: +0.3 mm anteroposterior (AP), ±2.2 mm mediolateral (ML), and −3.0 mm dorsoventral (DV) below dura and at +1.1 mm AP, ±1.7 mm ML, and −2.9 mm DV below dura.
6-OHDA mice.
Experimental parkinsonism was achieved through unilateral injection of 6-hydroxydopamine (6-OHDA, 3 µg/µL; Sigma) into the striatum as described previously (57). 6-OHDA was dissolved in saline containing 0.02% ascorbic acid and unilaterally injected in the striatum at the following two coordinates from bregma: 2 µL at +0.3 mm anteroposterior, +2.2 mm lateral, and −3.0 mm dorsoventral below dura and 2 μL at +1.1 mm anteroposterior, +1.7 mm lateral, and −2.9 mm dorsoventral. A total of 0.5 μL of concentrated virus (AAV10-mCerulean-βarr2 or AAV10-GFP) was injected following 6-OHDA injections at the same sites unilaterally. After 3 wk of recovery, animals underwent chronic injection of l-DOPA (20 mg/kg, s.c.) and benserazide (12.5 mg/kg, s.c.) once daily for 14 d for development of AIMs. The four AIMs categories (limb, axial, orolingual, and locomotive) were scored using a validated rating scale (56, 67) for 1 min every 20 min for 2 h by a blinded trained investigator.
Rat experimental schedule.
A total of 26 male Sprague-Dawley rats (Charles River Laboratories) were kept under regular lighting conditions (12 h light/dark cycle) and given food and water ad libitum. Experimental parkinsonism was achieved as previously described (68–71) through unilateral injection of (6-OHDA, 3 µg/µL; Sigma) into the medial forebrain bundle (MFB). 6-OHDA was dissolved in saline (NaCl 0.9% wt/vol) containing 0.1% ascorbic acid injected in the MFB according to the following coordinates from bregma: 2.5 µL at anteroposterior −3.7 mm, mediolateral 1.7 mm, and dorsoventral −7.5 mm below dura. To preserve the serotoninergic and noradrenergic neurons from the toxicity of 6-OHDA, rats were pretreated with the reuptake inhibitors citalopram (1 mg/kg, i.p.; Lundbeck) and desipramine hydrochloride (20 mg/kg, i.p.; Sigma). After 2 wk of recovery, animals underwent a chronic injection once daily with a solution containing l-DOPA (6 mg/kg, i.p.; Sigma) and benserazide (15 mg/kg, i.p.) for 21 d to induce a gradual development and a stable degree of AIMs. Then, dyskinetic rats were randomly sorted in three groups and subjected to striatal injections. Five microliters of concentrated viruses (AAV10-mCerulean-βarr2 or AAV10-GFP) or saline were injected at a flow rate of 0.25 µL/min into the striatum (ipsilateral to the lesion side) at coordinates anteroposterior +0.56, mediolateral −3.0, and dorsoventral −4.5 below dura. After 3 wk, l-DOPA treatment (6 mg/kg l-DOPA plus 15 mg/kg benserazide, i.p.) resumed for 7 d. AIMs were scored at days 3 and 7. This protocol allowed us to study the impact of βarr2 overexpression upon already established dyskinesia. The four AIM categories [limb, axial, orolingual (ALO) and locomotive] were scored using a validated rating scale (56, 67) for 1 min every 20 min for 3 h by a trained investigator as previously described (29, 30).
Monkey experimental schedule.
Three male macaques (Macaca fascicularis; aged 5 ± 1 y; weight = 5.1 ± 0.9 kg) were housed in individual primate cages allowing visual contacts and interactions with monkeys housed in adjacent cages, under controlled conditions. Food and water were available ad libitum. Animal care was supervised daily by veterinarians skilled in the healthcare and maintenance of nonhuman primates. The MPTP intoxication protocol, the chronic l-DOPA treatment, the clinical assessments, the terminal procedure, and the characterization of the extent of nigrostriatal denervation were conducted as previously published (29–31, 72–74). Animals were first rendered parkinsonian with MPTP-hydrochloride (0.2 mg/kg, i.v.; Sigma) dissolved in saline (30, 31, 72–74). Daily (9:00 AM) assessment of parkinsonism was performed in home cages for 30 min by two blinded observers using a validated rating scale (75) assessing tremor, general level of activity, body posture (flexion of spine), vocalization, freezing, and frequency of arm movements and rigidity (for each upper limb). Following stabilization of the MPTP-induced syndrome (3 mo), animals received twice daily 20 mg/kg l-DOPA p.o. for 3 mo and developed severe and reproducible dyskinesia, presenting choreic–athetoid (characterized by constant writhing and jerking motions), dystonic, and sometimes ballistic movements (large-amplitude flinging, flailing movements). Once animals were stably dyskinetic, striatal stereotactic delivery of viral vector was conducted under isoflurane anesthesia as previously described (29–31). Horsley-Clarke stereotaxic technique coupled with ventriculography was used to determine the position of left and right putamen. A total volume of 120 µL of concentrated virus (AAV10-mCerulean-βarr2) was injected bilaterally into each animal (60 µL per side at three rostrocaudal and two dorsoventral sites (AP −4, −1, and 1; ML ±14; DV 0 and 3 from anterior commissura) with a Hamilton syringe mounted into a microinjector system (WPI).
Behavioral assessment.
Monkeys’ response to their tailored dose of l-DOPA/carbidopa (ranging between 15 and 20 mg/kg for maximal reversal of PD symptoms) was defined before AAV intracerebral injections. Starting at 4 wk postsurgery, animals were repeatedly assayed 2 d apart for behavioral responses to 100% of the tailored dose of l-DOPA [per os (p.o.)], alone or in combination with amantadine (p.o. 20 mg/kg). The monkeys’ behavior was first recorded in the OFF state for 60 min in an observation cage (dimensions: 1.1 m × 1.5 m × 1.1 m). Drugs were then administered, and the monkeys’ behavior was recorded for a further 240 min in the observation cage as per guidelines (76) and as previously described (29–31, 72). The total duration of observation was 300 min including drug administration. The parkinsonian condition (and its reversal) was assessed on a parkinsonian monkey rating scale using videotape recordings of monkeys. A score of 0 corresponds to a normal animal and a score above 6, to a parkinsonian animal. The severity of dyskinesia was rated using the Dyskinesia Disability Scale: 0, dyskinesia absent; 1, mild, fleeting, and rare dyskinetic postures and movements; 2, moderate, more prominent abnormal movements, but not interfering significantly with normal behavior; 3, marked, frequent and, at times, continuous dyskinesia intruding on the normal repertoire of activity; or, 4, severe, virtually continuous dyskinetic activity replacing normal behavior and disabling to the animal. Locomotor activity was concomitantly monitored with infrared activity monitors, providing a mobility count every 5 min. The duration of antiparkinsonian action, i.e., on-time, was defined as the number of minutes for which bradykinesia was absent, i.e., score equal to zero. In addition, the duration of on-time associated with dyskinesia of varying severity was defined as follows (76): “good” quality on-time represents the number of minutes for which bradykinesia was zero while dyskinesia was either absent or of mild or moderate severity (0–2). Meanwhile, “bad” quality on-time represents the number of minutes for which bradykinesia was zero while dyskinesia was either marked or severe.
Drugs.
l-DOPA, Benserazide, AMPT, amantadine, 6-OHDA, citalopram hydrobromide, and desipramine hydrocloride were purchased from Sigma and freshly prepared in saline (Sal) or saline containing 0.02% ascorbic acid. SL327 and SB216736 were purchased from Tocris Biosciences and dissolved in a minimal amount of Tween-20 and brought up to volume with water. Rapamycin (RAP) was purchased from LC Labs and dissolved in 5% (vol/vol) ethanol in water as recommended by the vendor. All drugs were injected i.p. at a volume of 5 μL/g animal weight and the doses chosen were based on previous studies.
Western Blot.
Western blot analyses were performed on mice upon completion of behavioral analyses (66). Briefly, striatum was rapidly dissected from mouse brains on ice and tissue samples were immediately lysed in ice-cold RIPA buffer solution. Loading buffer was added to the samples, which were loaded onto 10% SDS gels (Nupage; Invitrogen), transferred to nitrocellulose membranes, and exposed to antibodies to phospho-ERK and ERK (1:500, Cell Signaling Technology), Phospho-S6 ribosomal protein and S6 ribosomal protein (1:500, Cell Signaling Technology), myc (1:500, Santa Cruz Biotechnology), βarr2 (1:500, Cell Signaling), GFP (1:2,000, Abcam), β-arrestin1/2 (A2CT clone, a generous gift from Robert J. Lefkowitz, Duke University, Durham, NC) and GAPDH (1:5,000, Millipore). Primary antibody was followed by infrared secondary antibody (LICOR) and the blots were developed using a LICOR Odyssey detection system.
Immunohistochemistry.
Upon completion of the behavioral testing, all animals (mice, rats, and monkeys) were deeply anesthetized and transcardially perfused for immunohistochemistry experiments with formalin. Brains were removed and postfixed overnight in formalin at 4 °C. Striatum vibratome or cryostat-cut free-floating sections (30 µm for mice, 50 µm for rats and monkeys) were processed for immunohistochemical analyses as described previously for mice (66), rats, and monkeys (29, 30). Whereas the whole striatum was investigated in rodents, immunohistochemical staining was measured in the putamen in monkeys, i.e., where the vector was injected. Images were acquired with Hamamatzu Nanozoomer 2.0 HT. TH-immunoreactive fiber density was analyzed using ImageJ software (NIH). For each animal, optical density was calculated as the mean of the five striatal levels and corrected for nonspecific background, measured in the corpus callosum. After tissue processing for GFP staining, coronal sections of AAV-injected animals were sampled throughout the striatum and for rats and monkeys transduction volume was calculated with Cavalieri’s principle using the Mercator image analysis system (Explora Nova) (29, 77).
Adeno-Associated Virus.
AAV10 virus was generated as described previously using an AAV 2/10 rep/cap plasmid. pAAV plasmids with CMV-GFP or EF1a-βarr2-myc tag-p2A-mCerulean were used to generate viral particles according to previously published methods (78, 79).
Radioligand Binding Assay.
Binding assay was performed as described previously (66).
Statistical Analyses.
Data were analyzed by a standard one-way or two-way ANOVA test for comparison between genotypes, treatments, or doses. Individual genotypes, treatments, or doses were compared using a post hoc Bonferroni test or by Sidak’s multiple comparisons test. For nonhuman primates data were analyzed using Friedman’s nonparametric one-way ANOVA followed by post hoc Dunn’s test. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Q. Li, H. Li, Y. Chen, W. Chu, W. Li, and R. Xing for their expert experimental support and Xiuqin Zhang and Wendy Roberts for maintenance of the mouse colony. This work was supported in part by a target validation grant from the Michael J. Fox Foundation (to N.M.U. and M.G.C.), National Institutes of Health Grant 5R37MH073853 (to M.G.C.) and K01DA024763 (to C.E.B.). The continued support of the Pall Family Foundation is greatly appreciated as is the support from the Sydney R. Bear Prize (to N.M.U.). For rat and monkey experiments, the University of Bordeaux and the Centre National de la Recherche Scientifique provided infrastructural support. This work was also supported in part by Agence Nationale de la Recherche Grant ANR-12-BSV4-0001-01 (to E.B.) and by a grant from LABEX BRAIN ANR-10-LABX-43 (to E.B.). S.B. is a Michael J. Fox-supported postdoctoral researcher. R.R.G. is supported by a Grant 14-50-00069 from the Russian Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502740112/-/DCSupplemental.
References
- 1.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294(5544):1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28(4):166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Attramadal H, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267(25):17882–17890. [PubMed] [Google Scholar]
- 6.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 7.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 9.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 10.Laporte SA, et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96(7):3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 12.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 13.Xiao K, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104(29):12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27(4):881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36(3):551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovoor A, et al. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25(8):2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold SJ, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27(52):14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerfen CR, Paletzki R, Worley P. Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase. J Neurosci. 2008;28(28):7113–7120. doi: 10.1523/JNEUROSCI.3952-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavón N, Martín AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59(1):64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27(26):6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorentini C, Savoia P, Savoldi D, Barbon A, Missale C. Persistent activation of the D1R/Shp-2/Erk1/2 pathway in l-DOPA-induced dyskinesia in the 6-hydroxy-dopamine rat model of Parkinson’s disease. Neurobiol Dis. 2013;54:339–348. doi: 10.1016/j.nbd.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 24.Hallett PJ, et al. Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Neuropharmacology. 2005;48(4):503–516. doi: 10.1016/j.neuropharm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Silverdale MA, et al. Synaptic recruitment of AMPA glutamate receptor subunits in levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate. Synapse. 2010;64(2):177–180. doi: 10.1002/syn.20739. [DOI] [PubMed] [Google Scholar]
- 26.Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: Potential for new therapies. Nat Rev Neurosci. 2001;2(8):577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- 27.Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9(9):665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- 28.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 29.Porras G, et al. PSD-95 expression controls L-DOPA dyskinesia through dopamine D1 receptor trafficking. J Clin Invest. 2012;122(11):3977–3989. doi: 10.1172/JCI59426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed MR, et al. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson’s disease. Sci Transl Med. 2010;2(28):28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasano S, et al. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc Natl Acad Sci USA. 2010;107(50):21824–21829. doi: 10.1073/pnas.1012071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feyder M, et al. A role for mitogen- and stress-activated kinase 1 in L-DOPA-induced dyskinesia and FosB expression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibbiani F, et al. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol. 2003;184(1):285–294. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M. Japanese Istradefylline Study Group Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: A randomized, controlled study. Mov Disord. 2010;25(10):1437–1443. doi: 10.1002/mds.23107. [DOI] [PubMed] [Google Scholar]
- 35.Rylander D, et al. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39(3):352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Rascol O, et al. Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Parkinsonism Relat Disord. 2014;20(9):947–956. doi: 10.1016/j.parkreldis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Stocchi F, et al. AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord. 2013;28(13):1838–1846. doi: 10.1002/mds.25561. [DOI] [PubMed] [Google Scholar]
- 38.Ory-Magne F, et al. NS-Park CIC Network Withdrawing amantadine in dyskinetic patients with Parkinson disease: The AMANDYSK trial. Neurology. 2014;82(4):300–307. doi: 10.1212/WNL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen Metman L, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50(5):1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 40.Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26(12):1017–1032. doi: 10.1007/s40263-012-0016-z. [DOI] [PubMed] [Google Scholar]
- 41.Sotnikova TD, Caron MG, Gainetdinov RR. DDD mice, a novel acute mouse model of Parkinson’s disease. Neurology. 2006;67(7) Suppl 2:S12–S17. doi: 10.1212/wnl.67.7_suppl_2.s12. [DOI] [PubMed] [Google Scholar]
- 42.Sotnikova TD, et al. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3(8):e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bezard E, et al. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol Dis. 2005;18(2):323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Price DL, et al. Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy—implications for excitotoxicity. PLoS ONE. 2010;5(11):e14020. doi: 10.1371/journal.pone.0014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106(24):9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajagopal K, et al. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103(44):16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102(5):1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 49.Johnston TH, Lee J, Gomez-Ramirez J, Fox SH, Brotchie JM. A simple rodent assay for the in vivo identification of agents with potential to reduce levodopa-induced dyskinesia in Parkinson’s disease. Exp Neurol. 2005;191(2):243–250. doi: 10.1016/j.expneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Ding Y, et al. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci USA. 2011;108(2):840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beaulieu JM, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281(43):32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- 53.Robertson HA. Synergistic interactions of D1- and D2-selective dopamine agonists in animal models for Parkinson’s disease: Sites of action and implications for the pathogenesis of dyskinesias. Can J Neurol Sci. 1992;19(1) Suppl:147–152. [PubMed] [Google Scholar]
- 54.White FJ. D-1 dopamine receptor stimulation enables the inhibition of nucleus accumbens neurons by a D-2 receptor agonist. Eur J Pharmacol. 1987;135(1):101–105. doi: 10.1016/0014-2999(87)90764-3. [DOI] [PubMed] [Google Scholar]
- 55.White FJ, Wang RY. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci. 1986;6(1):274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundblad M, et al. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15(1):120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 57.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16(1):110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Lundblad M, et al. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194(1):66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Birkmayer W, Hornykiewicz O. [The L-dihydroxyphenylalanine (L-DOPA) effect in Parkinson's syndrome in man: On the pathogenesis and treatment of Parkinson akinesis]. Arch Psychiatr Nervenkr. Z Gesamte Neurol Psychiatr. 1962;203:560–574. doi: 10.1007/BF00343235. [DOI] [PubMed] [Google Scholar]
- 60.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 61.Fisone G, Bezard E. Molecular mechanisms of l-DOPA-induced dyskinesia. Int Rev Neurobiol. 2011;98:95–122. doi: 10.1016/B978-0-12-381328-2.00004-3. [DOI] [PubMed] [Google Scholar]
- 62.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107(33):14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macpherson T, Morita M, Hikida T. Striatal direct and indirect pathways control decision-making behavior. Front Psychol. 2014;5:1301. doi: 10.3389/fpsyg.2014.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 66.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3β in D2R-expressing neurons reveals distinct roles for β-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109(50):20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cenci MA, Lee CS, Björklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10(8):2694–2706. [PubMed] [Google Scholar]
- 68.Schuster S, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson’s disease. J Neurosci. 2008;28(17):4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuster S, et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65(6):518–526. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Berthet A, et al. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 2009;29(15):4829–4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charron G, et al. Endogenous morphine-like compound immunoreactivity increases in parkinsonism. Brain. 2011;134(Pt 8):2321–2338. doi: 10.1093/brain/awr166. [DOI] [PubMed] [Google Scholar]
- 72.Bézard E, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9(6):762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- 73.Aubert I, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57(1):17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- 74.Santini E, et al. Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS ONE. 2010;5(8):e12322. doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bezard E, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21(17):6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fox SH, Johnston TH, Li Q, Brotchie J, Bezard E. A critique of available scales and presentation of the Non-Human Primate Dyskinesia Rating Scale. Mov Disord. 2012;27(11):1373–1378. doi: 10.1002/mds.25133. [DOI] [PubMed] [Google Scholar]
- 77.Engeln M, et al. Selective inactivation of striatal FosB/DeltaFosB-expressing neurons alleviates L-Dopa-induced dyskinesia. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Bass CE, et al. Optogenetic control of striatal dopamine release in rats. J Neurochem. 2010;114(5):1344–1352. doi: 10.1111/j.1471-4159.2010.06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.