Significance
The molecular pathophysiology associated with depression remains largely unknown. Recent evidence suggests that signaling pathways downstream of mechanistic target of rapamycin complex 1, such as p70 S6 kinase 1 (S6K1), can lead to structural changes in the medial prefrontal cortex (mPFC) of rats, which are associated with antidepressant responses. We used a viral-mediated approach to control S6K1 activity in the mPFC. Enhanced S6K1 activity produced antidepressant-like effects and resilience to chronic stress, whereas decreased S6K1 activity produced prodepressive behavior. Together, these studies demonstrate that aberrant activity of protein synthesis pathways may underlie the pathology of depression, and demonstrate that direct modulation of this pathway can control depressive behavior in a bidirectional manner. Further understanding of these signaling pathways may contribute to improved treatments for major depressive disorder.
Keywords: prefrontal cortex, translation, antidepressant, synapse, rapamycin
Abstract
Current treatments for major depressive disorder (MDD) have a time lag and are ineffective for a large number of patients. Development of novel pharmacological therapies requires a comprehensive understanding of the molecular events that contribute to MDD pathophysiology. Recent evidence points toward aberrant activity of synaptic proteins as a critical contributing factor. In the present studies, we used viral-mediated gene transfer to target a key mediator of activity-dependent synaptic protein synthesis downstream of mechanistic target of rapamycin complex 1 (mTORC1) known as p70 S6 kinase 1 (S6K1). Targeted delivery of two mutants of S6K1, constitutively active or dominant-negative, to the medial prefrontal cortex (mPFC) of rats allowed control of the mTORC1/S6K1 translational pathway. Our results demonstrate that increased expression of S6K1 in the mPFC produces antidepressant effects in the forced swim test without altering locomotor activity. Moreover, expression of active S6K1 in the mPFC blocked the anhedonia caused by chronic stress, resulting in a state of stress resilience. This antidepressant response was associated with increased neuronal complexity caused by enhanced S6K1 activity. Conversely, expression of dominant-negative S6K1 in the mPFC resulted in prodepressive behavior in the forced swim test and was sufficient to cause anhedonia in the absence of chronic stress exposure. Together, these data demonstrate a critical role for S6K1 activity in depressive behaviors, and suggest that pathways downstream of mTORC1 may underlie the pathophysiology and treatment of MDD.
Major depressive disorder (MDD) affects nearly one-fifth of the US population (1) and represents the second-leading cause of disability worldwide. Despite its prevalence, successful treatment of MDD is hindered by a lack of understanding of the molecular mechanisms underlying the disorder. Recent evidence points to a critical role for the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, which controls synaptic protein synthesis in mediating antidepressant behavior.
Drugs that demonstrate rapid antidepressant activity in preclinical models of depression, such as the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine, the NR2B-selective antagonist Ro 25-6981, the muscarinic antagonist scopolamine, and the metabotropic glutamate receptor 2/3 (mGluR2/3) antagonist LY341495, all share a common signaling profile through cellular protein synthesis cascades. All of these drugs increase activity of mTORC1 and its downstream substrates p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (2–4). Pharmacological inhibition of mTORC1 in the medial prefrontal cortex (mPFC) of rats completely blocks the behavioral effects of these drugs (2–4). These data suggest that enhanced signaling through the mTORC1 pathway is required for rapid antidepressant responses. It remains unknown, however, whether direct modulation of this translational pathway is sufficient to control depressive behavior, and which downstream targets of mTORC1 play a critical role.
To address this question, we used a viral-mediated approach to target S6K1 in discrete brain regions to assess depressive behavior. We demonstrate that targeted activation or suppression of S6K1 activity in the mPFC is sufficient to produce antidepressant or depressive behavior, respectively. These data demonstrate that manipulations of S6K1 in the mPFC can bidirectionally control depressive behavior, and suggest that aberrant activity of synaptic protein synthesis pathways may underlie the pathophysiology of MDD.
Materials and Methods
Animals.
Male Sprague–Dawley rats weighing 275–350 g were used for all behavioral experiments (Charles River). Animals were maintained on a 12-h light/dark cycle in a temperature- and humidity-controlled facility with ad libitum access to food and water. All experiments were in accordance with US National Institutes of Health guidelines and approved by the Yale University Institutional Animal Care and Use Committee.
Design of AAV2-S6K1 Constructs.
Constitutively active (S6K1-T389E-ΔCT) and kinase-inactive (S6K1-K100R) mutants of S6K1 were developed in the laboratory of John Blenis, Harvard Medical School, Boston (Addgene; 8993 and 8985) (5). Constructs were cloned from plasmids using PCR for insertion into a pAAV2 vector containing two independent CMV promoters to drive constitutive expression of S6K1 mutants and eGFP. Constructs were confirmed by sequencing and packaged into AAV2 viral vectors using a triple-transfection, helper-free method as previously described (6). Briefly, HEK293 cells were transfected with pHelper, pAAV-RC, and pAAV-S6K1 constructs. Cells were grown for 48 h and collected for purification of viral particles using HiTrap heparin columns (GE Healthcare; 17-0406-01).
Surgery and Viral Infusions.
Rats were anesthetized with ketamine/xylazine solution (80/6 mg/kg, i.p.). Previous studies in our laboratory have demonstrated that ketamine has no effects on mTOR/S6K signaling or antidepressant behavior at this anesthetic dose. Rats were placed into a stereotaxic frame and 1 μL of viral solution was infused bilaterally into the prelimbic region of the mPFC using the following coordinates: anterior-posterior (AP) +3.5, medial-lateral (ML) ±0.5, dorsal-ventral (DV) −4.0. For infusion into the dorsal striatum, the following coordinates were used: AP +1.6, ML ±2.8, DV −4.4. Following surgery, rats received carprofen (5 mg/kg, i.p.) and topical antibiotic ointment for 3 d. All tissue collection and behavioral testing commenced 21 d after surgery to allow expression of viral constructs.
Tissue Collection.
Rats were killed by rapid decapitation, and whole brains were immediately removed and frozen on dry ice. Brains were sectioned at 200 µm on a cryostat, and GFP was illuminated using a NIGHTSEA BlueStar flashlight to determine the location of viral infusion. Viral-infected regions were dissected using a tissue punch and frozen at −80 °C until use. Only visible GFP+ regions were collected and quantified using Western blot analysis.
SUnSET Measurement of Protein Synthesis.
SUnSET was performed as previously described (7). Briefly, HEK293 cells were plated on six-well plates and transfected with control, constitutively active S6K1 (S6K1CA), or kinase-inactive S6K1 (S6K1KI) constructs and allowed 48 h for construct expression. To measure increased translation by S6K1CA, cells were serum-starved overnight. To measure decreased translation by S6K1KI, cells were not serum-starved. The following day, cells were treated with 10 μg/mL final concentration of puromycin (Sigma-Aldrich) for 10 min. Cells were then washed once with ice-cold PBS and lysed with lysis buffer [50 mM Tris⋅HCl, 150 mM NaCl, 0.1% SDS, 1% Triton, 1 mM NaVO3, 10 mM NaF, and protease inhibitor mixture (Roche)]. Proteins were resolved by 4–20% PAGE, transferred to a nitrocellulose membrane, and blocked with 5% (wt/vol) milk, and puromycin was detected using Western blotting with an anti-puromycin antibody (Kerafast; 1:1,000). Proteins were detected using enhanced chemiluminescence (Western Lightning Plus ECL; PerkinElmer), quantified with Bio-Rad Image Lab software, and normalized to GAPDH (Advanced ImmunoChemical).
Western Blotting.
Protein concentrations were measured using a BCA Kit (Thermo Scientific), and 10–15 μg of protein was loaded onto 7.5% polyacrylamide gels for electrophoresis. Proteins were transferred to a nitrocellulose membrane and blocked for 1 h with 2% (wt/vol) BSA. Membranes were incubated overnight with an anti–phospho-S6 (Cell Signaling; 4858) antibody at 4 °C. The next day, membranes were washed three times with PBS-T (1 × PBS with 0.1% Tween 20) and incubated for 1 h with peroxidase-labeled anti-rabbit secondary antibody (1:10,000) in 5% (wt/vol) milk at room temperature, and proteins were detected with enhanced chemiluminescence. Bands were quantified using Bio-Rad Image Lab software and normalized to GAPDH.
Chronic Unpredictable Stress.
Following recovery from surgery (1 wk), rats were randomly assigned to control or chronic unpredictable stress (CUS) groups and exposed to CUS for 35 d. In brief, rats were exposed to two stressors per d consisting of overnight isolation, light on overnight, 3-h light off during the light cycle, stroboscopic light overnight, overnight food and water deprivation, overnight crowding, overnight cage tilt, overnight wet bedding, 1-h restraint, 10-min swim in cold water (18 °C), 1-h cold exposure (4 °C), or 1-h shaking on a rotator. Control rats were handled daily.
Behavioral Testing.
Locomotor activity.
Rats were placed in clean testing cages (46 × 23 × 20 cm) for 30 min, during which time the number of beam breaks was quantified using MED-PC software (Med Associates).
Elevated plus maze.
Rats were placed on a Plexiglas elevated plus maze apparatus consisting of two closed arms (10 × 51 × 40 cm) and two open arms (10 × 51 × 0.5 cm) for 10 min. The total time and number of entries into open arms were scored using ANY-maze software (Stoelting).
Novelty-suppressed feeding.
Rats were food-deprived for 18 h and placed into a novel open field chamber (76.5 cm × 76.5 cm × 40 cm, Plexiglas) with rat chow placed in the center of the arena. Testing was performed under red light and the latency to feed was measured.
Forced swim test.
Rats were placed into a Plexiglas cylinder (65-cm-height, 30-cm-diameter) filled to a height of 45 cm water (25 °C) and exposed to a 15-min test swim. Total time spent immobile was scored by an experimenter blind to treatment groups.
Sucrose preference.
Rats were exposed to a two-bottle choice test, in which rats could drink plain water or water sweetened with 1% sucrose for 1 h. Total milliliters of fluids consumed was measured.
Ketamine Experiment.
A cohort of rats was infused with control or S6K1KI virus in the mPFC as described above. Three weeks later, rats were tested in the forced swim test as follows. On experiment day 1, rats were exposed to a 15-min swim test as described above. Day 1 immobility time was scored to confirm our previous findings of increased immobility due to S6K1KI expression. Twenty-four hours after the day 1 swim, rats were counterbalanced for immobility time and divided into groups for i.p. treatment with either vehicle (saline) or ketamine (10 mg/kg). Twenty-four hours after drug treatment, rats were exposed to a 5-min test swim and minutes 2–5 were scored for immobility time, because this is a time often used for antidepressant drug screening. Ketaset (ketamine⋅HCl) was purchased from Henry Schein Animal Health and diluted to 10 mg/mL in 0.9% saline.
Primary Neuronal Cultures.
Cortices from embryonic day 18 embryos were dissected and incubated in trypsin⋅EDTA (0.25%; Gibco) for 10 min, after which neurons were dissociated and plated at 0.3 million cells per well on glass coverslips in DMEM (Gibco) containing 10% (vol/vol) FBS and 1% penicillin/streptomycin. The following day, medium was changed to a serum-free medium containing Neurobasal and B27 (Gibco), which was changed every 5 d. Cells were maintained at 37 °C, 5% CO2, and 95% humidity. For neuronal morphology experiments, neurons were infected with control or S6K1CA virus at day 3 in vitro. Two weeks after infection, neurons were fixed in 3.7% (wt/vol) paraformaldehyde, and coverslips were mounted on slides and imaged for endogenous expression of eGFP.
Data Analysis.
For behavioral testing and Western blot analysis, control vs. treatment groups were compared using a Student’s t test. For forced swim test time course, repeated measures ANOVA (time x virus) was used. For the CUS/sucrose preference experiment and the S6K1KI ± ketamine experiment, data were analyzed using a two-way ANOVA (stress x virus or virus x drug, respectively) with least significant difference post hoc tests where appropriate. For cell-culture experiments, repeated measures ANOVA (treatment x distance from soma) was used. Significance was determined at P < 0.05. All data are represented as mean ± SEM.
Results
Constitutively Active and Kinase-Inactive S6K1 Mutants Regulate S6 Phosphorylation and Protein Synthesis.
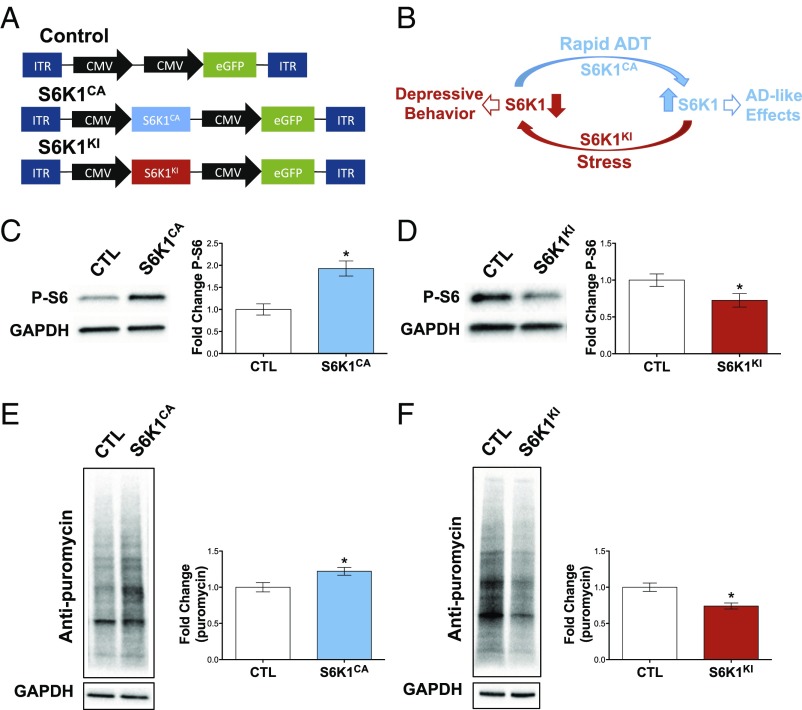
To control S6K1 activity in a region-specific manner, we used a viral-mediated gene transfer approach. To enhance S6K1, we used a pAAV2 vector to express a constitutively active S6K1 (S6K1CA) mutant (5) and eGFP under the control of independent CMV promoters. A schematic of pAAV control and S6K1CA constructs is depicted in Fig. 1A. To validate the functional activity of this construct, we microdissected mPFC tissue from rats infused with S6K1CA virus, and phosphorylation of S6 ribosomal protein, the major downstream substrate of S6K1, was determined. Viral expression of S6K1CA significantly increased levels of phospho-S6 in mPFC lysates compared with infusions of control virus (Fig. 1C). In HEK293 cells, expression of S6K1CA did not influence levels of total S6 protein (Fig. S1). Increased S6K1 expression and subsequent S6 activation should enhance protein synthesis. To measure this and further validate the activity of S6K1CA, we used a SUnSET technique (7). For this approach, transfected HEK293 cells were incubated with low doses of puromycin, which incorporates into newly synthesized polypeptide chains. Lysates from cells expressing S6K1CA showed significantly enhanced rates of protein synthesis (Fig. 1E). We chose to use HEK293 cells due to their ability to withstand transfection with large amounts of plasmid DNA, which is necessary to achieve a measurable increase in puromycin incorporation due to the limitations of the SUnSET assay. Transfection of primary neuronal cultures produces deleterious effects on neuron health and therefore interfered with protein synthesis rates. Conversely, viral infection of primary neurons did not yield robust expression and did not influence puromycin incorporation in the SUnSET assay. Due to the highly localized viral infection we see in vivo, we were unable to perform SUnSET experiments with in vivo tissue.
Fig. 1.
Constitutively active and kinase-inactive mutants of S6K1 control protein synthesis. (A) Depiction of AAV2 constructs. (B) Schematic model of increased and decreased S6K1 activity, leading to antidepressant or depressive behavioral responses, respectively. (C and E) S6K1CA increased phosphorylation of S6 ribosomal protein in vivo (C) (t7 = 4.2, P < 0.01; n = 4–5) and increased protein synthesis rates in HEK293 cells (E) (t10 = 2.6, P < 0.05; n = 6). (D and F) S6K1KI decreased S6 phosphorylation in vivo (D) (t11 = 2.2, P < 0.05; n = 6–7) and reduced protein synthesis rates in HEK293 cells (F) (t6 = 3.6, P < 0.01; n = 4). ADT, antidepressant treatment; CTL, control; ITR, inverted terminal repeat. Error bars represent mean ± SEM. *P < 0.05.
To suppress S6K1 activity, we used a kinase-inactive construct containing a K100R mutation that abolishes the ATP binding required for kinase activity (5). This construct was cloned into an identical pAAV2 vector (S6K1KI) (Fig. 1A). In contrast to S6K1CA, viral expression of S6K1KI decreased phosphorylation of S6 protein in the mPFC compared with control virus (Fig. 1D). In HEK293 cells, expression of S6K1KI did not influence levels of total S6 protein (Fig. S1). In addition, the ability of the kinase-inactive mutant to inhibit protein synthesis was confirmed in cultured cells. Using the SUnSET method, overexpression of S6K1KI decreased rates of protein synthesis compared with control (Fig. 1F). Together, these data demonstrate that S6K1CA and S6K1KI can increase or decrease S6K1 activity, respectively.
Increased S6K1 Activity in the mPFC Produces Antidepressant Effects.
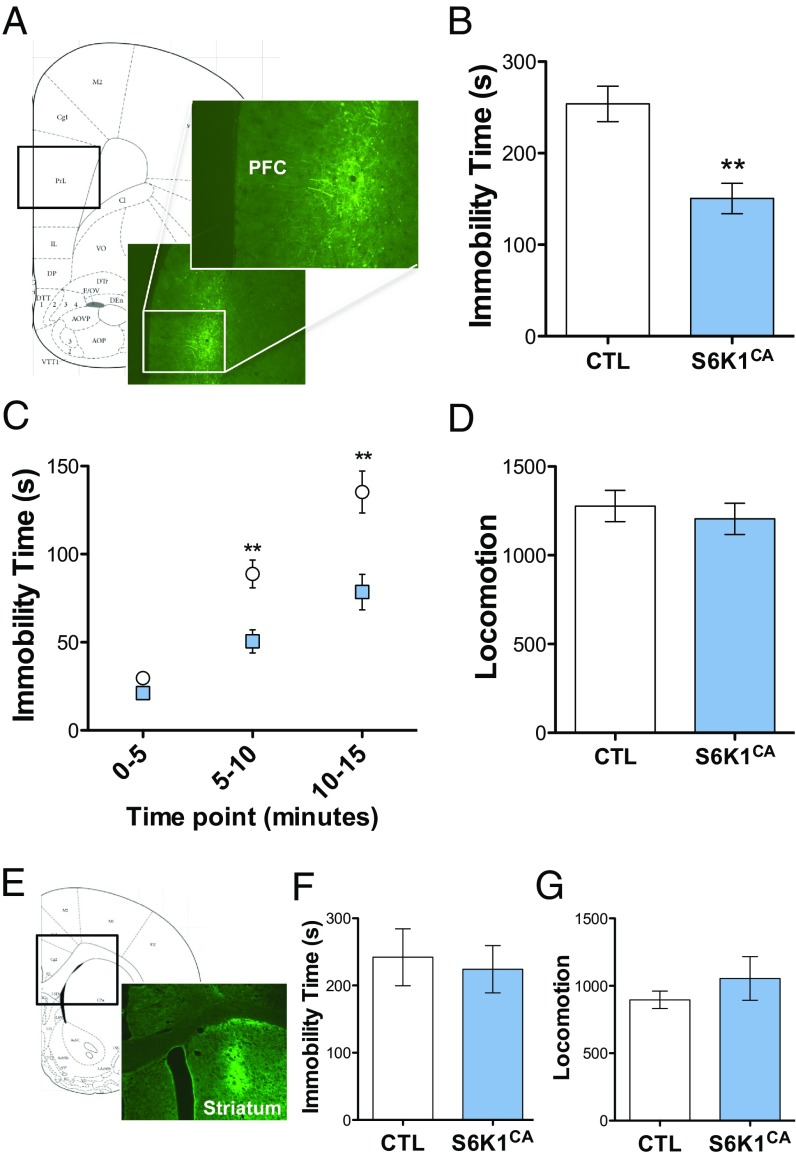
To determine the influence of S6K1 activity on depressive behavior, we infused rats with control or S6K1CA in the mPFC (Fig. 2A). The mPFC was targeted because in our previous studies we found that blockade of mTORC1 in the mPFC by microinfusion of rapamycin was sufficient to block the behavioral actions of systemic ketamine (2). Following 21 d to allow for expression of viral constructs, rats were tested in measures of depression and anxiety. Behavior was examined in the forced swim test (FST), a model of despair in which immobility is used as a standard screen for antidepressants. Rats infused with S6K1CA demonstrated lower immobility times, an antidepressant response, compared with control rats during a 15-min test swim (Fig. 2B). When measured in 5-min time bins, repeated measures ANOVA revealed a significant interaction between time and viral treatment (Fig. 2C). This effect on immobility is not due to a generalized increase in ambulation, as there were no differences in locomotor activity between the two groups (Fig. 2D).
Fig. 2.
Increased S6K1 activity in the mPFC, but not dorsal striatum, produces antidepressant behavior. (A) Representative viral expression in the mPFC of rats (5× and 10×). (B and C) S6K1CA decreased immobility time in the forced swim test (t16 = 3.9, **P < 0.01; time x virus interaction, F2,32 = 7.7, **P < 0.01; n = 8–10). (D) S6K1CA did not alter locomotor activity (t18 = 0.6, P = 0.5744; n = 9–11). (E) Representative viral expression in the dorsal striatum (5×). (F) Infusions of S6K1CA into the dorsal striatum had no effect on immobility time in the forced swim test (t10 = 0.3, P = 0.7531; n = 6). (G) S6K1CA did not affect locomotor activity when infused in the dorsal striatum (t10 = 0.9, P = 0.3848; n = 6). Error bars represent mean ± SEM.
The influence of S6K1CA was also tested in models of anxiety. The novelty-suppressed feeding test (NSFT) has been used for studies of antidepressants because anxiety in this model, measured as latency to feed in an open field, is decreased by chronic but not acute administration of a typical 5-hydroxytryptamine selective reuptake inhibitor (8). However, viral expression of S6K1CA in the mPFC had no effect on latency to feed (Fig. S2A). In the elevated plus maze (EPM), an additional measure of anxiety behavior, S6K1CA rats also showed no differences in time spent or entries into the open arms compared with control rats (Fig. S2 B and C).
To examine the role of increased S6K1 activity in a brain region not considered to be associated with an mTORC1-dependent antidepressant response, we infused a separate cohort of rats with control or S6K1CA virus in the dorsal striatum (Fig. 2E). After 21 d of viral expression, immobility time of rats infused with S6K1CA into the dorsal striatum did not differ from control rats (Fig. 2F). There were no differences in locomotor activity between these two groups (Fig. 2G). Together, these data demonstrate that increased S6K1 activity in the mPFC, but not dorsal striatum, is sufficient to drive antidepressant behavior, without effects on basal anxiety.
Decreased S6K1 Activity in the mPFC Produces Prodepressive Behavior.
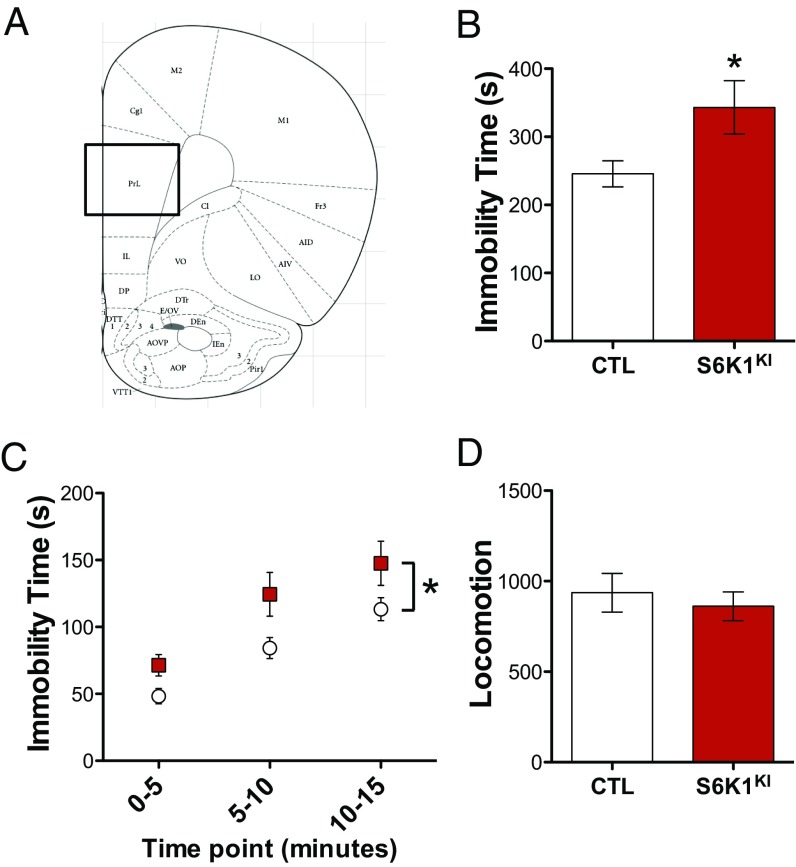
To determine the influence of S6K1 inhibition, we infused rats with control or S6K1KI in the mPFC and performed behavioral testing similar to experiments using S6K1CA. In the FST, S6K1KI rats had higher immobility time compared with control rats, indicative of increased behavioral despair (Fig. 3B). Repeated measures ANOVA revealed a main effect of virus but no significant interaction when compared in 5-min time bins (Fig. 3C). S6K1KI did not affect locomotor behavior, indicating that the blockade S6K1 does not lead to a general effect on ambulation (Fig. 3D).
Fig. 3.
Decreased S6K1 activity in the mPFC produces prodepressive behavior. (A) Virus was infused into the mPFC. (B and C) S6K1KI increased immobility time in the forced swim test (t14 = 2.2, *P < 0.05; time × virus interaction, F2,28 = 1.0, P = 0.3660; n = 8). (D) S6K1KI did not alter locomotor activity (t14 = 0.6, P = 0.5842; n = 8). Error bars represent mean ± SEM.
We also examined the influence of S6K1 inhibition on models of anxiety. S6K1KI infusions into the mPFC did not alter behavioral responses in the NSFT or EPM (Fig. S3), indicating that there were no significant effects on measures of anxiety, similar to the negative effects on anxiety behavior of increasing S6K1 activity. To determine whether S6K1KI and inhibition of downstream signaling impair neuronal health or survival, we performed immunofluorescence labeling of cleaved Caspase 3, a marker of cell apoptosis. Confocal imaging confirmed that GFP-labeled cells from animals infused with control or S6K1KI virus were not colabeled with activated Caspase 3 (Fig. S4). Moreover, the labeled cells from control and S6K1KI virus-infused animals had normal morphology and dendrite arbor (Fig. S4). These data demonstrate that impaired S6K1 activity in the mPFC is sufficient to drive prodepressive behavior in the FST.
S6K1 Inhibition in the mPFC Blocks the Antidepressant Effects of Ketamine.
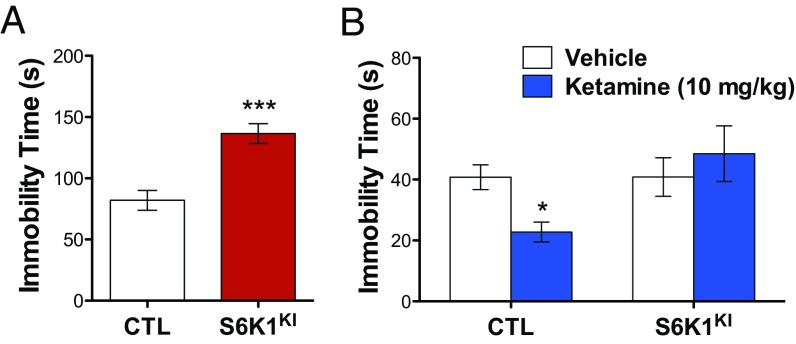
To further explore the role of S6K1 inhibition in mediating depressive behavior, we sought to determine whether S6K1KI expression would block the antidepressant effects of ketamine. As expected, expression of S6K1KI in the mPFC produced an increase in immobility time 3 wk after viral infusion, replicating our previous results (Fig. 4A). Rats were then divided into four groups, counterbalanced for immobility time, for subsequent treatment with vehicle or ketamine (10 mg/kg) 24 h after the initial swim. Twenty-four hours after vehicle or ketamine treatment, rats were exposed to a second test swim. The results demonstrate that ketamine produced the expected decrease in immobility time in rats infused with control virus. In rats infused with S6K1KI, the antidepressant effects of ketamine were completely blocked (Fig. 4B). These data further highlight the importance of the mTORC1/S6K1 pathway for mediating rapid antidepressant responses.
Fig. 4.
Suppression of S6K1 activity in the mPFC blocks the antidepressant effects of ketamine. (A) S6K1KI expression in the mPFC increases immobility time in the forced swim test (t26 = 4.8, ***P < 0.0001). (B) Decreased immobility produced by ketamine is blocked by S6K1KI expression (virus x drug interaction, F1,27 = 4.3, *P < 0.05). Error bars represent mean ± SEM. *P < 0.05 compared with CTL/vehicle.
S6K1 Activity in the mPFC Modulates the Effects of Chronic Stress.
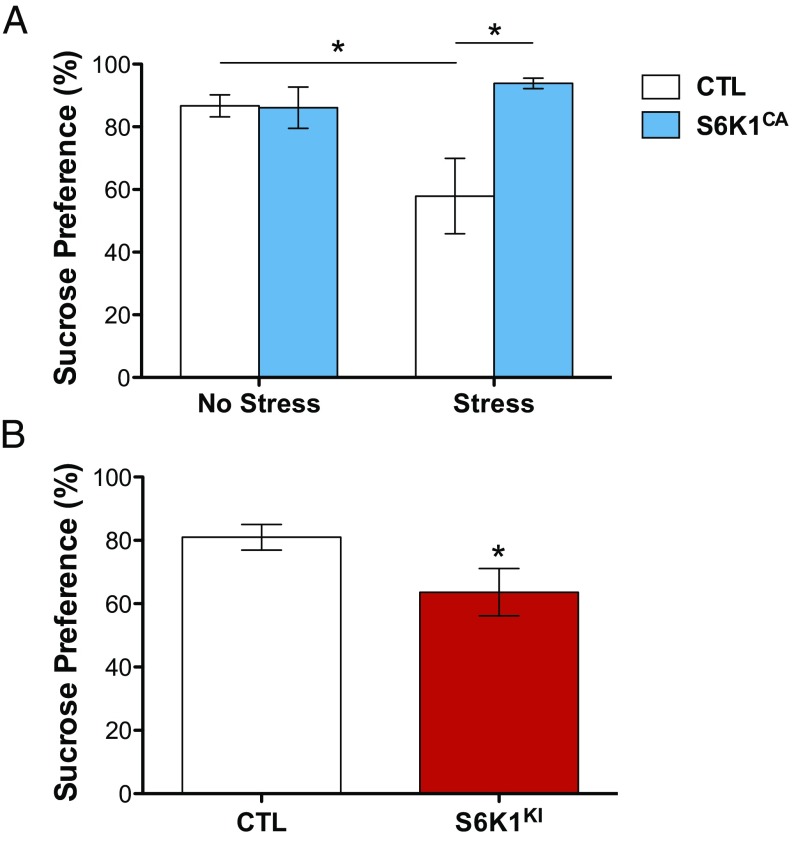
To examine what role increased S6K1 activity has on resilience to chronic stress, we used a chronic unpredictable stress paradigm. In this model, exposure to CUS over the course of several weeks causes anhedonia, a core symptom of depression, measured by preference for a sweetened solution (9). Rats were infused with control or S6K1CA virus and exposed to 35 d of CUS or control handling. At the end of CUS, rats were tested for preference of a 1% sucrose solution vs. plain water. CUS exposure produced an expected decrease in sucrose preference in rats infused with control virus. In contrast, in rats that received infusions of S6K1CA, exposure to CUS did not cause a decrease in sucrose preference, indicating resilience to CUS (Fig. 5A). These data indicate that increased S6K1 in the mPFC is sufficient to produce a state of resilience to the detrimental behavioral effects produced by CUS.
Fig. 5.
S6K1 activity in the mPFC modulates responses to chronic stress. (A) S6K1CA expression in the mPFC produces resilience to chronic stress in the sucrose preference test (stress x virus interaction, F1,26 = 6.1, *P < 0.05; n = 7–8). (B) S6K1KI expression in the mPFC produces anhedonia in the absence of chronic stress (t27 = 2.1, *P < 0.05; n = 14–15). Error bars represent mean ± SEM.
To determine the effect of decreased S6K1 activity on anhedonic behavior, we infused rats with control virus or S6K1KI in the mPFC and then examined anhedonic behavior in the sucrose preference test in the absence of CUS. Rats infused with S6K1KI had lower preference for sucrose compared with control rats, mimicking the effect of chronic stress exposure (Fig. 5B). These data indicate that impaired S6K1 activity in the mPFC is sufficient to produce anhedonic behavior.
Increased S6K1 Activity Increases Neuronal Complexity.
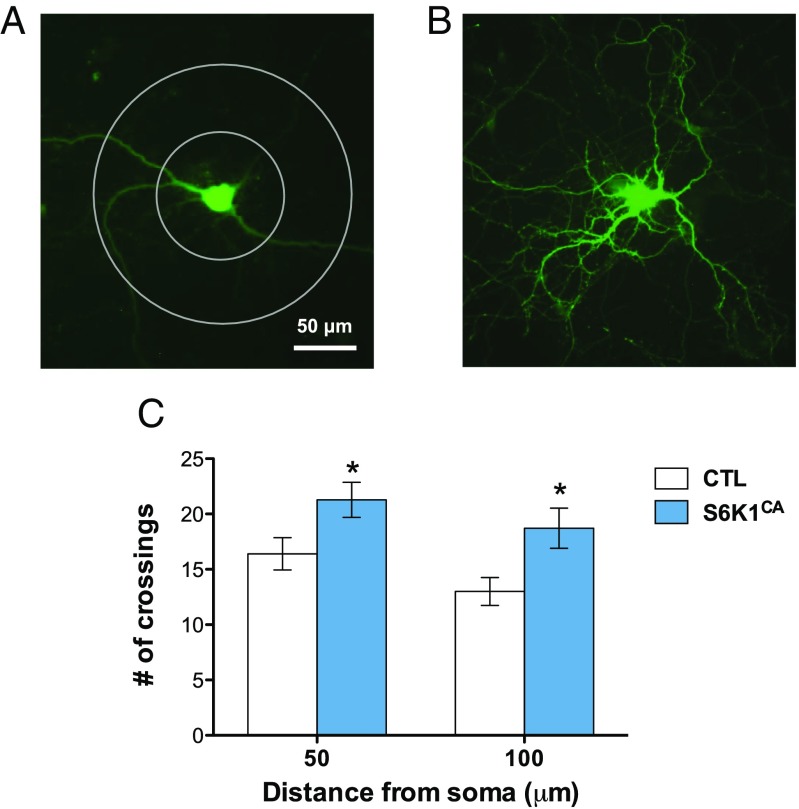
Rapid antidepressant compounds known to activate the mTORC1/S6K1 pathway produce increased neuronal connectivity (2). To measure the influence of enhanced S6K1 activity on neuronal morphology, we infected rat primary cortical cultures with control or S6K1CA virus and measured neuronal branching after 2 wk of viral expression. Neurons infected with S6K1CA demonstrated significantly higher neuronal complexity compared with control infection (Fig. 6). Sholl analysis demonstrates that viral expression of S6K1CA increased the number of dendrite branch crossings by 30–35% relative to control virus at both 50- and 100-µm distances from the neuronal soma.
Fig. 6.
Increased S6K1 activity increases neuronal complexity in cultured cells. (A and B) Representative images of an eGFP-expressing rat primary cortical neuron 2 wk after infection with (A) control virus and (B) S6K1CA virus. Concentric circles with 100- and 200-μm diameter were drawn around the cell soma for Sholl analysis. (C) S6K1CA expression increases neuronal complexity in primary cortical neurons (F1,15 = 9.5, *P < 0.05; n = 7–10). Error bars represent mean ± SEM.
Discussion
Our results demonstrate that altering the expression and function of S6K1 can bidirectionally control depressive behavior. We found that increasing S6K1 activity in the mPFC, but not the dorsal striatum, produces antidepressant effects in the FST and blocks the anhedonic behavior caused by CUS exposure, resulting in a state of stress resilience. Conversely, impaired S6K1 activity in the mPFC drives prodepressive behavior in the FST and is sufficient to cause anhedonia in the absence of chronic stress. Together, these data point toward aberrant S6K1 signaling as a potential molecular mechanism underlying the pathology of MDD.
Recent evidence has further implicated the mTORC1/S6K1 pathway in mediating depressive behavior, demonstrating that CUS decreases activity of mTOR and downstream substrates S6K1 and S6 ribosomal protein (10, 11). Decreased expression and function of the mTORC1/S6K1 pathway could contribute to dendritic reorganization and loss of spine synapses in the mPFC, as well as the hippocampus, leading to impairments in synaptic connectivity (12–17). Indeed, a similar loss of spine synapses is observed in MDD patients (18). The possibility that loss of synapses in depression results from aberrant activity of translational pathways is supported by a report that levels of mTOR and S6K1 are decreased in postmortem PFC of depressed subjects (19). These stress-induced impairments in synaptic connections within the mPFC may then lead to depressive behavior due to loss of mPFC circuit interactions with brain regions that control mood and emotion, such as the amygdala. Fast-acting antidepressants, such as ketamine, rapidly reverse these synaptic deficits via induction of mTORC1/S6K1 signaling, and could thereby reinstate synaptic connectivity that is required for control of emotion and mood (2, 20). Indeed, we demonstrate here that enhanced S6K1 activity is associated with increased neuronal complexity in cultured cortical neurons. Future experiments will be necessary for a comprehensive examination of the role of S6K1 modulation in synaptic connectivity, dendritic organization, and spine density in vivo.
Our results also demonstrate a specific role for S6K1 in mediating depressive, but not anxiety-related, behavior. The NSFT is sensitive to chronic, but not acute, treatment with typical antidepressants (8). In this model, ketamine produces rapid anxiolytic effects that are sensitive to mTORC1 inhibition. Pretreatment with rapamycin in the mPFC completely blocks ketamine’s rapid anxiolytic effect in the NSFT (2). Therefore, our results suggest that ketamine-induced mTORC1 activation may have multiple downstream targets driving different behavioral phenotypes. It is possible that another mTORC1 effector mediates ketamine’s anxiolytic effects. However, it is also possible that ketamine produces different behavioral outputs via actions on discrete brain regions within the mPFC. Indeed, expression of fear behavior, as well as extinction, can be differentially mediated by different mPFC subregions, such as the prelimbic and infralimbic regions (21, 22). It will be important in future studies to assess the effects of translational control on synapses in these regions, as well as other regions that may be associated with despair and anhedonic behavior, such as the lateral habenula, hippocampus, and dorsal raphe.
In conclusion, our data demonstrate an important role for the mTORC1/S6K1 translational pathway in mediating depressive behaviors. Further understanding the molecular signaling events impaired in MDD and reversal of these effects by antidepressant agents will be critical to the development of novel medications for affective disorders.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grants MH093897 (to R.S.D.) and MH102004 (to J.M.D.), the National Science Foundation Graduate Research Fellowship Program (J.M.D.), and the state of Connecticut.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505289112/-/DCSupplemental.
References
- 1.Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 2012;15(4):429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voleti B, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12(8):632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- 6.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9(12):1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6(4):275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 8.Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97(2):277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 9.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 10.Zhong P, et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39(7):1763–1776. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota KT, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20(5):531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 13.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105(1):359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588(2):341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 17.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 18.Kang HJ, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jernigan CS, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.