Significance
The clustered, regularly interspaced, short palindromic repeats associated endonuclease, Cas9, has quickly become a revolutionary tool in genome engineering. Utilizing small guiding RNAs, Cas9 can be targeted to specific DNA sequences of interest, where it catalyzes DNA cleavage. We now demonstrate that Cas9 from the Gram-negative bacterium Francisella novicida (FnCas9) can be reprogrammed to target a specific RNA substrate, the genome of the +ssRNA virus, hepatitis C virus, in eukaryotic cells. Further, this targeting results in inhibition of viral protein production. Overall, programmable Cas9-mediated viral RNA targeting likely represents one of myriad potential applications of FnCas9 in RNA targeting in eukaryotic cells.
Keywords: Cas9, CRISPR-Cas, hepatitis C virus, RNA targeting, Francisella
Abstract
Clustered, regularly interspaced, short palindromic repeats–CRISPR associated (CRISPR-Cas) systems are prokaryotic RNA-directed endonuclease machineries that act as an adaptive immune system against foreign genetic elements. Using small CRISPR RNAs that provide specificity, Cas proteins recognize and degrade nucleic acids. Our previous work demonstrated that the Cas9 endonuclease from Francisella novicida (FnCas9) is capable of targeting endogenous bacterial RNA. Here, we show that FnCas9 can be directed by an engineered RNA-targeting guide RNA to target and inhibit a human +ssRNA virus, hepatitis C virus, within eukaryotic cells. This work reveals a versatile and portable RNA-targeting system that can effectively function in eukaryotic cells and be programmed as an antiviral defense.
Clustered, regularly interspaced, short palindromic repeats–CRISPR associated (CRISPR-Cas) systems act as a prokaryotic adaptive immune system against foreign genetic elements (1–3). These RNA-directed endonuclease machineries use small CRISPR RNAs (crRNAs) that provide sequence specificity and Cas proteins to recognize and degrade nucleic acids (4–7). Our recent work revealed a unique form of prokaryotic gene regulation, whereby Cas9 from Francisella novicida (FnCas9) targets a bacterial mRNA, leading to gene repression (8). Given the ability of specific Cas9 proteins to be reprogrammed to target and cleave DNA in numerous biological systems (7, 9, 10), we hypothesized that FnCas9 could be retargeted to a distinct RNA in eukaryotic cells and lead to its inhibition. To eliminate any confounding interactions of FnCas9 with DNA, we targeted FnCas9 to the +ssRNA virus, hepatitis C virus (HCV), which has no DNA stage in its lifecycle. HCV is an important human pathogen associated with liver fibrosis, cirrhosis, and hepatocellular carcinoma and is the leading cause of liver transplantation (11, 12).
Results
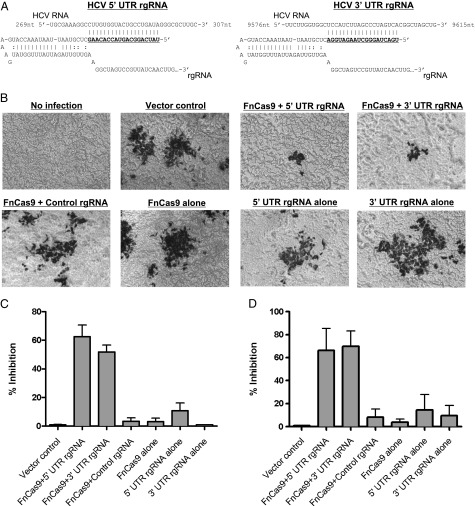
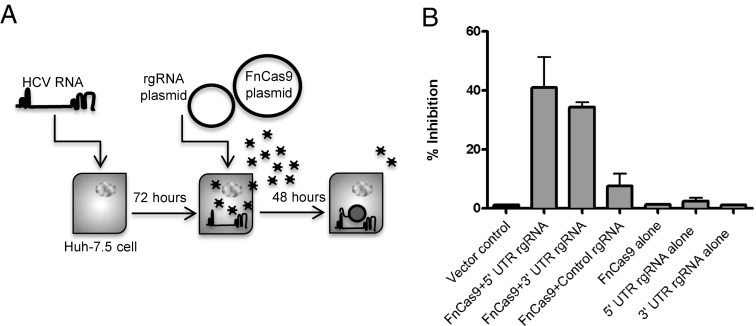
To target the RNA of HCV, we engineered a small RNA, which we term an RNA-targeting guide RNA (rgRNA). The rgRNA is similar in structure to that naturally created by the F. novicida tracrRNA (transactivating CRISPR RNA) and scaRNA (small CRISPR-Cas associated RNA), which are used for endogenous mRNA targeting (8). It consists of a dsRNA region thought to be important for interaction with Cas9, and a ssRNA-targeting sequence complementary to a portion of the highly conserved HCV 5′ untranslated region (UTR), involved in both translation of the viral polyprotein and replication of the viral RNA (Fig. 1A and Fig. S1). Vectors encoding either this rgRNA or FnCas9 (Fig. S2 and Dataset S1) were transfected into human hepatocellular carcinoma cells (Huh-7.5) and subsequently infected with a previously described cell culture derived HCV (HCVcc) genotype 2a recombinant virus encoding Renilla luciferase (13). Expression of both the 5′ UTR-targeting rgRNA and FnCas9 together reduced the levels of viral proteins, as measured by immunostaining for the E2 glycoprotein (Fig. 1 B and C) or quantification of luciferase production (Fig. 1D). Conversely, expression of either the rgRNA or FnCas9 alone had no effect (Fig. 1 B–D), nor did expression of a nonspecific rgRNA and FnCas9 (Fig. 1 B–D), demonstrating the specificity of this system. Additionally, an rgRNA complementary to a portion of the 3′ UTR, necessary for replication of viral RNA, decreased viral protein levels similarly (Fig. 1 A–D), demonstrating that the effect was not specific to a single site in the HCV genome. Therefore, FnCas9 can be programmed by a single rgRNA to target the RNA of a human virus in eukaryotic cells, leading to viral inhibition.
Fig. 1.
FnCas9 can be reprogrammed to inhibit viral protein production in eukaryotic cells. (A) rgRNA schematic with targeting sequences (gray highlight) against the 5′ or 3′ UTR of HCV genomic RNA. (B) Huh-7.5 cells were transfected with the indicated combinations of FnCas9 and rgRNA and infected 48 h later with HCV encoding Renilla luciferase. At 72 h, cells were fixed and stained with anti-E2 antibody and imaged. (C) E2-positive foci from B were quantified and plotted as percent inhibition compared with the vector control. (D) Quantification of viral luciferase production displayed as percent inhibition compared with the vector control (n = 3; bars represent the SEM; data are representative of at least six experiments).
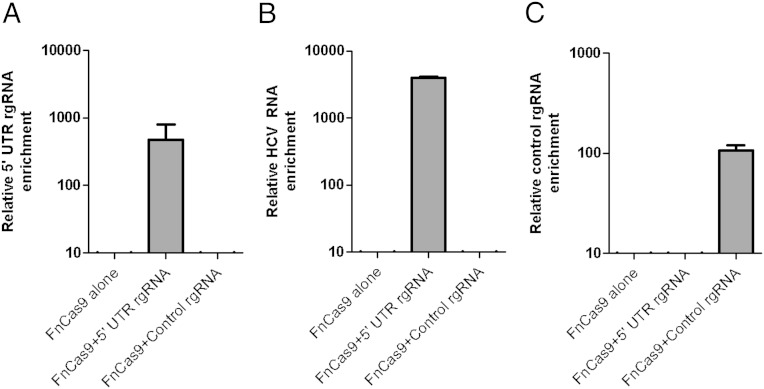
To determine if FnCas9 was directly associated with HCV RNA, we performed coimmunoprecipitation experiments. We transfected cells with an HA epitope-tagged version of the protein [which maintained its ability to inhibit HCV (Fig. S3 A and B)] as well as the 5′ UTR-targeting rgRNA and subsequently infected the cells with HCV. FnCas9 was immunoprecipitated from cell lysates, associated RNA was purified, and quantitative real-time PCR was performed for the rgRNA and HCV RNA. The rgRNA was present in association with FnCas9 (Fig. 2A), as was HCV RNA (Fig. 2B), suggesting that HCV RNA was directly targeted by the FnCas9:rgRNA complex. A nonspecific rgRNA did not facilitate this interaction (Fig. 2B) but did associate with FnCas9 (Fig. 2C). Thus, FnCas9 can be targeted to associate with viral RNA within eukaryotic cells.
Fig. 2.
FnCas9 targets and associates with HCV RNA. Huh-7.5 cells producing an HA epitope-tagged FnCas9 alone, or with either the 5′ UTR targeting rgRNA or the control rgRNA, were infected with HCV. At 72 h postinfection, lysates were immunoprecipitated with anti-HA. Coprecipitating RNA was purified and analyzed by quantitative real-time PCR to detect the relative enrichment of the (A) 5′ UTR rgRNA, (B) HCV RNA, or (C) control rgRNA, normalizing to gapdh mRNA levels (n = 4; bars represent the SEM; data are representative of three experiments).
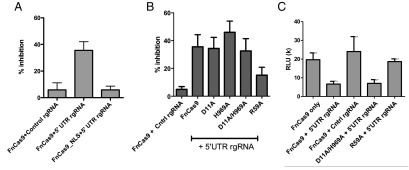
We next sought to determine how FnCas9 inhibited HCV, testing whether its endonucleolytic activity was required and if it inhibits translation and/or viral replication. As a first control, we found that addition of a nuclear localization signal (NLS) to FnCas9 abrogated its repression of viral protein production (Fig. 3A and Fig. S4), in line with its targeting of cytosolic HCV RNA. Because Cas9 proteins including FnCas9 are known to cleave DNA through two conserved structural domains, the RuvC and HNH endonuclease domains (7), we considered that these regions might be important for inhibiting HCV. We therefore generated alanine point mutations in the conserved RuvC and HNH active sites of FnCas9 (D11A and H969A, respectively). Despite mutation in one or both of these domains, FnCas9 maintained its ability to inhibit HCV (Fig. 3B). However, mutation in the RNA-binding arginine-rich motif (ARM; R59A), which is necessary for the interaction of Cas9 with nucleic acids (8, 14, 15), resulted in diminished HCV inhibition (Fig. 3B). Although these data do not exclude a model whereby accessory cellular RNases are recruited to the target by FnCas9, or that FnCas9 possesses an additional domain with endonucleolytic activity, these data do suggest that endonuclease activity of the canonical DNA cleavage motifs are dispensable for FnCas9 inhibition of HCV.
Fig. 3.
Molecular requirements for FnCas9-mediated HCV inhibition. (A) Huh-7.5 cells were transfected with FnCas9 ± NLS, the 5′ UTR-targeting rgRNA, and HCV. At 72 h, viral luciferase was quantified and the percent inhibition compared with the nontargeting rgRNA is displayed (n = 8; data compiled from three independent experiments). (B) Experiments were performed as above, using alanine point mutants in the RuvC domain (D11A), HNH domain (H969A), the double mutant (D11A/H969A), or the ARM (R59A) (n = 8; data compiled from three independent experiments). (C) Rabbit reticulocyte lysate in vitro translation assays of HCV luciferase were performed using the indicated Cas9 and RNAs and viral luciferase measured (n = 4; data are representative of four experiments).
We subsequently tested whether FnCas9 could inhibit translation of HCV RNA. We performed an in vitro translation reaction using immunoprecipitated FnCas9 from transfected Huh-7.5 cells, purified RNA from cells transfected with either the 5′ UTR-targeting rgRNA or the nontargeting RNA, as well as an in vitro transcribed HCV genomic RNA. Addition of both FnCas9 and the 5′ UTR-targeting rgRNA resulted in decreased translation of the HCV genome, as measured by viral luciferase production (Fig. 3C). Furthermore, a catalytically inactive FnCas9 (D11A/H969A) maintained its ability to inhibit translation of HCV, whereas the ARM (R59A) mutant displayed less translational inhibition (Fig. 3C). Taken together, these data suggest that FnCas9 is capable of directly inhibiting translation of target RNA. To determine whether FnCas9 also inhibits replication of HCV RNA, we targeted the negative-sense strand of the 5′ UTR (generated during replication and which is untranslated). Such targeting resulted in inhibition of HCV (Fig. S5). Together with the targeting of the 3′ UTR (which is involved in viral replication, but not translation), this suggests that FnCas9 is capable of inhibiting viral replication as well. Overall, these data strongly support a model whereby FnCas9 binds HCV RNA and inhibits the function of both translational and replication machineries.
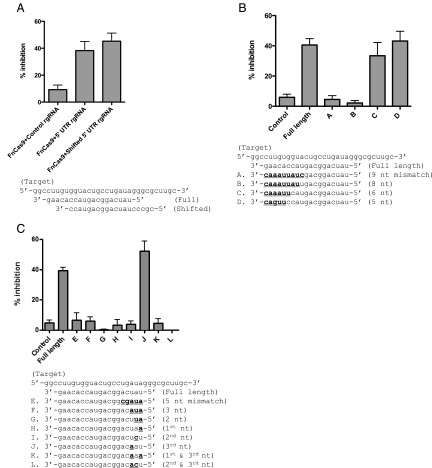
We subsequently tested the sequence requirements for RNA targeting. Cas9 proteins require a short sequence adjacent to the targeted region, called a proto-spacer adjacent motif (PAM), to cleave DNA (14). We sought to determine if a similar conserved adjacent region was necessary for HCV inhibition. A 5′ UTR-targeting rgRNA shifted to lack similar adjacent sequences still inhibited HCV (Fig. 4A). In fact, no common features are observed in the sequences adjacent to the targets of rgRNAs used in this study (Fig. S6A). In contrast, DNA targeting by FnCas9 endogenously within F. novicida absolutely requires a PAM (Fig. S6 B and C). Thus, these data demonstrate that FnCas9-mediated inhibition of HCV is independent of sequences adjacent to the targeted region.
Fig. 4.
RNA sequence requirements for FnCas9 inhibition of HCV. (A) Huh-7.5 cells were transfected with FnCas9 using the rgRNA mutants in the indicated shifted alignments. At 72 h, viral luciferase was quantified and the percent inhibition compared with the nontargeting rgRNA is displayed (n = 12; data are compiled from three independent experiments). (B) Experiments were performed as above, using the mutants in the 3′ region indicated in the alignment below the figure (n = 12; bars represent the SEM; data are compiled from three independent experiments). (C) Experiments were performed as above, using the mutants in the 5′ region indicated in the alignment below the figure (n = 12; bars represent the SEM; data are compiled from three independent experiments).
DNA-targeting RNAs used by Cas9 require a 3′ seed sequence within the targeting region, and even a single mismatch in this region can abrogate function (16, 17). To test if there was a similar requirement for RNA targeting, we generated a panel of rgRNA mutants containing mismatches within the targeting sequence. We found that mutation of up to six bases within the 3′ targeting region of the rgRNA was tolerated without loss of HCV inhibition (Fig. 4B). Longer regions of mismatched bases at the 3′ end resulted in a loss of activity (Fig. 4B). Specific mismatches in the 5′ region of rgRNA were also nonfunctional (Fig. 4C). A single mismatch in either the first or second base was sufficient to abrogate viral inhibition (Fig. 4C). However, a mismatch in the third base alone did not lead to a loss of activity (Fig. 4C). These data strongly suggest that unlike DNA targeting by other Cas9 proteins, FnCas9 inhibition of HCV requires a critical sequence in the 5′ end rather than the 3′ end of the targeting region of the guiding RNA (16, 17).
The previous experiments demonstrated that FnCas9 could target an RNA and facilitate resistance to HCV infection. We next tested whether FnCas9 could be introduced following an established viral infection and inhibit the virus (Fig. 5A). Transfection of HCV-infected Huh-7.5 cells with FnCas9 and 5′ or 3′ UTR-targeting rgRNAs resulted in decreased viral protein production (Fig. 5B), whereas expression of either FnCas9 or rgRNAs alone was not sufficient to inhibit HCV infection (Fig. 5B). Thus, the FnCas9:rgRNA machinery can combat both new and established viral infections.
Fig. 5.
FnCas9 can inhibit an established viral infection. (A) Experimental outline. HCV-transfected Huh-7.5 cells were transfected with FnCas9 and the indicated targeting RNAs, after 72 h postinfection. (B) Quantification of viral luciferase production, displayed as percent inhibition compared with the vector control (n = 3; bars represent the SEM; data are representative of at least 12 experiments).
Discussion
These data demonstrate the successful adaptation of the CRISPR-Cas prokaryotic immune system as an intracellular eukaryotic antiviral defense. Although other CRISPR-Cas systems can target RNA in archaea (18–20) and bacteria (21), and recently Streptococcus pyogenes Cas9 (SpCas9) has been shown to cleave RNAs in vitro (22), this work demonstrates the reprogramming of a Cas protein (FnCas9) to target RNA within a eukaryotic cell. Intriguingly, we find that orthologous Cas9 proteins from diverse type II CRISPR-Cas families, including S. pyogenes, Streptococcus thermophilus, and Neisseria meningitidis, are also capable of inhibiting HCV during cellular infection (Figs. S7 and S8). This suggests a broader capability of diverse Cas9 proteins to target and associate with RNAs of interest. Our results further demonstrate that FnCas9 inhibition of HCV is PAM-independent, unlike the in vitro RNA-targeting ability of SpCas9, which requires exogenous PAM-encoding oligomers (22). Thus, this method of RNA inhibition may be more flexible in its targeting. Importantly, expression of FnCas9 and either the HCV targeting or control rgRNA did not lead to significant changes in host cell gene expression compared with cells expressing a vector control, demonstrating the specificity of these complexes (Fig. S9 and Dataset S2). Furthermore, the presence of FnCas9 in the cytosol is necessary to inhibit HCV, in contrast to DNA targeting by Cas9 that relies on nuclear localization (23). Cytosolic RNA targeting would potentially limit FnCas9 off-target effects on host DNA.
Because the lifecycles of viruses with both RNA and DNA genomes require an RNA stage (generated during transcription, replication, or both), and FnCas9 can target both negative- and positive-sense strands of RNA and inhibit by blocking both viral translation and replication machineries, it is likely that the FnCas9:rgRNA machinery can be used to target diverse viruses, including both +ssRNA viruses (such as flavivirus, poliovirus, and rhinovirus) and –ssRNA viruses (such as filovirus, paramyxovirus, and orthomyxovirus). Furthermore, some eukaryotic viruses have mechanisms to circumvent eukaryotic RNA-targeting antiviral defenses, such as classical RNAi systems (24–26); however, these viruses have not evolved in the presence of Cas9, so it is unlikely that they have Cas9 evasion strategies. Thus, the FnCas9:rgRNA machinery could facilitate the targeting of viruses as soon as their genome sequences are available, without knowledge of the virus lifecycle or host receptors.
Given the vast success of Cas9 as a mediator of genome engineering in a multitude of species (7, 9, 10, 16, 27–33), our data suggest that FnCas9 could be used in a broad range of systems, representing an innovative paradigm in Cas9-mediated genetic engineering. This work demonstrates a portable, interdomain machinery capable of viral inhibition, likely just one of myriad potential biotechnological and medical applications of Cas9-mediated RNA targeting.
Materials and Methods
Plasmid Construction.
FnCas9 was amplified using the primers found in Table S1 and cloned into the XbaI and PmeI sites of pcDNA3.3 (Invitrogen). FnCas9 point mutants were amplified from strains published previously (8). SpCas9, StCas9, and NmCas9 were amplified from Addgene vectors 48669, 41815, and 47872, respectively. To create rgRNA vectors, F. novicida CRISPR repeat sequences and the indicated targeting sequence were cloned into the gRNA-encoding plasmid from the Church Lab (Addgene 41824) (9), within the pCR-Blunt-II (Invitrogen) backbone, using overlapping PCR and the primers indicated in Table S1.
Cell Lines and Culture Conditions.
Huh-7.5 cells were maintained in Dulbecco’s modified Eagle medium (DMEM; BioWhittaker) containing 10% (vol/vol) FBS (HyClone) and 100 μg/ mL of penicillin/streptomycin (Cellgro) at 37° in 5% CO2.
Plasmid Transfection.
Huh-7.5 cells were seeded to 80% confluence in 24-well plates in DMEM without antibiotics the day before transfection. We transfected 800 ng of total plasmid DNA using Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. Six hours following transfection, cell supernatants were aspirated and replaced with DMEM supplemented with FBS and antibiotics.
Virus Transcription and Transfection.
Rluc virus used for luciferase assays encodes the Renilla luciferase gene between the p7 and NS2 coding sequences of the previously described J6/JFH genotype 2a infectious clone Cp7 (13). Rluc and Cp7 viral RNA were prepared as previously described (13). Purified plasmid encoding the cDNA copy of the full-length viral genome was linearized, and 5′ overhangs were digested with Mung Bean Nuclease (New England Biolabs). Linearized DNA was purified with phenol–chloroform extraction and ethanol precipitation. Transcription of the linearized DNA template was performed using a MEGAscript T7 kit, and the linear template was treated with DNase I (Ambion). RNA was purified with phenol–chloroform extraction and isopropanol precipitation, and concentration and purity were determined using a Nanodrop spectrophotometer and standard agarose gel electrophoresis.
Huh-7.5 cells were grown to 70% confluence, trypsinized, and washed twice in PBS. We mixed 10 µg of purified RNA with 0.4 mL Huh-7.5 cells suspended at a concentration of 2 × 107 cells/mL. Samples were electroporated in BTX 2-mm gap cuvettes (Harvard Apparatus, Inc.) using an ECM 830 apparatus (BTX Genetronics) with five pulses of 99 μs at 820 V over 1.1 s. Cells were suspended in 25 mL complete DMEM, and virus was harvested and stored at –80 °C 3 d following transfection.
Immunoprecipitation.
Anti-HA IP was performed according to the manufacturer’s instructions from lysates of Huh-7.5 cells infected with HCVcc and transfected with FnCas9 and rgRNA expression vectors as indicated (Sigma-Aldrich). Total RNA was extracted from the precipitated fraction using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions.
In Vitro Translation Assay.
Immunoprecipitated FnCas9 was incubated with 1 µg HCV RNA, and 500 ng RNA from Huh-7.5 cells transfected with either the 5′ UTR-targeting rgRNA or the control rgRNA, in conjunction with the rabbit reticulocyte lysate in vitro translation kit (Life Technologies) according to the manufacturer’s instructions. Translated viral luciferase was measured as described below.
Quantitative RT-PCR.
Quantitative reverse transcription reactions were performed using Taqman One Step RT-PCR Master Mix Reagent (Applied Biosystems) and primers specific for the 5′ UTR of HCV (Table S1). Sample analysis was performed on an Applied Biosystems 7500 apparatus. rgRNAs were quantified via Syber Green One Step RT-PCR, with primers found in Table S1.
Luciferase Assays.
Huh-7.5 cells in a 96-well plate format were lysed and analyzed for relative light activity using the Renilla Luciferase Assay System (Promega) according to the manufacturer’s instructions. Briefly, 50 μL of Renilla substrate in assay buffer was added to 50 μL of cell lysate, and relative light units were quantitated on a Clarity 4.0 microplate luminometer (Biotek).
Immunohistochemistry.
Six thousand Huh-7.5 cells per well were plated in collagen-coated 96-well plates. The following day, cells were infected with the J6/JFH genotype 2a virus Cp7. Following 3 d of incubation, cells were fixed with methanol, washed twice with PBS, and permeabilized with PBS containing 0.1% Tween-20 (PBS-T). Fixed cells were incubated in blocking buffer containing 1% BSA and 0.2% skim milk for 30 min. Endogenous peroxidase was blocked using 3% (vol/vol) H2O2, and then cells were washed twice with PBS and once with PBS-T. Cells were then incubated with the 2C1 monoclonal antibody to HCV E2 glycoprotein for 1 h at room temperature. After two washes with PBS and one with PBS-T, cells were incubated with ImmPress anti-mouse HRP (Vector Laboratories), washed, and developed using DAB substrate (Vector Laboratories).
Plasmid Inhibition Transformation Assay.
The spacer sequence from the F. novicida crRNA#1 (nucleotides 818163–818196 in the F. novicida U112 genome) was cloned into the pBAV plasmid (KanR) (Addgene 26702) using the overlapping PCR primers found in Table S1 to create a dsDNA target for FnCas9. The pBAV plasmids were transformed individually (250 ng plasmid/transformation) into chemically competent U112 (300 µL) as described previously (4). Transformants were selected for on TSA containing 0.2% cysteine and 30 µg/mL kanamycin.
RNA-Seq Preparation and Analysis.
Two sets of Huh-7.5 cells transfected with Cas9 + 5′ UTR-targeting rgRNA, Cas9 + Control rgRNA, or vector only were sorted by GFP on a FACS Aria. Total RNA was prepared using the QIAGEN RNEasy Micro Kit. Libraries were generated from 500 pg of total RNA using the CLONTECH SMARTer Ultralow V3 kit, and barcoding and sequencing primers were added using the Illumina NexteraXT DNA kit. Libraries were assessed by Bioanalyzer capillary electrophoresis, quantified, pooled, and sequenced on an Illumina MiSeq system as 150-base single-read reactions. Average read depth was 5.8 M, and all samples had Q30 scores >93.9%. Transcripts were aligned to the hg19 reference genome using the STAR v1.4.0g1 software (34). Transcript quantitation and differential expression were performed with the Cufflinks suite of tools and Cuffdiff v2.1.1 (35). Sequenced reads were deposited into the Sequence Read Archive database.
Supplementary Material
Acknowledgments
We thank Rafi Ahmed for helpful discussions, Sarkis Mazmanian for critical support, Yerkes Flow Cytometry Core for technical support, and Steven Bosinger of the Yerkes Genomics Core for performance of RNAseq and its analysis. This project was supported by National Institutes of Health (NIH) Grants R01-AI11070 and U54-AI057157 (Southeastern Regional Center of Excellence for Emerging Infections and Biodefense), the Burroughs Wellcome Fund (to D.S.W.), and Yerkes Research Center Base Grant P51RR-000165 and NIH Grants R01-AI070101 and R01-DK083356 (to A.G.). T.R.S. was supported by the National Science Foundation Graduate Research Fellowship Program and the Achievement Rewards for College Scientists Foundation.
Footnotes
Conflict of interest statement: The authors have filed a related patent.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the NCBI BioProjects database (accession no. PRJNA281426).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422340112/-/DCSupplemental.
References
- 1.Sorek R, Lawrence CM, Wiedenheft B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem. 2013;82:237–266. doi: 10.1146/annurev-biochem-072911-172315. [DOI] [PubMed] [Google Scholar]
- 2.Makarova KS, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9(6):467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 4.Brouns SJ, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321(5891):960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westra ER, et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell. 2012;46(5):595–605. doi: 10.1016/j.molcel.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA. 2012;109(39):E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson TR, Saroj SD, Llewellyn AC, Tzeng YL, Weiss DS. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497(7448):254–257. doi: 10.1038/nature12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 12.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19(7):837–849. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateu G, Donis RO, Wakita T, Bukh J, Grakoui A. Intragenotypic JFH1 based recombinant hepatitis C virus produces high levels of infectious particles but causes increased cell death. Virology. 2008;376(2):397–407. doi: 10.1016/j.virol.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinek M, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343(6176):1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimasu H, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156(5):935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31(3):233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenova E, et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad Sci USA. 2011;108(25):10098–10103. doi: 10.1073/pnas.1104144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale CR, et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell. 2009;139(5):945–956. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, White MF. Hot and crispy: CRISPR-Cas systems in the hyperthermophile Sulfolobus solfataricus. Biochem Soc Trans. 2013;41(6):1422–1426. doi: 10.1042/BST20130031. [DOI] [PubMed] [Google Scholar]
- 20.Zebec Z, Manica A, Zhang J, White MF, Schleper C. CRISPR-mediated targeted mRNA degradation in the archaeon Sulfolobus solfataricus. Nucleic Acids Res. 2014;42(8):5280–5288. doi: 10.1093/nar/gku161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staals RH, et al. Structure and activity of the RNA-targeting Type III-B CRISPR-Cas complex of Thermus thermophilus. Mol Cell. 2013;52(1):135–145. doi: 10.1016/j.molcel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connell MR, et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516(7530):263–266. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan CS, Ganem D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J Virol. 2005;79(12):7371–7379. doi: 10.1128/JVI.79.12.7371-7379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh G, et al. Suppression of RNA silencing by Flock house virus B2 protein is mediated through its interaction with the PAZ domain of Dicer. FASEB J. 2009;23(6):1845–1857. doi: 10.1096/fj.08-125120. [DOI] [PubMed] [Google Scholar]
- 26.Chao JA, et al. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat Struct Mol Biol. 2005;12(11):952–957. doi: 10.1038/nsmb1005. [DOI] [PubMed] [Google Scholar]
- 27.Mali P, et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi LS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bikard D, et al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41(15):7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou Z, et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc Natl Acad Sci USA. 2013;110(39):15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang N, et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 2013;23(4):465–472. doi: 10.1038/cr.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gratz SJ, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194(4):1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Pinera P, et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods. 2013;10(10):973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trapnell C, et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31(1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.