A hallmark of animal life is the ability to move through the environment to catch prey, avoid predators, or find mates. Animals achieve this using a staggering diversity of locomotor strategies despite having similar body shape and being subjected to similar physics—e.g., gazelles pronk and cheetahs gallop. These differences in strategy may allow animals to fill different ecological niches by affording more (or less) stability, maneuverability, speed, efficiency, and stealth. Many animals rely on specialized appendages—limbs, fins, and wings—that reciprocate to produce forward motion. However, some organisms move using a completely different strategy that involves the generation of undulatory traveling waves that propagate along the body or a specialized elongated fin. Most studies of such undulatory locomotion have focused on the role of a single, in-plane wave that travels from head-to-tail to produce forward thrust, as seen for example in aquatic animals such as eels, lampreys, and leeches (1, 2). In PNAS, Astley et al. (3) present behavioral data that suggest a role for multiplane body undulations in sidewinding snakes to achieve turning maneuvers. Specifically, they observe that rattlesnakes adjust the relative amplitude and timing of the horizontal and vertical waves and that these changes are, in turn, correlated with shallow and sharp turning. Of course, correlations do not prove a mechanistic relationship, so the investigators looked for a complementary approach to determine whether these shifts in the traveling waves are indeed responsible for the animal's extraordinary maneuverability. A natural approach for understanding such biomechanical mechanisms is the use of models—either computational (simulations) or physical (robots).
Complex and nonlinear interactions between an animal and its environment are often difficult to capture using computer simulations. Even as computers get faster, predicting the motion of a robot or animal subject to these complex interactions is akin to predicting the weather. This might be in part due to a lack of understanding of individual “components” (muscles, limbs, substrate) or due to lack of knowledge of [and extreme sensitivity to (4)] the detailed interactions between multiple components. These complexities can be addressed using experimental robotics, i.e., physical models.
Recent improvements in the mechanical design and manufacturing of robots and the ability to reproduce naturalistic movements and morphologies (i.e., improved “biofidelity”) has increased the success of using robots to understand biological systems (4–9). In particular, strides have been made toward using robots to understand the interactions between the body, sensorimotor control, and environment. Moreover, despite the apparent complexity of animal behaviors, many of the behaviors seem to result from comparatively simple, low-dimensional patterns of movement. Low-dimensional, task-specific models for the locomotor behavior of interest, called “templates” (10, 11), enable the application of control systems analysis to locomotion (12, 13). These simplified models of behavior are essential for understanding stability, maneuverability, efficiency, and the underlying control schemes in biological systems and bioinspired robots.
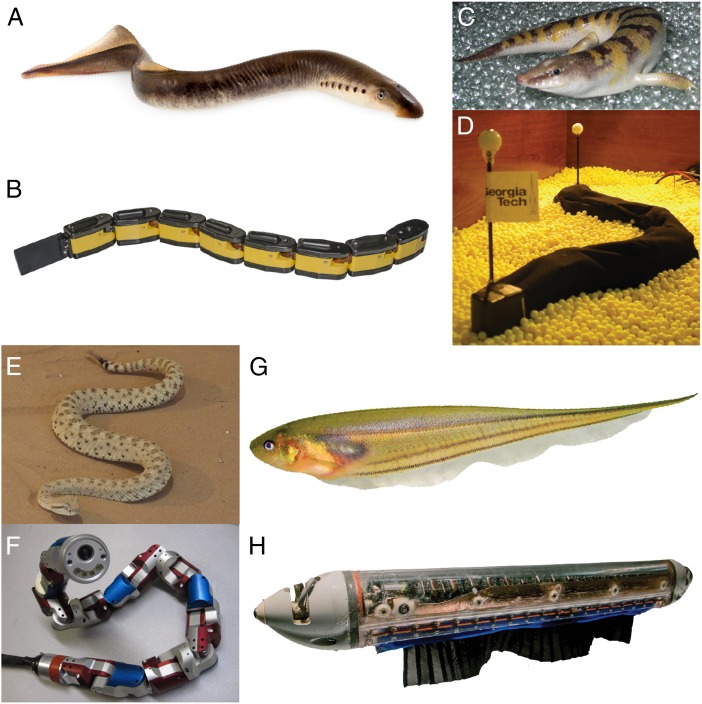
Experiments with biomimetic robots, sometimes in conjunction with simplified task-level template models, play a critical role in understanding undulatory locomotion (Fig. 1) (7, 14–16). Within undulatory movements, there is a wide range of behavioral strategies. Most of the prior work has focused on traveling waves in a single dimension, but even when restricted to one dimension, there are still multiple control strategies observed in nature. For example, lamprey swim (Fig. 1 A and B) through water and sandfish lizards (Fig. 1 C and D) swim through granular media using a single traveling wave along the body (15, 16). By contrast, knifefish (Fig. 1 G and H) partition their undulatory ribbon-fin motion into two in-plane inward-counterpropagating waves (17), a mechanism that enhances the stability and maneuverability of these fish (7).
Fig. 1.
(A and B) Lamprey and its biomimetic robot, Amphibot. Reproduced with permission from (A) © Can Stock Photo Inc./Arsty and (B) Biorobotics Laboratory, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland. (C and D) Sandfish lizard and its biomimetic robot that swim through granular media using body undulations. Reproduced with permission from Royal Society. (E and F) Rattlesnake sidewinder and a modular snake robot that exhibit maneuverability during turning by modulating the amplitude and phase of two orthogonal traveling waves along the body (3). Reproduced with permission from ref. 3. (G and H) Glass knifefish and a biomimetic robot with motorized fin that achieve fore–aft maneuverability and stability by partitioning the fin into two inward-counterpropagating waves. Reproduced with permission from ref. 7.
Multiplane undulatory motions have been far less studied. As Astley et al. describe (3), sidewinding is a particular mode of locomotion in certain snakes that emerges from the superposition of horizontal and vertical traveling waves. Indeed, the ground couples vertical waves—which define what parts of the body are in contact with the ground—and horizontal waves—which are ultimately responsible for generating forces for movement. Based on their biological observations, they put forth a simple two-wave template control scheme that exploits this coupling to produce turning behaviors in sidewinders. They hypothesize that the commonly observed turning behaviors in rattlesnakes, namely “differential turning” (gradual changes in direction per cycle of undulation) and “reversal turning” (sudden and rapid changes in direction) are generated by amplitude and phase (timing) modulations, respectively, of the horizontal and vertical waves. To support this hypothesis, they implemented their two-wave control template in a modular biomimetic snake robot and showed similar maneuverability during turning to the biological snake (Fig. 1 E and F). The robotic data reveal that differential turning behavior is achieved through an amplitude modulation in the horizontal wave, whereas reversal turning is achieved through
Astley et al. present behavioral data that suggest a role for multiplane body undulations in sidewinding snakes to achieve turning maneuvers.
a 180° phase shift of the vertical wave. The use of a modular snake robot as the physical model is advantageous over a simulation approach because the substrate–body interactions are not yet fully understood.
The combination of animal and robotic studies is a powerful tool. The richness and diversity of animal behavior provides an extensive resource for inspiration in engineering design of robots. Conversely, even though biomimetic and bioinspired robots still lag behind their biological counterparts in terms of robustness, sensing, maneuverability, etc., robots can be used to test hypotheses in biology and neuromechanics. Critically, the use of physical models allows for experiments that are nearly impossible in animals due to the difficulty in making quantifiable measurements or manipulations to the morphology or control scheme, manipulations that are routine with robotic platforms.
Synergistically, the use of robots for understanding biology can also lead to strategies that have not been observed in nature. In the paper by Astley et al. (3), it is revealed that the snake-like robot can also produce a third type of turn, termed “frequency turning,” which is not observed in biological snakes. This behavior was elicited by systematically varying the spatial frequency of the horizontal and vertical waves. It is unclear why the snakes do not perform such turns—perhaps it is simply a behavioral preference of the animal or there could be an underlying biological limitation. On the other hand, demonstration of the two-wave template for controlling the modular snake robot offers new insight into developing simplified control strategies for limbless mobile robots that exhibit high maneuverability and robustness in challenging substrates such as sand and mud. This highlights a key feature of an animal–robot experimental approach: they are complementary experimental approaches and can be used to inform and build upon one another, with significant benefits to both fields.
Acknowledgments
This work was supported by a James S. McDonnell Foundation Complex Systems Scholar Award.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6200.
References
- 1.Colgate JE, Lynch KM. Mechanics and control of swimming: A review. IEEE J Oceanic Eng. 2004;29(3):660–673. [Google Scholar]
- 2.Tytell ED, Hsu C-Y, Williams TL, Cohen AH, Fauci LJ. Interactions between internal forces, body stiffness, and fluid environment in a neuromechanical model of lamprey swimming. Proc Natl Acad Sci USA. 2010;107(46):19832–19837. doi: 10.1073/pnas.1011564107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astley HC, et al. Modulation of orthogonal body waves enables high maneuverability in sidewinding locomotion. Proc Natl Acad Sci USA. 2015;112:6200–6205. doi: 10.1073/pnas.1418965112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Umbanhowar PB, Komsuoglu H, Koditschek DE, Goldman DI. Sensitive dependence of the motion of a legged robot on granular media. Proc Natl Acad Sci USA. 2009;106(9):3029–3034. doi: 10.1073/pnas.0809095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saranli U, Buehler M, Koditschek DE. Rhex: A simple and highly mobile hexapod robot. Int J Robot Res. 2001;20(7):616–631. [Google Scholar]
- 6.Wood RJ. The first takeoff of a biologically inspired at-scale robotic insect. IEEE Trans Robot. 2008;24(2):341–347. [Google Scholar]
- 7.Sefati S, et al. Mutually opposing forces during locomotion can eliminate the tradeoff between maneuverability and stability. Proc Natl Acad Sci USA. 2013;110(47):18798–18803. doi: 10.1073/pnas.1309300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mongeau J-M, Demir A, Lee J, Cowan NJ, Full RJ. Locomotion- and mechanics-mediated tactile sensing: Antenna reconfiguration simplifies control during high-speed navigation in cockroaches. J Exp Biol. 2013;216(Pt 24):4530–4541. doi: 10.1242/jeb.083477. [DOI] [PubMed] [Google Scholar]
- 9.Ijspeert AJ. Biorobotics: Using robots to emulate and investigate agile locomotion. Science. 2014;346(6206):196–203. doi: 10.1126/science.1254486. [DOI] [PubMed] [Google Scholar]
- 10.Full RJ, Koditschek DE. Templates and anchors: Neuromechanical hypotheses of legged locomotion on land. J Exp Biol. 1999;202(Pt 23):3325–3332. doi: 10.1242/jeb.202.23.3325. [DOI] [PubMed] [Google Scholar]
- 11.Holmes P, Full RJ, Koditschek D, Guckenheimer J. The dynamics of legged locomotion: Models, analyses, and challenges. SIAM Rev. 2006;48(2):207–304. [Google Scholar]
- 12.Cowan NJ, et al. Feedback control as a framework for understanding tradeoffs in biology. Integr Comp Biol. 2014;54(2):223–237. doi: 10.1093/icb/icu050. [DOI] [PubMed] [Google Scholar]
- 13.Roth E, Sponberg S, Cowan NJ. A comparative approach to closed-loop computation. Curr Opin Neurobiol. 2014;25:54–62. doi: 10.1016/j.conb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Curet OM, Patankar NA, Lauder GV, MacIver MA. Mechanical properties of a bio-inspired robotic knifefish with an undulatory propulsor. Bioinspir Biomim. 2011;6(2):026004. doi: 10.1088/1748-3182/6/2/026004. [DOI] [PubMed] [Google Scholar]
- 15.Crespi A, Badertscher A, Guignard A, Ijspeert AJ. AmphiBot I: An amphibious snake-like robot. Robot Auton Syst. 2005;50(4):163–175. [Google Scholar]
- 16.Maladen RD, Ding Y, Umbanhowar PB, Kamor A, Goldman DI. Mechanical models of sandfish locomotion reveal principles of high performance subsurface sand-swimming. J R Soc Interface. 2011;8(62):1332–1345. doi: 10.1098/rsif.2010.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curet OM, Patankar NA, Lauder GV, MacIver MA. Aquatic manoeuvering with counter-propagating waves: A novel locomotive strategy. J R Soc Interface. 2011;8(60):1041–1050. doi: 10.1098/rsif.2010.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]