Significance
The skin and intestinal barrier are controlled by signaling scissors, termed ADAM17 (a disintegrin and metalloprotease 17), that reside in the membrane on the surface of cells. The main purpose of these signaling scissors is to liberate growth factors from their membrane anchor, allowing them to activate their receptors, including the epidermal growth factor receptor (EGFR). The ADAM17/EGFR signaling axis is tightly regulated, yet little is known about the underlying mechanism. Here we use genetic, cell biological, and biochemical approaches to identify two membrane proteins termed iRhoms 1 and 2 (inactive Rhomboid-like proteins) as crucial upstream regulators of ADAM17-dependent EGFR signaling. This uncovers the iRhoms as attractive novel targets to treat ADAM17/EGFR-dependent diseases such as cancer.
Keywords: inactive Rhomboid proteins, a disintegrin and metalloprotease 17, epidermal growth factor receptor, heparin-binding epidermal growth factor, transforming growth factor alpha
Abstract
The metalloproteinase ADAM17 (a disintegrin and metalloprotease 17) controls EGF receptor (EGFR) signaling by liberating EGFR ligands from their membrane anchor. Consequently, a patient lacking ADAM17 has skin and intestinal barrier defects that are likely caused by lack of EGFR signaling, and Adam17−/− mice die perinatally with open eyes, like Egfr−/− mice. A hallmark feature of ADAM17-dependent EGFR ligand shedding is that it can be rapidly and posttranslationally activated in a manner that requires its transmembrane domain but not its cytoplasmic domain. This suggests that ADAM17 is regulated by other integral membrane proteins, although much remains to be learned about the underlying mechanism. Recently, inactive Rhomboid 2 (iRhom2), which has seven transmembrane domains, emerged as a molecule that controls the maturation and function of ADAM17 in myeloid cells. However, iRhom2−/− mice appear normal, raising questions about how ADAM17 is regulated in other tissues. Here we report that iRhom1/2−/− double knockout mice resemble Adam17−/− and Egfr−/− mice in that they die perinatally with open eyes, misshapen heart valves, and growth plate defects. Mechanistically, we show lack of mature ADAM17 and strongly reduced EGFR phosphorylation in iRhom1/2−/− tissues. Finally, we demonstrate that iRhom1 is not essential for mouse development but regulates ADAM17 maturation in the brain, except in microglia, where ADAM17 is controlled by iRhom2. These results provide genetic, cell biological, and biochemical evidence that a principal function of iRhoms1/2 during mouse development is to regulate ADAM17-dependent EGFR signaling, suggesting that iRhoms1/2 could emerge as novel targets for treatment of ADAM17/EGFR-dependent pathologies.
ADAM17 (a disintegrin and metalloprotease 17) is a membrane-anchored metalloproteinase that controls two major signaling pathways with important roles in development and disease, the EGF receptor (EGFR) pathway and the proinflammatory tumor necrosis factor α (TNF-α) pathway (1–5). Mice lacking ADAM17 resemble mice with defects in EGFR signaling in that they have open eyes at birth, enlarged semilunar heart valves, and enlarged hypertrophic zones in long bone growth plates, most likely caused by a lack of ADAM17-dependent release of the EGFR ligands transforming growth factor α (TGF-α) and heparin-binding epidermal growth factor (HB-EGF) (3, 6–14). In humans, defects in skin and intestinal barrier protection have been reported in a patient lacking ADAM17 (15) and in patients treated with EGFR inhibitors (16, 17), and similar skin defects were recently identified in a patient with defective EGFR signaling (18). Mouse models of ADAM17/EGFR signaling appear to recapitulate these mechanisms, because defects in skin barrier protection can be observed by inactivating either ADAM17 or the EGFR in keratinocytes (19), as well as in mice expressing very low levels of ADAM17, which also have increased susceptibility to intestinal inflammation (20). A hallmark feature of ADAM17 is its rapid response to various activators of cellular signaling pathways (21–23), which is presumably important to allow a rapid response to injury and to maintain the skin and intestinal barrier. The rapid activation of ADAM17 is controlled by its transmembrane domain whereas the cytoplasmic domain is dispensable in this context (22), suggesting that ADAM17 is regulated by one or more other membrane proteins, yet the underlying mechanism has remained enigmatic.
Recent studies have shown that the maturation and function of ADAM17 in myeloid cells depend on inactive Rhomboid 2 (iRhom2), a catalytically inactive member of the Rhomboid family of seven membrane-spanning intramembrane serine proteinases (24–28). Myeloid cells lacking iRhom2 release very little TNF-α in response to activation of Toll-like receptor 4 by lipopolysaccharide (LPS) (24, 26, 28). Therefore, mice lacking iRhom2 are protected from the detrimental effects of TNF-α in mouse models for septic shock and inflammatory arthritis, similar to conditional knockout mice lacking ADAM17 in myeloid cells (11, 26, 29). However, iRhom2−/− (iR2−/−) mice are viable with no evident spontaneous pathological phenotypes (26, 29), whereas Adam17−/− (A17−/−) mice die shortly after birth (3). A major unresolved question has therefore been whether iRhom2 and the related iRhom1 are the long-sought-after regulators of the function of ADAM17-dependent EGFR signaling in vivo. Here we generate iRhom1−/− (iR1−/−) mice, which are viable and healthy, and report that iR1/2−/− double knockout mice closely resemble mice lacking ADAM17 or the EGFR, providing the first genetic evidence, to our knowledge, that the principal function of iRhoms1/2 during mouse development is to control ADAM17/EGFR signaling.
Results
Generation and Characterization of Mice Lacking iRhom1.
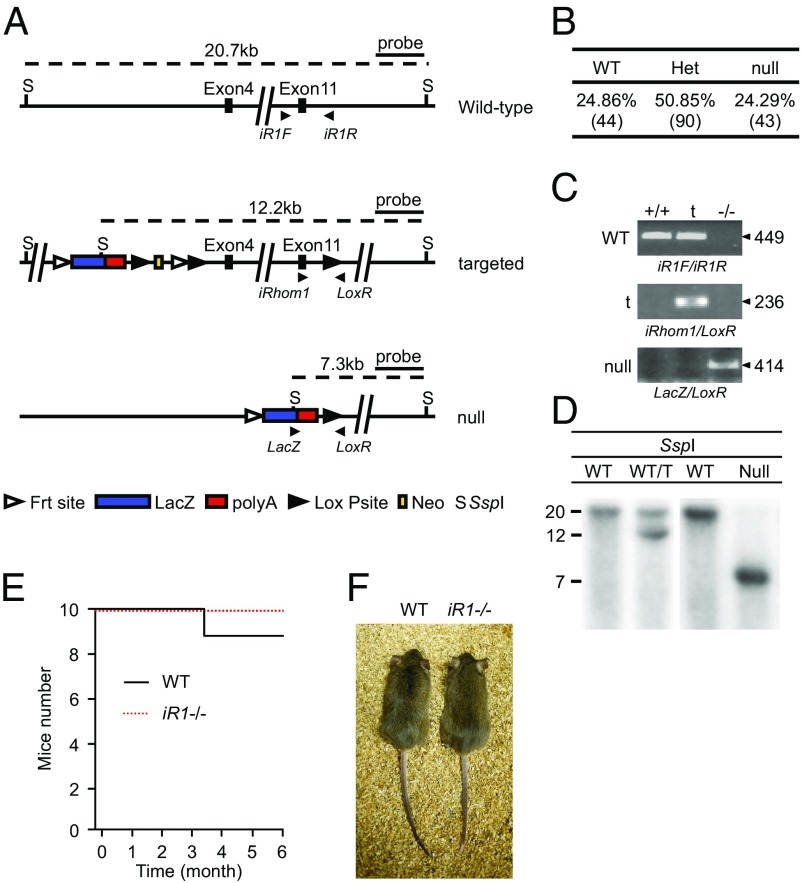
Mice lacking iRhom1 were generated using embryonic stem cells provided by the European Conditional Mouse Mutagenesis Program consortium. Animals carrying the targeted allele of iR1 were mated with mice expressing Cre under the control of the adenovirus EIIa promoter to delete exons 4–11 of iR1 in the germ line (Fig. 1A and Fig. S1A). Matings of the resulting heterozygous iR1+/− mice produced iR1−/− offspring at the expected Mendelian ratio (Fig. 1B), as assessed by genotyping through genomic PCR (Fig. 1C) and confirmed by Southern blot analysis (Fig. 1D). Characterization of the iR1 mRNA produced in these animals demonstrated that exons 1/2 could be detected in iR1−/− mouse embryonic fibroblasts (mEFs) by quantitative (q)PCR, whereas exons 4/5, 12/13, and 16/17 were not detectable (Fig. S1 B–E). Thus, any protein fragment, if produced in the mutant mice, would lack all transmembrane domains and most of the N-terminal cytoplasmic domain of iRhom1. iR1−/− mice were indistinguishable from their wild-type littermate controls during routine handling, appeared normal, survived up to at least 6 mo of age (Fig. 1 E and F), and had no evident spontaneous pathological phenotypes as adults (see Materials and Methods and SI Materials and Methods for details).
Fig. 1.
Generation and initial characterization of iR1−/− mice. (A) Diagram of the wild-type mouse iRhom1 locus from exon 4 to exon 11 (Top), the targeted (iR1t/+) allele with loxP sites flanking exon 4 to exon 11 (Middle), and the null allele resulting from recombination by EIIa-Cre (Bottom). (B) Ratio of offspring from iR1+/− x iR1+/− matings. (C) PCR genotyping results using DNA generated from wild-type, iR1t/+, and iR1−/− mice. t, targeted. (D) Southern blot analysis of genomic DNA from mice carrying the targeted allele of iRhom1 (A, Middle) or the null allele (A, Bottom). (E) The survival of 10 wild-type and iR1−/− mice was monitored over 6 mo, during which time one wild-type mouse died whereas all iR1−/− mice survived and appeared indistinguishable from their wild-type controls. (F) Photo of a 6-mo-old adult male iR1−/− mouse next to an age-matched male wild-type control.
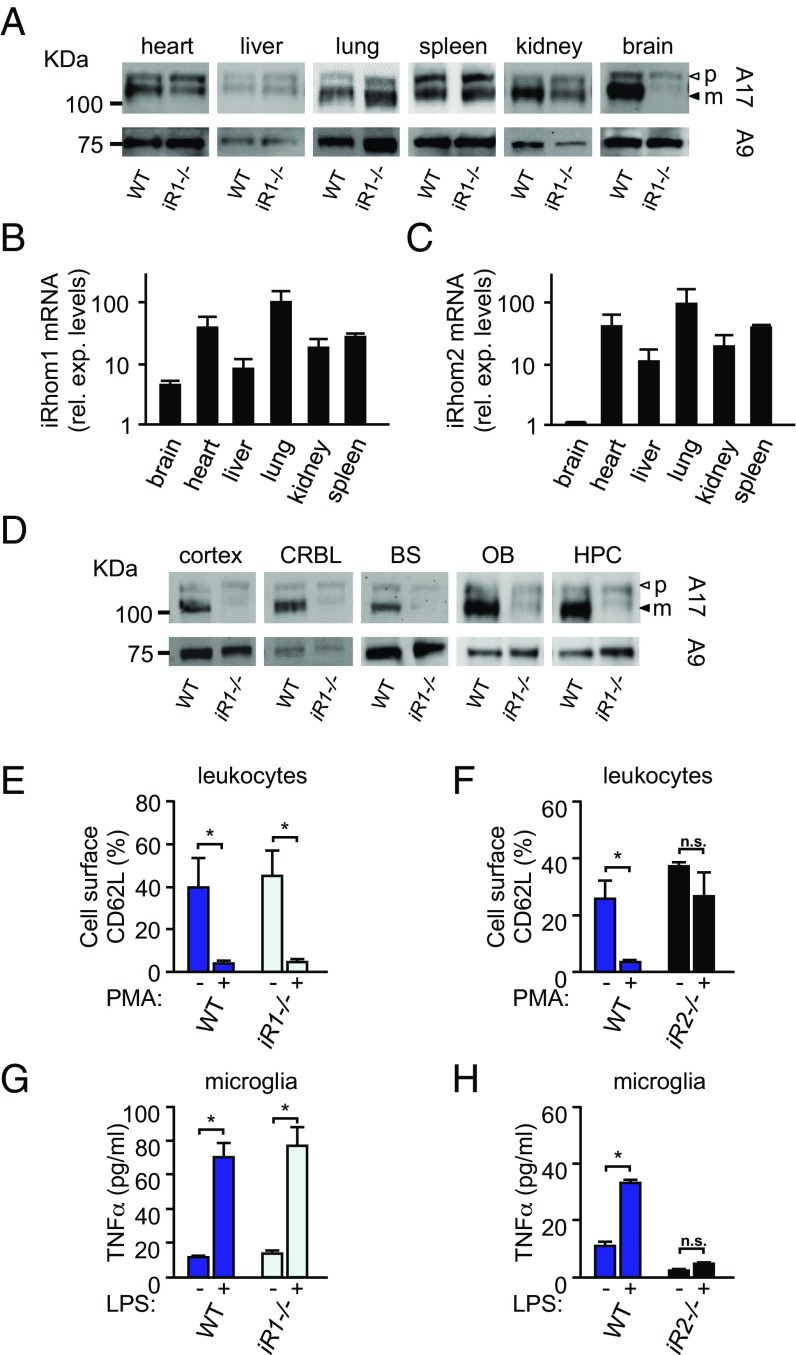
A Western blot analysis of ADAM17 in different tissues showed largely normal levels of mature ADAM17 in the heart, liver, lung, and spleen of iR1−/− mice compared with wild-type controls but slightly lower levels in the kidney and a strong reduction in the brain (Fig. 2A; quantification is shown in Fig. S2A). A qPCR analysis of iRhom 1 and 2 transcripts in different tissues of wild-type mice demonstrated that both iRhoms were expressed across all tissues examined, with the exception of the brain, where iRhom1 was easily detected but levels of iRhom2 were very low (Fig. 2 B and C) (biogps.org/#goto=genereport&id=217344) (30). Western blots of different parts of the brain of 8-wk-old iR1−/− mice showed barely detectable mature ADAM17 in the cortex, cerebellum, brainstem, olfactory bulb, and hippocampus, in sharp contrast to wild-type mice (Fig. 2D; quantification is shown in Fig. S2B). Because iRhom2 is required for the maturation and function of ADAM17 in myeloid cells, we isolated CD45high leukocytes from the brain to assess the function of ADAM17 by measuring the phorbol ester (phorbol 12-myristate 13-acetate; PMA)-stimulated shedding of CD62L (l-selectin) (31). We found that the PMA-stimulated down-regulation of CD62L was comparable in wild-type and iR1−/− brain leukocytes (Fig. 2E) but almost completely abolished in iR2−/− brain leukocytes (Fig. 2F). Similar experiments with resident microglia [CD45lowCD11bhighF4/80+Ly6Clow cells (32)] showed normal LPS-stimulated release of the ADAM17 substrate TNF-α from iR1−/− microglia compared with controls (Fig. 2G), whereas TNF-α shedding was almost undetectable from iR2−/− microglia (Fig. 2H), demonstrating that the functions of ADAM17 in microglia and circulating leukocytes depend on iRhom2 and not iRhom1.
Fig. 2.
ADAM17 Western blots and qPCR for iRhom1 and iRhom2 expression in different tissues of iR1−/− mice and controls, and analysis of the role of iR1 and iR2 in regulating the function of ADAM17 in microglia. (A) Representative Western blots of ADAM17 expression in tissues of iR1−/− and control mice. (B and C) Representative qPCR analysis of the expression of iRhom1 (B) or iRhom2 (C) in different tissues (representative of three experiments; please note that the scale is logarithmic). (D) Representative Western blots of ADAM17 in extracts from different brain areas from iR1−/− mice and controls (BS, brainstem; CRBL, cerebellum; HPC, hippocampus; OB, olfactory bulb). Open arrowheads in A and D indicate pro-ADAM17 (p); black arrowheads indicate mature ADAM17 (m). ADAM9 (A9) Western blots served as loading control in A and D; blots are representative of three separate experiments (quantification is shown in Fig. S2 A and B). (E–H) Circulating CD45high leukocytes (E and F) and CD45lowCD11bhighF4/80+Ly6Clow resident microglia (G and H) were isolated from brains of iR1−/− mice or iR2−/− mice or their wild-type control littermates. (E and F) The down-regulation of the substrate CD62L following stimulation with PMA was normal in circulating CD45high leukocytes from iR1−/− mice compared with controls (E), but was abolished in circulating CD45high leukocytes from iR2−/− mice compared with controls (F). (G and H) The function of ADAM17 in microglia was monitored by measuring LPS-stimulated release of TNF-α, which was normal in microglia isolated from iR1−/− mice but strongly reduced in microglia from iR2−/− mice compared with controls. *P < 0.05; ±SD (n = 3). n.s., not significant.
Generation and Characterization of iR1/2−/− Double Knockout Mice.
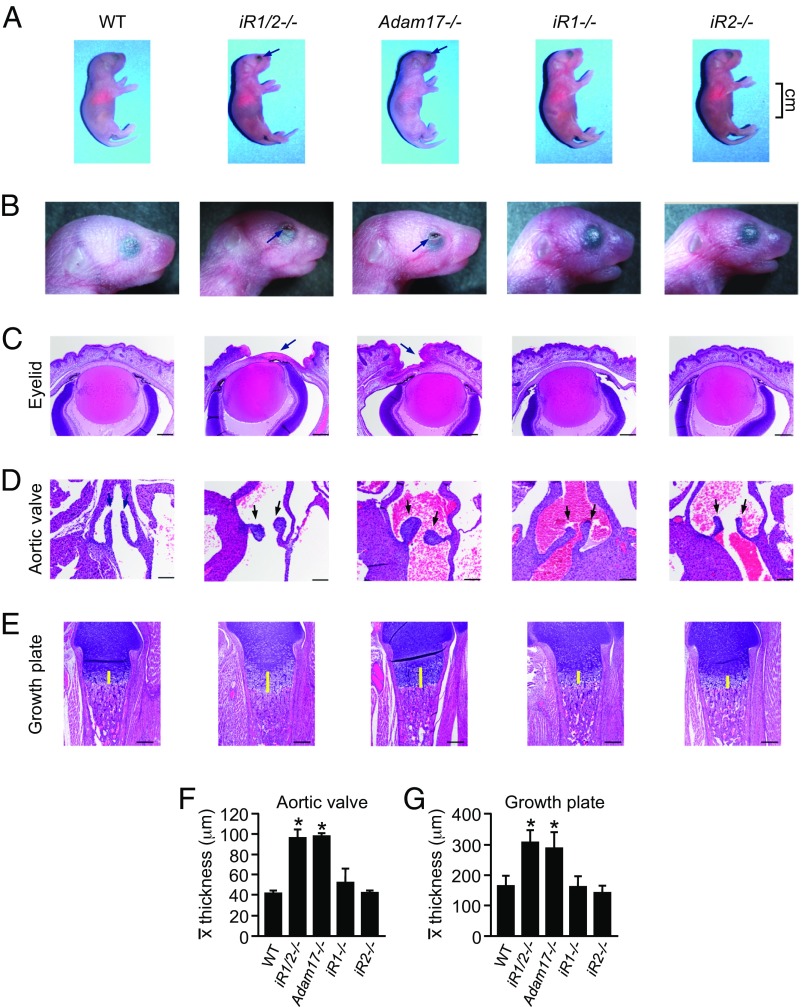
The normal appearance and behavior of iR1−/− mice (this study) or iR2−/− mice (24, 26, 28) and the finding that treatment of iR2−/− mEFs with iRhom1 siRNA reduced the function of ADAM17 (27, 29) led us to examine possible compensatory or redundant roles of iRhoms 1 and 2 during mouse development by simultaneously inactivating both iRhoms. Matings of iR1+/−iR2−/− mice yielded iR1/2−/− double knockout offspring at the expected Mendelian ratio (Fig. S3A). However, iR1/2−/− mice were born with open eyes (Fig. 3 A and B) and suffered perinatal lethality, just like A17−/− mice (3, 11). A histopathological analysis of iR1/2−/− mice showed failure of eyelid closure (Fig. 3C), enlarged aortic, pulmonic, and tricuspid heart valves (but normal mitral valves), and an enlarged zone of hypertrophic chondrocytes in the femoral and humoral growth plates, closely resembling defects in newborn A17−/− mice (Fig. 3 D–G and Fig. S3 B–E) (3, 10, 12). In iR1−/− or iR2−/− single knockout mice, these and other tissues were indistinguishable from wild-type controls (Fig. 3 and Fig. S3 B–E).
Fig. 3.
iR1/2−/− double knockout mice closely resemble Adam17−/− mice. (A) A comparison of newborn (P1) wild-type, iR1/2/−/−, Adam17−/−, iR1−/−, or iR2−/− mice, which appeared similar except for the open eyes at birth (OEB) in iR1/2−/− and Adam17−/− mice (arrows). (B and C) The OEB phenotype (arrows) is shown on macroscopic images of the head (B) and histological sections (C). (D) Aortic valve sections (arrows). (E) Sections through the femoral growth plate (the thickness of the hypertrophic zone is indicated by yellow lines). In all cases, the defects observed in iR1/2−/− mice (OEB, enlarged aortic valve, thickened zone of hypertrophic chondrocytes) closely resembled those in Adam17−/− mice, whereas iR1−/− and iR2−/− mice appeared normal. (F and G) Quantification of the average width relative to the length of individual aortic valve leaflets (F) or of the length of the growth plates (G) for each genotype (see Materials and Methods for details). All sections are representative of three mice examined. [Scale bars, 200 µm (C and E) and 100 µm (D).] , average. *P < 0.05; ±SD (n = 3).
Analysis of the Maturation of ADAM17 in Tissues and Cells from Newborn iR1/2−/− Mice.
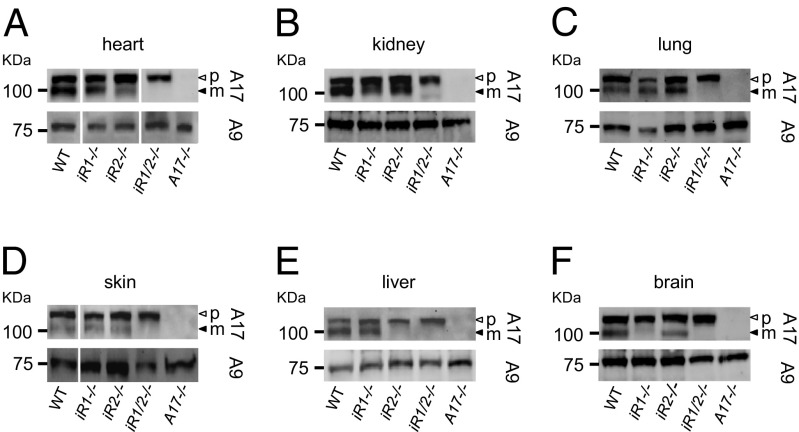
In a Western blot analysis of several tissues, we found no detectable mature ADAM17 in the heart, kidney, lung, skin, liver, or brain of newborn iR1/2−/− mice, although the proform was always present (Fig. 4; tissues from newborn wild-type or Adam17−/− mice served as positive and negative controls, respectively). Mature ADAM17 was also present in the heart, kidney, lung, and skin of iR1−/− and iR2−/− mice (Fig. 4 A–D) but strongly reduced in the liver of iR2−/− mice (Fig. 4E) and the brain of iR1−/− mice (Fig. 4F), consistent with similar findings in adult iR1−/− and iR2−/− mice (Fig. 2A; see also ref. 29; quantification of Fig. 4 is shown in Fig. S4). Taken together, these results demonstrate that either or both iRhoms 1 and 2 regulate the maturation and function of ADAM17 in all tissues examined during mouse development.
Fig. 4.
Western blots of ADAM17 in different tissues of newborn mice. Different tissues were harvested from newborn (P1) mice of the indicated genotypes [heart (A); kidney (B); lung (C); skin (D); liver (E); brain (F)] and subjected to Western blot analysis to determine the levels of pro- and mature ADAM17 in each sample (the open arrowheads point to pro-ADAM17; the closed arrowheads point to mature ADAM17), with ADAM9 serving as loading control. All blots are representative examples of at least three separate experiments (quantification is shown in Fig. S4).
EGFR Ligand Shedding from iR1/2−/− mEFs and EGFR Phosphorylation in iR1/2−/− Tissues.
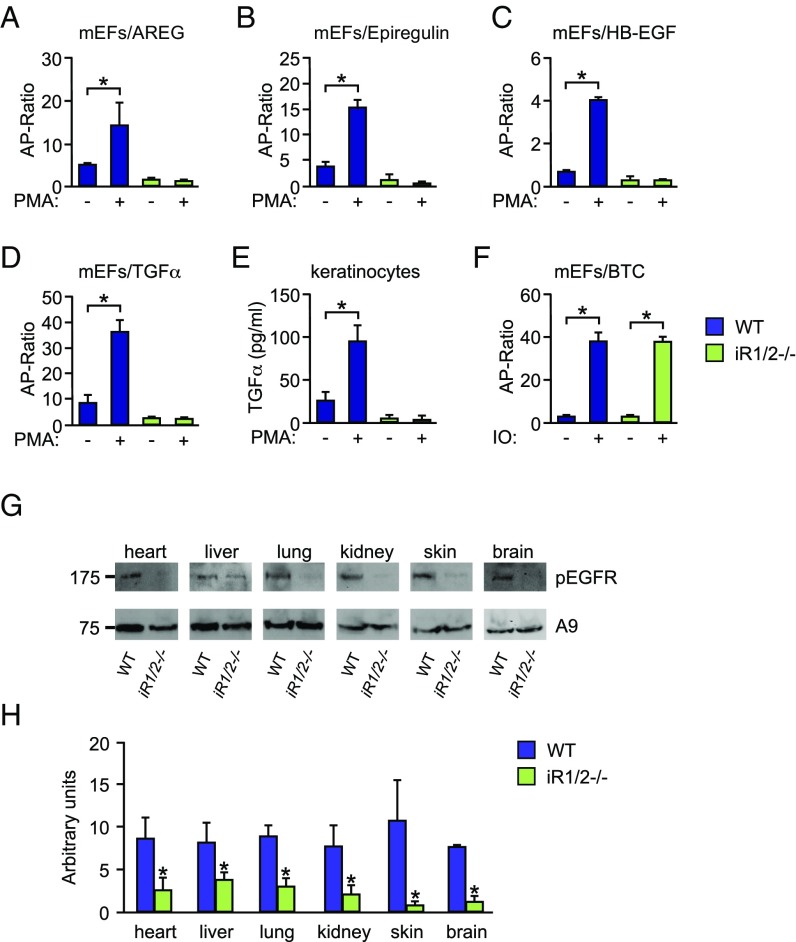
Previous experiments had uncovered a role for iRhom2 in regulating the substrate selectivity of stimulated ADAM17-dependent shedding events in mEFs (27). To test how inactivation of iRhom1 or both iRhoms 1 and 2 affects the function of ADAM17 in cell-based assays, we performed shedding experiments with iR1−/− mEFs, which have marginally reduced levels of mature ADAM17 compared with wild-type controls, or iR1/2−/− mEFs, which have no mature ADAM17 (Fig. S5 A and B; see also refs. 27 and 30). Immortalized iR1−/− mEFs showed comparable constitutive or PMA-stimulated shedding of the EGFR ligands epiregulin and HB-EGF and a slight decrease in TGF-α and amphiregulin shedding compared with wild-type controls (Fig. S6 A–D). However, iR1/2−/− mEFs resembled Adam17−/− mEFs (4, 27) in that they showed almost no constitutive or PMA-stimulated shedding of amphiregulin, epiregulin, HB-EGF, or TGF-α (Fig. 5 A–D). Moreover, constitutive and PMA-stimulated shedding of endogenous TGF-α from primary iR1/2−/− keratinocytes was also strongly reduced compared with wild-type controls (Fig. 5E). The ionomycin-stimulated shedding of the ADAM10 substrate betacellulin was normal in iR1/2−/− and iR1−/− mEFs, arguing against a role of iRhoms 1 and 2 in ADAM10-dependent ectodomain shedding (Fig. 5F and Fig. S6E). Because a principal function of ADAM17 is to promote EGFR signaling during development, we determined how the lack of mature ADAM17 affects the phosphorylation of the EGFR in different tissues of iR1−/−, iR2−/−, and iR1/2−/− mice compared with wild-type controls. We found no significant differences in EGFR phosphorylation in tissues of iR1−/− or iR2−/− mice compared with controls (Fig. S6F). However, the levels of phosphorylated EGFR were strongly reduced in all tissues harvested from viable newborn iR1/2−/− mice compared with wild-type tissues, providing direct evidence for an in vivo defect in EGFR signaling in iR1/2−/− mice (Fig. 5 F and G).
Fig. 5.
ADAM17-dependent EGFR ligand shedding is abolished in iR1/2−/− mEFs, and EGFR phosphorylation is strongly reduced in iR1/2−/− tissues. (A–F) The constitutive and PMA-stimulated shedding of amphiregulin (AREG) (A), epiregulin (B), HB-EGF (C), and TGF-α (D) is almost completely abolished in iR1/2−/− mEFs compared with wild-type controls, as is the constitutive and PMA-stimulated shedding of endogenous TGF-α from primary iR1/2−/− keratinocytes (E), whereas the constitutive and ionomycin (IO)-stimulated shedding of the ADAM10 substrate betacellulin (BTC) from iR1/2−/− mEFs is normal (F). (G and H) The levels of phosphorylated EGFR are strongly reduced in extracts of heart, liver, lung, kidney, skin, and brain of newborn iR1/2−/− mice compared with wild-type controls (quantification is shown in H). *P < 0.05; ±SD (n = 3).
Discussion
The ADAM17/EGFR signaling pathway can be rapidly activated within minutes in a manner that depends on the transmembrane domain of ADAM17, which is distal from its catalytic site (22, 23). This raises interesting questions about putative interacting regulatory membrane proteins. The discovery of iRhom2 as a crucial regulator of ADAM17 in hematopoietic cells (24, 26, 29) and in vitro studies in mouse embryonic fibroblasts demonstrating that iRhom2 controls the substrate selectivity of ADAM17-dependent shedding (27) and that iRhoms 1 and 2 are required for the function of ADAM17 in mouse embryonic fibroblasts (27, 30) raised the possibility that iRhom2 and the related iRhom1 could be the long-sought-after membrane regulators of ADAM17-dependent EGFR signaling. However, this hypothesis had not been previously corroborated by in vivo genetic studies. The close resemblance of iR1/2−/− double knockout mice and Adam17−/− mice reported here and the lack of functional ADAM17 as well as lack of EGFR phosphorylation in iR1/2−/− tissues provide genetic, cell biological, and biochemical evidence that the primary function of iRhoms 1 and 2 during development in vivo is to control ADAM17-dependent activation of the EGFR. Therefore, these studies strongly support a model in which the iRhoms are crucial upstream regulators of ADAM17 and the EGFR signaling pathway.
We also report that mice lacking iRhom1 appear normal and have no evident pathological or histopathological defects. Interestingly, iRhom1 is essential for the maturation of ADAM17 in the brain, likely because iRhom2 expression in the brain is too low to independently support the maturation of ADAM17 (biogps.org/#goto=genereport&id=217344) (30). We have previously found that iRhom1/ADAM17-dependent shedding has a significantly more limited substrate repertoire compared with iRhom2/ADAM17-dependent shedding (27). The unique importance of iRhom1 for ADAM17 maturation and function in brain cells may have evolved because the more limited substrate repertoire of iRhom1/ADAM17 could have advantages in the central nervous system. Very little is currently known about the function of ADAM17 in the brain, and a patient who is deficient in ADAM17 has no reported cognitive deficits (15). However, the targeted deletion of ADAM17 in oligodendrocytes leads to defects in myelination and in exploratory behavior in mice (33), and inactivation of the EGFR results in progressive neurodegeneration (34). Because iR1−/− mice are indistinguishable from their wild-type littermates during routine handling and do not display evident histopathological brain defects, future studies will be necessary to learn more about the function of ADAM17 and iRhom1 in the brain and whether iR1−/− mice could have subtle defects in the brain or cognitive or behavioral abnormalities that were not evident in the analysis performed here.
A recent study identified a strong association between Alzheimer’s disease and changes in methylation of iRhom2 in patient brains (35). Presumably, the change in methylation of iRhom2 (RHBDF2) leads to an increased expression of iRhom2 in microglia or brain leukocytes or both cell types, which could enhance their capacity to trigger proinflammatory injury in the brain. Our results suggest that the function of ADAM17 in resident microglia and circulating leukocytes infiltrating the brain depends on iRhom2, just as in peripheral myeloid cells (29). Decreasing iRhom2 activity in the brain would selectively inhibit ADAM17 in microglia and infiltrating leukocytes, likely without altering the function of ADAM17 in other cell types in the brain. Therapeutically targeting iRhom2 in the brain could thus be beneficial for prevention or treatment of Alzheimer’s disease, particularly in patients with increased expression of iRhom2 (35).
The phenotype of the iR1−/− and iR1/2−/− mice described here is different from the more severe phenotype of iR1−/− mice and the early embryonic lethality of iR1/2−/− mice reported by Christova et al. (30). This could be caused by the larger deletion generated by Christova et al. (exons 2–18 versus exons 4–11 in our iR1−/− mice), which could potentially affect regulatory elements for other genes or the presence of unidentified proximal mutations segregating with the targeted allele. We cannot rule out a contribution of subtle differences in genetic background, or that the first two exons in our iR1−/− mice (encoding amino acid residues 1–82 of iRhom1) could have functions that are lacking in the iR1−/− mice generated by Christova et al. (see SI Materials and Methods for details). However, because all transmembrane domains of iRhom1 are absent in the iR1−/− mice used here and we find striking similarity between iR1/2−/− mice and ADAM17−/− mice, the difference in phenotypes is most likely not related to additional iRhom1/2-regulated client membrane proteins that interact with these transmembrane domains (30).
The identification of iRhoms 1 and 2 as key regulators of ADAM17-dependent EGFR signaling in mice highlights an important difference compared with EGFR signaling in Drosophila melanogaster, which depends on active Rhomboid proteinases to catalyze EGFR ligand release (36, 37). Because other components of the EGFR signaling pathway are largely conserved between Drosophila and mammals (38), it is interesting to consider the potential evolutionary basis for why the release of EGFR ligands, which is crucial for activating the EGFR (reviewed in ref. 21), is accomplished by active Rhomboids in flies, whereas it requires one or both inactive Rhomboids and ADAM17 in mammals. The principal functions of ADAM17 in vivo appear to be protection of the skin and intestinal barrier through activating the EGFR (15, 19, 20). A unique property of ADAM17 (not observed for Drosophila Rhomboids) is its ability to be rapidly and posttranslationally activated within minutes by many signaling pathways (22, 23, 27, 39, 40). Therefore, it is tempting to speculate that this dedicated system, consisting of iRhoms 1 and 2 and ADAM17, evolved because of the advantage provided by a rapid EGFR activation to protect the skin and intestinal barrier (17) as well as release of TNF-α to activate innate immune responses, two major functions of ADAM17 (3, 11, 15, 19, 20). Taken together with previous studies demonstrating that mutations in the cytoplasmic domain of iRhom2 result in activation of ADAM17, leading to esophageal cancer with palmar-plantar keratosis (41), and our previous observation that iRhom2 controls the substrate selectivity of ADAM17-dependent shedding (27), our findings support a model in which the principal purpose of iRhoms 1 and 2 is to control the maturation and function of ADAM17, which specifically and rapidly engages the EGFR- and TNF-α–dependent signaling pathways. The interaction between iRhoms and ADAM17 thus provides novel therapeutic opportunities for selective and simultaneous inactivation of two major signaling pathways with important roles in development and disease, the EGFR signaling pathway, a well-established target for treatment of cancer, and the TNF-α pathway, which is a target for treatment of autoimmune diseases such as rheumatoid arthritis.
Materials and Methods
Additional details are provided in SI Materials and Methods.
Materials.
All materials were from Sigma-Aldrich unless noted otherwise. Restriction enzymes were from New England BioLabs, and dNTPs for qPCR were from Qiagen. The rabbit antibodies against phospho-EGFR were from Cell Signaling Technology and the secondary anti-rabbit HRP-labeled antibodies were from Promega.
Genetically Modified Mice.
Adam17−/− mice (A17−/−) and iRhom2−/− mice (iR2−/−) have been described previously (11, 26) and were of mixed genetic background (129Sv,C57BL/6). iRhom1−/− mice (iR1−/−) were generated from a conditionally targeted C57BL/6N ES cell line obtained from the European Conditional Mouse Mutagenesis Program (EPD0577_2_H04). iR1/2−/− double knockout mice were of mixed genetic background (129Sv,C57BL/6N,FVB). All animal experiments were approved by the Internal Animal Use and Care Committee of the Hospital for Special Surgery.
Southern Blot Analysis.
Genomic DNA isolated from mouse tails was purified and subsequently digested overnight with SspI (New England BioLabs), separated on a 1% agarose gel, and transferred to a Biodyne B membrane (Pall). Immobilized DNA was detected with a 32P-labeled DNA probe (see Fig. 1A for localization of the probe and the expected band size for the wild-type, targeted, and null alleles). Hybridization was performed for 14 h at 65 °C, and then the membranes were washed in 0.1× SSC/0.1% SDS and subsequently exposed to HyBlot CL film (Denville).
Western Blot Analysis.
For Western blots of the pro- and mature forms of ADAM17 in different tissues of adult iRhom1−/− mice and wild-type controls, the heart, liver, lung, spleen, kidney, brain, or different areas of the brain were isolated and processed as previously described (29), and the same approach was used to prepare samples for Western blots of different tissues in newborn mice [postnatal day (P)1].
Quantitative PCR Analysis.
Total RNA was extracted from heart, liver, lung, spleen, kidney, and brain using the RNeasy Mini Kit (Qiagen). RNA was reverse-transcribed by M-MuLV Reverse Transcriptase (New England BioLabs). qPCR (SYBR Green; ABI PRISM 7900HT; Applied Biosystems) was normalized to GAPDH.
Isolation of Microglia for TNF-α ELISA and Circulating Brain Leukocytes for CD62L FACS Analysis.
Mice were euthanized and perfused with PBS before isolation of whole brains. Brains were minced and digested with 1 mg/mL collagenase D (Roche Life Science) at 37 °C for 30 min. After passage through a cell strainer, cells were centrifuged in 40% (vol/vol) Percoll at 1,000 × g at room temperature for 15 min with low acceleration and no brake. Enriched microglia were collected at the bottom of the tube. Cells were stained with FITC-conjugated anti-mouse CD45 (clone 30-F11; BioLegend), PE-conjugated anti-mouse CD11b (clone M1/70; BioLegend), APC-conjugated anti-mouse F4/80 (clone BM8; BioLegend), and PerCp/Cy5.5-conjugated anti-mouse Ly6C Abs (clone HK1.4; BioLegend) for 30 min on ice. CD45lowCD11bhighF4/80+Ly6Clow microglia (32) were sorted on a FACSVantage cell sorter (Becton Dickinson) with >95% purity. Microglia (1 × 105) were cultured in the presence or absence of LPS (1 µg/mL) in a 96-well U-bottom plate at 37 °C for 2 h. Secreted TNF-α levels in the culture supernatant were measured with a TNF-α ELISA Kit (eBiocience). For analyzing CD62L shedding on circulating leukocytes in the brain, leukocytes enriched from whole mouse brain were left untreated or were treated with 25 ng/mL PMA for 20 min at 37 °C. Cell-surface expression of CD62L on CD45high circulating leukocytes was analyzed by FACS on an upgraded 11-color FACSCalibur machine (Becton Dickinson).
Histopathological Analysis.
Following euthanasia of 13.5-wk-old iR1−/− and wild-type mice (two of each genotype) by carbon dioxide (according to the guidelines of the American Veterinary Medical Association), all organs were examined grossly and fixed in 10% neutral buffered formalin or 4% paraformaldehyde. Tissues were processed routinely for histology and embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined.
Generation of Mouse Embryonic Fibroblasts.
Embryonic fibroblasts were isolated from embryonic day (E)13.5 iR1−/− and iR1/2−/− embryos to generate primary mEFs for immortalization as previously described (4).
Transfection and Ectodomain Shedding Assays.
iR1−/−, iR1/2−/−, and wild-type control mEFs were grown to 80% confluency and then transfected with plasmids encoding alkaline phosphatase-tagged EGF receptor ligands (4) using Lipofectamine 2000 (Invitrogen). One day after transfection, cells were washed in Opti-MEM (Gibco) for 1 h. Then, fresh Opti-MEM with or without 25 ng/mL phorbol 12-myristate 13-acetate or 2.5 µM ionomycin was added to the cells for 45 min to stimulate shedding, as indicated. After incubating with the alkaline phosphatase substrate 4-nitrophenyl phosphate, the alkaline phosphatase activity in the supernatant and lysate was measured at A405. Three identical wells were prepared, and the ratio of alkaline phosphatase activity in the supernatant and that of the cell lysate plus supernatant was calculated. Each experiment was conducted at least three times.
Statistical Analysis.
All values are expressed as means ± SD (with data from at least three independent experiments). The assumptions for normality (Kolmogorov–Smirnov test) and equal variance (Levene median test) were verified with SigmaStat 3.1 software (SYSTAT). The analysis of variance was performed with one-way analysis of variance. Statistics following a Student’s t distribution were generated using a two-tailed Student’s t test. Multiple parametric statistical comparisons between experimental groups versus a control group were accomplished by Dunnett’s method. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Elin Mogollon for technical assistance, and Alex Wolujuczyk for preparing the sections for histological analysis. This work was funded by NIH GM64750 (to C.P.B.), NIH CA159222 (to H.C.C.), Heinrich Heine University Grant 9772555 and Canadian Institutes of Health Research (CIHR) fellowship 201210MFE-289576-150035 (to D.R.M.), CIHR-MOP123276 (to T.W.M.), and NIH CA008748 (to S.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505649112/-/DCSupplemental.
References
- 1.Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 2.Moss ML, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 3.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 4.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunnarborg SW, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277(15):12838–12845. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen PJ, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376(6538):337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 7.Threadgill DW, et al. Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science. 1995;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 8.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269(5221):234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 9.Sibilia M, et al. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130(19):4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- 10.Hall KC, et al. ADAM17 controls endochondral ossification by regulating terminal differentiation of chondrocytes. Mol Cell Biol. 2013;33(16):3077–3090. doi: 10.1128/MCB.00291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiuchi K, et al. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179(5):2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LF, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22(11):2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mine N, Iwamoto R, Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132(19):4317–4326. doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto R, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA. 2003;100(6):3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaydon DC, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365(16):1502–1508. doi: 10.1056/NEJMoa1100721. [DOI] [PubMed] [Google Scholar]
- 16.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenberger BM, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013;5(199) doi: 10.1126/scitranslmed.3005886. 199ra111. [DOI] [PubMed] [Google Scholar]
- 18.Campbell P, et al. Epithelial inflammation resulting from an inherited loss-of-function mutation in EGFR. J Invest Dermatol. 2014;134(10):2570–2578. doi: 10.1038/jid.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzke CW, et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med. 2012;209(6):1105–1119. doi: 10.1084/jem.20112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalaris A, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–1624. doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blobel CP. ADAMs: Key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 22.Le Gall SM, et al. ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J Cell Sci. 2010;123(Pt 22):3913–3922. doi: 10.1242/jcs.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maretzky T, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011;2:229. doi: 10.1038/ncomms1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenthaler SF. Sheddase gets guidance. Science. 2012;335(6065):179–180. doi: 10.1126/science.1216815. [DOI] [PubMed] [Google Scholar]
- 26.McIlwain DR, et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science. 2012;335(6065):229–232. doi: 10.1126/science.1214448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maretzky T, et al. iRhom2 controls the substrate selectivity of stimulated ADAM17-dependent ectodomain shedding. Proc Natl Acad Sci USA. 2013;110(28):11433–11438. doi: 10.1073/pnas.1302553110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siggs OM, et al. iRhom2 is required for the secretion of mouse TNFα. Blood. 2012;119(24):5769–5771. doi: 10.1182/blood-2012-03-417949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Issuree PD, et al. iRHOM2 is a critical pathogenic mediator of inflammatory arthritis. J Clin Invest. 2013;123(2):928–932. doi: 10.1172/JCI66168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christova Y, Adrain C, Bambrough P, Ibrahim A, Freeman M. Mammalian iRhoms have distinct physiological functions including an essential role in TACE regulation. EMBO Rep. 2013;14(10):884–890. doi: 10.1038/embor.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Brazzell J, Herrera A, Walcheck B. ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood. 2006;108(7):2275–2279. doi: 10.1182/blood-2006-02-005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palazuelos J, et al. TACE/ADAM17 is essential for oligodendrocyte development and CNS myelination. J Neurosci. 2014;34(36):11884–11896. doi: 10.1523/JNEUROSCI.1220-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibilia M, Steinbach JP, Stingl L, Aguzzi A, Wagner EF. A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 1998;17(3):719–731. doi: 10.1093/emboj/17.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Jager PL, et al. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci. 2014;17(9):1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban S, Lee JR, Freeman M. Drosophila Rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107(2):173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- 37.Urban S, Lee JR, Freeman M. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 2002;21(16):4277–4286. doi: 10.1093/emboj/cdf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blobel CP, Carpenter G, Freeman M. The role of protease activity in ErbB biology. Exp Cell Res. 2009;315(4):671–682. doi: 10.1016/j.yexcr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prenzel N, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 40.Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31(Pt 6):1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 41.Blaydon DC, et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet. 2012;90(2):340–346. doi: 10.1016/j.ajhg.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.