Monitoring the immune system is complicated by its enormous cellular heterogeneity and by the continuous flux in the phenotypes and numbers of immune cells throughout the body. Measurements can be performed on tissue biopsies or peripheral blood, but these methods do not provide whole-body systemic information and may suffer from sampling errors. Noninvasive imaging techniques could provide an alternative strategy to sampling-based approaches and are being developed to allow for whole-body measurements of immune cell locations and changes (1). In particular, positron emission tomography (PET) is a highly sensitive imaging modality that provides three-dimensional and quantitative information on the accumulation of radiolabeled probes specific for either intracellular proteins or cell surface markers. The versatility of PET comes from the possibility of using radiolabeled small molecules, peptides, or proteins of interest as molecular probes, allowing for measurements of a wide range of biological processes in vivo (2). However, in practice, canonical approaches to produce libraries of PET probes specific for various cellular immune subsets are lacking, and many clinical applications of this imaging platform rely on a very small subset of probes, in particular the fluorinated glucose analog [18F]-2-fluorodeoxyglucose ([18F]FDG). In PNAS, Rashidian et al. (3) describe a potentially generalizable solution to the current limited availability of PET imaging assays for cell surface markers. The authors focused on alpaca-derived antibody fragments comprised of VHHs [the variable domain (VH) of a camelid heavy-chain–only antibody]. VHHs consist of single-domain variable regions (12–15 kDa) isolated from antibodies found in camels, alpacas, and sharks that are able to bind to a specific antigen with high affinity (Fig. 1A) (4). Traditional antibodies, isolated from species such as mice, rats, and humans contain a variable region that consists of two chains VH and VL creating the binding specificity. If the antibody variable region is isolated and expressed similar to VHHs, the resulting antibody fragment is ∼25-kDa fragment (scFv) or double the size of VHHs (Fig. 1A). Similar to scFv and other antibody fragments, VHHs do not contain an Fc domain, thereby removing all Fc-mediated functions such as antibody-dependent cell-mediated cytotoxicity (4). VHHs can provide reagents toward previously hard-to-target proteins or may bind to regions that are not typically accessible to traditional antibodies (such as catalytic sites in enzymes) (4). Additionally, VHHs that target alternate peptide motifs could be used as companion imaging agents toward protein targets of traditional antibodies, ideally without blocking the therapeutic efficacy.
Fig. 1.
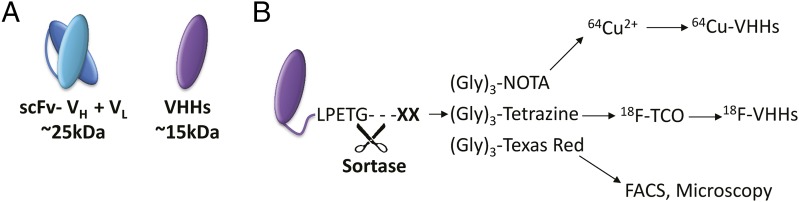
(A) The smallest traditional antibody fragment is composed of a binding domain containing two chains (VH and VL). The smallest binding domain of a camelid heavy-chain only antibody is the variable heavy domain (VHHs). (B) VHHs contain a sortase recognition motif. Incubation with sortase allows for cleavage between T/G residues and addition of the multi-modality imaging agents. Creating VHH-LPET(G)3-(agent of interest).
In the current study, VHHs were raised against mouse MHC class II (VHH7) and the myeloid surface marker CD11b (VHHDC13) (3, 5). In both preclinical and clinical PET, there is an abundant need to track myeloid cells to better understand the cell locations and dynamics of the innate response. The most commonly used PET probe, [18F]FDG, accumulates in most tumor cells, but also in myeloid cells making it difficult to distinguish between inflammation and malignant transformation in some instances (6–8). Furthermore, cancer therapies, such as immune-modulating drugs or ionizing radiation can increase tumor infiltration by immune cells (9, 10). For this reason, [18F]FDG enhancement following some treatments may be due to inflammation, and not additional tumor growth termed pseudoprogression (11, 12). Follow-up scans with VHH7 or VHHDC13 in preclinical models could begin to delineate pseudoprogression or identify patients who are responding to treatment without the need for tumor biopsies. In addition, the small size of VHHs is favorable for these applications and allows for rapid blood clearance, high tissue penetrance, and should reduce any enhanced permeability and retention effect that may occur with full-length antibodies (13). These properties are advantageous for imaging and reduce the time from injection to scanning. With the described VHHs, the blood half-life is ∼20 min and animals were scanned as early as 1 h after injection with labeled VHH7 or VHHDC13 allowing for high-contrast PET (with 18F) images (3).
To enable multimodality imaging applications, the VHHs were engineered to contain a C-terminal sortase recognition motif. Upon incubation with sortase A, the threonine–glycine is broken allowing for site-specific modifications (Fig. 1B) (5). VHHs were then conjugated with fluorochromes (for FACS and intravital microscopy applications), and with various glycine-based linkers to enable the attachment of PET radioisotopes: (Gly)3-Tetrazine (for 18F labeling), or (Gly)3-NOTA (for 64Cu labeling) (Fig. 1B). For PET imaging, the radiolabeling was fast and efficient with a 20-min labeling time and ∼70% (18F) and 90% (64Cu) decay-corrected radiochemical yield (3). These imaging agents were easily produced, therefore demonstrating how sortase allows for a broad range of selective modifications to engineered VHHs to generate a variety of multimodality imaging agents. Rashidian et al. then obtained PET scans of CD11b, or MHC class II-expressing cells with increased signal seen in the lymph nodes, spleen, and either the tumor or site of induced inflammation. For VHH7, the specificity of the probe was demonstrated by imaging MHC class II−/− mice that had no observable signal in lymphoid tissue. The sensitivity of 18F-VHH7 PET was demonstrated by obtaining scans at 6-d post tumor implantation, before the tumor reaching a palpable size. In a comparison of VHH7, VHHDC13, and FDG for monitoring inflammation, the accumulation of VHHDC13 was significantly higher in the inflamed footpad.
Although Rashidian et al. demonstrate two examples of VHH imaging agents, several questions remain to be addressed in future studies. These VHHs come from alpacas and are likely immunogenic. Although future clinical applications will certainly require “humanized” reagents (4), will the same hold true for preclinical studies? Without understanding the immunogenicity, sequential imaging with immune-specific VHHs may cause artifacts due to immune responses to the injected antigen. 18F radiolabeling
Rashidian et al. describe a potentially generalizable solution to the current limited availability of PET imaging assays for cell surface markers.
allowed for images to be obtained at 1–2 h postinjection. In the 64Cu labeling, scans were performed up to 24 h after injection with a reduced tumor/blood ratio compared with earlier time points. Will all VHHs have optimal imaging at earlier time points? Additionally, does binding of the VHHs increase internalization/turnover of targeted epitope and will this change the image? Last, what is the sensitivity of these probes: (i) how many epitopes are needed per cell, (ii) how many cells are needed per area, and (iii) will location and/or cell type change either of these parameters? In total, imaging with VHHs has the potential to have a broad impact by creating targeted imaging agents for almost any defined extracellular antigen. We anticipate this strategy will be adapted for multiple applications including those outside of the cancer/immunology field.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6146.
References
- 1.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8(11):677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins GD, Miller MA, Soon VC, Receveur T. Small animal PET imaging. ILAR J. 2008;49(1):54–65. doi: 10.1093/ilar.49.1.54. [DOI] [PubMed] [Google Scholar]
- 3.Rashidian M, et al. Noninvasive imaging of immune responses. Proc Natl Acad Sci USA. 2015;112:6146–6151. doi: 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Meyer T, Muyldermans S, Depicker A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014;32(5):263–270. doi: 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Witte MD, et al. Preparation of unnatural N-to-N and C-to-C protein fusions. Proc Natl Acad Sci USA. 2012;109(30):11993–11998. doi: 10.1073/pnas.1205427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair-Gill E, et al. PET probes for distinct metabolic pathways have different cell specificities during immune responses in mice. J Clin Invest. 2010;120(6):2005–2015. doi: 10.1172/JCI41250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimori T, et al. Increased 18F-FDG uptake in a model of inflammation: Concanavalin A-mediated lymphocyte activation. J Nucl Med. 2002;43(5):658–663. [PubMed] [Google Scholar]
- 8.Radu CG, Shu CJ, Shelly SM, Phelps ME, Witte ON. Positron emission tomography with computed tomography imaging of neuroinflammation in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2007;104(6):1937–1942. doi: 10.1073/pnas.0610544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day SE, et al. Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1-13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn Reson Med. 2011;65(2):557–563. doi: 10.1002/mrm.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang H, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42(9):1412–1417. [PubMed] [Google Scholar]
- 13.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17-18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]


