Significance
Most fishes are cold-blooded, but tunas and some sharks (e.g., white sharks) maintain their exercising muscles warmer than ambient waters. This ability is a remarkable example of convergent evolution because bony and cartilaginous fishes diverged as long as 450 million years ago. What are the ecological benefits driving the evolution of warm muscles in fishes? Despite extensive discussion, no previous studies have tested a simple possibility that fishes with warm muscles may swim faster in nature. We demonstrate that fishes with warm muscles swim faster and perform larger-scale annual migrations than similar-sized cold-blooded fishes. Our results suggest that warm muscles enhance power output and, thus, cruising speeds, which may enable longer-distance migrations and potentially greater access to seasonally available resources.
Keywords: marine predator, swim speed, migration, body temperature
Abstract
Despite long evolutionary separations, several sharks and tunas share the ability to maintain slow-twitch, aerobic red muscle (RM) warmer than ambient water. Proximate causes of RM endothermy are well understood, but ultimate causes are unclear. Two advantages often proposed are thermal niche expansion and elevated cruising speeds. The thermal niche hypothesis is generally supported, because fishes with RM endothermy often exhibit greater tolerance to broad temperature ranges. In contrast, whether fishes with RM endothermy cruise faster, and achieve any ecological benefits from doing so, remains unclear. Here, we compiled data recorded by modern animal-tracking tools for a variety of free-swimming marine vertebrates. Using phylogenetically informed allometry, we show that both cruising speeds and maximum annual migration ranges of fishes with RM endothermy are 2–3 times greater than fishes without it, and comparable to nonfish endotherms (i.e., penguins and marine mammals). The estimated cost of transport of fishes with RM endothermy is twice that of fishes without it. We suggest that the high energetic cost of RM endothermy in fishes is offset by the benefit of elevated cruising speeds, which not only increase prey encounter rates, but also enable larger-scale annual migrations and potentially greater access to seasonally available resources.
In 1835, the British physician John Davy reported that skipjack tuna have body temperatures 10 °C higher than ambient waters and considered this fish an exception to the general rule that fishes are cold-blooded (1). It is currently known that at least 14 species of tuna (family Scombridae) and five species of shark (four species in the family Lamnidae and one species in the family Alopiidae) have the ability to retain metabolic heat via vascular countercurrent heat exchangers, and to maintain the temperature of slow-twitch, aerobic red muscle (hereafter denoted RM) significantly above that of the ambient water (2–7). This “RM endothermy” (see SI Materials and Methods for terminology) in fishes represents a remarkable example of convergent evolution, because bony fishes and cartilaginous fishes diverged as long as 450 million years ago (8). In addition to elevated RM temperature, tunas and endothermic sharks share a number of morphological (e.g., medially located RM), physiological (e.g., high metabolic rates), and ecological (e.g., highly mobile and predatory lifestyle) characteristics (9).
RM endothermy is an energetically expensive thermal strategy (9), and its convergent evolution indicates that the extra energetic costs incurred by RM endothermy can be outweighed by some ecological advantages. This topic has been discussed intensively, and two primary, nonmutually exclusive hypotheses have been proposed: expansion of the thermal niche and elevated cruising speeds (2). The thermal niche hypothesis states that fishes with RM endothermy can tolerate a broader range of water temperatures and, thus, can expand their geographic niche. An increasing suite of evidence supports this hypothesis; tunas and endothermic sharks often range widely and dive well beneath the thermocline and, consequently, experience a broad temperature range (e.g., more than 20 °C in some species; refs. 10 and 11). However, some ectothermic species (e.g., blue shark) experience similar temperature ranges by diving deep (11, 12), suggesting that other factors may also affect the thermal preference and tolerance of pelagic fishes.
The elevated cruising speed hypothesis states that elevated RM temperature enhances the power output of RM and, thereby, increases cruising speed of the fishes (2). This hypothesis is reasonable, because the contraction speed and power output of the isolated RM (13) and the sustained swim speed of ectothermic fishes in captivity (14) all increase with temperature within a species, at least within their normal temperature range. Surprisingly, however, a previous laboratory study found no differences in the sustained swim speeds between two Scombridae species with and without RM endothermy (15). As a result, evidence for the hypothesis is still lacking.
If fishes with RM endothermy are shown to cruise faster in nature, what ecological benefits could they achieve from doing so? Fishes can increase prey encounter rates and, thus, potential energy gains by cruising faster (16); however, this benefit may be counteracted if energetic costs incurred by cruising faster and being endothermic are high. It is therefore important to examine whether the cost of transport (i.e., the energy needed to move a unit body mass over a unit distance) at their cruising speeds is higher for fishes with RM endothermy.
In addition to the benefit of increased prey encounter rates, fishes with RM endothermy may be able to move greater distances in a given time period, such as a year, because of their fast cruising speed. Annual migrations are common in fishes, often between foraging grounds and reproductive habitats (10, 11, 17); therefore, it is hypothesized that fishes with RM endothermy exhibit annual migrations over larger spatial scales than fishes without it. If such difference is observed, large-scale migration could be an ecological advantage, because it allows the fishes with RM endothermy to better exploit seasonal peaks of resource abundance and avoid seasonal resource depression (18).
Because of the rapid development and improvement of various data-recording or transmitting tags, information on fish movements in the wild is increasingly available, both from fine-scale (e.g., recording swim speed; ref. 19) and long-term (e.g., recording migration path; ref. 11) animal-tracking studies. Such information has provided much insight into the ecology of many species; however, no previous studies have examined the possible differences in the movement patterns or swimming energetics in nature between fishes with and without RM endothermy. In this study, therefore, we compiled data on cruising swim speed and migration range of fishes, recorded by various animal-tracking tools, both from the literature and our own fieldwork. We also estimated the cost of transport for each species swimming at each cruising speed. Using phylogenetically informed allometry, we examined whether fishes with RM endothermy (i) swim faster, (ii) have higher cost of transport, and (iii) exhibit larger-scale annual migrations.
Results
Cruising Speed.
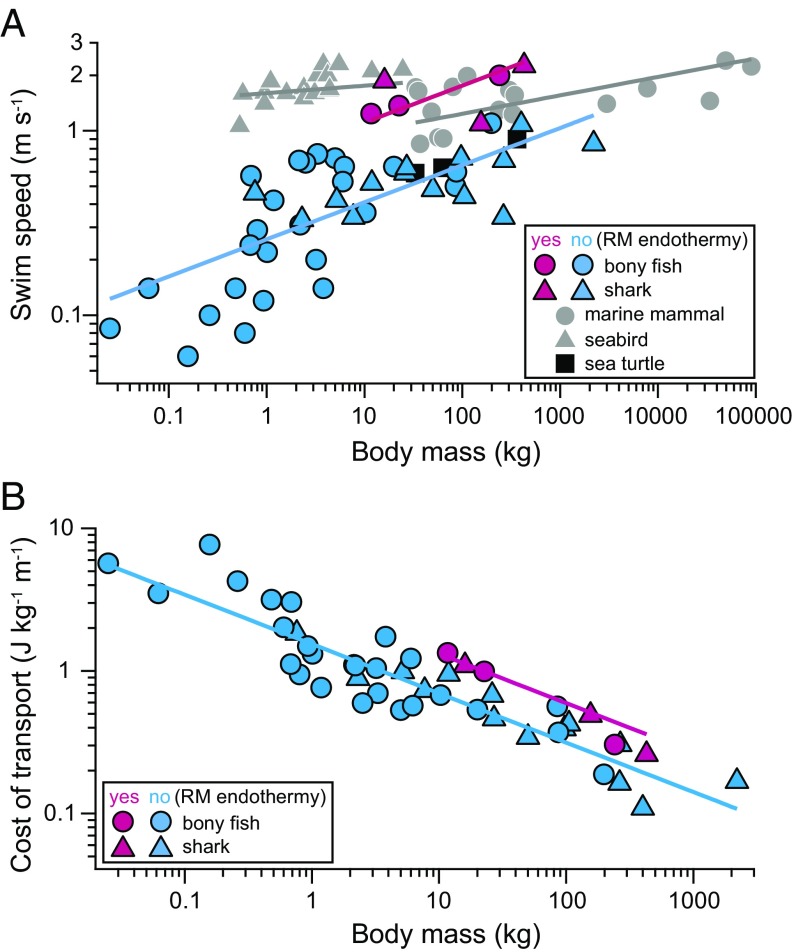
We compiled cruising speed data for 46 fish species with body masses ranging from 0.025 to 2,200 kg, including six species with RM endothermy (three sharks and three tunas) (Table S1). Phylogenetically informed regressions (20) (see Fig. S1 for the phylogenetic tree), coupled with model selection analysis based on Akaike information criterion (AIC), showed that cruising speed is best explained by body mass, body temperature, and whether the fish has RM endothermy (R2 = 0.66, Akaike weight = 0.73) (Table 1). Body temperature was set at specific values based on previous measurements for fishes with RM endothermy, and at ambient temperature of the swimming depths for fishes without RM endothermy. For a given body mass, cruising speed was 2.7 times faster for fishes with RM endothermy (speed = 0.70 × mass0.20) than fishes without it (speed = 0.26 × mass0.20) (Fig. 1A). For a given body mass and body temperature, cruising speed was 2.4 times faster for fishes with RM endothermy (speed = 0.29 × mass0.20 × temp0.28) than fishes without it (speed = 0.12 × mass0.20 × temp0.28).
Table 1.
Fitting of phylogenetic regression models
| Model | R2 | AIC | ΔAIC | wAIC |
| Swim speed ∼ Body mass + Body temp + Endothermy | 0.66 | −4.5 | 0 | 0.73 |
| Swim speed ∼ Body mass + Endothermy | 0.60 | −2.4 | 2.1 | 0.26 |
| Swim speed ∼ Body mass + Body temp | 0.51 | 3.4 | 7.9 | 0.01 |
| Swim speed ∼ Body mass | 0.44 | 6.5 | 11.1 | <0.01 |
| Swim speed ∼1 | 0 | 29.8 | 34.4 | <0.01 |
| COT ∼ Body mass + Body temp + Endothermy | 0.90 | −53.3 | 0 | 0.88 |
| COT ∼ Body mass + Body temp | 0.88 | −49.2 | 4.1 | 0.12 |
| COT ∼ Body mass + Endothermy | 0.84 | −34.4 | 18.9 | <0.01 |
| COT ∼ Body mass | 0.8 | −25.5 | 27.8 | <0.01 |
| COT ∼1 | 0 | 45.9 | 99.2 | <0.01 |
| Migration range ∼ Body mass + Endothermy | 0.67 | 0.3 | 0 | 0.99 |
| Migration range ∼ Body mass | 0.35 | 9.7 | 9.4 | <0.01 |
| Migration range ∼1 | 0 | 16.2 | 15.9 | <0.01 |
The best models are shown in bold. wAIC, Akaike weight.
Fig. 1.
Cruising speed and the cost of transport as a function of body mass. (A) Cruising speed of fishes with RM endothermy (pink) and fishes without it (light blue) recorded in the wild, with other vertebrates swimmers [seabirds, marine mammals (gray), and sea turtles (black)] for comparison. (B) Cost of transport (i.e., the energy needed to move a unit body mass over a unit distance) estimated for each fish species with each cruising speed. See main text for the equations of phylogenetically informed regression lines shown in the figure, except for seabirds (speed = 1.60 × mass0.04) and marine mammals (speed = 0.78 × mass0.10) (21) in A.
The cruising speeds of fishes were compared with those of other vertebrate swimmers (seabirds, marine mammals, and sea turtles) compiled previously (21) (Fig. 1A). There was considerable variation in swim speed for a given body mass in each group, and the slopes of the phylogenetic regression lines were steeper for fishes (exponent, 0.20) than seabirds (0.04) and marine mammals (0.10). Nevertheless, cruising speeds of fishes with RM endothermy were closer to those of nonfish endotherms (seabirds and marine mammals) than to fishes without RM endothermy. The speeds of sea turtles (ectotherms, except for the leatherback turtle; ref. 22) were close to those of fishes without RM endothermy.
Cost of Transport.
Cost of transport (COT) estimated for each species with each cruising speed (Table S1) was also best explained by body mass, body temperature, and whether the fish has RM endothermy (R2 = 0.90, Akaike weight = 0.88) (Table 1). For a given body mass, COT was 1.9 times higher for fishes with RM endothermy (COT = 2.92 × mass−0.35) than fishes without it (COT = 1.54 × mass−0.35) (Fig. 1B). For a given body mass and body temperature, COT was 1.5 times higher for fishes with RM endothermy (COT = 0.78 × mass−0.36 × temp0.40) than fishes without it (COT = 0.53 × mass−0.36 × body0.40).
Migration Range.
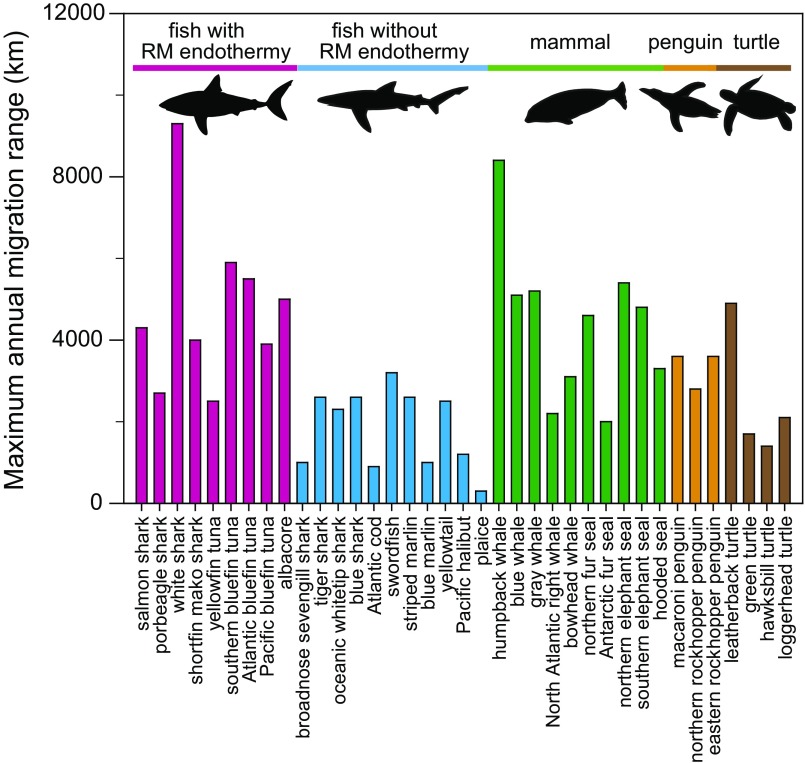
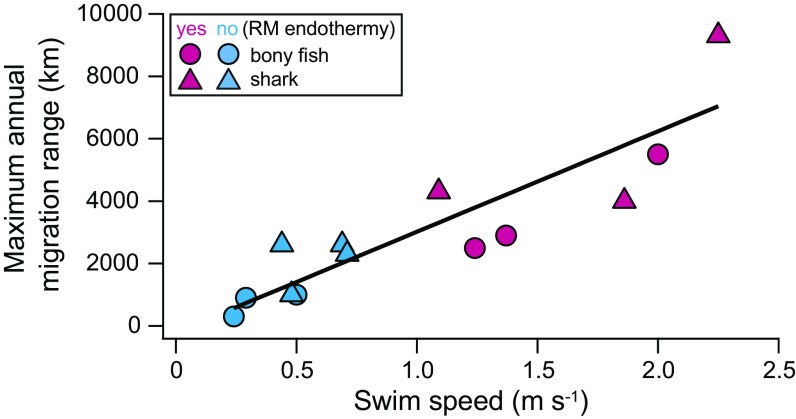
Annual migration patterns vary greatly among individuals, ages, and populations in some species (11, 23), and the “average” migration pattern might not be informative. Therefore, the maximum, rather than average, annual migration range was compiled for 20 fish species with body masses ranging from 0.6 to 315 kg, including nine species with RM endothermy (four sharks and five tunas), and 17 species of nonfish vertebrate swimmers (Fig. 2 and Table S2). The migration range of fishes was best explained by body mass and whether the fish has RM endothermy (R2 = 0.67, Akaike weight = 0.99) (Table 1; see also Fig. S2 for the phylogenetic tree). For a given body mass, maximum migration range was 2.5 times larger for fishes with RM endothermy (range = 1,754 × mass0.23) than fishes without it (range = 707 × mass0.23) (Fig. S3). In 13 species for which both maximum migration ranges and cruising speeds were available, the two parameters were linearly related (R2 = 0.77) (Fig. 3).
Fig. 2.
The maximum annual migration ranges for various vertebrate swimmers. The range for a species is measured from long-term animal-tracking studies as the maximum value (among individuals) of linear distances along the Earth’s surface between the two furthest points on an individual’s annual migration loop.
Fig. 3.
The relationship between cruising speed and maximum annual migration range in fishes. Among the species shown in Figs. 1A (cruising speed) and 2 (maximum annual migration range), only 13 species for which both parameters are available are used in this figure. The least-squares regression line is as follows: range = 3,095 × speed – 117.
Maximum migration ranges of fishes with RM endothermy were closer to those of marine mammals, penguins, and leatherback turtles than to fishes without RM endothermy (Fig. 2).
Finally, we repeated the regression analyses of cruising speed, COT, and migration range by using ordinary least-squares (i.e., nonphylogenetic) method and found a similar amount of support (R2 value and Akaike weight) for each candidate model (Table S3). Thus, our results are not specific to our choice of evolutionary process models (Materials and Methods).
Discussion
Fast Cruising Speed.
We show that fishes with RM endothermy cruise at faster speeds than fishes without it. Our result is inconsistent with a previous laboratory experiment (15) that swam two Scombridae species with and without RM endothermy and found no support for the elevated cruising speed hypothesis. However, because of the size limitation of the water tunnel, the fishes swum in that study were small juveniles (<300 g). RM endothermy develops gradually with body size (24), and potential differences in the swimming performance might not have been detected in that study. In contrast, we took a phylogenetic comparative approach by using data for a range of wild fishes, including adult tunas (up to 240 kg) and endothermic sharks (up to 428 kg), and provided a statistically robust result.
Our result indicates that convergent evolution of RM endothermy in fishes has not only allowed the expansion of thermal niche, as shown in previous studies (10, 11), but also enhanced cruising speeds, as shown here for the first time to our knowledge. Fishes with RM endothermy are faster even after the effect of body temperature is controlled for, suggesting that their elevated cruising speeds are not simply a result of thermal effects on RM, but are enhanced by other characteristics shared by these fishes. Such characteristics include high metabolic rates (higher than what would be predicted for their body temperature based on the Q10 effect from the metabolic rates of ectothermic fishes; ref. 2) and thunniform swimming mode (where lateral movements are largely confined to the caudal region) associated with a unique force-transmission system from RM to the caudal region (25).
Interestingly, the cruising speeds of fishes with RM endothermy are close to those of nonfish endotherms (seabirds and marine mammals), whereas speeds of fishes without RM endothermy are comparable to sea turtles. We suggest that thermal strategy (endothermy or ectothermy) is a major determinant of cruising speed not only in fishes, but also in marine vertebrates in general, presumably through its strong effect on metabolic rates and muscle contractile properties.
High Energetic Cost.
Elevated cruising speed of fishes with RM endothermy should enable increased prey encounter rates and, thus, increased potential energy gains, as shown by a predator–prey interaction model in 3D space (16). However, we also show that COT of fishes with RM endothermy is approximately twice that of fishes without it. The high COT can be attributed to their high standard metabolic rates (9) and the exponential increase in energy requirements with swim speed (26). A previous comparison of swimming energetics of fishes studied in water tunnels provided a similar result, but with a smaller dataset including only bony fishes (27). Although our estimates for COT are inevitably based on many assumptions (e.g., a universal Q10 value among fishes, and the extrapolation of scaling relationship of basal metabolic rate in large species; Materials and Methods), our analyses indicate that any energetic benefit of increased prey encounter rates is, at least partly, counteracted by the higher energetic costs incurred by swimming faster and being endothermic. High energetic costs may also be linked to fast somatic and gonadal growth and elevated digestion rates in tunas (28), although the link is less clear in endothermic sharks (29).
Large-Scale Migration.
In addition to the benefit of increased prey encounter rates, we find that the maximum annual migration range of a species is larger for fishes with RM endothermy than fishes without it, even after controlling for the effect of body size. Notably, all sharks with RM endothermy in our dataset (salmon, porbeagle, white, and shortfin mako sharks) are capable of larger scale migrations than any ectothermic sharks (broadnose sevengill, tiger, oceanic whitetip, and blue sharks), despite their similar ecological niches as upper trophic-level predators and relatively large numbers of tracking records available.
Our result can partly be explained by greater tolerance to broad temperature ranges in species with RM endothermy, because long, latitudinal migrations involve significant changes in water temperature. However, the migration range of fishes with RM endothermy is 2.5 times larger than similar-sized fishes without RM endothermy, a similar value to what we find for cruising speeds (2.7 times). Moreover, the relationship between maximum migration ranges and cruising speeds across fishes is linear. Together, these results indicate that the spatial scale of annual migration of fishes is strongly affected by their cruising speed.
Intriguingly, the maximum migration ranges of fishes with RM endothermy are closer to those of nonfish, endothermic swimmers than to fishes without RM endothermy. For example, the Atlantic bluefin tuna (30), salmon shark (11), blue whale (31), northern fur seal (32), and leatherback turtle (a turtle with warmed body core) (33) migrate between temperate and tropical (or subpolar and subtropical) habitats, and no fishes without RM endothermy perform migrations over similar spatial scales. Another notable example is the swordfish, which migrates between temperate and tropical habitats (34), a spatial scale that is the largest among fishes without RM endothermy (Fig. 2). RM of this species is not significantly warmer than surrounding waters (35), but located medially in the body (similarly to fishes with RM endothermy) with a simple form of heat exchangers present, a morphology that suggests the ability to reduce heat loss from RM during steady swimming (36). Overall, our finding suggests that, in marine vertebrates in general, thermal strategy (endothermy or ectothermy) is a major determinant of the spatial scales of annual migrations through its effect on cruising speeds and tolerance to broad temperature ranges.
In conclusion, our comparative analyses indicate that a potential ecological advantage of RM endothermy in fishes is the ability to cruise faster, which not only increases prey encounter rates, but also enables larger-scale annual migrations and greater access to seasonally available resources. We suggest that this advantage, coupled with the previously recognized benefit of thermal niche expansion, could outweigh high energetic costs incurred by RM endothermy and, thus, has facilitated the radiation and diversification of tunas and endothermic sharks. Our analyses also indicate that fishes with RM endothermy are similar to birds and mammals in many respects, including not only high metabolic rates (2) and temperature dependence of muscle function (37), but also fast cruising speeds and the capabilities of large-scale migrations.
Materials and Methods
Cruising Speed.
Mean speeds of fishes swimming freely in the wild were compiled from the literature and our own field experiments (Table S1). For the published sources, the main methods of recording swim speed were (i) attaching a speed sensor (a propeller in most cases) directly to the fish (“Speed sensor” in the method column of Table S1); and (ii) tracking the fish with an acoustic transmitter attached and recording its movement path over a time period (“Acoustic tracking 2D” or “Acoustic tracking 3D”; see SI Materials and Methods for details). In addition, we accepted swim speed data from (iii) flow-speed measurements taken on the boat that was driven alongside the surface-swimming fish (“Boat”), (iv) vertical speed detected by depth sensors and divided by the sine of the pitch angle estimated from acceleration records (“Pitch”), and (v) the tail-beat frequency of the fish in the wild detected by attached magnetic sensors, coupled with the linear relationship between swim speed and tail-beat frequency examined in captivity (“Tailbeat”).
Our field experiments recorded swim speeds for the salmon shark Lamna ditropis (July 2012, in Prince Williams Sound in Alaska), oceanic whitetip shark Carcharhinus longimanus (May 2013 and April–May 2014, off Cat Island, The Bahamas), blacktip reef shark Carcharhinus melanopterus and gray reef shark Carcharhinus amblyrhynchos (July 2013, at Palmyra Atoll). Sharks were hooked and restrained alongside a boat, except for the salmon shark that was hooked and then lifted in a stretcher up on the deck of a boat. A PD3GT logger (21-mm diameter, 115-mm length, and 60 g; Little Leonardo) was incorporated into a package for the instrument recovery (38), which was composed of a time-scheduled release mechanism (Little Leonardo), float, very high frequency (VHF) radio transmitter (Advanced Telemetry Systems), and Argos transmitter (Wildlife Computers). The package was attached to the dorsal fin of the sharks, before the sharks were released. Once the package detached from the animals after a 1–4 d free-swimming period, it was located by using VHF and Argos signals and recovered by a boat. The logger recorded relative swim speed as the number of rotations of a propeller at 1-s interval, as well as depth, temperature (at 1-s interval), and three-axis accelerations (at 1/16-s interval). The propeller rotation values were converted to the actual swim speeds (m⋅s−1) by using the equation from a previous calibration experiment (39).
For comparative analyses of swim speeds, body mass and body temperature were estimated for each species in the dataset. See SI Materials and Methods for details.
COT.
COT (i.e., the energy required to move a unit body mass over a unit distance) (J⋅kg−1⋅m−1) was computed for each species in our dataset by estimating its routine metabolic rate (W) and dividing it by its cruising speed (m⋅s−1) and body mass (kilograms) (Table S1). Routine metabolic rate was assumed to be composed of basal (or standard) metabolic rate (BMR) and the net locomotion cost at the cruising speed.
BMR of ectothermic fishes was estimated from body mass and body temperature, using the scaling relationship (BMR = 0.224 × mass0.879 at 38 °C, where BMR is in mL of O2⋅h−1 and mass is in grams) and Q10 value (1.65) reported in fishes (40). Because the maximum body mass is only 2 kg in the published source (40), we had to extrapolate the scaling relationship of BMR for the species with larger body mass. Fishes with RM endothermy have higher BMR (2) and were considered separately. BMR reported for yellowfin tuna (91 mg of O2⋅kg−1⋅h−1 for 5.4 kg of body mass in 20 °C water) (41) was adjusted for body mass and water temperature [using the slope of the scaling relationship (0.879) and Q10 value (1.65); ref. 40] to estimate BMR of this species in our dataset. Similarly, BMR reported for Pacific bluefin tuna (120 mg of O2⋅kg−1⋅h−1 for 8.3 kg of body mass in 20 °C water) (41) and shortfin mako shark (124 mg of O2⋅kg−1⋅h−1 for 6.1 kg of body mass in 18 °C water) (42) were used to estimate BMR of two bluefin tuna species (Pacific and Atlantic) and three lamnid shark species (salmon, white, and shortfin mako) in our dataset, respectively. BMR expressed in the volume of oxygen consumed per unit time was converted to the physical unit (W), by assuming that 1 mol oxygen occupies 22.4 L and equates to the utilization of 434 kJ.
The net locomotion cost (i.e., the elevation of metabolic cost above BMR during steady swimming) has been measured in water tunnels for many fish species. When oxygen consumption rate (in log scale) is plotted against swim speed (in linear scale, in body length⋅s−1), the relationships are linear, and the slopes are similar among different species with different body sizes (26). That is, for a unit increase in swim speed (in body length⋅s−1), there is approximately a corresponding 2.3-fold elevation in metabolic rates for many species. Therefore, we used this value and the relative cruising speed of each species in our dataset [cruising speed (m⋅s−1) divided by body length (meters); Table S1] to estimate its net locomotion cost.
Migration Range.
The maximum annual migration range for a species was examined from the literature that tracked marine vertebrates (fishes, penguins, seals, whales, and sea turtles) for a long period (Table S2). Among seabirds, only penguins (which migrate by swimming) were considered, because we focused on swimming behavior rather than flight. The main methods of recording migration paths in the collected literature were (i) satellite tracking by using Argos transmitting tags (“Argos tag” in the method column of Table S2), (ii) light-level–based geolocation by using archival tags (“Archival tag”), and (iii) the combination of Argos satellite tracking and light-level–based geolocation, using pop-up archival transmitting tags (“Pop-up tag”). In addition, the data from (iv) the tidal location method (based on the time of high water and tidal range measured by depth sensors attached to benthic fish) (“Tidal location”), (v) the photo identification (“Photo ID”), and (vi) the conventional radio tracking (“Radio tag”) used for some whales were also included.
Any migration paths making a complete loop in a year were accepted as a candidate. For species known to return to the same area every year, paths representing an incomplete loop (i.e., in the case that tracking ended long before a year) were also accepted. For each species, the maximum value (among individuals) of the linear distances along the Earth’s surface between two furthest points on an individual’s migration loop was measured by using Google Earth. Incomplete loops only available for some species, and the photo identification and radio tracking methods used for some whales (in which individual animals are located only limited times), precluded us from estimating the distance traveled along the migration loops.
For comparative analyses of migration ranges, body mass was estimated for each species in the dataset. See SI Materials and Methods for details.
Data Analyses.
Phylogenetic trees for the species in our dataset were created by using the software Mesquite (43), with the published sources for the phylogenetic relationships among species (44–47) and an arbitrary branch length (48) (Figs. S1 and S2). The trees were transferred to the software Matlab (MathWorks), where further regression analyses were conducted by using the Regressionv2.m program (20).
All continuous variables (i.e., swim speed, cost of transport, migration range, body mass, and body temperature) were log10 transformed to improve linearity of relationships among the variables, and whether the fish have RM endothermy was input as a categorical value (Table 1). The regression equation, the coefficient of determination (R2 value), and AIC for each model was computed under the Ornstein–Uhlenbeck evolutionary process model, and the model with best support was determined based on Akaike weights. To examine the robustness to our choice of evolutionary process model, the procedure was repeated by using ordinary least-squares (i.e., nonphylogenetic) regression method.
Supplementary Material
Acknowledgments
We thank D. Abercrombie, S. Anderson, T. Bacon, M. Bond, D. Bradley, A. Brooks, E. Brooks, A. Carlisle, S. Genereaux, L. Howey-Jordan, L. Jordan, C. Lowe, J. Musick, J. Salamone, and S. Williams for their help in the field; T. Garland for providing the Regressionv2.m program; B. Mate for providing additional information for the published tracking data of blue whales; and N. Payne, A. Takahashi, and two anonymous reviewers for helpful comments on the draft. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science Grant 25850138 (to Y.Y.W.), and Grants-in-Aids from the Alaska Department of Fish and Game (to K.J.G.), the Marisla Foundation (to J.E.C.), and the Moore Bahamas Foundation and the Save Our Seas Foundation (to D.D.C.), and the Marine Alliance for Science and Technology for Scotland (Y.P.P.). The production of this paper was supported by a National Institute of Polar Research (NIPR) publication subsidy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500316112/-/DCSupplemental.
References
- 1.Davy J. On the temperature of some fishes of the genus Thunnus. Philos Trans R Soc Lond B Biol Sci. 1835;•••:327–328. [Google Scholar]
- 2.Dickson KA, Graham JB. Evolution and consequences of endothermy in fishes. Physiol Biochem Zool. 2004;77(6):998–1018. doi: 10.1086/423743. [DOI] [PubMed] [Google Scholar]
- 3.Carey FG, Teal JM. Heat conservation in tuna fish muscle. Proc Natl Acad Sci USA. 1966;56(5):1464–1469. doi: 10.1073/pnas.56.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal D, Sepulveda CA. Evidence for temperature elevation in the aerobic swimming musculature of the common thresher shark, Alopias vulpinus. Copeia. 2005;2005(1):146–151. [Google Scholar]
- 5.Block BA, Finnerty JR, Stewart AFR, Kidd J. Evolution of endothermy in fish: Mapping physiological traits on a molecular phylogeny. Science. 1993;260(5105):210–214. doi: 10.1126/science.8469974. [DOI] [PubMed] [Google Scholar]
- 6.Goldman KJ. Regulation of body temperature in the white shark, Carcharodon carcharias. J Comp Physiol B Biochem. Syst Environ Physiol. 1997;167(6):423–429. [Google Scholar]
- 7.Goldman KJ, Anderson SD, Latour RJ, Musick JA. Homeothermy in adult salmon sharks, Lamna ditropis. Environ Biol Fishes. 2004;71(4):403–411. [Google Scholar]
- 8.Ravi V, Venkatesh B. Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev. 2008;18(6):544–550. doi: 10.1016/j.gde.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Bernal D, Dickson KA, Shadwick RE, Graham JB. Review: Analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comp Biochem Physiol A Mol Integr Physiol. 2001;129(2-3):695–726. doi: 10.1016/s1095-6433(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 10.Block BA, et al. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science. 2001;293(5533):1310–1314. doi: 10.1126/science.1061197. [DOI] [PubMed] [Google Scholar]
- 11.Weng KC, et al. Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science. 2005;310(5745):104–106. doi: 10.1126/science.1114616. [DOI] [PubMed] [Google Scholar]
- 12.Queiroz N, Humphries NE, Noble LR, Santos AM, Sims DW. Spatial dynamics and expanded vertical niche of blue sharks in oceanographic fronts reveal habitat targets for conservation. PLoS ONE. 2012;7(2):e32374. doi: 10.1371/journal.pone.0032374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rome LC, Swank DM, Coughlin DJ. The influence of temperature on power production during swimming. II. Mechanics of red muscle fibres in vivo. J Exp Biol. 2000;203(Pt 2):333–345. doi: 10.1242/jeb.203.2.333. [DOI] [PubMed] [Google Scholar]
- 14.Bennett AF. Thermal dependence of locomotor capacity. Am J Physiol. 1990;259(2 Pt 2):R253–R258. doi: 10.1152/ajpregu.1990.259.2.R253. [DOI] [PubMed] [Google Scholar]
- 15.Sepulveda C, Dickson KA. Maximum sustainable speeds and cost of swimming in juvenile kawakawa tuna (Euthynnus affinis) and chub mackerel (Scomber japonicus) J Exp Biol. 2000;203(Pt 20):3089–3101. doi: 10.1242/jeb.203.20.3089. [DOI] [PubMed] [Google Scholar]
- 16.Gerritsen J, Strickler JR. Encounter probabilities and community structure in zooplankton: A mathematical model. J Fish Res Board Can. 1977;34(1):73–82. [Google Scholar]
- 17.Howey-Jordan LA, et al. Complex movements, philopatry and expanded depth range of a severely threatened pelagic shark, the oceanic whitetip (Carcharhinus longimanus) in the western North Atlantic. PLoS ONE. 2013;8(2):e56588. doi: 10.1371/journal.pone.0056588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alerstam T, Hedenstrom A, Akesson S. Long-distance migration: Evolution and determinants. Oikos. 2003;103(2):247–260. [Google Scholar]
- 19.Watanabe YY, Lydersen C, Fisk AT, Kovacs KM. The slowest fish: Swim speed and tail-beat frequency of Greenland sharks. J Exp Mar Biol Ecol. 2012;426:5–11. [Google Scholar]
- 20.Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T., Jr Morphometrics of the avian small intestine compared with that of nonflying mammals: A phylogenetic approach. Physiol Biochem Zool. 2008;81(5):526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe YY, et al. Scaling of swim speed in breath-hold divers. J Anim Ecol. 2011;80(1):57–68. doi: 10.1111/j.1365-2656.2010.01760.x. [DOI] [PubMed] [Google Scholar]
- 22.Casey JP, James MC, Williard AS. Behavioral and metabolic contributions to thermoregulation in freely swimming leatherback turtles at high latitudes. J Exp Biol. 2014;217(Pt 13):2331–2337. doi: 10.1242/jeb.100347. [DOI] [PubMed] [Google Scholar]
- 23.Block BA, et al. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434(7037):1121–1127. doi: 10.1038/nature03463. [DOI] [PubMed] [Google Scholar]
- 24.Dickson K. Tunas as small as 207 mm fork length can elevate muscle temperatures significantly above ambient water temperature. J Exp Biol. 1994;190(1):79–93. doi: 10.1242/jeb.190.1.79. [DOI] [PubMed] [Google Scholar]
- 25.Donley JM, Sepulveda CA, Konstantinidis P, Gemballa S, Shadwick RE. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature. 2004;429(6987):61–65. doi: 10.1038/nature02435. [DOI] [PubMed] [Google Scholar]
- 26.Beamish F. Swimming capacity. In: Hoar WS, Randall DJ, editors. Locomotion, Fish Physiology. Vol 7. Academic; London: 1978. pp. 101–187. [Google Scholar]
- 27.Korsmeyer KE, Dewar H. Tuna metabolism and energetics. In: Block BA, Stevens ED, editors. Tuna: Physiology, Ecology, and Evolution, Fish Physiology. Vol 19. Academic; San Diego: 2001. pp. 35–78. [Google Scholar]
- 28.Brill RW. Selective advantages conferred by the high performance physiology of tunas, billfishes, and dolphin fish. Comp Biochem Physiol. 1996;113A(1):3–15. [Google Scholar]
- 29.Goldman KJ, Musick JA. Growth and maturity of salmon sharks (Lamna ditropis) in the eastern and western North Pacific, and comments on back-calculation methods. Fish Bull. 2006;104(2):278–292. [Google Scholar]
- 30.Galuardi B, et al. Complex migration routes of Atlantic bluefin tuna (Thunnus thynnus) question current population structure paradigm. Can J Fish Aquat Sci. 2010;67(6):966–976. [Google Scholar]
- 31.Bailey H, et al. Behavioural estimation of blue whale movements in the Northeast Pacific from state-space model analysis of satellite tracks. Endanger Species Res. 2009;10:93–106. [Google Scholar]
- 32.Ream RR, Sterling JT, Loughlin TR. Oceanographic features related to northern fur seal migratory movements. Deep Sea Res. II. 2005;52(5-6):823–843. [Google Scholar]
- 33.James MC, Ottensmeyer CA, Myers RA. Identification of high-use habitat and threats to leatherback sea turtles in northern waters: New directions for conservation. Ecol Lett. 2005;8(2):195–201. [Google Scholar]
- 34.Neilson JD, et al. Investigations of horizontal movements of Atlantic swordfish using pop-up satellite archival tags. In: Nielsen JL, et al., editors. Tagging and Tracking of Marine Animals with Electronic Devices. 2009. pp. 145–159. [Google Scholar]
- 35.Carey FG. A brain heater in the swordfish. Science. 1982;216(4552):1327–1329. doi: 10.1126/science.7079766. [DOI] [PubMed] [Google Scholar]
- 36.Block BA. Endothermy in fish: Thermogenesis, ecology and evolution. In: Hochachka PW, Mommsen TP, editors. Biochemistry and Molecular Biology of Fishes. Vol 1. Elsevier; New York: 1991. pp. 269–311. [Google Scholar]
- 37.Bernal D, Donley JM, Shadwick RE, Syme DA. Mammal-like muscles power swimming in a cold-water shark. Nature. 2005;437(7063):1349–1352. doi: 10.1038/nature04007. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Baranov EA, Sato K, Naito Y, Miyazaki N. Foraging tactics of Baikal seals differ between day and night. Mar Ecol Prog Ser. 2004;279:283–289. [Google Scholar]
- 39.Watanabe Y, et al. Swimming behavior in relation to buoyancy in an open swimbladder fish, the Chinese sturgeon. J Zool (Lond) 2008;275(4):381–390. [Google Scholar]
- 40.White CR, Phillips NF, Seymour RS. The scaling and temperature dependence of vertebrate metabolism. Biol Lett. 2006;2(1):125–127. doi: 10.1098/rsbl.2005.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blank JM, Farwell CJ, Morrissette JM, Schallert RJ, Block BA. Influence of swimming speed on metabolic rates of juvenile Pacific bluefin tuna and yellowfin tuna. Physiol Biochem Zool. 2007;80(2):167–177. doi: 10.1086/510637. [DOI] [PubMed] [Google Scholar]
- 42.Sepulveda CA, Graham JB, Bernal D. Aerobic metabolic rates of swimming juvenile mako sharks, Isurus oxyrinchus. Mar Biol. 2007;152(5):1087–1094. [Google Scholar]
- 43.Maddison WP, Maddison DR. 2011 Mesquite: A modular system for evolutionary analysis. Version 2.75. Available at mesquiteproject.org. Accessed January 1, 2015.
- 44.Vélez-Zuazo X, Agnarsson I. Shark tales: A molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes) Mol Phylogenet Evol. 2011;58(2):207–217. doi: 10.1016/j.ympev.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Betancur-R R, et al. The tree of life and a new classification of bony fishes. PLoS Curr. 2013;5:5. doi: 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow S, Nakagawa T, Suzuki N, Takeyama H, Matsunaga T. Phylogenetic relationships among Thunnus species inferred from rDNA ITS1 sequence. J Fish Biol. 2006;68:24–35. [Google Scholar]
- 47.Phillips RB, Matsuoka MP, Konkol NR, McKay S. Molecular systematics and evolution of the growth hormone introns in the Salmoninae. Environ Biol Fishes. 2004;69(1-4):433–440. [Google Scholar]
- 48.Grafen A. The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci. 1989;326(1233):119–157. doi: 10.1098/rstb.1989.0106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.