Significance
Osteoblasts produce bone matrix and contribute to bone remodeling. We have established a procedure to directly convert human fibroblasts into osteoblasts by transducing some defined factors and culturing in osteogenic medium. Osteoblast-specific transcription factors, Runx2 and Osterix, in combination with Oct4 and L-Myc, drastically induced fibroblasts to produce calcified bone matrix and express osteoblast-specific markers. The directly converted osteoblasts (dOBs) showed similar gene expression profiles as normal osteoblasts and contributed to bone repair after transplantation into mice with bone defects. Furthermore, dOBs did not require continuous expression of the exogenous genes to maintain their phenotype. These findings strongly suggest successful direct reprogramming of fibroblasts into osteoblasts, which may be applicable to bone regeneration therapy.
Keywords: osteoblasts, regenerative medicine, direct reprogramming
Abstract
Osteoblasts produce calcified bone matrix and contribute to bone formation and remodeling. In this study, we established a procedure to directly convert human fibroblasts into osteoblasts by transducing some defined factors and culturing in osteogenic medium. Osteoblast-specific transcription factors, Runt-related transcription factor 2 (Runx2), and Osterix, in combination with Octamer-binding transcription factor 3/4 (Oct4) and L-Myc (RXOL) transduction, converted ∼80% of the fibroblasts into osteocalcin-producing cells. The directly converted osteoblasts (dOBs) induced by RXOL displayed a similar gene expression profile as normal human osteoblasts and contributed to bone repair after transplantation into immunodeficient mice at artificial bone defect lesions. The dOBs expressed endogenous Runx2 and Osterix, and did not require continuous expression of the exogenous genes to maintain their phenotype. Another combination, Oct4 plus L-Myc (OL), also induced fibroblasts to produce bone matrix, but the OL-transduced cells did not express Osterix and exhibited a more distant gene expression profile to osteoblasts compared with RXOL-transduced cells. These findings strongly suggest successful direct reprogramming of fibroblasts into functional osteoblasts by RXOL, a technology that may provide bone regeneration therapy against bone disorders.
Osteoblasts play a central role in bone formation and remodeling by producing type I collagen, osteopontin, osteocalcin, and bone sialoprotein (BSP), and calcifying these bone matrixes (1). They are also involved in hematopoiesis, phosphate metabolism, and glucose metabolism (2). Osteoblasts are derived from mesenchymal progenitor cells that are common precursors shared by chondrocytes, adipocytes, and myoblasts (3). The differentiation of osteoblasts is regulated by various transcription factors, including Runt-related transcription factor 2 [Runx2, also known as core-binding factor subunit α-1 (Cbfα-1)] (4, 5), Osterix (6, 7), Distal-less homeobox 5 (Dlx5) (8), and activation transcription factor 4 (ATF-4) (8). A functional decline in osteoblasts relative to osteoclasts results in imbalance between bone formation and resorption and may cause osteolytic pathological conditions, such as osteoporosis (9), alveolar bone resorption associated with periodontitis (10), and bone lysing associated with bone tumors, including multiple myeloma (11).
It has been demonstrated that forced expression of combinations of some transcription factors, such as Octamer-binding transcription factor 3/4 (Oct4), Sox2, Klf-4, and c-Myc (reprogramming factors), induces immortality and pluripotency in mammalian somatic cells (12, 13). The generation of induced pluripotent stem (iPS) cells clearly indicates that genome-wide epigenetic programming can be drastically changed in somatic cells by a small number of transcription factors that may have key regulatory roles in cell fate decisions (14, 15).
Recent studies have reported that direct conversion, or direct reprogramming, of somatic cells into another differentiated lineage can be achieved without passing an intermediate pluripotent stage by introducing a set of transcription factors that are pivotal for development of the destination cells. These conversions include direct conversion of murine fibroblasts into cardiomyocytes (16, 17), neurons (18–20), chondrocytes (21), and hepatocytes (22, 23), as well as of human fibroblasts into cardiomyocytes (24, 25), neurons (19, 26), and hematopoietic cells (27). Although the efficiencies of conversion are generally low at present, with only 0.005–30% of fibroblasts successfully converted into the desired cells (16–20, 22–24, 26), this technology may become quite useful for regenerative therapy against a variety of human diseases.
To date, however, there have been no reports on the induction of human osteoblasts from another differentiated somatic cell lineage by means of direct reprogramming procedures. If this can be achieved, then a sufficient number of osteoblasts may be obtained from the somatic cells of a patient with osteolytic disease and then transplanted back into this patient. Moreover, such cell-based therapies might be applied to other clinical settings as well, such as repair of large bone defect caused by trauma or by surgical resection of osteosarcoma or metastatic bone tumors.
In the present study, we attempted to directly convert human fibroblasts into osteoblasts by genetically introducing osteoblast-specific transcription factors and reprogramming factors. We also assessed the capability of the resulting directly converted osteoblasts (dOBs) to contribute to bone formation in vivo.
Results
Human Fibroblasts Were Induced to Produce Calcified Bone Matrix by Defined Factors.
We focused on two transcription factors, Runx-2 (R) and Osterix (X), which are critical regulators of osteoblast differentiation (8, 28), along with three reprogramming factors, Oct4 (O), L-Myc (L), and c-Myc (M), because of their potential to reprogram cell fates (13, 18, 20, 21, 29). Retrovirus vectors encoding these factors were infected into human gingival fibroblasts either individually or in various combinations. The cells were cultured in osteogenic medium, followed by analyses of osteoblast-like characteristics, including mRNA expression of alkaline phosphatase (ALP) and osteocalcin and staining properties by the von Kossa method. As shown in Fig. 1, Oct4 alone moderately induced the osteoblast-like phenotypes. Similar results were also obtained by the two-factor combinations containing Oct4 (e.g., Runx2 plus Oct4, Osterix plus Oct4). Therefore, Oct4 may play a crucial role in induction of the osteoblast-like phenotype. Among the two-factor combinations, Oct4 plus L-Myc (OL) induced high levels of both ALP and osteocalcin mRNA, and evoked massive production of calcified bone matrix. The most remarkable phenotypic change was elicited by a four-factor combination: Runx2, Osterix, Oct4, and L-Myc (RXOL); therefore, we analyzed RXOL and OL in the subsequent experiments.
Fig. 1.
Some combinations of defined factors induced osteoblast-like phenotypes in human fibroblasts. Human gingival fibroblasts were seeded in 35-mm dishes and infected with mixtures of the indicated retrovirus vectors (day 0). (A) After culturing for 14 d, mRNA levels for ALP and osteocalcin genes were quantified by real-time RT-PCR. The relative mRNA levels were calculated as follows: Relative mRNA level (fold) = [(target gene mRNA level in sample)/(β-actin gene mRNA level in sample)]/[(target gene mRNA level in nontransduced control)/(β-actin gene mRNA level in nontransduced control)]. Values are mean ± SD (n = 3). *P < 0.05, **P < 0.01 vs. noninfected control. (B) On day 28, the cells were stained by the von Kossa method as described in SI Materials and Methods.
RXOL- and OL-Transduced Cells Significantly Produced Bone Matrix and Expressed Osteoblast-Related Genes.
Human gingival fibroblasts were transduced with RXOL and OL retrovirus vectors. ALP staining showed that transduction with RXOL induced ALP activity in ∼80% of the fibroblasts at 14 d after infection, and OL infection also induced this osteoprogenitor marker (Fig. 2A, Left). Alizarin Red S staining showed that the RXOL transduction induced apparent calcium deposition on day 14, and the calcified body occupied almost the entire surface of the culture dish on day 28 (Fig. 2A, Right).
Fig. 2.
Osteoblast-like features of the human gingival fibroblasts infected with RXOL and OL. Human gingival fibroblasts seeded in 35-mm dishes were infected with RXOL or OL (day 0), with some aliquots of cells left uninfected (−). (A) After culturing for the indicated days, cells were stained with ALP (Left) or Alizarin Red S (Right). Normal human osteoblasts were stained as controls. (Original magnification: Upper, 1×; Lower, 40×.) (B) RNA was obtained from the cells at 14 d after gene transfer, as well as from MSCs and MSC-OBs. mRNA levels for the indicated genes were analyzed by real time-RT-PCR. The relative mRNA levels were calculated as in Fig. 1A. *P < 0.05, **P < 0.01 vs. noninfected control; #P < 0.05, ##P < 0.01 vs. osteoblasts. Values are mean ± SD (n = 4).
We examined whether fibroblasts of other origins were also induced to produce bone matrix. Human dermal fibroblasts infected with RXOL or OL were positive for ALP activity at 14 d after gene transfer, and Alizarin Red S and von Kossa staining revealed calcium deposition and bone formation nodules on day 28 (Fig. S1A).
To further elucidate osteoblast-like characteristics of the RXOL- and OL-transduced cells, we infected gingival fibroblasts with the retrovirus vectors and tested for mRNA expression of osteoblast-related genes. As shown in Fig. 2B, RXOL induced expression of osteocalcin, ALP, and Runx2 mRNA at levels comparable to those in normal osteoblasts and mesenchymal stem cell (MSC)-derived osteoblasts (MSC-OBs). The RXOL-transduced cells expressed osteopontin, BSP, and Osterix mRNA at levels between those of osteoblasts and MSC-OBs. The transduction of OL significantly induced osteocalcin, osteopontin, BSP, and ALP mRNA. OL-transduced cells expressed Runx2 at a low level and did not significantly express mRNA for the Osterix gene (Fig. 2B).
Human dermal fibroblasts infected with RXOL and OL also expressed mRNA for osteocalcin, ALP, and BSP genes at significant levels (Fig. S1B).
RXOL-Transduced Cells Shared Similar Global Gene Expression Signature with Osteoblasts.
We performed DNA microarray analysis of mRNA from RXOL-transduced, OL-transduced, and untransduced fibroblasts, osteoblasts, MSCs, and MSC-OBs. In hierarchical clustering analysis, we focused on osteoblast-related differentiation and signaling genes, and found that the expression patterns of these osteoblast-related genes in RXOL-transduced cells were the most similar to those in osteoblasts (Fig. 3A). Genome-wide gene expression profiles also indicated that RXOL-transduced cells were most similar to osteoblasts among all the cells tested (including MSC-OBs), and vice versa (Fig. 3B).
Fig. 3.
Characteristics of dOBs induced from fibroblasts. (A) RNA extracted from the indicated cells was subjected to DNA microarray analysis. Heat map and hierarchical clustering analysis of the DNA microarray data showing the genes for transcription factors and signaling molecules involved in osteoblast differentiation. RXOL and OL represent human fibroblasts infected with the corresponding retrovirus vectors. In the heat map, genes with increased expression are colored red, and genes with decreased expression are in blue (see the color range below the heat map). The expression level of each gene was normalized to median signal intensity. Clusters indicate that RXOL-transduced cells showed the greatest similarity to osteoblasts. (B) RNA obtained from the indicated cells were analyzed by the GeneChip human Gene 1.0 ST (Affymetrix), consisting of 29,096 probes. (Upper) In the heat map, red color indicates high correlation between the two cells, whereas black color shows low correlation (see color range). (Lower) Correlation coefficient values. (C) DNA extracted from human gingival fibroblasts, dOBs (RXOL-transduced fibroblasts), and osteoblasts were tested for CpG methylation at the osteocalcin gene upstream region.
These findings strongly suggest that the RXOL-transduced cells share similar gene expression profiles with osteoblasts. Meanwhile, OL-transduced cells were less similar to osteoblasts than RXOL-transduced cells in terms of the expression patterns of both osteoblast-related genes (Fig. 3A) and whole genes (Fig. 3B). Based on these findings, RXOL-transduced cells were used in further detailed analyses as representative directly converted osteoblasts (dOBs).
Epigenetic Status and Immunostaining Properties of dOBs.
In experiments performed to assess the epigenetic status of dOBs, we found that in osteoblasts, genomic DNA was mostly unmethylated at CpG dinucleotides at the osteocalcin gene upstream region, whereas in fibroblasts, the CpG sequences were heavily methylated (Fig. 3C). These results are consistent with previous reports on epigenetic markers at the osteocalcin gene loci in rat osteoblasts (30) and mouse osteoblasts (31). dOBs showed an intermediate level of CpG methylation at this osteoblast-specific gene locus, suggesting that the epigenetic status of dOBs had drastically changed from that of fibroblasts, but had not reached that of osteoblasts.
Immunostaining of cells indicated that dOBs produced osteocalcin and osteopontin (Fig. 4A). On day 21 after the transduction, the proportion of the osteocalcin-positive cells among the RXOL-infected cells was 79.9 ± 3.5%, whereas that among gingival fibroblasts was only 0.47 ± 0.18% (Fig. 4 B and C).
Fig. 4.
Approximately 80% of fibroblasts were converted into dOBs. Human fibroblasts were infected with RXOL to obtain dOBs. (A) At 21 d after infection, cells were immunostained with anti-human osteocalcin and osteopontin antibodies. (B) Cells were dual-stained with anti-osteocalcin antibody and DAPI. (C) The proportion of osteocalcin producers was determined as follows: % Osteocalcin-producing cells = the number of osteocalcin(+)DAPI(+) cells per total number of DAPI(+) cells × 100. (Original magnification: 100× in A and B.) Values in C are mean ± SD (n = 5). **P < 0.01.
Fibroblasts Were Converted into dOBs Without Passing Through an Intermediate Pluripotent Stage.
Human fibroblasts infected with RXOL were immunostained with anti-Nanog antibody every 2 d from day 1 to day 15 after infection. More than 1,000 cells were observed at each time point under fluorescent microscopy. No significant expression of Nanog, which is an indispensable transcription factor for pluripotency, was detected in any cell over the entire experimental period (Fig. S2A). These results demonstrate that fibroblasts were directly converted into dOBs without passing through an immature pluripotent stage.
We also estimated mRNA expression for the Rex-1 and Nanog genes during the conversion from fibroblasts to dOBs. Real-time RT-PCR analysis revealed no detectable expression of these pluripotent stem cell-specific genes in fibroblasts or in RXOL-infected fibroblasts at 7, 14, and 21 d after transduction (Fig. S2B).
We considered the possibility that the osteoblast-like cells could have arisen from MSCs that were a contaminating factor in the original fibroblast population. The fibroblasts were cultured under adipogenic, osteogenic, and chondrogenic conditions. Adipocytes, osteoblasts, and chondrocytes were not induced from the fibroblasts, whereas adipose-derived MSCs cultured as a positive control were induced into these mesenchymal lineages under the corresponding conditions (Fig. S3). The results and the aforementioned high efficiency of conversion (with ∼80% of fibroblasts converted to dOBs) clearly indicate that the dOBs were not derived from MSCs that had contaminated the fibroblast population.
Transient Expression of RXOL Was Sufficient to Induce dOBs.
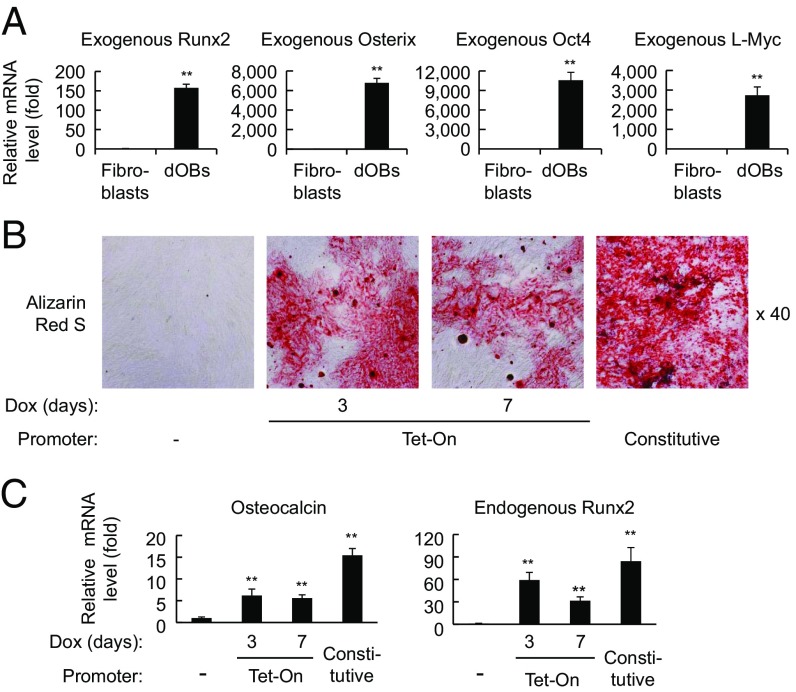
The dOBs strongly expressed retroviral transgenes (Fig. 5A). It may be possible that the continuous expression of transgenes is required for the dOBs to maintain their osteoblast-like phenotypes. Alternatively, once established by the transduced factors, the converted cells may remain stably osteoblast-like even in the absence of sustained expression of the exogenous genes.
Fig. 5.
dOBs were induced by transient expression of RXOL. (A) RNA was extracted from fibroblasts and RXOL-transduced cells at 21 d after infection. Exogenous Runx2, Osterix, Oct4, and L-Myc mRNA levels were analyzed by real-time RT-PCR using transgene-specific primers. The relative mRNA levels were calculated as in Fig. 1A. **P < 0.01 vs. untransduced fibroblasts (n = 3). (B) Cells were infected with retrovirus vectors containing RXOL driven by the Tet-On promoter. The cells were cultured in complete medium supplemented with doxycycline (1 μg/mL) during the first 3 or 7 d, followed by further culturing in osteogenic medium without doxycycline. Other cells were infected with standard RXOL retrovirus vectors [in which retroviral long terminal repeat (LTR) constitutively drove the transgenes] or left uninfected (−). At 28 d after infection, cells were stained with Alizarin Red S. (C) Measurement of osteocalcin and endogenous Runx2 mRNA. The relative mRNA levels were calculated as in Fig. 1A. *P < 0.05, **P < 0.01 vs. fibroblasts (n = 4).
To address this issue, we constructed retroviral vectors that express R, X, O, L, and GFP under control of the tetracycline-inducible (Tet-On) promoter. After infection, doxycycline was added to the culture during the first 3 or 7 d, followed by further culturing without doxycycline. Osteogenic medium was used only after the withdrawal of doxycycline. The cells showed significant production of calcified bone matrix and expression of osteocalcin mRNA (Fig. 5 B and C) on day 28, when the transgene was no longer expressed (Figs. S4 and S5).
dOBs Contributed to Bone Repair After Transplantation into Mice at an Artificial Bone Defect Region.
To examine whether dOBs can enhance bone regeneration, we transplanted dOBs and fibroblasts into bone defect lesions surgically created at the femurs of NOD/SCID mice. Bone repair was estimated by macroscopic, radiographic, biomechanical, and histological examinations at 21 d after transplantation. Macroscopic observation of the femur showed that the defect parts were totally ossified in dOB-transplanted bones, whereas large defects remained unossified in the fibroblast-transplanted bones (Fig. S6). Radiographically, the defect parts in dOB-transplanted femurs were covered with bridging callus formation, which was not seen in the fibroblast-transplanted femurs (Fig. 6A and Fig. S7A). The dOB transplantation resulted in a significantly higher degree of callus formation compared with fibroblast transplantation (Fig. 6B). Micro computed tomography (μCT) revealed that the callus was composed of regularly aligned bone trabeculae, with no signs of pseudoarthrosis. Furthermore, the radiopacity of the defective area was higher in dOB-transplanted femurs than in fibroblast-transplanted femurs, as demonstrated by the μCT transmission images (Fig. S7B).
Fig. 6.
dOBs facilitated bone repair in vivo. (A) Human fibroblasts and dOBs (14 d after RXOL infection) were transplanted to an artificial segmental bone defect in femoral diaphysis in NOC/SCID mice. Mice were killed at 21 d after transplantation, and μCT imaging of the femur was performed. Serial 10-μm slices are shown. Triangles indicate bone defect lesions; arrowheads, regenerated bone tissue. (B) Percent callus formation was evaluated as described in SI Materials and Methods. Mean ± SD values are plotted (n = 3). **P < 0.01. (C) Human fibroblasts and dOBs (at 14 d after RXOL infection) were transplanted into NOC/SCID mice at the bone defect lesion, and a control group was implanted with Matrigel alone. Twenty-one days later, the mice were killed and femurs excised. Serial sections of the femur tissue were stained with Alizarin Red S (Upper) or H&E (Lower). (Original magnification: 40×.) (D) Transplantation was performed as above, but with dOBs infected with a GFP retrovirus vector before transplantation. Sections were stained with anti-human osteocalcin antibody and DAPI. (Original magnification: 40× and 100×.)
Histological analysis also showed bridging callus formation at the defective lesion in the dOB-transplanted femurs, but only partially formed callus in the fibroblast-transplanted femurs (Fig. 6C). Alizarin Red S staining confirmed ossification at the callus of the dOB-transplanted femurs (Fig. 6C). These results indicate that local transplantation of dOBs accelerated bone healing at bone defect sites in NOD/SCID mouse femurs.
We next assessed the distribution of, and bone matrix production by, the dOBs in the regenerated bone tissue. GFP-labeled dOBs were transplanted into the bone defect lesions, and human osteocalcin was visualized by immunohistochemical staining. GFP-positive cells were located on the surface of the callus and in the thickened periosteum, whereas human osteocalcin was widely distributed in the callus and periosteum, as demonstrated by staining with an antibody that specifically recognized human, but not mouse, osteocalcin (Fig. 6D). Sox9-positive GFP-negative chondrocytes were present in bone marrow and callus (Fig. S8). These results strongly suggest that the donor cells retained the osteoblast-like feature and produced bone matrix in vivo.
Discussion
In the present study, we accomplished direct conversion of human fibroblasts into osteoblasts that produced mineralized bone matrix at high efficiency by introducing four transcription factor genes (R, X, O, and L) and then culturing in osteogenic medium. The resultant osteoblasts facilitated bone repair in vivo after transplantation into immunodeficient mice at the bone defect lesions. The dOBs induced by RXOL may be epigenetically reprogrammed cells that significantly expressed endogenous Runx2 and Osterix.
Interestingly, we also found that RXOL was not the minimum essential combination of factors to achieve some degree of osteoblast-like conversion of fibroblasts. A smaller number of factors, such as OL and even Oct4 alone, was sufficient to induce bone matrix production in fibroblasts (Fig. 1). Among the four factors, only Oct4 may have an essential role, and the other factors might not be indispensable for the induction of bone matrix production. Nevertheless, the difference between RXOL- and OL-transduced cells strongly suggests important roles of Runx2 and Osterix in converting fibroblasts into such osteoblasts that express endogenous Runx2 and Osterix and have similar global gene expression profiles as bone-derived osteoblasts (Figs. 2 and 3).
Runx2, a master regulator of osteoblast development (4, 5, 32), is a member of the Runx family of transcription factors that is specifically expressed in cells of osteoblastic lineage at various differentiation stages, from immature mesenchymal osteochondroprogenitors to mature osteoblasts. Runx2 expression is both necessary and sufficient for the differentiation of mesenchymal progenitor cells toward the osteoblastic lineages (4, 5). Runx2 enhances the transcription of all major osteoblast-related genes, such as osteoclacin, by specifically binding to the genes’ regulatory regions (28, 31). Runx2-null mice lack ossification owing to the maturational arrest of osteoblasts (33). A previous study found that Runx2 transfection accelerates osteoblastic differentiation from iPS cells (34). In other studies, forced expression of Runx2 induced osteocalcin in rodent fibroblasts (35, 36), but not in human fibroblasts (Fig. 1).
Osterix, a zinc-finger transcription factor, is specifically expressed in osteoblasts at a later stage of differentiation than Runx2-expressing cells (6, 7). Expression of Osterix depends on Runx2, whereas Osterix acts downstream of Runx2. Osterix-null mice also lack bone formation (6). The present study indicates that Runx2 and Osterix have important roles in the direct conversion of osteoblasts, consistent with previous reports demonstrating indispensable roles of lineage-specific transcription factors in direct reprogramming of the corresponding tissue cells (16–18, 21, 23).
Oct4 plays central roles in maintaining pluripotency and reprogramming differentiated cells into pluripotent stem cells (12, 13, 37). Shu et al. (38) recently reported that Oct4 and Sox2 present mesendodermal and ectodermal differentiation cues, respectively, and that the balance between these counteracting cues may facilitate reprogramming. In the present study, we found that Oct4 is indispensable for the direct reprogramming of osteoblast-like cells (Fig. 1), strongly suggesting that the mesendodermal skewing induced by Oct4 may be crucially involved in the mechanisms underlying the lineage conversion toward osteoblasts.
The proto-oncogene c-Myc is another reprogramming factor, although it is not a prerequisite for iPS cell generation (39). Recent studies indicate that L-Myc may substitute for c-Myc (29). In our experiments, L-Myc was superior to c-Myc in terms of efficiency of osteoblast induction. L-Myc may have lower tumorigenecity compared with c-Myc, which would be a significant advantage in future clinical applications of dOBs. In addition, c-Myc is considered a crucial factor in the direct reprogramming of mouse fibroblasts into neural stem cells (18, 20) and chondrocytes (21).
Transduction of OL induced osteoblast-like phenotypes in fibroblasts (Figs. 1, 2, and 3). The OL-transduced cells moderately expressed endogenous Runx2 (Fig. 2B), strongly suggesting important roles of endogenous Runx2 in the cell type conversion caused by OL. The OL-transduced cells showed less similarity to osteoblasts compared with RXOL-transduced cells in terms of genome-wide gene expression profiles (Fig. 4 A and B). This “incomplete conversion” may be related to the low expression of endogenous Osterix (Fig. 2B), strongly suggesting a crucial role of Osterix in “full conversion”. The detailed molecular mechanisms involved in this cell conversion remain to be elucidated, however.
Transplantation of autologous bone marrow cells and MSCs obtained from the bone marrow or adipose tissue has been performed to treat bone loss after surgical resection of bone tumors or large bone defects caused by trauma (40). This transplantation may facilitate bone repair, probably because osteoblasts may be originated from the graft and contribute to bone regeneration. The clinical application of autologous MSC transplantation to bone regeneration faces some obstacles, however, especially in elderly patients, including limited numbers of stem cells, age-related decrease in potential for differentiation into osteoblasts, and invasion during the cell isolation procedure (41, 42). Such obstacles may be avoided by applying the direct reprogramming technology, in which a large number of functional osteoblasts can be obtained from fibroblasts isolated from patients or allogenic donors by a less-invasive procedure (43, 44). Fibroblasts have a high proliferative capacity, which does not decline with increasing donor age (43, 45). Thus, direct reprogramming may provide another option for such patients in whom MSC transplantation might not be an appropriate therapy.
Osteoblasts induced from a patient’s own somatic cells or allogenic cells also may be applicable to cell-based therapy against bone resorptive conditions associated with osteoporosis, bone disruption caused by rheumatoid arthritis, and alveolar bone resorption due to periodontitis. This therapy may help increase the patient’s quality of life and capacity to perform activities of daily living.
Materials and Methods
For direct reprogramming, human fibroblasts were retrovirally transduced with transcriptional factors and cultured in DMEM supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerol phosphate, 100 nM dexamethasone, and 10% FBS (osteogenic medium). NHost cells (normal human osteoblasts derived from femur of a 39-y-old male) were purchased from Lonza. Detailed information on cells, retroviral vectors, infection, induction into osteoblasts, cell staining, real-time RT-PCR, immunostaining, DNA microarray analysis, bisulfite sequencing, surgical procedure, cell transplantation, radiographic and histological assessment, and data analysis is provided in SI Materials and Methods. The sequences of real-time RT-PCR primers are provided in Table S1.
Supplementary Material
Acknowledgments
We thank Dr. Wilfred Germeraad for a critical reading of the manuscript and Hiroaki Ichioka and Ken-ichi Honjo for technical support. This work was supported by grants from the Japan Science and Technology Agency (PRESTO and A-Step); the Japanese Ministry of Education, Culture, Sports, Science and Technology; and the Suzuken Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE52817).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420713112/-/DCSupplemental.
References
- 1.Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011;343(2):289–302. doi: 10.1007/s00441-010-1086-1. [DOI] [PubMed] [Google Scholar]
- 2.Bethel M, Srour EF, Kacena MA. Hematopoietic cell regulation of osteoblast proliferation and differentiation. Curr Osteoporos Rep. 2011;9(2):96–102. doi: 10.1007/s11914-011-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Dalle Carbonare L, Innamorati G, Valenti MT. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev. 2012;8(3):891–897. doi: 10.1007/s12015-011-9337-4. [DOI] [PubMed] [Google Scholar]
- 5.Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339(1):189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 7.Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem. 2013;114(5):975–984. doi: 10.1002/jcb.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15(5):208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and the future. Lancet. 2011;377(9773):1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartold PM, Cantley MD, Haynes DR. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000. 2010;53:55–69. doi: 10.1111/j.1600-0757.2010.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Buckle CH, et al. Soluble rank ligand produced by myeloma cells causes generalised bone loss in multiple myeloma. PLoS ONE. 2012;7(8):e41127. doi: 10.1371/journal.pone.0041127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010;28(10):1079–1088. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- 15.Papp B, Plath K. Reprogramming to pluripotency: Stepwise resetting of the epigenetic landscape. Cell Res. 2011;21(3):486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagawa K, Ieda M. Direct reprogramming of mouse fibroblasts into cardiac myocytes. J Cardiovasc Transl Res. 2013;6(1):37–45. doi: 10.1007/s12265-012-9412-5. [DOI] [PubMed] [Google Scholar]
- 18.Han DW, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10(4):465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22(5):778–784. doi: 10.1016/j.conb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10(4):473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu K, et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011;121(2):640–657. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang P, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 23.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475(7356):390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 24.Nam YJ, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110(14):5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada R, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA. 2013;110(31):12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang ZP, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo E, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468(7323):521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 28.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci USA. 2010;107(32):14152–14157. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villagra A, et al. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J Cell Biochem. 2002;85(1):112–122. [PubMed] [Google Scholar]
- 31.Xiao G, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005;280(35):30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- 32.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233–1239. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 33.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 34.Tashiro K, et al. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009;27(8):1802–1811. doi: 10.1002/stem.108. [DOI] [PubMed] [Google Scholar]
- 35.Phillips JE, Guldberg RE, García AJ. Dermal fibroblasts genetically modified to express Runx2/Cbfa1 as a mineralizing cell source for bone tissue engineering. Tissue Eng. 2007;13(8):2029–2040. doi: 10.1089/ten.2006.0041. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T. Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcif Tissue Int. 2011;88(4):336–347. doi: 10.1007/s00223-011-9461-9. [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Shu J, et al. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013;153(5):963–975. doi: 10.1016/j.cell.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Wu J, Zhu Y, Han J. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2014;14(1):13–24. doi: 10.1007/s10238-012-0218-1. [DOI] [PubMed] [Google Scholar]
- 41.Kim M, et al. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133(5):215–225. doi: 10.1016/j.mad.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhou S, et al. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huschtscha LI, et al. Enhanced isolation of fibroblasts from human skin explants. Biotechniques. 2012;53(4):239–244. doi: 10.2144/0000113939. [DOI] [PubMed] [Google Scholar]
- 44.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 45.Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: A reevaluation. Proc Natl Acad Sci USA. 1998;95(18):10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.