Significance
The composition of 170-y-old champagne samples found in a shipwreck in the Baltic Sea constitutes a remarkable and unprecedented example of long-term combinatorial chemistry, which can occur in such sealed 750-mL microlaboratories. Multiple analytical tools, including metabolomics, metallomics, and sensory analysis, were combined to characterize the molecular diversity of these champagnes having aged in close-to-perfect conditions at the bottom of the sea. The analyzed champagnes retained intrinsic features allowing us to shed light on the winemaking practices in use in the middle of the 19th century. Therefore, this archeochemistry approach enabled us to rewrite a piece of our cultural heritage.
Keywords: metabolomics, archaeochemistry, champagne, wine
Abstract
Archaeochemistry as the application of the most recent analytical techniques to ancient samples now provides an unprecedented understanding of human culture throughout history. In this paper, we report on a multiplatform analytical investigation of 170-y-old champagne bottles found in a shipwreck at the bottom of the Baltic Sea, which provides insight into winemaking practices used at the time. Organic spectroscopy-based nontargeted metabolomics and metallomics give access to the detailed composition of these wines, revealing, for instance, unexpected chemical characteristics in terms of small ion, sugar, and acid contents as well as markers of barrel aging and Maillard reaction products. The distinct aroma composition of these ancient champagne samples, first revealed during tasting sessions, was later confirmed using state-of-the-art aroma analysis techniques. After 170 y of deep sea aging in close-to-perfect conditions, these sleeping champagne bottles awoke to tell us a chapter of the story of winemaking and to reveal their extraordinary archaeometabolome and elemental diversity in the form of chemical signatures related to each individual step of champagne production.
Discovering ancient objects from excavation sites or simply at the back of a cellar has always piqued human interest because of the messages from the past they may contain. Unsurprisingly, our interest increases even more when exhuming old bottles or even jars that seem to have contained grapes or wine (1–3), giving a glimpse into the little-known history of winemaking. When divers first discovered bottles in a shipwreck off the Finnish Åland archipelago in the Baltic Sea (4) in July 2010 (Fig. 1) and tasted one of them on site, they realized that they were most likely drinking a century-old champagne. The numerous echoes that have since resounded in the international media highlight the worldwide interest in such stories.
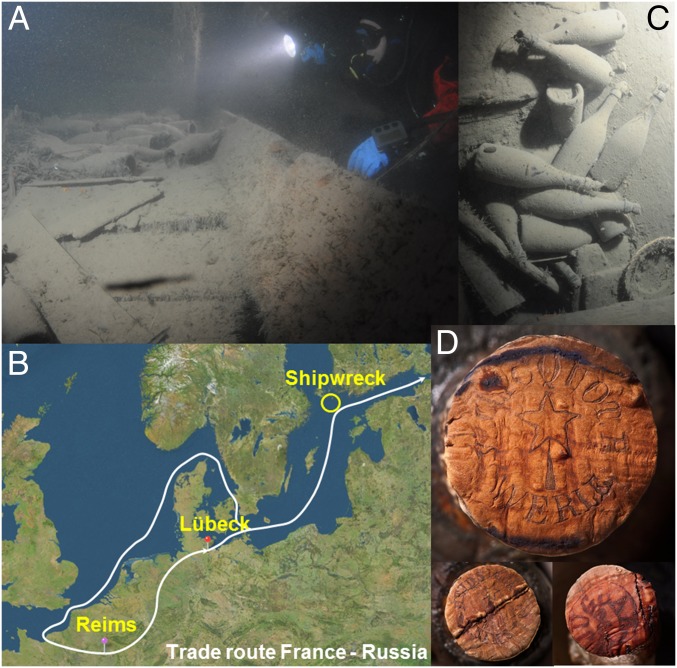
Fig. 1.
(A) Discovery of champagne bottles in a shipwreck off the Finnish Åland archipelago, in July 2010. The ship was a two-masted schooner (21.5 m long × 6.5 m broad) around 200 y old (location: south of the municipality of Föglö, Åland). Copyright, Anders Näsman/The Government of Åland. (B) One possible planned itinerary for the boat. (C) A few of the 168 bottles found in the Baltic Sea at a depth of about 50 m, in nearly ideal slow-aging conditions in terms of temperature (2–4 °C), darkness, low salinity, and high pressure. (D) Branded engravings on the cork surface that is in contact with the wine (mirroir in French). The representation of the 1811 great meteorite attests to the bottles having been corked after 1811. (C and D) Courtesy of Visit Åland.
A total of 168 bottles were retrieved from the shipwreck (Fig. 1). None of the labels remained, but bottles were later identified as champagnes from the Veuve Clicquot Ponsardin (VCP), Heidsieck, and Juglar (known as Jacquesson since 1832) champagne houses thanks to branded engravings on the surface of the cork that is in contact with the wine. A few of the recovered bottles lay horizontally in close-to-perfect slow-aging conditions, kept in total darkness at a fairly constant temperature (2–4 °C) and in conditions of low salinity (<10 g/kg NaCl), typical of the depth of about 50 m where they were found.
These bottles of champagne, while perhaps not the oldest to have made it to our time, most likely contain the oldest champagne to ever have been tasted. However, in the midst of the initial excitement raised by this discovery, it became clear that many questions remained: When were these wines produced, and what winemaking processes were in use at the time? Were they traveling on a regular trade route, and what was their final destination? One could expect to begin to answer these questions and many others by simply comparing the current champagne elaboration process to historical records in conjunction with straightforward basic analyses of these 170-y-old champagne samples. However, it proved difficult to draw any conclusions about these decisively enigmatic bottles. Fewer than 200 bottles were found in the wreck, which raises questions as to their intended use and/or destination. Based on the site of discovery, one could suppose that they were en route for the Russian Empire. Indeed, Madame Clicquot did strive to please the Russian taste for sweet wines from as early as 1814. Nevertheless, the frequent correspondence with her agent in Saint Petersburg testifies to the customers’ distinctive request for a very specific sugar dosage of nearly 300 g/L: “Here they always have some sugar on any table close to their wine glass, for they add sugar not only to red wine but also to champagne” (5). Thus, the relatively low sugar levels of the shipwrecked bottles, less than 150 g/L, suggest that they might instead have been intended for the customers in the Germanic Confederation. Even dating these bottles proved difficult, as no information could be inferred from the shipwreck itself, and the bottles gave contradictory clues. Based on historical records, the characteristic branding found on the bottom face of the corks, known in French as the “mirroir,” indicated that the VCP bottles had been sealed after 1841, but the Juglar bottles must have been produced before 1832, when the house was renamed Jacquesson. Interestingly, bottles of beer were also found in the same shipwreck that did not bring any clue as to either the destination or the age of the champagne bottles (6). Therefore, various questions remain, the answers to which could shed light not only on the history of the commerce of champagne in the beginning of the 19th century but also on the winemaking practices of the time.
Archaeochemistry, as the application of the most recent analytical techniques to ancient samples, now provides an unprecedented understanding of human culture throughout history (1–3). Indeed, these methods recently demonstrated the introduction of viniculture in France (7) and have identified the earliest known winemaking activities as having occurred in the Middle East, thousands of years B.C.E. Similarly, a combination of various chromatographic techniques and antioxidant activity measurements applied to 600-y-old fermented fruit juice found in amphorae from the vestiges of a ship discovered off the Sicilian coast in 2000 (8) demonstrated that under such storage conditions, this juice retained significant health-protecting properties. The application of a combination of targeted and nontargeted modern chemical analytical approaches to such historical samples can now provide unique characterization in terms of chemical diversity and molecular resolution, therefore elucidating various aspects of the winemaking practices of their time.
Using such an approach, three of the “Baltic champagne” samples (named A11, A33, and B17) were tasted and subsequently analyzed in comparison with three modern champagne samples from VCP (see Materials and Methods and Table S1). Various straightforward observations based on expert sensory analyses confirmed that these Baltic samples exhibited characteristics of very old champagnes. Furthermore, elemental analyses, alongside metabolomics (9, 10) and aroma analyses (11), provided a significant amount of data, which, once compiled and analyzed, shed light on the champagne making process used in the 19th century and defined its most characteristic steps (12).
For instance, the Baltic champagne samples displayed unusually high metallic cation concentrations. Iron and copper reached unexpectedly high levels compared with those commonly found in modern champagne samples. Iron levels in the Baltic champagnes reached up to 13–118 mg/L compared with 1–4.6 mg/L in modern samples, and copper levels attained 100–1,400 μg/L in the recovered champagnes vs. 27–78 μg/L in the modern analogs (Table S2). At the estimated time of production of these ∼170-y-old bottles of champagne, the use of proper picking and pressing methods (13) ensured high-quality musts (the extracted grape juice) that would allow for the subsequent production of light and delicate still white wines to be converted into champagne during the second fermentation (12). An example of an important practice to ensure this quality is the fractionation of the juice during pressing of the grapes between the cuvée, considered to be the finest fraction, and the following taille. Compared with the cuvée, the taille is characterized by a lower total acidity and increased pH as well as mineral and phenolic concentrations (14, 15). Therefore, one could speculate that the high concentrations in metallic cations in the Baltic champagnes may be the result of the use of a significant proportion of taille having been incorporated into the blend. However, such concentrations could just as easily be explained by a combination of increased cation extraction from the grapes (16) together with the use of metal-containing vessels during the winemaking process. Indeed, consistent with our knowledge of the pinot noir phenology at the time (17), the smaller berries harvested in the 19th century would have exhibited a higher skin-to-pulp mass ratio, hence a higher ratio of dry matter to grape juice volume (18), and thus a greater concentration of both endogenous cations extracted from the skin and exogenous cations from treatment residues present on the grape skin. In fact, although the term “bouillie bordelaise” or “Bordeaux mixture” was probably coined later (around 1883–1885), copper sulfate was already used at the time to protect the grapevine against fungal pathogens. Furthermore, the use of iron nails in the assembly of barrels and iron rods to hold sulfur wicks during the sulfurization of these barrels could most certainly have contributed to the increased iron concentration, since these iron elements would easily have been oxidized during winemaking and/or attacked by sulfuric acid-containing vapors.
Following the harvesting and pressing of the grapes, the must would have undergone its first alcoholic fermentation (AF). Historical records indicate that in the late 1830s, this step took place later in the year than it does now, hence under colder temperatures, and it was carried out by native yeast (17), which, it is reasonable to assume, would have been less efficient than modern selected yeast. Therefore, incomplete AFs may have been a common occurrence (17). Furthermore, one could suppose that the slightly colder climate of the 19th century would have retarded grape maturation, leading to an overall reduced sugar content than is seen today. All of these elements are likely to have contributed to the significantly lower alcohol level of the Baltic specimens (9.34–9.84% alcohol by volume) compared with the modern ones (12.33% alcohol by volume) (Tables S1 and S3) as determined by conventional analyses and mouthfeel, and further confirmed by NMR. Moreover, the low alcohol content would suggest that these wines did not undergo chaptalization, that is, the addition of sugar to must to increase the alcohol level of the finished wine. Nonetheless, it is difficult to ascertain this point, as the Baltic champagnes may have undergone a subsequent dilution when the liqueur d’expédition was added, as is later discussed herein. Finally, wood markers such as 5-carboxyvanillic acid and castalin (a wood ellagitannin) (19) were systematically found in the Baltic samples using Fourier transform ion cyclotron resonance/mass spectrometry (FTICR/MS) (Fig. S1) and suggest that AF took place in barrels.
Another striking difference between the Baltic and modern champagne specimens can be seen in their Na+, Cl−, and Br− concentrations. In fact, with 0.4–1 g/L of Na+, 0.92–1.5 g/L of Cl−, and 2–4 mg/L of Br−, the Baltic samples displayed significantly higher concentrations than their modern analogs that contained 8–12 mg/L of Na+, 6–12 mg/L of Cl−, and no detectable Br− (Table S2). Given the circumstances, seawater contamination was initially suspected but immediately ruled out. In fact, the A33 sample, which was identified as contaminated by seawater upon tasting (Table S2), provided standard values for some major sea-derived compound ratios in the case of contamination by the Baltic Sea, the composition of which is known to be stable throughout open seas and oceans. Consequently, if all of the samples had been contaminated, one would have expected to find the same relative proportion of Cl and Br in A11 and B17 as in A33. However, the Cl/Br ratios for the former samples, respectively 444 and 240, were significantly different from that of A33 (Cl/Br = 645) (see Table S2 for further discussion). The aforementioned high sodium and chlorine concentrations in the champagne samples from the Baltic Sea are thus more likely to be related to enological practices used at the time of their production. Wine clarification to control colloidal instability can be carried out using fining agents such as sodium chloride or sodium chloride-containing gelatin. Although VCP’s archives do not report the use of sodium chloride as a clarifying agent, Madame Clicquot's correspondence does mention the use of gelatin, the preparation of which required sodium chloride to facilitate protein dissolution (20). Moreover, Etienne (17) states that, at the time, clarification used to be done two or three times over the course of the winemaking process, hence possibly contributing to an accumulation of Na+ and Cl− in the Baltic samples.
Today, tartaric stabilization of champagne (to avoid precipitation of tartaric acid salts inside the bottle) is done after blending by chilling the wine to −4 °C (21). Based on historical records, including the writings of Dom Pérignon, we know that the practice of blending wines, that is, the mixing of wines from different grape varieties, origins, and years (assemblage), which is essential to maintaining the quality and style of each house, predates the 19th century. Even so, it is likely that at the time the Baltic samples were produced, tartaric acid salt stabilization occurred naturally during winter and thus before blending, which normally takes place just before spring. Indeed, the similarity between the Baltic and modern champagne samples regarding the average concentrations of the two cations central to tartaric acid salt precipitation, Ca2+ and K+ (Table S2), indicates that the Baltic samples did undergo proper tartaric stabilization. It is thus reasonable to assume that the wine experienced a slow cold precipitation while stored in wooden barrels exposed to naturally low wintertime temperatures. Tartaric acid salt precipitates were most likely removed during subsequent racking and filtration operations. This would explain the slight haze observed inside the bottles but the lack of proper precipitates even after the 170 y that this wine remained under the sea. By the beginning of spring, increasing temperatures would have allowed for completion of AF, in cases where it had been stopped prematurely by the winter cold. Concomitantly with the end of AF, uncontrolled malolactic fermentation (MLF) might have occurred in the barrels. MLF consists of a biological deacidification of wines resulting in the transformation of sharp, green-tasting malic acid into the softer lactic acid. Nowadays, this process usually occurs after AF and is closely monitored to complete malic acid consumption by the lactic acid bacteria Oenococcus oeni (21), as illustrated by the NMR measurements obtained for the modern samples (Table S3). Conversely, champagne samples from the Baltic Sea exhibited high malic acid contents with malic acid/lactic acid ratios in the range of 0.46–0.81. These results underscore the fact that, although now widely used in champagne production, MLF was left uncontrolled during the 19th century, leading to partial MLF probably occurring in the bottle.
According to VCP historical archives, the second AF (or prise de mousse) took place in April, in capped bottles, after the addition of sugar in the liqueur de tirage (the use of this name came into practice by the middle of the century) and carried out by indigenous yeast, since the use of prepared cultures was not introduced until the end of the 19th century. During the second AF, carbon dioxide is produced, leading to the effervescence characteristic of champagne. In the early 1840s, the second AF would have enabled the production and dissolution of about 10 g of CO2 per liter of wine. Nevertheless, cork being a porous material, over 170 y, the CO2 slowly diffused (along its inverse pressure gradient) out of the bottleneck and into the sea, so much so that even with undamaged corks, the retrieved bottles contained far less dissolved CO2 than the characteristic 10 g/L of their youth. Expert tasters noticed that, whereas no bubbles were observed upon pouring, a slight tingling effect was felt upon tasting. This is a clear indication that dissolved CO2 fell below the critical concentration required to enable heterogeneous bubble nucleation (on the order of 2.5 g/L at 10 °C) (22) but nevertheless still exceeded the several tenths of a milligram per liter needed to stimulate both trigeminal receptors and gustatory receptors, via the conversion of dissolved CO2 into carbonic acid (23).
Possibly the most striking feature of the Baltic champagne samples is their extraordinarily high sugar content (over 140 g/L) (Table S1), when nowadays the amount of sugar added before corking via the liqueur d’expédition (a mixture of mature wine, sugar, and antioxidants) generally varies from none at all for some extra brut wines to 50 g/L for demi-sec, with the champagne doux (greater than 50 g/L) having been more or less abandoned. From the location of the shipwreck, one is naturally tempted to suggest that the champagne bottles from the Baltic Sea were being shipped to Russia. However, the dosage (amount of added sugar) usually practiced by VCP for Russia in the middle of the 19th century was considerably higher, with levels approaching 300 g/L, and thus warranting the creation of a specific category of champagne known as Champagne à la Russe. According to VCP’s archives, a dosage of around 150 g/L was desired by the French and the German markets, while British and Americans preferred a lower sugar content (22–66 g/L).
The liqueur d’expédition can be prepared from sugar beet, sugar cane, or grape syrup. Thanks to her correspondence, we know that Madame Clicquot was reluctant to use sugar beets for the preparation of her liqueur d’expédition, and recommended sugar cane instead (24). Interestingly, the NMR profile of the Baltic samples reveals the presence of pentoses such as ribose, which were also seen in grape juice but neither in cane syrup nor in modern champagne specimens. This observation suggests that grape syrup may have been used for the liquor preparation, albeit possibly in combination with sugar cane syrup (Fig. S2).
Nevertheless, the question of how winemakers could achieve such high sugar concentrations remains. In the 19th century and for the aforementioned reasons, it is unlikely that champagne grapes reached a sugar content of more than 180 g per liter of juice; thus the addition of a disproportionate volume of this grape juice to the wine would have been necessary to reach the final sugar concentration of around 150 g/L observed in the Baltic samples. Although it is difficult to evaluate to what extent, such an addition would have undoubtedly caused a significant dilution of the alcohol content of the wine, hence concealing a potential earlier chaptalization of the must. In addition, the Baltic champagne samples exhibited relatively high concentrations of furfural derivatives such as 5-hydroxymethylfurfural (5-[hydroxymethyl]-2-furaldehyde) (25), which originates from fructose during the caramelization that occurs via Maillard reactions in acidic conditions (Fig. 2). Similarly, the presence of difructose dianhydrides, which are key markers of caramelization processes, was proven by their detection as chlorine adducts by FTICR/MS (26, 27) (Fig. S3). Consequently, the evidence seems to point toward the use of grape juice concentrated by heating to produce grape syrup with over 700 g of sugar per liter as the base for the liqueur d’expédition. This hypothesis was nicely confirmed by the detection of the same furfural derivatives in heated grape juice, but not in heated cane syrup or unheated grape juice (Fig. 2 and Table S3).
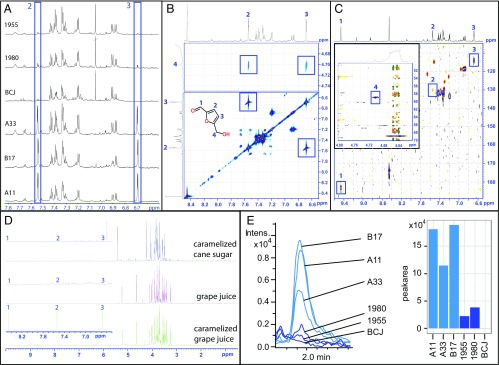
Fig. 2.
Characterization of 5-hydroxymethylfurfural (HMF) in the Baltic Sea champagne samples. (A) 1H NMR spectra (spectral region 7.8–6.6 ppm) illustrating the presence or absence of HMF in the six different champagne samples (blue boxes). (B) Molecular structure of HMF and carbon annotation. (B and C) Identification of HMF via (B) 2D-1H-1H DIPSI and (C) 2D-1H-13C-NMR (blue boxes) (D) Determination of the possible origin of HMF in the Baltic champagne samples. Absence of HMF (with focus on carbons 1, 2, and 3) in caramelized cane sugar and unheated grape juice vs. presence of HMF in heat-treated grape juice. (E) Quantification of HMF by UPLC-MS. High-resolution–extracted ion chromatogram of mass 125.024418 ± 0. 005 Da corresponding to [M-H]− ion of hydroxymethylfurfural in different champagne samples analyzed under reversed-phase conditions and peak area of the chromatographic peak of hydroxymethylfurfural.
FTICR/MS further provided comprehensive chemical signatures for each of the samples, ancient and modern. The Baltic champagne fingerprints were typical of wines that have undergone slow molecular diagenesis over time, with molecular masses that are, on average, significantly elevated and a lower chemical diversity. Indeed, the Baltic champagne specimens seem to have undergone a greater loss of peptidic fractions, as illustrated in the compositional networks by an overall lower number of nitrogen-containing compounds compared with the modern samples (Fig. 3 and Figs. S4 and S5).
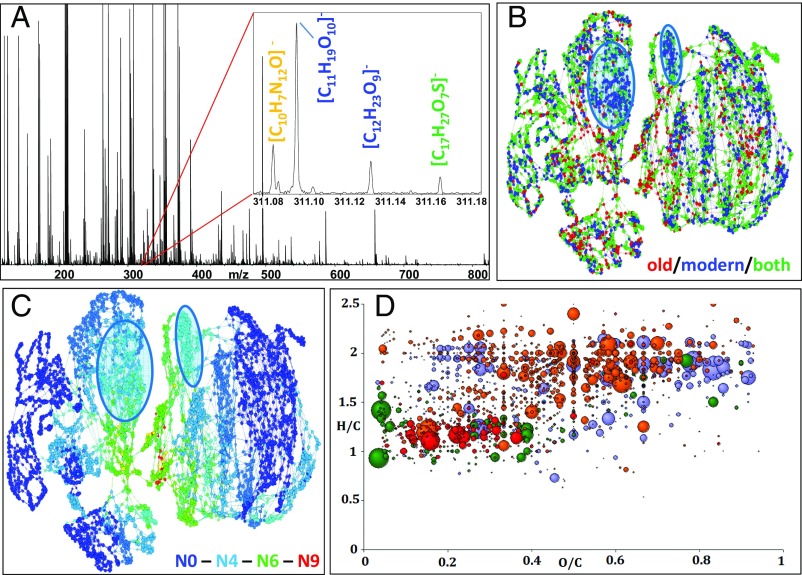
Fig. 3.
Compositional space of champagne samples as analyzed with FTICR/MS. (A) Mass spectrum of sample A33 in the m/z range from 150 to 800; 10-millimass-range spectrum details (331.08–311.18) with elemental composition assignment are shown. (B) Compositional similarity network (CSN) of all 4,196 features highlighting the m/z specific of old (Baltic Sea) (red) and modern (blue) champagne samples; two zones specific to the modern champagne specimens are highlighted in blue. (C) Nitrogen compositional space showing the distribution of compounds according to the number of nitrogen atoms they contain, ranging from none (N0, dark blue) to 9 (color gradient). (D) Typical electrospray ionization (+)-FTICR/MS van Krevelen diagram of sample A33 with CHO (blue), CHNO (orange), CHOS (green), and CHNOS (red) compounds; circle size is proportional to signal intensity.
In today’s context of growing concerns about food safety, wine producers take particular care to ensure that their wines are free of any bacterial contamination, through the use of antimicrobial compounds such as sulfites and good hygiene practices. We are often led to believe that hygiene is a modern concept, so it was inspiring to realize that the 170-y-old champagne samples presented very low concentrations of acetic acid, a sign of wine spoilage (21). Acetic acid levels were similar to those found in the modern specimens (Tables S1 and S3). Moreover, the concentrations of anions such as SO42− were also similar between the Baltic and modern champagnes. These anions are oxidation products of sulfites (Table S2), and the similar levels attest to the likelihood of microbiological stabilization already being in use in the 19th century.
In addition to the scientific and historical value of the chemical analysis of these ancient samples, there remains also the crucial question of their taste. Here we attempted to compare the information collected during expert tastings with the analytical data. Of the 40 aromas identified by GC/MS, 26 of these compounds showed concentrations higher than their known detection thresholds (Tables S4 and S5), and the correlation between the compiled descriptors and the aromas considered detectable is compelling.
At first, the Baltic samples were described using terms such as “animal notes,” “wet hair,” “reduction,” and sometimes “cheesy.” Animal notes are unequivocally related to the presence of volatile phenols, particularly 3-ethyl- and 3-propyl-phenol as identified by GC/MS. FTICR/MS analyses also revealed the presence of p-coumaroyltartaric acid and feruloyltartaric acid (Fig. S6), the respective precursors of 4-vinylphenol, which is responsible for empyreumatic notes, and 4-ethylphenol, which would contribute to the animal notes (28). Production of volatile phenols is classically attributed to Brettanomyces contamination (28). However, acid phenol decarboxylases and the corresponding vinylphenol reductases involved in volatile phenol biogenesis could also derive from Saccharomyces cerevisiae and lactic acid bacterial activity, albeit limited, resulting in the significant production of these compounds over time. “Reduction” and “wet hair” descriptors were to be expected for a wine that had spent such a long time sheltered from any oxygen source, and they were justified by the presence of light sulfurous compounds such as hydrogen sulfide, methanethiol, and dimethyldisulfide (29). Finally, the term “cheesy” is related to butanoic and octanoic acids (21) as well as, to a lesser extent, to isoamyl lactate (30) (creamy character), all present in the tasted wine, the latter being a sign of an incomplete malolactic fermentation having taken place inside the bottle. Upon swirling the wine in the glass to oxygenate it, the aroma became far more pleasant, with the main aromas described as empyreumatic, grilled, spicy, smoky, and leathery, together with fruity and floral notes. The ferulic acid released after hydrolysis of the corresponding ester (see above) would most likely have generated 4-vinylguaiacol and 4-ethylguaiacol, which are indeed described as spicy, smoky, and grilled (28). The “fruity” character of the Baltic champagne samples can unambiguously be attributed to the presence of some ethyl esters of fatty acids (31) (ethyl hexanoate/octanoate), which are the main aroma compounds derived from fermentation in white wines, but also to isoamyl acetate (30) and diethyl succinate (32), while ethyl dihydrocinnamate (33), octan-1-ol (21), and 2-phenylethanol (34) are clearly responsible for the floral notes. Finally, cis-oak and trans-oak lactones were also detected, and their presence further confirms the use of wooden barrels in the production of the Baltic Sea champagnes.
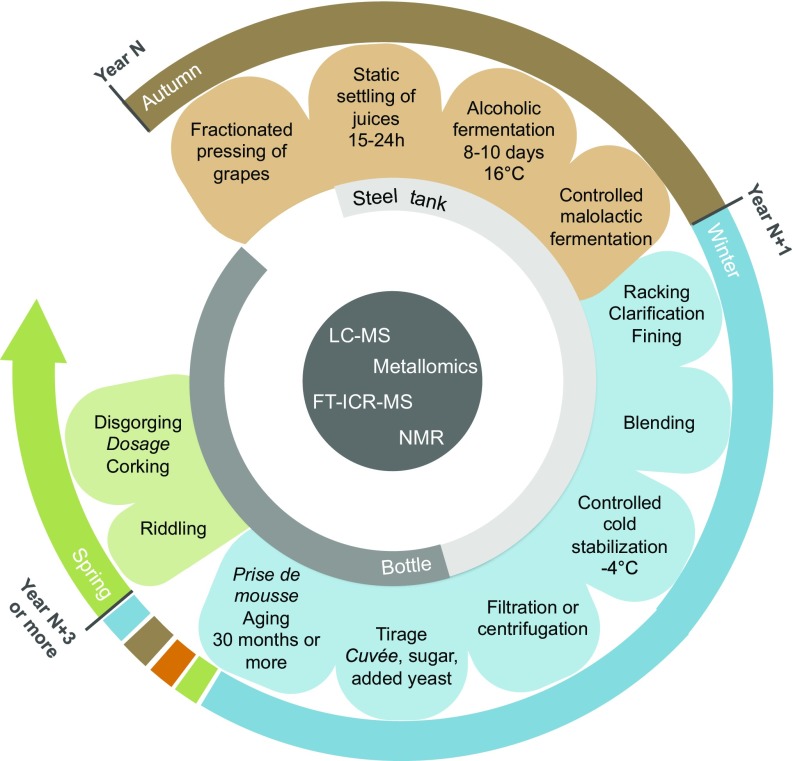
Owing to molecular insights acquired by comparing the Baltic champagnes to modern ones, we managed to decipher a clearer view of the winemaking practices used in Champagne at the beginning of the 19th century (Fig. 4) and to compare it to the modern winemaking process (Fig. 5). In addition, our nontargeted enolomic analysis shows a good conservation of the organoleptic quality of the champagne over almost two centuries, raising the question of the intrinsic qualities of a vibration-free and isothermal marine environment for long-term wine conservation.
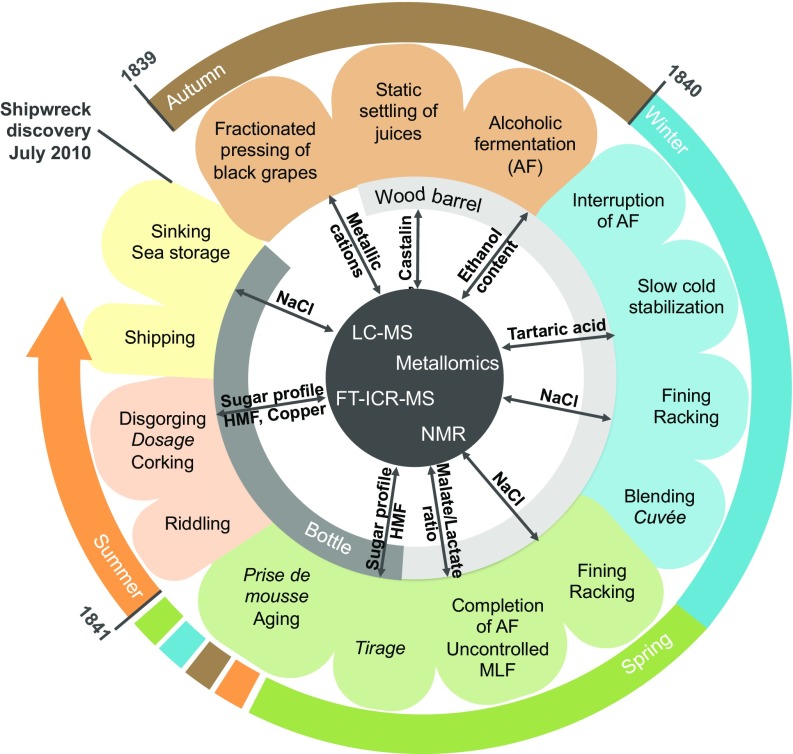
Fig. 4.
Representation of the putative champagne elaboration process in place at the beginning of the 19th century.
Fig. 5.
Representation of the modern champagne-making process.
Materials and Methods
Six different champagne samples were used for this set of experiments: three discovered in the Baltic Sea (A11, A33, and B17) and three modern ones dating from 1980 (1980), 1955 (1955), and 2011 (BCJ) and provided by the Veuve Clicquot Ponsardin (VCP) house. A11 and A33 were two VCP champagnes, with the latter being contaminated by seawater, whereas the B17 sample was a Juglar champagne. Quantification of elements was done using inductively coupled plasma atomic emission spectroscopy (ICP-AES). In case of elements close to- or below their detection limit, samples were reanalyzed by inductively coupled plasma sector field mass spectrometry (ICP-sf-MS). Metabolomics data were obtained using FTICR/MS (9, 10). From the resulting datasets, up to thousands of formulas containing C, H, O, N, P, and S elements were calculated and then represented using 2D van Krevelen diagram projections, which sort them along two axes according, for instance, to H/C and O/C atomic ratios. Metabolites were also separated and analyzed using reversed phase (RP) and hydrophilic interaction (HILIC)–ultrahigh-performance liquid chromatography coupled to quadrupole time of flight mass spectrometry (RP- and HILIC-UPLC-Q-ToF-MS) (10). For a quantitative nontargeted overview of abundant metabolites, one-dimensional and multidimensional NMR spectra were generated. One-dimensional spectra (1H) served for quantitative comparison of metabolites between the samples, and 2D experiments [1H-1H-DIPSI (decoupling in the presence of scalar interactions), 1H-1H-J-resolved, 1H-13C-heteronuclear single quantum coherence, 1H-13C-heteronuclear multiple bond correlation] helped with metabolite identification. Aroma analyses were performed through stir bar sorptive extraction-liquid desorption-gas chromatography-mass spectrometry (SBSE-LD-GC-MS) (11, 35–37).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500783112/-/DCSupplemental.
References
- 1.McGovern PE, Glusker DL, Exner LJ, Voigt MM. Neolithic resinated wine. Nature. 1996;381(6582):480–481. [Google Scholar]
- 2.McGovern PE, Mirzoian A, Hall GR. Ancient Egyptian herbal wines. Proc Natl Acad Sci USA. 2009;106(18):7361–7366. doi: 10.1073/pnas.0811578106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalieri D, McGovern PE, Hartl DL, Mortimer R, Polsinelli M. Evidence for S. cerevisiae fermentation in ancient wine. J Mol Evol. 2003;57(Suppl 1):S226–S232. doi: 10.1007/s00239-003-0031-2. [DOI] [PubMed] [Google Scholar]
- 4.Leppäranta M, Myrberg K. Physical Oceanography of the Baltic Sea. Springer; New York: 2009. [Google Scholar]
- 5. Veuve Clicquot Archives (1810−1840) Correspondence exchanged by Madame Clicquot with Louis Bohne and Louis Boissonnet, 1810s through 1840s (Veuve Clicquot Archives, Reims, France)
- 6.Londesborough J, et al. Analysis of beers from an 1840s’ shipwreck. J Agric Food Chem. 2015;63(9):2525–2536. doi: 10.1021/jf5052943. [DOI] [PubMed] [Google Scholar]
- 7.McGovern PE, et al. Beginning of viniculture in France. Proc Natl Acad Sci USA. 2013;110(25):10147–10152. doi: 10.1073/pnas.1216126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertelli AA, Morelli R, Lo Scalzo R, Ferrara F. Long-lasting antioxidant activity in a 600-year-old fermented fruit juice. Antioxid Redox Signal. 2004;6(5):934–940. doi: 10.1089/ars.2004.6.934. [DOI] [PubMed] [Google Scholar]
- 9.Liger-Belair G, et al. Unraveling different chemical fingerprints between a champagne wine and its aerosols. Proc Natl Acad Sci USA. 2009;106(39):16545–16549. doi: 10.1073/pnas.0906483106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roullier-Gall C, Witting M, Gougeon RD, Schmitt-Kopplin P. High precision mass measurements for wine metabolomics. Front Chem. 2014;2:102. doi: 10.3389/fchem.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steyer D, et al. QTL mapping of the production of wine aroma compounds by yeast. BMC Genomics. 2012;13:573. doi: 10.1186/1471-2164-13-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeandet P, Charters S. 2011. Producing champagne. The Business of Champagne, a Delicate Balance, Routledge Studies of Gastronomy, Food and Drink, ed Charters S (Routledge, London), pp 15–24.
- 13.Yokotsuka K. Effect of press design and pressing pressures on grape juice components. J Ferment Bioeng. 1990;70(1):15–21. [Google Scholar]
- 14.Valade M, Blanck G. Evolution des paramètres analytiques au cours du pressurage en Champagne. Rev. Fr. Oenol. 1989;118:23–27. [Google Scholar]
- 15.Cheynier V, Masson G, Rigaud J, Moutounet M. Estimation of must oxidation during pressing in Champagne. Am J Enol Vitic. 1993;44(4):393–399. [Google Scholar]
- 16.Kristl J, Veber M, Slekovec M. The contents of Cu, Mn, Zn, Cd, Cr and Pb at different stages of the winemaking process. Acta Chim Slov. 2003;50:123–136. [Google Scholar]
- 17.Etienne M. 1994. Veuve Clicquot Ponsardin: Aux Origines d’un Grand Vin de Champagne [Veuve Clicquot Ponsardin: At the Origins of a Great Champagne Wine] (Economica, Paris). French.
- 18.Bessis R, Fournioux JC, Jeandet P. The climate in relation to the quality/typicity of the wines of Burgundy: The 1995 vintage. J Wine Res. 1996;7(2):125–129. [Google Scholar]
- 19.Sanz M, et al. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal Chim Acta. 2012;732:33–45. doi: 10.1016/j.aca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 20. Veuve Clicquot Archives (1837, 1839), Copies de lettres françaises [copies of French letters] 1A 1E 018, p. 213, 22 July 1837; Copies de lettres françaises [copies of French letters] 1A 1E 019, p. 531, September 1839 (Veuve Clicquot Archives, Reims, France). French.
- 21.Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D. 2000. Handbook of Enology. The Chemistry of Wine, Stabilization and Treatments (Wiley, Chichester), Vol 2.
- 22.Liger-Belair G. How many bubbles in your glass of bubbly? J Phys Chem B. 2014;118(11):3156–3163. doi: 10.1021/jp500295e. [DOI] [PubMed] [Google Scholar]
- 23.Chandrashekar J, et al. The taste of carbonation. Science. 2009;326(5951):443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veuve Clicquot Archives (1837), Copies de lettres françaises [copies of French letters], 1A 1E 018, p.220, 3 August 1837 (Veuve Clicquot Archives, Reims, France). French.
- 25.Serra-Cayuela A, et al. Kinetics of browning, phenolics, and 5-hydroxymethylfurfural in commercial sparkling wines. J Agric Food Chem. 2014;62(5):1159–1166. doi: 10.1021/jf403281y. [DOI] [PubMed] [Google Scholar]
- 26.Ratsimba V, García Fernández JM, Defaye J, Nigay H, Voilley A. Qualitative and quantitative evaluation of mono- and disaccharides in D-fructose, D-glucose and sucrose caramels by gas-liquid chromatography-mass spectrometry. Di-D-fructose dianhydrides as tracers of caramel authenticity. J Chromatogr A. 1999;844(1-2):283–293. doi: 10.1016/s0021-9673(99)00322-2. [DOI] [PubMed] [Google Scholar]
- 27.Boutegrabet L, et al. Attachment of chloride anion to sugars: Mechanistic investigation and discovery of a new dopant for efficient sugar ionization/detection in mass spectrometers. Chemistry. 2012;18(41):13059–13067. doi: 10.1002/chem.201103788. [DOI] [PubMed] [Google Scholar]
- 28.Chatonnet P, Dubourdieu D, Boidron JN, Lavigne V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J Sci Food Agric. 1993;62(2):191–202. [Google Scholar]
- 29.Siebert TE, Solomon MR, Pollnitz AP, Jeffery DW. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. J Agric Food Chem. 2010;58(17):9454–9462. doi: 10.1021/jf102008r. [DOI] [PubMed] [Google Scholar]
- 30.Morakul S, et al. A dynamic analysis of higher alcohol and ester release during winemaking fermentations. Food Bioprocess Technol. 2013;6(3):818–827. [Google Scholar]
- 31.Gallart M, Francioli S, Viu-Marco A, López-Tamames E, Buxaderas S. Determination of free fatty acids and their ethyl esters in musts and wines. J Chrom A. 1997;776(2):283–291. [Google Scholar]
- 32.Camara JS, Alves MA, Marques JC. Changes in volatile composition of Madeira wines during their oxidative ageing. Anal Chim Acta. 2006;563(1-2):188–197. [Google Scholar]
- 33.López R, Ferreira V, Hernández P, Cacho JF. Identification of impact odorants of young red wines made with Merlot, Cabernet Sauvignon and Grenache grape varieties: A comparative study. J Sci Food Agric. 1999;79(11):1461–1467. [Google Scholar]
- 34.Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Camara JS. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorant compounds. Anal Chim Acta. 2006;563(1-2):154–164. [Google Scholar]
- 35.Culleré L, et al. Characterisation of aroma active compounds in black truffles (Tuber melanosporum) and summer truffles (Tuber aestivum) by gas chromatography-olfactometry. Food Chem. 2010;122(1):300–306. [Google Scholar]
- 36.Pino JA, Tolle S, Gök R, Winterhalter P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012;132(3):1436–1444. doi: 10.1016/j.foodchem.2011.11.133. [DOI] [PubMed] [Google Scholar]
- 37.Csoka M, Amtmann M, Sardy DN, Kallay M, Korany K. GC-MS description of the primary aroma structure of two Kadarka wines considered indigenous in Hungary. J Appl Bot Food Qual. 2013;86:104–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.