Significance
One of the important impacts of marine phytoplankton on climate systems is the geophysical feedback by which chlorophyll and the related pigments in phytoplankton absorb solar radiation and then change sea surface temperature. Yet such biogeophysical impact is still not considered in many climate projections by state-of-the-art climate models, nor is its impact on the future climate quantified. This study shows that, by conducting global warming simulations with and without an active marine ecosystem model, the biogeophysical effect of future phytoplankton changes amplifies Arctic warming by 20%. Given the close linkage between the Arctic and global climate, the biologically enhanced Arctic warming can significantly modify future estimates of global climate change, and therefore it needs to be considered as a possible future scenario.
Keywords: biogeophysical feedback, phytoplankton−climate interaction, Arctic climate changes
Abstract
Phytoplankton have attracted increasing attention in climate science due to their impacts on climate systems. A new generation of climate models can now provide estimates of future climate change, considering the biological feedbacks through the development of the coupled physical–ecosystem model. Here we present the geophysical impact of phytoplankton, which is often overlooked in future climate projections. A suite of future warming experiments using a fully coupled ocean−atmosphere model that interacts with a marine ecosystem model reveals that the future phytoplankton change influenced by greenhouse warming can amplify Arctic surface warming considerably. The warming-induced sea ice melting and the corresponding increase in shortwave radiation penetrating into the ocean both result in a longer phytoplankton growing season in the Arctic. In turn, the increase in Arctic phytoplankton warms the ocean surface layer through direct biological heating, triggering additional positive feedbacks in the Arctic, and consequently intensifying the Arctic warming further. Our results establish the presence of marine phytoplankton as an important potential driver of the future Arctic climate changes.
Phytoplankton, aquatic photosynthetic microalgae, play a key role in marine ecology, forming the foundation of the marine food chain. Besides this ecological importance, the climatic importance of phytoplankton is also evident, given their role in carbon fixation, which potentially reduces human-induced carbon dioxide (CO2) in the atmosphere (1–3). The great strides made in the field of biogeochemical modeling have improved climate models, enabling the investigation of carbon−climate feedback, such as diagnosing the strength of biogeochemical feedback and quantifying its importance in total carbon cycle responses. In fact, several modeling groups provide future climate projections including the biogeochemical process, as seen in a recent version of the Coupled Model Intercomparison Project (CMIP), i.e., CMIP5.
In addition to their biogeochemical feedback, phytoplankton also modify physical properties of the ocean. Chlorophyll and related pigments in phytoplankton affect the radiant heating in the ocean by decreasing both the ocean surface albedo and shortwave penetration (4–6). Thus, higher phytoplankton biomass generally results in warmer ocean surface layer. This biogeophysical feedback is known to significantly impact the global climate (7–10) and large-scale climate variability, such as the El Niño–Southern Oscillation (11–13) and Indian Ocean dipole (14). Unlike biogeochemical feedback, however, biogeophysical feedback has been overlooked in many future climate projections simulated by state-of-the-art climate models, even in projections by so-called Earth System Models that include interactive marine ecosystem components.
Greenhouse warming generally involves changes in physical fields that inevitably affect growth factors of phytoplankton such as temperature, light, and nutrients. Hence, they lead to changes in phytoplankton responding to future climate warming. Recent analyses based on the historical observation of phytoplankton and the future phytoplankton population estimated by modeling works have suggested substantial future changes in global phytoplankton, with the opposite sign of their trends in different regions (15–17). Such climate change-induced phytoplankton response would impact climate systems, given the aforementioned biological feedbacks.
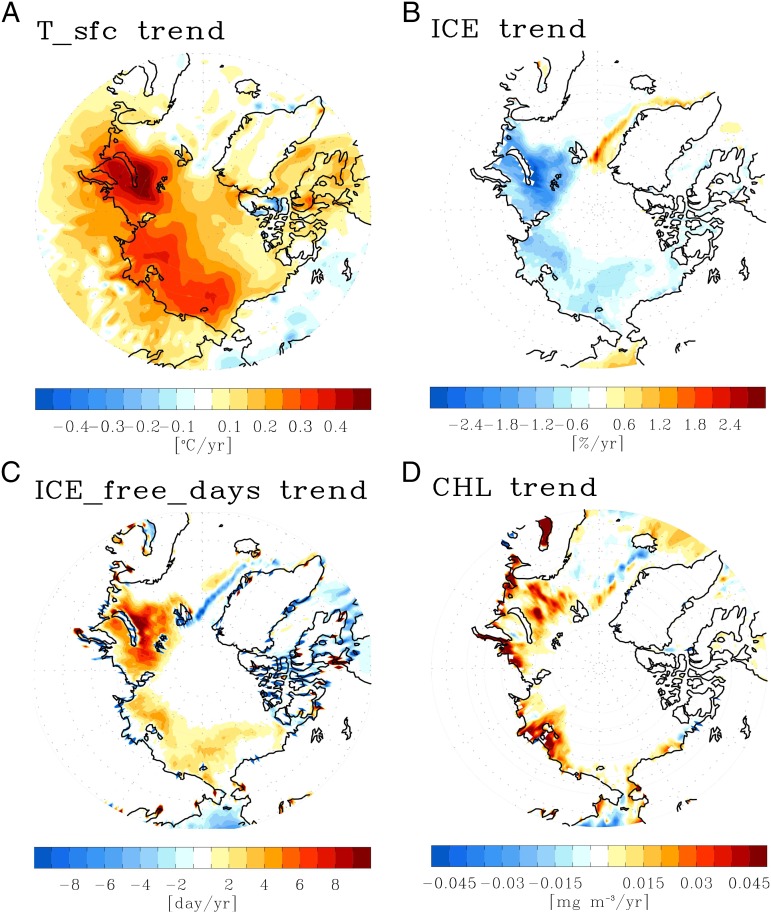
Previous studies discovered an increase in the annual area-integrated primary production in the Arctic, which is caused by the thinning and melting of sea ice and the corresponding increase in the phytoplankton growing area (18, 19). Considering that the biogeophysical feedback of phytoplankton leads to a warmer ocean surface layer, the increased area of Arctic phytoplankton bloom can induce first-order warming in the Arctic Ocean. The Arctic is a particularly vulnerable region where various positive feedback processes are involved, such as ice albedo feedback, temperature lapse rate feedback, and water vapor/cloud feedback (20–22). Thus, Arctic warming is generally greater than the globally averaged warming, a phenomenon known as Arctic amplification (23). Due to such strong positive feedback processes, an initial warming induced by the Arctic phytoplankton increase can be amplified and can potentially contribute to the Arctic amplification. In this sense, recent trends in annual mean surface temperature, sea ice, and chlorophyll may suggest the potential role of biogeophysical feedback on the current Arctic warming trend (Fig. 1). The strong trends of surface warming and the related sea ice reduction appear over Barents, Kara, Laptev, and Chukchi Seas. The pattern of increased annual mean chlorophyll, which can be caused by stronger Arctic phytoplankton bloom or by extended growing season, is closely linked with that of surface warming and ice melting trends. Although, in this observational result, the causality of their relationship and the quantitative impact of phytoplankton cannot be established, the similar patterns between the different variables suggest the possibility of interactive phytoplankton−climate feedback in the Arctic.
Fig. 1.
Observational Arctic climate trends in recent warming. Annual mean trends of observational (A) surface temperature, (B) sea ice concentration, (C) ice-free days, and (D) chlorophyll over the period 1998–2013 when the satellite-retrieved chlorophyll data are available. The ice-free days are defined as the number of days when sea ice concentration is lower than 5%.
The same feedback loop may hold for the future. If global warming continues with increased ice-free regions as projected by most climate models, the phytoplankton growing area would increase in the Arctic Ocean, and this, in turn, may intensify the Arctic amplification. However, most future climate projections have been produced without considering the impact of biogeophysical feedback, as mentioned earlier. Therefore, the effects of phytoplankton feedback need to be investigated in the context of the phytoplankton−Arctic warming hypothesis.
Results
To examine the biological impact on the climate responses to greenhouse warming, a coupled circulation−ecosystem model is used. Two greenhouse warming experiments (1%/y CO2 increase runs to double CO2) are performed respectively with and without the biogeophysical feedback by future phytoplankton changes. The shortwave penetration, which determines oceanic vertical shortwave heating, depends on the vertical distribution of chlorophyll in both experiments. In one experiment, named ECO.on, the chlorophyll concentration is calculated from the interactive marine ecosystem model. In the other experiment, named ECO.off, the marine ecosystem model is turned off, and instead, the chlorophyll concentration is prescribed with the long-term climatology of the present climate simulation. Therefore, ECO.off cannot consider future changes in marine phytoplankton, and thus the difference between the two experiments implies the impact of biogeophysical feedback on future climate projections.
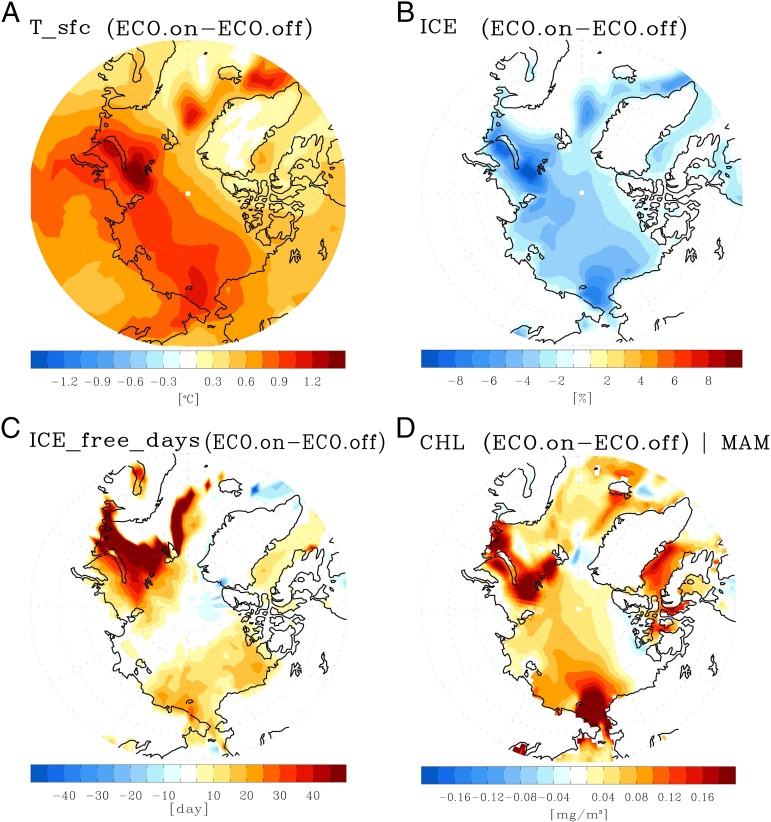
The five-member ensemble mean of two experiments shows a stronger Arctic surface warming in ECO.on compared with ECO.off (Fig. 2A). Although ECO.on results in a globally warmer climate than ECO.off, the relative warming in ECO.on is most evident in Arctic regions (SI Appendix, Fig. S2). Interestingly, the Arctic surface warming pattern with the most remarkable warming near the Kara and Chukchi Seas is consistent with the observational results (compare Figs. 2A and 1A). Moreover, the patterns of additional sea ice melting, extended open water season, and chlorophyll increase in ECO.on coincide with the temperature increase, which is also in agreement with the observational trend (compare Figs. 2 B−D and 1 B−D). Since the only difference between the two experiments is the presence of interactive biogeophysical feedback, the amplified Artic warming in ECO.on is attributable to the direct or indirect influence of future phytoplankton change simulated by the ecosystem model. We found that the enhanced Arctic warming is presumably induced by biological feedbacks confined to Arctic regions, not just mediated by other processes from lower latitudes (SI Appendix, Fig. S3). Therefore, these results indicate the role of ice−chlorophyll−shortwave heating feedback in contributing to the additional future Arctic warming.
Fig. 2.
Modified Arctic climate projection by future phytoplankton change. The ensemble mean difference of (A) surface temperature, (B) sea ice concentration, (C) ice-free days, and (D) spring chlorophyll concentration between two warming experiments, ECO.on and ECO.off, with and without interactive biogeophysical feedback to oceanic shortwave heating.
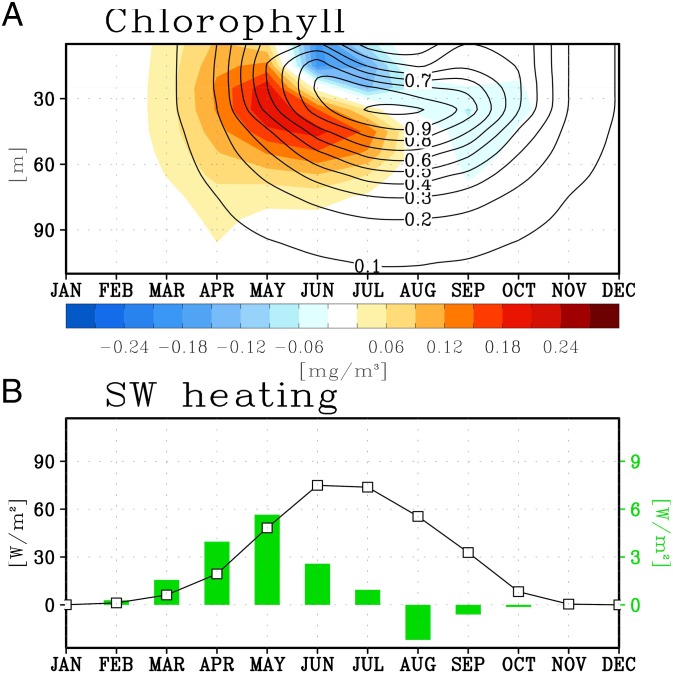
Further evidence for biological feedback to the amplified Arctic warming can be obtained by analyzing the physiology of Arctic phytoplankton. One of the important limiting factors of phytoplankton growth in the Arctic, particularly in the ice-covered ocean, is light, because of the insufficient amount of incoming sunlight and the high surface reflectance by sea ice. Thus, the phytoplankton bloom (chlorophyll concentration >2 mg/m3) starts in spring when the day length begins to increase, and it reaches its maximum in summer with a local minimum at the ocean surface (contour in Fig. 3A), probably due to typical summer oligotrophic conditions caused by increased stratification. Future global warming can modify the seasonal evolution of phytoplankton. The future warming generally causes sea ice reduction and extended open water season in the Arctic, which allows more solar radiation to penetrate into the ocean. Consequently, the increased ingress of sunlight results in an earlier phytoplankton bloom in spring by about 2 wk. The resultant phytoplankton increase, mostly in the spring season, is similar to the recent observational finding of the massive Arctic phytoplankton bloom in the context of ice-melting impact on marine biology (19).
Fig. 3.
Future phytoplankton change and its impact on ocean radiant heating. (A) Climatological mean of Arctic (30°W−210°E, 65°N−90°N) chlorophyll prescribed in the experiment without interactive biogeophysical feedback, ECO.off (contour), and mean chlorophyll difference between two experiments with and without biogeophysical feedback, ECO.on and ECO.off (shading). (B) Same as A but for shortwave heating averaged in the upper 30-m ocean (line, climatology from ECO.off; bar, mean difference between ECO.on and ECO.off).
The Arctic phytoplankton increase in spring under global warming can produce additional warming through biologically induced heating. In general, the climatological shortwave heating in the upper 30-m ocean starts to increase in spring and has its maximum in June, as simulated by ECO.off (line in Fig. 3B). In the case of ECO.on (bar in Fig. 3B) including the future phytoplankton increase, however, a stronger shortwave heating compared with ECO.off occurs mostly in spring, which is consistent with the earlier and extended spring phytoplankton bloom. The oceanic shortwave heating in our model is parameterized by a function of chlorophyll concentration and incoming shortwave radiation. Thus, the additional shortwave heating in ECO.on is attributed to either the direct impact of biological shortwave heating or the indirect impact of biologically induced mean climate change in the Arctic, such as changes in surface albedo and cloud cover, or both. Regardless of its causes, this possible impact by phytoplankton change on future Arctic climate cannot be captured if biogeophysical feedback is not implemented in models, similar to future projections provided by most of the current climate models.
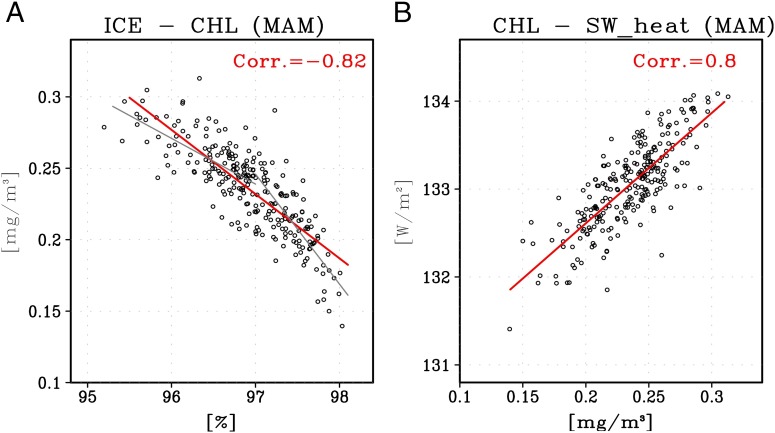
The ice−chlorophyll−shortwave heating feedback is further examined for the robustness and quantification of the components’ relationship. Firstly, the relationship between sea ice concentration and chlorophyll concentration in the Arctic is shown in Fig. 4B. The chlorophyll concentration averaged over the Arctic Ocean increases up to twice its value, depending on ice concentration. Note that the area-averaged chlorophyll concentration used here may represent the increase both in integrated phytoplankton over the Arctic Ocean (i.e., integrated biomass) and in phytoplankton per unit area (i.e., specific biomass) (SI Appendix, Fig. S7). This result supports that the mechanism of Arctic phytoplankton increase by sea ice reduction is plausible, and that the relationship between them is robust not only for long-term climate changes but also for interannual variability. We also found that the relationship is most pronounced in regions where open waters start to form (sea ice concentration >0.9). Due to the dramatic summer retreat of Arctic sea ice and increased ice-free regions, available solar radiation for phytoplankton growth is already sufficient during summer and fall. Hence, the impact of sea ice melting on phytoplankton growth in summer and fall is not as noteworthy as that in spring, which is consistent with the future phytoplankton response shown in Fig. 3A. In fact, a steeper chlorophyll increase by 1% of sea ice reduction in the higher ice coverage regime (>97%) than in the lower ice coverage regime (<97%) implies such nonlinear response of chlorophyll to sea ice melting (gray fitted lines in Fig. 4A).
Fig. 4.
Relationship among sea ice, chlorophyll, and ocean shortwave heating. (A) Scatter plot between sea ice concentration and chlorophyll (averaged in the upper 30-m ocean) in the Arctic Ocean (30°W−210°E, 65°N−90°N) simulated from five ensemble runs of ECO.on. Each dot represents the seasonal mean value from an individual member of the ensemble run. The red line represents a linear fit, and the two gray lines are linear fits for lower (<97%) and higher (>97%) ice coverage regimes. (B) Same as A, but for chlorophyll and shortwave heating in the upper 30-m ocean.
The chlorophyll concentration is also strongly correlated with shortwave heating (Fig. 4B). To quantify the direct biological heating effect, the shortwave heating used here is derived from the ratio of absorbed shortwave radiation in the upper ocean to incoming shortwave radiation. Thus, this result shows the biologically induced direct heating in the ocean, without indirect influences such as cloud cover and surface albedo. We found that phytoplankton exert more than 2 W/m2 of radiative forcing on the upper ocean. Considering that the global radiative forcing by the doubled CO2 concentration is ∼3.7 ± 0.74 W/m2 as estimated by CMIP5 models (24), the biologically induced radiative forcing could be an influential factor in the upper ocean heat budget. In particular, if this direct biological heating triggers a positive feedback through changes in surface albedo and cloud cover in the Arctic, the impact can be further enhanced. This is why the intensified springtime shortwave heating in ECO.on compared with ECO.off is nearly twice the direct biological heating (Fig. 3B). Eventually, the biological impact can be strong enough to substantially modify the future projection of Arctic climate, resulting in amplified future Arctic warming and decreased sea ice concentration (Fig. 2). The length of the open water season also considerably increases in most geographic sectors in the Arctic Ocean (SI Appendix, Table S1).
Another important consequence of biological feedback is that the rate of sea ice decline simulated by ECO.on is faster than those simulated by ECO.off. Hence, the simulated evolution of Arctic sea ice in ECO.on compares better with the recent abrupt decline in observed sea ice cover. Our future warming experiments are forced with prescribed carbon dioxide, increased by 1% per year from the level of 1990 to double its initial concentration. Thus, we may compare the simulated sea ice decreasing rate with the observed rate in recent decades, although an exact comparison requires a more detailed modeling framework. Interestingly, ECO.on is better able to represent the observed trend of Arctic sea ice decline (0.67% loss per year during 1990–2010) than ECO.off (SI Appendix, Fig. S8). Given that most climate models tend to underestimate the recent observational sea ice decline (25), this result suggests that the inclusion of interactive biogeophysical feedback in climate models may lead to better representation of historical sea ice reduction.
Discussion
This work, for the first time to our knowledge, quantifies the potential implication of future Arctic phytoplankton changes under greenhouse warming, which should be distinguished from previous works that explore a biogeophysical feedback in the present Arctic (7, 8, 10). Although the previous studies suggest a possible modification of Arctic climate due to interactive biogeophysical feedback in the present-day climate, our supplementary present-day simulations with and without interactive biogeophysical feedback do not show any significant Arctic temperature difference (SI Appendix, Fig. S11). The inconsistency in the present-climate simulations probably arises from the way the control experiments were set up (i.e., ECO.off). Previous studies used a constant light attenuation depth in control simulations that did not consider the temporal or spatial variations of biooptical properties. Thus, their results cannot separate the effects of interactive versus climatological biology, which actually limits the assessment of biogeophysical feedbacks. In our experiments, however, the control simulation is prescribed by long-term mean climatological chlorophyll calculated from the present-climate simulation with the marine ecosystem model turned on. Therefore, the greenhouse-warming-induced future phytoplankton mean change simulated in the ecosystem model, rather than the interannually varying phytoplankton, is the key factor that triggers the additional future Arctic warming.
Although our global warming experiments show an increase in future Arctic phytoplankton and the multimodel ensemble mean projection also shows a general increase in Arctic primary production, the range of future Arctic production change in the late twenty-first century compared with the late twentieth century is still highly uncertain. A previous study shows that many Earth system models in CMIP5 predict an increase in Arctic primary production by the end of the twenty-first century, while some models predict a decreased production below the level in the late twentieth century (26). This uncertainty is primarily driven by different future evolutions of the limiting factors for Arctic phytoplankton growth (27, 28). For example, the model predicting decreased future Arctic phytoplankton exhibits a steady increase in Arctic primary production for the first half of the twenty-first century due to less perennial sea ice (i.e., decreased light limitation), with a decrease during the late twenty-first century due to increased ocean stratification and nitrate reductions under recurrent ice-free conditions (i.e., increased nutrient limitation). Given that the timing of oligotrophy onset, which determines the sign of future Arctic production change, is largely dependent on future emission scenarios and that all Earth system models consistently feature an increase in Arctic primary production before a perennial ice-free condition (SI Appendix, Fig. S9), the future increase in Arctic phytoplankton is probably a more plausible scenario, at least in the next century. In fact, even a model that predicts a decrease in future Arctic production under the strongest climate change scenario shows an increase under a medium emission scenario (SI Appendix, Fig. S10). Therefore, the amplified Arctic warming by increasing Arctic phytoplankton biomass is valid in the context of climate projections on a century time scale.
The role of marine biology in the climate system is becoming conspicuous in the field of climate modeling. Our results show an emerging role for future phytoplankton in amplifying Arctic temperature in a warming climate. This finding is also confirmed by another state-of-the-art climate model with a similar experimental setup (SI Appendix, Fig. S12), which demonstrates that the biogeophysical impact of phytoplankton is an important contributor to future Arctic warming. In fact, biologically induced Arctic warming could be enhanced further if the biogeophysical impact of decreasing ocean surface albedo is also included (4), given that this study only considers the biogeophysical impact on the vertical distribution of oceanic shortwave heating. One of the common deficiencies of state-of-the-art climate models is that they tend to underestimate recent observational sea ice decline (25). In regard to such a shortcoming, our results suggest the potential role of interactive biogeophysical feedback in improving the representation of observed sea ice reduction. Therefore, a fully interactive and costly ecosystem model coupled with physical ocean−atmosphere models could be necessary for a more reliable estimate of the future Arctic climate. In addition, determining the factors that are responsible for Arctic phytoplankton change would be a critical step in improving the short-term to long-term prediction of Arctic climate.
Methods
The observational data analyzed in Fig. 1 are obtained from different sources: The surface temperature is from ERA-interim (29), the monthly sea ice concentration is from Hadley Centre sea ice and sea surface temperature (HadISST) datasets (30), the daily sea ice data are from National Oceanic and Atmospheric Administration High-Resolution SST data products (31), and the chlorophyll concentration is from two satellite-based ocean color sensors—the Sea-viewing Wide Field-of-view Sensor (SeaWiFS) and the Moderate Resolution Imaging Spectroradiometer (MODIS) (32, 33). The original chlorophyll data binned to a 9-km latitude/longitude grid is interpolated onto the regular 1.0° × 1.0° grid for computational efficiency by using a bilinear interpolation method. The median value in each grid is used in the interpolation process due to a nearly log-normal distribution of ocean chlorophyll concentrations (34). All data are analyzed for the period 1998–2013 to match the period of satellite-retrieved chlorophyll.
The model developed by the Geophysical Fluid Dynamics Laboratory (GFDL CM2.1) is used for the double CO2 experiments (35). This climate model is coupled to a complex marine ecosystem model, called Tracers of Phytoplankton with Allometric Zooplankton (TOPAZ) (36). The ocean component of the coupled model is based on the Modular Ocean Model version 4, and the oceanic vertical mixing is determined by K-profile parameterization scheme (37). The experiment is forced with prescribed carbon dioxide (CO2), increased by 1% per year to double its initial concentration and to remain constant afterward. The initial CO2 concentration is 352.7 ppm, a level in the late twentieth-century, and the model is integrated for 100 y after a 450-y spin-up period. Thus, the simulation mimics the experiment for the twenty-first century warming projection. This warming experiment consists of a pair of two runs, named ECO.on and ECO.off. In ECO.on, the ecosystem model is turned on, and fully interactive chlorophyll is used to determine the shortwave heating in the ocean, following a recent parameterization of shortwave penetration (8). In ECO.off, the ecosystem model is turned off, inactivating the biogeophysical feedback. Instead, the present-day monthly climatology of chlorophyll is prescribed for the calculation of oceanic shortwave heating. The prescribed chlorophyll is the 3D (in longitude−latitude−depth) climatological field simulated by a 300-y-long present climate run (forced by fixed CO2 concentration of 352.7 ppm), with the ecosystem model turned on. Five ensemble runs are performed in all warming experiments, and each ensemble run is started every 50 y in the present climate run to discount the possible impact of long-term Arctic climate variability on our results. We only analyzed the last 50 y of each ensemble member.
In Fig. 4, we only consider the region where open waters start to form (sea ice concentration >0.9) due to the strongest response of chlorophyll after sea ice melts. The shortwave heating in Fig. 4B is defined, based on the absorbed shortwave fraction in the upper ocean, to only consider direct biological heating. That is, we first converted the oceanic shortwave heating into the absorbed shortwave fraction by dividing it by incoming shortwave radiation in each grid point. Through this method, we exclude possible indirect heating by mean climate change, such as mean cloud cover or surface ice concentration changes. Then, the shortwave fraction is multiplied by the mean shortwave radiation penetrating into the Arctic Ocean for the quantified analysis of biologically induced heating.
Supplementary Material
Acknowledgments
The authors are grateful to two anonymous reviewers for their valuable comments that greatly contributed to improving the paper. This work was supported by National Research Foundation (NRF-2014R1A2A2A01003827).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416884112/-/DCSupplemental.
References
- 1.Arrigo KR, et al. Phytoplankton community structure and the drawdown of nutrients and CO2 in the southern ocean. Science. 1999;283(5400):365–367. doi: 10.1126/science.283.5400.365. [DOI] [PubMed] [Google Scholar]
- 2.De Baar HJ, et al. Importance of iron for plankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature. 1995;373(6513):412–415. [Google Scholar]
- 3.Le Borgne R, et al. Pacific warm pool and divergence: Temporal and zonal variations on the equator and their effects on the biological pump. Deep Sea Res Part II. 2002;49(13-14):2471–2512. [Google Scholar]
- 4.Frouin R, Iacobellis SF. Influence of phytoplankton on the global radiation budget. J Geophys Res. 2002;107(D19):4377. [Google Scholar]
- 5.Lewis MR, Carr ME, Feldman GC, Esaias W, McClain C. Influence of penetrating solar radiation on the heat budget of the equatorial Pacific Ocean. Nature. 1990;347(6293):543–545. [Google Scholar]
- 6.Morel A. Optical modeling of the upper ocean in relation to its biogenous matter content (case I waters) J Geophys Res. 1988;93(C9):10749–10768. [Google Scholar]
- 7.Lengaigne M, et al. Bio-physical feedbacks in the Arctic Ocean using an Earth system model. Geophys Res Lett. 2009;36(21):L21602. [Google Scholar]
- 8.Manizza M, Le Quéré C, Watson AJ, Buitenhuis ET. Bio-optical feedbacks among phytoplankton, upper ocean physics and sea-ice in a global model. Geophys Res Lett. 2005;32(5):L05603. [Google Scholar]
- 9.Murtugudde R, Beauchamp J, McClain CR, Lewis M, Busalacchi AJ. Effects of penetrative radiation on the upper tropical ocean circulation. J Clim. 2002;15(5):470–486. [Google Scholar]
- 10.Patara L, Vichi M, Masina S, Fogli PG, Manzini E. Global response to solar radiation absorbed by phytoplankton in a coupled climate model. Clim Dyn. 2012;39(7-8):1951–1968. [Google Scholar]
- 11.Anderson W, Gnanadesikan A, Wittenberg A. Regional impacts of ocean color on tropical Pacific variability. Ocean Sci. 2009;5(3):313–327. [Google Scholar]
- 12.Jochum M, Yeager S, Lindsay K, Moore K, Murtugudde R. Quantification of the feedback between phytoplankton and ENSO in the Community Climate System Model. J Clim. 2010;23(11):2916–2925. [Google Scholar]
- 13.Timmermann A, Jin FF. Phytoplankton influences on tropical climate. Geophys Res Lett. 2002;29(23):2104. [Google Scholar]
- 14.Park J-Y, Kug J-S. Marine biological feedback associated with Indian Ocean Dipole in a coupled ocean/biogeochemical model. Clim Dyn. 2013;42(1-2):329–343. [Google Scholar]
- 15.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444(7120):752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 16.Boyce DG, Lewis MR, Worm B. Global phytoplankton decline over the past century. Nature. 2010;466(7306):591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 17.Henson SA, et al. Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences. 2010;7(2):621–640. [Google Scholar]
- 18.Arrigo KR, et al. Massive phytoplankton blooms under Arctic sea ice. Science. 2012;336(6087):1408. doi: 10.1126/science.1215065. [DOI] [PubMed] [Google Scholar]
- 19.Arrigo KR, van Dijken G, Pabi S. Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett. 2008;35(19):L19603. [Google Scholar]
- 20.Graversen RG, Wang M. Polar amplification in a coupled climate model with locked albedo. Clim Dyn. 2009;33(5):629–643. [Google Scholar]
- 21.Screen JA, Simmonds I. The central role of diminishing sea ice in recent Arctic temperature amplification. Nature. 2010;464(7293):1334–1337. doi: 10.1038/nature09051. [DOI] [PubMed] [Google Scholar]
- 22.Winton M. Amplified Arctic climate change: What does surface albedo feedback have to do with it? Geophys Res Lett. 2006;33(3):L03701. [Google Scholar]
- 23.Holland MM, Bitz CM. Polar amplification of climate change in coupled models. Clim Dyn. 2003;21(3-4):221–232. [Google Scholar]
- 24.Intergovernmental Panel on Climate Change . Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; London: 2013. [Google Scholar]
- 25.Stroeve JC, et al. Trends in Arctic sea ice extent from CMIP5, CMIP3 and observations. Geophys Res Lett. 2012;39(16):L16502. [Google Scholar]
- 26.Vancoppenolle M, et al. Future Arctic Ocean primary productivity from CMIP5 simulations: Uncertain outcome, but consistent mechanisms. Global Biogeochem Cycles. 2013;27(3):605–619. [Google Scholar]
- 27.Popova EE, et al. What controls primary production in the Arctic Ocean? Results from an intercomparison of five general circulation models with biogeochemistry. J Geophys Res. 2012;117(C8):C00D12. [Google Scholar]
- 28.Tremblay J-É, Gagnon J. 2009. The effects of irradiance and nutrient supply on the productivity of Arctic waters: A perspective on climate change. Influence of Climate Change on the Changing Arctic and sub-Arctic Conditions, eds Nihoul JCJ and Kostianoy AG (Springer, New York), pp 73–93.
- 29.Dee D, et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q J R Meteorol Soc. 2011;137(656):553–597. [Google Scholar]
- 30.Rayner NA, et al. Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J Geophys Res. 2003;108(D14):4407. [Google Scholar]
- 31.Reynolds RW, et al. Daily high-resolution-blended analyses for sea surface temperature. J Clim. 2007;20(22):5473–5496. [Google Scholar]
- 32.Esaias WE, et al. An overview of MODIS capabilities for ocean science observations. IEEE Trans Geosci Remote Sens. 1998;36(4):1250–1265. [Google Scholar]
- 33.McClain CR, et al. Science quality SeaWiFS data for global biosphere research. Sea Technol. 1998;39(9):10–16. [Google Scholar]
- 34.Campbell JW. The lognormal distribution as a model for bio-optical variability in the sea. J Geophys Res. 1995;100(C7):13237–13254. [Google Scholar]
- 35.Delworth TL, et al. GFDL's CM2 global coupled climate models. Part I: Formulation and simulation characteristics. J Clim. 2006;19(5):643–674. [Google Scholar]
- 36.Dunne JP, Armstrong RA, Gnanadesikan A, Sarmiento JL. Empirical and mechanistic models for the particle export ratio. Global Biogeochem Cycles. 2005;19(4):GB4026. [Google Scholar]
- 37.Large WG, McWilliams JC, Doney SC. Oceanic vertical mixing: A review and a model with a nonlocal boundary layer parameterization. Rev Geophys. 1994;32(4):363–403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.