Abstract
Tocols induce high levels of granulocyte-colony-stimulating factor (G-CSF). G-CSF mobilises progenitors that allow mice that have been severely immunocompromised by exposure to acute, high-dose ionising irradiation to recover and to survive. The neutralisation of G-CSF abrogates the radioprotective efficacy of tocols. This article reviews studies in which CD2F1 mice were irradiated with sufficiently high doses to cause acute radiation syndrome symptoms and then administered (iv) progenitor-enriched whole blood or peripheral blood mononuclear cells from tocol- and AMD3100-injected donor mice (AMD3100 is a chemokine receptor antagonist used to improve the yield of mobilised progenitors). In some experiments, G-CSF was neutralised completely. Irradiated recipient mice were observed for 30 d post-irradiation for survival, a primary endpoint used for determining therapeutic effectiveness. Additionally, potential tocol-induced biomarkers (cytokines, chemokines and growth factors) were quantified. The authors suggest that tocols are highly effective agents for mobilising progenitors with significant therapeutic potential.
INTRODUCTION
Nuclear detonation either through military or terrorist action most likely would cause a mass-casualty scenario involving victims with varying degrees of exposure to ionising radiation(1). Exposure victims will show various signs and symptoms based on the level of radiation they received; these signs and symptoms collectively are known as acute radiation syndrome (ARS).
Natural products such as vitamins have become attractive targets for research(2). Vitamin E, well known for its health benefits, including antioxidant, neuroprotective and anti-inflammatory properties, acts to regulate peroxidation reactions and control free-radical production within the body. Investigations indicate that tocols, vitamin E isoforms, are not redundant with respect to their biological functions(3). Most of the biological properties of tocotrienols have been discovered only in the last 10 y, even though they were discovered a half century ago. Studies suggest that alpha-tocotrienol is highly neuroprotective(4) and delta-tocotrienol is effective in targeting prostate cancer stem cell-like populations(5), as well as effective against pancreatic carcinoma(6). Earlier studies suggest that there may be as much as a 30-fold difference between the abilities of alpha- and gamma-tocotrienol, to inhibit cholesterol biosynthesis via 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, a key target enzyme to reduce inflammation(7). Due to these properties, tocols and their derivatives have been evaluated for their radioprotective properties at the Armed Forces Radiobiology Research Institute (AFRRI) as well as at several other institutions(8).
Here, the authors summarise studies showing that tocols—specifically gamma-tocotrienol (GT3), delta-tocotrienol (DT3) and tocopherol succinate (TS, ester of alpha-tocopherol)—induce high levels of various cytokines, chemokines and growth factors including granulocyte colony-stimulating factor (G-CSF). G-CSF stimulates proliferation and differentiation of stem cells. Further, tocol-induced G-CSF mobilised progenitors and administration of this progenitor-enriched whole blood or isolated peripheral blood mononuclear cells (PBMCs) to irradiated recipient mice mitigated radiation injury. Additionally, administration of a G-CSF antibody neutralises tocol-induced G-CSF in peripheral blood and leads to the abrogation of tocols' radioprotective efficacy. These studies were completed for the purpose of advancing these agents under the Animal Efficacy Rule so that they may be developed for human use in the event of nuclear attack or accident. Overall, the reviewed studies examined tocols' effectiveness against radiation injury.
MATERIALS AND METHODS
Mice
Six- to eight-week-old male, specific pathogen-free CD2F1 mice were purchased from Harlan Laboratories (Indianapolis, IN, USA) and housed in an air-conditioned facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal procedures were performed according to a protocol approved by the AFRRI Institutional Animal Care and Use Committee. Research was conducted according to the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Research, US National Research Council.
Irradiation
Mice were placed in compartmentalised and ventilated Plexiglas boxes and exposed to bilateral 9.2-Gy gamma-irradiation (0.6 Gy min−1; LD90/30 dose for CD2F1 mice) in the AFRRI 60Co facility as described earlier(9). Radiation dosimetry was based primarily on the alanine/EPR (electron paramagnetic resonance) system(10, 11), currently accepted as one of the most accurate methods and used for comparison between national metrology institutions.
Drug preparation, cytokine analysis and G-CSF neutralisation
The optimal (previously determined) dose of tocol (200 mg kg−1) for cytokine induction was used for all three drugs(12). DT3 and GT3 formulations (5 % Tween-80 in saline) were purchased from Yasoo Health, Inc. (Johnson City, TN, USA). TS, administered at 400 mg kg−1 for blood mobilisation studies, was purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). Mice were administered tocols subcutaneously (sc) 24 h before blood harvest (for cytokine induction, n = 8) or irradiation (survival experiments, n = 16). Eight hours after tocol administration, the mice received 0.1 ml of either G-CSF antibody or isotype control intraperitoneally (ip; 600 or 1000 µg per mouse), as described earlier(13, 14). G-CSF antibody and isotype were tested and found negative for 12 viral agents by BioReliance (Rockville, MD, USA) using a MAP-IT (molecular antigen PCR-identification test for mice) assay. For cytokine analysis, blood was collected from terminally anaesthetised (isoflurane; Abbott Laboratories, Chicago, IL, USA) mice via the inferior vena cava 16 h after G-CSF antibody injection (24 h after tocol administration). The collected serum was stored at −70°C until analysed by Luminex 200 (Luminex Corp., Austin, TX, USA) to detect 40 cytokines as described earlier(12).
Mobilisation of progenitors by TS and transfusion of blood/PBMC
The efficacy of PBMC infusions after total-body irradiation was evaluated using donor and recipient mice. All donor mice received either TS or vehicle 72 h before harvest and received AMD3100 1 h before harvest (each in 0.1 ml, sc) to mobilise progenitors from the bone marrow into peripheral blood(15). Recipient mice were transfused with various volumes of blood or numbers of PBMCs at 2, 24 or 48 h after irradiation via the retro-orbital sinus (intravenously, iv) and were monitored for survival for 30 d.
Statistical analysis
For cytokines and G-CSF data analyses, mean values with standard errors (SE, when applicable) were reported. Analysis of variance with a Tukey's post hoc test was performed to determine whether and where significant differences were present between treatment groups. For survival data, a log-rank and Fisher′s exact tests were used to compare survival curves and rates. A Bonferroni correction controlled for type-I error if multiple comparisons were used. All statistical tests were two-sided with a 5 % significance level and conducted with IBM SPSS Statistics version 19.
RESULTS
Induction of various cytokines, chemokines and growth factors by tocols in mice
The authors compared the levels of various cytokines following administration of DT3, GT3 and TS. Their results suggest that tocols induce significantly higher levels of several cytokines compared with vehicle. These cytokines (40 evaluated) may play a critical role in the radioprotective efficacy of these tocols. Mice injected with DT3 had significantly higher levels of four cytokines (G-CSF, KC, MCP-1 and IL-17F). Mice injected with GT3 had significantly higher levels of seven cytokines (IL-9, G-CSF, KC, PDGF-bb, IL-17F, CD40L and MIP3-α). Mice injected with TS had higher levels of two cytokines (KC and MCP-1) (data not presented due to space limitations)(12).
Neutralisation of tocol-induced G-CSF by its antibody and effect of such G-CSF neutralisation on radioprotective efficacy in mice
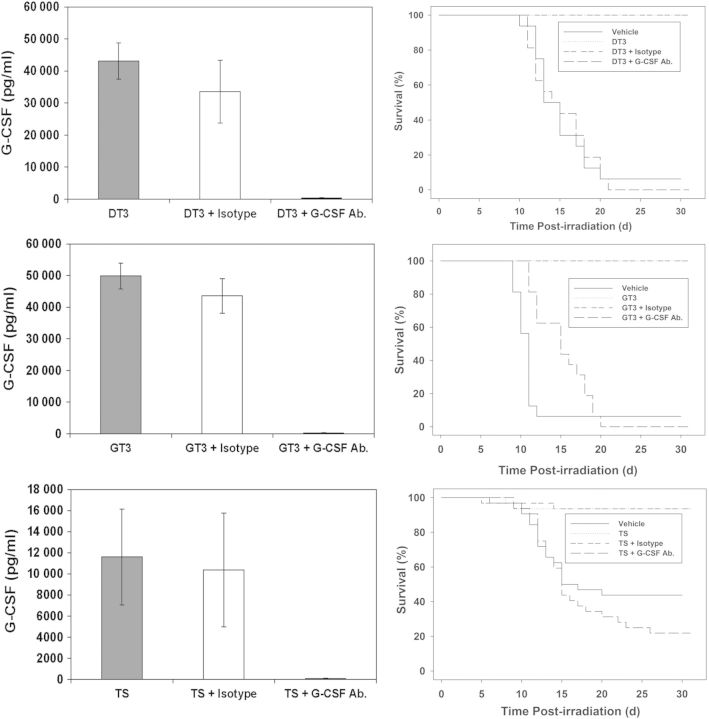
Three groups of mice (n = 8) were injected (sc) with tocols (200 mg kg−1) in three different experiments. One group received the G-CSF antibody 8 h after tocol injection and another group received its isotype ip. The third group did not receive a second injection (G-CSF antibody or isotype). Blood was harvested from mice 24 h after tocol injection. G-CSF antibody administration specifically and completely neutralised circulating G-CSF in peripheral blood (Figure 1).
Figure 1.
Neutralisation of tocol-induced G-CSF and abrogation of their radioprotective efficacy by G-CSF antibody administration in mice. Please note for each tocol, the survival curves for the groups that received tocol and tocol + isotype overlap. For G-CSF abrogation studies, n = 8; for survival studies, n = 16.
To determine whether G-CSF induction by tocols is a key factor in protection against radiation injury, the authors conducted an experiment to neutralise G-CSF, as stated earlier. Mice were exposed to gamma radiation 24 h post-tocol administration and observed for 30 d for survival. Methods and groups were as stated earlier. An additional group in each experiment received only vehicle. Data demonstrate that mice receiving only tocol or tocol plus the isotype were protected significantly from ionising radiation compared with vehicle control and tocol plus the G-CSF neutralising antibody (Figure 1). There is no significant difference between tocol-treated and tocol plus isotype-treated mice.
Efficacy of infusing whole blood or PBMCs from TS-injected donor mice on the survival of recipient mice exposed to gamma radiation
To determine the efficacy of whole blood or PBMCs on survival after whole-body irradiation, mice (n = 16) were transfused with different volumes of whole blood or different numbers of PBMCs. All donors received (sc) single doses of either TS (400 mg kg−1) or vehicle 72 h prior to blood harvest and AMD3100, 1 h before blood harvest. The recipients were irradiated (9.2 Gy) and subsequently transfused with whole blood or PBMCs via the retro-orbital sinus 24 h after radiation exposure (to learn whether transfusion of donor cells can be delayed) and monitored for survival over 30 d.
The authors’ results demonstrate that as small a quantity as 25 µl of blood from TS-injected mice protected a significant number of recipient mice against an LD90/30 dose of radiation (9.2 Gy; Table 1). PBMCs could be administered as late as 48 h after radiation exposure; however, with increasing numbers of transfused PBMCs, better survival outcomes were clearly noted. There was no significant difference between recipients of cells or blood from vehicle-injected mice and the irradiated control group. Higher numbers of early progenitors (c-kit or/and sca-1 positive cells) were present within the TS-mobilised blood/PBMCs samples compared with vehicle(16).
Table 1.
Summary of efficacy of whole blood or PBMC from TS-injected mice as a radiomitigator against several doses of total-body gamma-irradiation.
| Experiment | Mobilising agent | Blood volume (µl)/PBMC # (million–M) | Transfusion time in relation to irradiation (h) | Radiation dose of recipient (Gy) | Recipient % survival at Day 30 | Statistical significance |
|---|---|---|---|---|---|---|
| 1. | TS | Blood 25 µl | +2 | 9.2 | 26 | NS |
| Blood 50 µl | +2 | 9.2 | 89 | S | ||
| Blood 100 µl | +2 | 9.2 | 84 | S | ||
| Blood 150 µl | +2 | 9.2 | 95 | S | ||
| Vehicle | Blood 150 µl | +2 | 9.2 | 5 | ||
| 2. | TS | PBMC 0.5 M | +2 | 9.2 | 84 | S |
| Vehicle | PBMC 0.5 M | +2 | 9.2 | 50 | ||
| 3. | TS | PBMC 2 M | +2 | 9.2 | 100 | S |
| Vehicle | PBMC 2 M | +2 | 9.2 | 6 | ||
| 4. | TS | PBMC 2 M | +24 | 9.2 | 88 | S |
| Vehicle | PBMC 2 M | +24 | 9.2 | 50 | ||
| 5. | TS | Blood 100 µl | +2 | 11 | 100 | S |
| Vehicle | Blood 100 µl | +2 | 11 | 31 | ||
| TS | Blood 100 µl | +24 | 11 | 88 | S | |
| Vehicle | Blood 100 µl | +24 | 11 | 13 | ||
| 6. | TS | PBMC 5 M | +2 | 11 | 100 | S |
| Vehicle | PBMC 5 M | +2 | 11 | 31 | ||
| TS | PBMC 5 M | +24 | 11 | 94 | S | |
| Vehicle | PBMC 5 M | +24 | 11 | 38 | ||
| TS | PBMC 5 M | +48 | 11 | 69 | S | |
| Vehicle | PBMC 5 M | +48 | 11 | 0 | ||
| 7. | TS | PBMC 5 M | +24 | 11.5 | 94 | S |
| Vehicle | PBMC 5 M | +24 | 11.5 | 19 | ||
| TS | PBMC 5 M | +24 | 12 | 69 | S | |
| Vehicle | PBMC 5 M | +24 | 12 | 6 | ||
| TS | PBMC 5 M | +24 | 12.5 | 38 | NS | |
| Vehicle | PBMC 5 M | +24 | 12.5 | 0 | ||
| TS | PBMC 5 M | +24 | 13 | 0 | NS | |
| Vehicle | PBMC 5 M | +24 | 13 | 0 | ||
| TS | PBMC 5 M | +24 | 14 | 0 | NS | |
| Vehicle | PBMC 5 M | +24 | 14 | 0 |
Donor mice were administered TS and AMD3100 (72 and 1 h, respectively) before blood collection. PBMCs were isolated from donor blood samples. Recipient mice (n = 16 per treatment group) were irradiated with varying doses of radiation and then transfused with whole blood or PBMCs at different times after irradiation. S indicates a significant difference between the TS-treated group and the respective vehicle. NS indicates no significant difference.
DISCUSSION
Cytokines are highly potent molecules that in general are transiently expressed in response to various stimuli(17). Recently, the authors have demonstrated induction of high levels of selected cytokines by several radiation countermeasures(12–14, 18–20). These studies identified G-CSF to be a candidate biomarker for the radioprotective and radiomitigative efficacy of tocols. The authors’ results further support the role of G-CSF in radioprotection. The authors have demonstrated that all three tocol derivatives and radiation exposure individually induce G-CSF and administration of a G-CSF antibody to mice before radiation exposure exacerbates radiation injury, providing additional support that G-CSF provides an essential radioprotective effect(21). Recently, other investigators have demonstrated the radiomitigative potential of G-CSF in mouse, minipig and nonhuman primate models(22–25).
Recently, myeloid progenitor cell intravenous administration was used as bridging therapy in the experimental animal model of ARS. Such cells have been shown to be effective in protecting animals exposed to lethal doses of radiation(9). These myeloid progenitors (along with other haematopoietic progenitor cell types) can be mobilised out of the bone marrow into the blood for the reconstitution of haematopoiesis. As discussed, various tocols can be used to mobilise marrow progenitors, which are especially useful as a bridging therapy for radiation casualties. Further, the extent of progenitor mobilisation elicited by tocols, specifically TS, in mice is comparable with clinically used drugs such as recombinant granulocyte-colony-stimulating factor (rhG-CSF/Neupogen®) and the bicyclam AMD3100 (plerixafor/Mozobil)(13); therefore, the authors propose that TS be considered for further translational development and, ultimately, for use in humans.
FUNDING
This study was supported by Armed Forces Radiobiology Research Institute intramural awards (RAB2CZ, RBB2GQ, and RAB2HD) to V.K.S.
REFERENCES
- 1.DiCarlo A. L., Maher C., Hick J. L., Hanfling D., Dainiak N., Chao N., Bader J. L., Coleman C. N., Weinstock D. M. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med. Public Health Prep. 2011;5(Suppl 1):S32–S44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V. K., Beattie L. A., Seed T. M. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013;54:973–988. doi: 10.1093/jrr/rrt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesaretnam K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008;269:388–395. doi: 10.1016/j.canlet.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 4.Sen C. K., Khanna S., Roy S., Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 2000;275:13049–13055. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- 5.Luk S. U., et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer. 2011;128:2182–2191. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 6.Hussein D., Mo H. d-delta-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas. 2009;38:e124–e136. doi: 10.1097/MPA.0b013e3181a20f9c. [DOI] [PubMed] [Google Scholar]
- 7.Pearce B. C., Parker R. A., Deason M. E., Qureshi A. A., Wright J. J. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 8.Singh V. K., Ducey E. J., Brown D. S., Whitnall M. H. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Biol. 2012;88:296–310. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- 9.Singh V. K., Christensen J., Fatanmi O. O., Gille D., Ducey E. J., Wise S. Y., Karsunky H., Sedello A. K. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat. Res. 2012;177:781–791. doi: 10.1667/rr2894.1. [DOI] [PubMed] [Google Scholar]
- 10.Nagy V. V. Accuracy considerations in EPR dosimetry. Appl. Radiat. Isot. 2000;52:1039–1050. doi: 10.1016/s0969-8043(00)00052-x. [DOI] [PubMed] [Google Scholar]
- 11.ISO-ASTM Standard practice for use of an alanine dosimetry. 2004. ISO/ASTM International Standard 5167 (Geneva, Switzerland: ASTM International)
- 12.Singh V. K., Wise S. Y., Scott J. R., Romaine L. P., Newman V. L., Fatanmi O. O. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 2014;98:113–122. doi: 10.1016/j.lfs.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 13.Singh V. K., Brown D. S., Kao T. C. Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int. J. Radiat. Biol. 2010;86:12–21. doi: 10.3109/09553000903264515. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni S., Singh P. K., Ghosh S. P., Posarac A., Singh V. K. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–285. doi: 10.1016/j.cyto.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer H. E., et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh V. K., Singh P. K., Wise S. Y., Seed T. M. Mobilized progenitor cells as a bridging therapy for radiation casualties: a brief review of tocopherol succinate-based approaches. Int. Immunopharmacol. 2011;11:842–847. doi: 10.1016/j.intimp.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Schaue D., Kachikwu E. L., McBride W. H. Cytokines in radiobiological responses: a review. Radiat. Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krivokrysenko V. I., et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J. Pharmacol. Exp. Ther. 2012;343:497–508. doi: 10.1124/jpet.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakhov A. N., et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2) PLoS one. 2012;7:e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh V. K., Ducey E. J., Fatanmi O. O., Singh P. K., Brown D. S., Purmal A., Shakhova V. V., Gudkov A. V., Feinstein E., Shakhov A. CBLB613: A TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat. Res. 2012;177:628–642. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 21.Singh V. K., Fatanmi O. O., Singh P. K., Whitnall M. H. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine. 2012;58:406–414. doi: 10.1016/j.cyto.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Farese A. M., Cohen M. V., Katz B. P., Smith C. P., Gibbs A., Cohen D. M., MacVittie T. J. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat. Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farese A. M., Cohen M. V., Stead R. B., Jackson W., III, Macvittie T. J. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat. Res. 2012;178:403–413. doi: 10.1667/RR2900.1. [DOI] [PubMed] [Google Scholar]
- 24.Kim J. S., Ryoo S. B., Heo K., Kim J. G., Son T. G., Moon C., Yang K. Attenuating effects of Granulocyte-colony stimulating factor (G-CSF) in radiation induced intestinal injury in mice. Food Chem. Toxicol. 2012;50:3174–3180. doi: 10.1016/j.fct.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Moroni M., Ngudiankama B. F., Christensen C., Olsen C. H., Owens R., Lombardini E. D., Holt R. K., Whitnall M. H. The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:986–992. doi: 10.1016/j.ijrobp.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]