Abstract
Background
Anecdotal reports suggesting that survival rates among hospitalized patients with Ebola virus disease in Guinea are higher than the 29.2% rate observed in the current epidemic in West Africa.
Methods
Survival after symptom onset was determined using Kaplan Meier survival methods among patients with confirmed Ebola virus disease treated in Conakry, Guinea from March 25, 2014, to August 5, 2014. We analyzed the relationship between survival and patient factors, including demographics and clinical features.
Results
Of the 70 patients analyzed [mean age ± standard deviation (SD), 34 ± 14.1; 44 were men], 42 were discharged alive with a survival rate among hospitalized patients of 60% (95% confidence interval, 41.5–78.5%). The survival rate was 28 (71.8%) among 39 patients under 34 years of age, and 14 (46.7%) among 30 patients aged 35 years or greater (p = 0.034). The rates of myalgia (3 of 42 versus 7 of 28, p = 0.036) and hiccups (1 of 42 versus 5 of 28, p = 0.023) were significantly lower among patients who survived.
Conclusions
Our results provide insights into a cohort of hospitalized patients with Ebola virus disease in whom survival is prominently higher than seen in other cohorts of hospitalized patients.
On March 23, 2014, the World Health Organization (WHO) was notified as an outbreak1 and by June 18, 2014, it was considered the largest outbreak of Ebola virus disease.2 Ebola virus disease in the current epidemic has disproportionately affected the persons who are between 15 and 44 years of age (comprising 60.8% of cases, although this age group makes up only 44% of the population).1 The estimated case survival rate was 29.2% (95% confidence interval, 27.8–30.6%) among the persons with known clinical outcome of infection in an analysis of 3343 confirmed and 667 probable Ebola cases collected in Guinea, Liberia, Nigeria, and Sierra Leone.1 The case survival rate was higher among hospitalized patients (35.7%, 95% confidence interval 33.6–37.8%) than among all patients with definitive outcomes. There was a prominently higher case survival rate among health care workers in Guinea of 43.9% (95% confidence interval, 30.5–57.3%) as compared with the 20% (95% confidence interval, 15.1–24.9%) rate observed in Liberia.1 There were anecdotal reports suggesting that the case survival rates among hospitalized patients with Ebola virus disease in Guinea were much higher than the 20% rate observed in patients treated at Kikwit General Hospital during the 1995 outbreak.3 However, higher survival has not been confirmed and reasons underlying higher survival have not been identified.
We performed an analysis of patient level data collected at Donka National Hospital, Conakry, Guinea to determine the survival and associated factors among patients with Ebola virus disease hospitalized in Guinea.
Methods
Donka National Hospital, Conakry, Guinea serves as the predominant designated hospital for the treatment of Ebola virus infected patients in Guinea. Since March 2014, an isolation ward comprising a single-level building structure has been setup for management of confirmed Ebola virus disease patients. The presence of Ebola virus disease is confirmed by means of polymerase chain reaction test. The isolation unit comprises of small (two beds) and large rooms (three beds).
Acute care is provided in three shifts and by five teams consisting of doctors, nurses, psychologists, and hygienists, who staffs each shift. Vital signs are measured four times daily using cuff measurements and manual palpation. Antimalarial agents consisting of a combination of Artemether and Lumefantrine are routinely used in all patients. The treatment is focused on symptom relief, which includes oral paracetamol (acetaminophen), tramadol, and infrequently intravenous or intramuscular morphine sulphate for fever, headache, arthralgia, and myalgias. Oral hydration formulation is the preferred intervention, but intravenous hydration using lactated Ringers, dextrose-based fluids, and rarely normal saline is used for the treatment of volume depletion, hypotension, and malnutrition. Nutritional care is dependent on food provided by families. Patients who are not able to tolerate oral intake rely on caloric intake from intravenous dextrose containing fluids. Laboratory tests, such as complete blood count and basic metabolic panel, are not performed to avoid spread of infection during transport to routine laboratories in other parts of the hospital. Blood transfusions and central venous catheters are very rarely used. There are no intravenous vasopressors, parenteral nutrition formulations, and mechanical ventilators in use. The only option for respiratory distress is supplemental oxygen via facemask. There was no antiviral medication, vaccine, and exchange transfusion being used within the ward during the study period. If a patient is afebrile or asymptomatic for three days, a polymerase chain reaction test is performed and if negative, the patient is discharged.
The findings of each patient encounter that are recorded include vitals, medication administered, and any new development, which are dictated to a team member outside the isolation ward and archived in the paper files. Clinical and demographic data were collected from the paper files with the use of a standard case investigation form and entered in a database on confirmed, probable, and suspected Ebola virus disease cases. The study contained 54 variables, some of which were patient’s age, sex, signs and symptoms (present at admission to hospital), the date of symptom onset and of hospital admission, and the date of death or discharge. The only laboratory test recorded in the database was qualitative polymerase chain reaction test results. All records were deidentified consistent with the Privacy Rule (45 C.F.R. § 164.514(b)(2)(i))4 and met the definition of deidentified records provided by the Office of Human Research Protections.5
Statistical Analysis
The analysis was predominantly descriptive with categorical and continuous variables expressed as frequencies and means with SDs, respectively. We performed univariate analysis, chi-square test for categorical, and t-test for continuous variables to identify factors associated with survival, including age, gender, time interval between symptom onset and admission, and clinical features. The 21-day survival after the first day of hospitalization was estimated using Kaplan Meier survival methods. The survival rates were estimated with standard error (SE) to provide the precision of estimate. We attempted to identify a group of patients who were likely to survive based on a combination of risk factors identified in the univariate analysis. We analyzed various dichotomization points for age and selected the cutoff point where statistically significant difference in survival was evident. All analyses were performed using the SPSS statistics software (IBM Corp, Armonk, NY).
Results
There were 75 patients with confirmed Ebola virus disease entered in the database at the time of data censor (from March 25, 2014, to August 5, 2014). Care was ongoing in five patients and outcome was not determined. A total of 70 patients were included in the analysis (mean age ± SD, 34 ± 14.1; 44 were men). The mean ± SD time interval between symptom onset and admission was 5.7 ± 3.6 days and mean ± SD for duration of hospitalization was 7 ± 5 days. Of the 70 patients analyzed, 42 were discharged alive and free of infection providing a survival rate among hospitalized patients of 60% (95% CI 41.5–78.5%). The survival rate according to age strata was as follows: 28 (71.8%) among 39 patients under 34 years of age and 14 (46.7%) among 30 patients aged 35 years or greater (p = 0.034). Age was not available for one patient. The estimated 21-day survival after first day of hospitalization was 50% (SE 0.07).
Table 1 provides a comparison of demographic and clinical characteristics between those who survived and those who did not survive. The mean age (±SD) of patients who survived was nonsignificantly different than those who died (31.6 ± 13.9 years versus 37.9 ± 13.8 years, p = 0.068). There was a trend toward higher survival among women compared with men (19 of 26 versus 23 of 44, p = 0.086). The rates of myalgia (3 of 42 versus 7 of 28, p = 0.036) and hiccups (1 of 42 versus 5 of 28, p = 0.023) were significantly lower among patients who survived. There was no difference in rate of fever occurrence (27 of 42 versus 19 of 28, p = 0.758) and bleeding events (7 of 42 versus 6 of 28, p = 0.616) between those who survived and those who died. The mean length (±SD) of hospitalization was significantly longer among patients who survived compared with those who died (9.2 ±5 days versus 3.9 ± 2.9 days, p = <0.001).
Table 1. Factors associated with survival among patients with Ebola virus disease hospitalized in Donka National Hospital, Conakry, Guinea (March 25, 2014, to August 5, 2014).
| Patients who died (%) | Patients who survived (%) | p-Value | |
|---|---|---|---|
| Number of patients | 28 | 42 | |
| Time intervals* | |||
| Symptom onset to admission (days ±SD) | 5±2.9 | 6.2±4 | 0.188 |
| Length of hospitalization (days ±SD) | 3.9±2.9 | 9.2±5 | <0.001 |
| Symptom onset to discharge(days ±SD) | 8.9±3.8 | 15.6±6.2 | <0.001 |
| Demographic characteristics | |||
| Age (years ±SD)& | 37.9±13.8 | 31.6±13.9 | 0.068 |
| Men | 21(75) | 23(54.8) | 0.086 |
| Women | 7(25) | 19(45.2) | |
| Clinical features | |||
| Fever | 19(67.9) | 27(64.3) | 0.758 |
| Weakness | 23(82.1) | 32(76.2) | 0.552 |
| Anorexia | 16(57.1) | 18(42.9) | 0.241 |
| Skin rash | 0(0) | 1(2.4) | 0.411 |
| Nausea | 6(21.4) | 11(26.2) | 0.649 |
| Vomiting | 14(50) | 21(50) | 0.999 |
| Abdominal pain | 7(25) | 11(26.2) | 0.911 |
| Diarrhea | 13(46.4) | 17(40.5) | 0.622 |
| Myalgia | 7(25) | 3(7.1) | 0.036 |
| Arthralgia | 4(14.3) | 5(11.9) | 0.771 |
| Low back pain | 1(3.6) | 3(7.1) | 0.528 |
| Headache | 16(57.1) | 19(45.2) | 0.329 |
| Dyspnea | 0(0) | 0(0) | |
| Cough | 2(7.1) | 1(2.4) | 0.335 |
| Hiccups | 5(17.9) | 1(2.4) | 0.023 |
| Thoracic pain | 2(7.1) | 3(7.1) | 0.999 |
| Any bleeding$ | 6(21.4) | 7(16.7) | 0.616 |
| Hemoptysis | 0(0) | 2(4.8) | 0.241 |
| Conjunctivitis | 3(10.7) | 3(7.1) | 0.601 |
| Epistaxis | 0(0) | 0(0) | |
| Hematemesis | 1(3.6) | 1(2.4) | 0.770 |
| Melena | 1(3.6) | 3(7.1) | 0.528 |
| Rectal bleeding | 1(3.6) | 0(0) | 0.217 |
Data available for 64 patients. In six patients, time of symptom onset was not known.
One patient’s age was not recorded.
Individual bleeding numbers sum up greater than number of any bleeding events because some patients had more than one type of bleeding event.
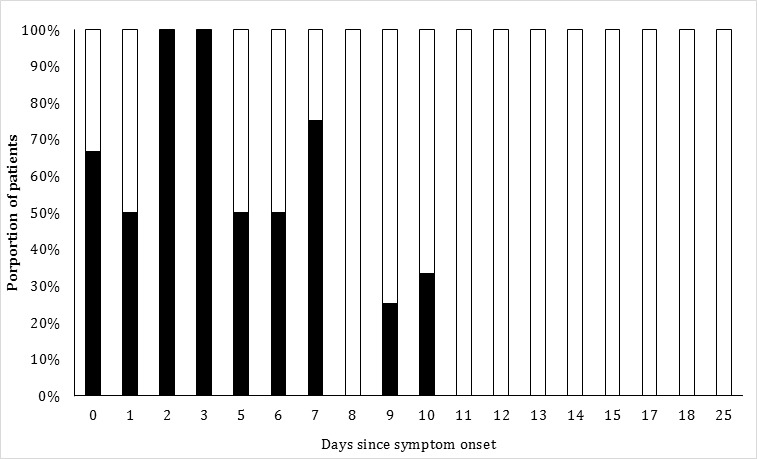
The time interval between the onset of symptoms and the outcome was not available for 6 of 70 patients. The mean interval (±SD) from symptom onset to outcome was 12.7 ± 6.2 days. The estimated 21-day survival after first day of hospitalization was 62% [SE 0.09] and 39% [SE 0.21] in patients aged <35 years and those aged ≥35 years, respectively (Figure 1). The estimated 21-day survival after first day of hospitalization was 62% [SE 0.09] and 20% [SE 0.10] in patients who developed or did not develop either hiccups or myalgias, respectively (Figure 2). The chances of survival increased as the days survived after symptom onset increased (see Figure 3). The mean time interval (±SD) from symptom onset to death was 8.9 ± 3.8. Half of all deaths occurred within 8 days and 90% of all deaths occurred within 13 days after symptom onset. The chance of survival was 64.7% in 51 patients who had survived 8 days or greater after symptom onset and 86.1% in 36 patients who had survived 12 days or greater after symptom onset.
Figure 1. Survival of patients with Ebola virus disease after first day of hospitalization according to age strata. Solid line: survival among patients aged <35 years. Dashed line: survival among those aged 35 years or greater.
Figure 2. Survival of patients with Ebola virus disease after first day of hospitalization according to the presence or absence of hiccups or myalgias. Solid line: survival among patients who did not develop either symptom. Dashed line: survival among those developed either hiccups or myalgias.
Figure 3. Proportion of patients with Ebola virus disease surviving according to time interval elapsed between symptom onset and day of discharge. Black and white components of the vertical bar represent proportion of patients who died or survived according to days past symptom onset, respectively.
Among patients aged ≥35 years, the survival was 33.3% among those who developed either hiccups or myalgias and 50% among those who did not develop either (p = 0.464). Among patients aged <35 years, the survival was 22.2% among those who developed either hiccups or myalgias and 86.7% (p <0.001) among those who did not develop either symptoms.
Discussion
The survival rate among patients with Ebola virus disease was 60% among the 70 patients admitted in the early part of the epidemic at Donka National Hospital, Guinea. The survival rate among patients with confirmed Ebola virus disease was higher than the 20% survival rate reported in Kikwit General Hospital during 1995.6 The survival at Donka National Hospital was also higher than the survival rate of 26% observed in Kenema Government Hospital in Sierra Leone between May 25, 2014, and June 18, 2014.7 Table 2 summarizes a comparison of the demographic and clinical features of the three cohorts of hospitalized patient admitted with confirmed Ebola virus disease.1 The mean age of the cohort was 34 years that was similar to the mean age of 34.7 years observed in patients admitted in Kikwit General Hospital.3 There was a higher rate of hemorrhagic complications and diarrhea seen in patients admitted in Kikwit General Hospital during the 1995 epidemic compared with patients admitted to Donka National Hospital, suggesting some difference in clinical manifestations, which may account for the differential survival. However, no such difference was evident in the demographic and clinical characteristics of patients admitted at Donka National and Kenema Government Hospitals. The clinical manifestations of fever (in 89% of the patients) and headache (in 80%) appeared more prevalent in the patient cohort from Kenema Government Hospital in Sierra Leone but symptoms, such as weakness (in 66%) and diarrhea (in 51%), were seen with equal frequency compared with Donka National Hospital.7
Table 2. Demographic and clinical characteristics of hospitalized patients with Ebola virus disease at Donka National Hospital, Conakry, Guinea (March 25, 2014, to August 5, 2014), Kenema Government Hospital, Sierra Leone (May 25, 2014, to June 18, 2014), and Kikwit General Hospital, Democratic Republic of Congo (January 6, 1995, to July 16, 1995).
| Characteristics | Donka National Hospital 2014 | Kenema Government Hospital 20147 | Kikwit General Hospital 19953 |
|---|---|---|---|
| Mean age (years) | 34 | NR | 34.7 |
| Men | 63 | 42 | 47 |
| Fever | 66 | 89 | NR |
| Headache | 50 | 80 | 73 |
| Weakness | 79 | 66 | 78 |
| Diarrhea | 43 | 51 | 74 |
| Abdominal pain | 26 | 40 | 56 |
| Vomiting | 50 | 35 | 70 |
| Conjunctivitis | 9 | 31 | 34 |
| Cough | 4 | 20 | NR |
| Rash | 1 | 3 | NR |
| Myalgia and/or arthralgia | 20 | NR | 51 |
| Anorexia | 49 | NR | 73 |
| Dyspnea | 0 | NR | 25 |
| Hiccup | 9 | NR | 14 |
| Any bleeding | 19 | NR | 40–5028 |
| Melena | 6 | NR | 14 |
| Hematemesis | 3 | NR | 13 |
Abbreviations used: NR, not recorded; all values are represented as percentages.
Variations in intensity of medical care may account for some of the observed differences in survival rates between the three cohorts of patients. A linear decrease in mortality was noted from 100% in January to 62% in June in 1995 within Kikwit General Hospital, Democratic Republic of the Congo suggesting a learning curve in patient management, which was probably not necessary at Donka National Hospital in Guinea. The last two decades have witnessed considerable advancement in medical care of sepsis and dysfunction involvement. However, targeted interventions, such as early resuscitation before the development of organ failure in severely ill patients, has resulted in an estimated 23% mortality reduction in previous studies,8 were not instituted at Donka National Hospital. There was a lack of interventions, such as early goal-directed resuscitation during the first six hours after recognition using crystalloid or colloid fluid resuscitation, vasopressors, and targeted hemoglobin levels recommended in the guidelines for management of severe sepsis and septic shock.9 Therefore, it appears unlikely that the higher survival rates in Donka National Hospital are attributed to early goal-directed resuscitation and other supportive care measures, such as provision of enteral nutrition and prevention of nosocomial infections, stress ulcers, skin breakdown, and deep venous thrombosis.10 The relatively low impact of advancements in medical care is also supported by the prominent differences in survival among contemporary cohorts in Guinea and Sierra Leone.7 Another explanation may be regional or hospital specific exposure to other pathogens, such as Lassa virus, malaria parasites, and gram-negative bacteria, in patients with Ebola virus disease,11, 12 and concurrent infections contributing to higher mortality. Evidence of altered immune regulation is evident in serum from Ebola virus disease patients that contain very low levels of circulating cytokines produced by T lymphocytes and low counts of CD3+CD4+ and CD3+CD8+ peripheral cells.13 Defective inflammatory responses and massive monocyte/macrophage activation were associated with fatal outcome in Ebola virus disease patients in two outbreaks in Gabon14 and the higher mortality in part maybe related to increased vulnerability to other infections.
The predictors of survival among Ebola virus disease patients have been investigated in previous studies. Younger age at time of infection consistently correlates with a higher survival in all studies.1,3,7 It remains unclear whether the lower survival associated with increased age is similar to the relationship seen between survival and age in sepsis from bacteremia.15, 16 However, the prominent decline in survival is seen in elderly patients (aged ≥65 years) among patients with bacteremia associated sepsis and is related to coexistence of co morbidities.15, 16 However, in Ebola virus disease patients, the mortality increased among patients aged ≥35 years suggesting other reasons for higher mortality than existence of comorbidities. T cell and B cell proliferative responses are reduced with aging resulting in a general decline in immune function, commonly known as immune senescence that contributes to the increased susceptibility to pathogens.17 Age-related decrease in interferon response through STAT1, IRF1, and IRF7 signaling following infection with West Nile Virus has been previously demonstrated.18 Dendritic cell counts decline with increasing age reducing the antigen-presenting cell populations.19 Therefore, the immune system’s ability to respond effectively to Ebola virus infection may decline with increasing age.
Absence of clinical features, such as fever, weakness, dizziness, and diarrhea, has been associated with higher survival in some studies.7 Significant risk factors for death included a number of general symptoms (diarrhea, conjunctivitis, difficulty breathing or swallowing, confusion or disorientation, and coma) and hemorrhagic symptoms (unexplained bleeding, bleeding gums, epistaxis, bleeding at the injection site, and bleeding from the vagina).1 Some of the symptoms maybe manifestation of end stage clinical deterioration, such as confusion or disorientation and coma. Absence of myalgias and hiccups was associated with higher survival in patients admitted at Donka National Hospital. Hiccups have been seen in patients with brainstem lesions,20 in patients with uremia, 21,22 and in those with gastritis or esophagitis.23 Myalgias secondary to muscle inflammation and myonecrosis is not uncommon in patients with viral infections.24–27 Acute kidney failure is a commonly associated factor in patients with virus infection who develop myalgias.24,26 Therefore, the most likely reasons for association of hiccups and myalgias with mortality may be attributed to such manifestations being a marker of severe acute kidney failure in patients with Ebola virus disease. We observed that the highest value of such symptoms in identifying patients with anticipated higher survival is among those who are aged <35 years. Interestingly, the survival chances continue to improve with each continuing day of survival after symptom onset. Approximately 90% of all deaths occurred within 13 days after symptom onset. The chance of survival was 86.1% in 36 patients who had survived 12 days or greater after symptom onset.
One of the limitations of mortality assessment based on hospitalized patients is the lack of detection of outside hospital mortality associated with Ebola virus disease and mortality associated with undiagnosed infection. The WHO Ebola response team1 calculated the case fatality rate as the percentage of fatal Ebola virus disease cases among reported cases with a known definitive clinical outcome and estimated a rate of 70.8%. The case fatality rate among hospitalized patients was 64.3% possibly attributed to exclusion of deaths in nonhospitalized patients. When case fatality rate was calculated only based on the ratio of reported deaths to reported cases, including in the denominator cases for which the clinical outcome is unknown, this resulted in an estimate of 38%. There is also a time trend, which we were not able to study that demonstrates a reduction in mortality from 71.3% before August 18 to 59.9% in the period between August 18 and September 14 in the current Ebola virus disease epidemic in West Africa.1 The small sample size provided by the single center design reduced the precision of survival estimates and limited our ability to detect smaller magnitude differences between survivors and those who died. The definitions of certain variables, such as clinical symptomology, were based on chart annotation and lacked standardized definitions. However, the single-center design also provides homogeneity in medical care and data collection and ascertainment, and thus reduced the biases introduced by differences in case definition and patient management.
Our results provide insights into a cohort of hospitalized patients with Ebola virus disease in whom survival is prominently higher than seen in other cohorts of hospitalized patients. Further studies focused on such cohorts may identify strategies to improve survival among patients with Ebola virus disease.
References
- Ebola virus disease in West Africa--the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MG, Schafer IJ. Centers for Disease C, Prevention. Ebola viral disease outbreak--West Africa, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:548–51. [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S76–86. doi: 10.1086/514306. Commission de Lutte contre les Epidemies a Kikwit. [DOI] [PubMed] [Google Scholar]
- Rothstein MA. Is deidentification sufficient to protect health privacy in research? The Am J Bioeth. 2010;10:3–11. doi: 10.1080/15265161.2010.494215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHRP - Guidance on Research Involving Coded Private Information or Biological Specimens. [Nov 2;2014 ]; Available at: http://www.hhs.gov/ohrp/policy/cdebiol.html.
- Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S177–87. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- Schieffelin JS, Shaffer JG, Goba A, et al. Clinical Illness and Outcomes in Patients with Ebola in Sierra Leone. N Engl J Med. 2014. [DOI] [PMC free article] [PubMed]
- Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–92. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–14. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- Schoepp RJ, Rossi CA, Khan SH, Goba A, Fair JN. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg Infect Dis. 2014;20:1176–82. doi: 10.3201/eid2007.131265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuels B, Wichmann D, Emmerich P, et al. A Case of Severe Ebola Virus Infection Complicated by Gram-Negative Septicemia. N Engl J Med. 2014. [DOI] [PubMed]
- Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baize S, Leroy EM, Georges AJ, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–8. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello M, Trecarichi EM, Caira M, et al. Derivation and validation of a scoring system to identify patients with bacteremia and hematological malignancies at higher risk for mortality. PloS one. 2012;7:e51612. doi: 10.1371/journal.pone.0051612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta JE, Joshi KP, Beeson L, Nguyen HB. Patient and hospital characteristics associated with inpatient severe sepsis mortality in California, 2005-2010. Crit Care Med. 2012;40:2960–6. doi: 10.1097/CCM.0b013e31825bc92f. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Vomaske J, Totonchy T, et al. Chikungunya virus infection results in higher and persistent viral replication in aged rhesus macaques due to defects in anti-viral immunity. PLoS Negl Trop Dis. 2013;7:e2343. doi: 10.1371/journal.pntd.0002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Delroux K, Wang X, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–23. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–21. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Z, Tsuchiya K, Uchihara T, et al. Intractable hiccup caused by medulla oblongata lesions: a study of an autopsy patient with possible neuromyelitis optica. J Neurol Sci. 2009;285:241–5. doi: 10.1016/j.jns.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Krahn A, Penner SB. Use of baclofen for intractable hiccups in uremia. Am J Med. 1994;96:391. doi: 10.1016/0002-9343(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Chang JM, Hwang SJ, Kuo HT, et al. Fatal outcome after ingestion of star fruit (Averrhoa carambola) in uremic patients. Am J Kidney Dis. 2000;35:189–93. doi: 10.1016/s0272-6386(00)70325-8. [DOI] [PubMed] [Google Scholar]
- Launois S, Bizec JL, Whitelaw WA, Cabane J, Derenne JP. Hiccup in adults: an overview. Eur Respir J. 1993;6:563–75. [PubMed] [Google Scholar]
- Huang SY, Lee IK, Liu JW, Kung CT, Wang L. Clinical Features of and Risk Factors for Rhabdomyolysis Among Adult Patients with Dengue Virus Infection. Am J Trop Med Hyg. 2014. [DOI] [PMC free article] [PubMed]
- Geissler AL, Thorp E, Van Houten C, et al. Infection with colorado tick Fever virus among humans and ticks in a national park and forest, wyoming, 2010. Vector Borne Zoonotic dis. 2014;14:675–80. doi: 10.1089/vbz.2013.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latus J, Kitterer D, Kimmel M, Alscher MD, Braun N. The case: fever, myalgia, visual disorders, and acute kidney failure in a pregnant woman. Diagnosis: Severe acute hantavirus infection. Kidney Int. 2013;84:629–31. doi: 10.1038/ki.2013.49. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Yamakawa T, Nagasawa H, et al. Epidemic myalgia associated with human parechovirus type 3 infection among adults occurs during an outbreak among children: findings from Yamagata, Japan, in 2011. J Clin Virol. 2013;58:188–93. doi: 10.1016/j.jcv.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]




