ABSTRACT
Dynamic reciprocity (DR) refers to the ongoing, bidirectional interaction between cells and their microenvironment, specifically the extracellular matrix (ECM). The continuous remodeling of the ECM exerts mechanical force on cells and modifies biochemical mediators near the cell membrane, thereby initiating cell-signaling cascades that produce changes in gene expression and cell behavior. Cellular changes, in turn, affect the composition and organization of ECM components. These continuous interactions are the fundamental principle behind DR, and its critical role throughout development and adult tissue homeostasis has been extensively investigated. While DR in the mammary gland has been well described, we provide direct evidence that similar dynamic interactions occur in other areas of reproductive biology as well. In order to establish the importance of DR in the adaptive functioning of the female reproductive tract, we present our most current understanding of DR in reproductive tissues, exploring the mammary gland, ovary, and uterus. In addition to explaining normal physiological function, investigating DR may shed new light into pathologic processes that occur in these tissues and provide an exciting opportunity for novel therapeutic intervention.
Keywords: breast, dynamic reciprocity (DR), extracellular matrix (ECM), fibroids, folliculogenesis, mechanotransduction (MT), ovary, ovulation, pathogenesis, uterine leiomyoma
INTRODUCTION
Throughout each reproductive cycle, as well as throughout life, the female reproductive system undergoes extensive and dynamic structural remodeling [1–4]. There are complex biochemical signals that initiate and regulate remodeling that affect both the extracellular matrix (ECM) and the cell, resulting in tissue organization. This impressive dynamism is achieved by changes in the ECM that lead to mechanotransduction (MT), the cellular processes that translate mechanical stimuli into biochemical signals, as well as soluble biochemical signaling through hormones and cytokines, allowing cells to adapt to their changing physical environment. These rapid, transient cell-cell and cell-matrix interactions are bidirectional and referred to as dynamic reciprocity (DR) [5–7]. Mammoto and Ingber [8] as well as Mammoto et al. [9] substantiated this concept in developmental biology, proving the role of mechanical force is as critical as biochemical signaling in embryogenesis, thereby transforming how we view the extracellular environment. Mechanical signals are relayed through the membrane and cytoskeleton to the nucleus by integrins, cell adhesion molecules, cytoskeletal filaments, and signaling cascades resulting in changes in gene transcription and chromatin remodeling [10]. Furthermore, cell-cell and cell-matrix interactions create intracellular contractile forces that place the cell in a state of tension and can act to modify cell form and function [11]. In this manner, cellular mechanochemical processes and changes in the ECM microenvironment govern tissue morphogenesis and adult organ homeostasis.
Bissell and colleagues [5, 12, 13] extrapolated a role for DR in reproductive biology by studying normal mammary gland development and the progression of cell events leading to malignancy. In addition, a number of earlier studies indicated that stretching uterine tissues induced protein synthesis and changes in cellular function and was significant in parturition, validating a role for mechanical signaling in these tissues and demonstrating how it contributed to cell form and behavior [14–16]. More recently, there has been extraordinary expansion in the field of matrix biology that has led to new insights into DR and reproduction [9, 17–20]. Biochemical signaling alone is not sufficient to explain the complexities that occur in development and function of the breast, ovary, and uterus, and there is now persuasive evidence for the critical role of mechanical signaling in these tissues [21, 22]. Through further advancement and integration of our understanding of mechanochemical transduction events, we can gain valuable insights into both normal and tumorigenic behavior of cells, tissues, and organs and develop effective interventions for reproductive tract functions and disease. Toward this goal, this is the first review to focus on the significance of DR in the reproductive system. An overview of MT, a concept critical to appreciating DR, will be discussed first. We next explore the mammary gland where a more comprehensive picture of DR has been demonstrated and highlight those studies that have contributed most to our appreciation of this concept. We then review other reproductive organs where an appreciation of DR is emerging, focusing on the processes of folliculogenesis, ovulation, and uterine fibroids (leiomyomas).
THE PROCESS OF MT
In the broader field of cell biology, Ingber and colleagues [23, 24] described the importance of the cell cytoskeleton in mechanical signaling and developed the concept of tensegrity (tensorial integrity) to describe how this signaling exhibits the properties of an integrated system. They demonstrated that mechanical strain placed on integrins, located in the cell membrane, immediately changed the shape and organization of the nuclei, demonstrating a MT process dependent on intermediate filaments and microfilaments [25, 26]. Integrins are not static proteins but are expressed transiently on the cell surface in a dynamic fashion and are involved in bidirectional signaling from the cell to its microenvironment [27, 28]. Mechanical forces drive tissue homeostasis through cell-cell junctions and cell-matrix adhesions mediated by integrins [8]. These forces can be quite small, resulting in compression or stretch, external to the cell, such as movement or gravity or generated through changes in cell contractility or shape. Force changes are sensed or perceived by the cells as signals (transduction) that produce changes in intracellular biochemistry and ultimately gene expression and chromatin remodeling [9].
Many molecular pathways are signaled by the mechanical forces exerted on the cell. Force transmitted across cell surfaces by integrins is relayed to the cell cytoskeleton by focal adhesions, an anchoring complex that functions as a mechanochemical-signaling center [26]. This signaling complex can transmit both internal and external forces and will assemble or separate depending on the presence of stress [29, 30]. Other integrin interacting-signaling molecules, including TRPV4 and talin, undergo conformational changes to mediate downstream signals [31, 32]. Mechanical stress acting through focal adhesion kinase (FAK), a nonreceptor kinase in the cytoplasm, activates the mitogen-activated protein kinase (MAPK) pathway, leading to upregulation of collagen type I and other critical ECM proteins that are involved in matrix composition and remodeling [33–35]. Annexin A2, a multifunctional bridging protein, conducts bidirectional informational flow and is regulated by changes in the ECM and intracellular calcium flux [36]. Thus MT, one aspect of DR, is a dynamic process with many critical signaling factors responding to mechanical force that play a role in the cell. In this manner, all components of the system, both biomechanical and biochemical, and not merely one paramount molecule, influence cell behavior (Fig. 1A).
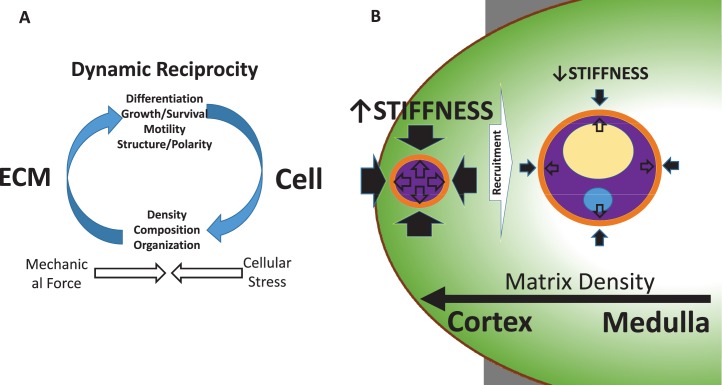
FIG. 1.
A) Schematic diagram of the bidirectional interaction between the cell and its environment, specifically the ECM, demonstrating the concept of DR. Mechanical force from the ECM is sensed by the cell and leads to changes in cell structure and function. These changes, in combination with mechanical signaling, can alter gene expression and epigenetic remodeling of the cell nucleus, leading to changes in ECM content, composition, and organization and an overall remodeling of the matrix. Cells sense the mechanical force and counter it with intracellular contractile tension, creating cellular stress. In this manner, force-generated mechanochemical signaling affects both the cell and its environment. B) An example of DR in folliculogenesis. Mechanical forces (closed arrows) in the stiff outer ovarian cortex act on the primordial follicle, contributing to its quiescent state. Tensional forces (open arrows) within the follicle counter this. Recruitment to the more pliable inner medulla relieves the mechanical strain and permits the follicle to proceed through folliculogenesis. Factors that determine progression from the cortex to the medulla are unknown. Arrow size is proportional to the amount of perceived force.
DYNAMIC RECIPROCITY IN THE MAMMARY GLAND: INSIGHTS INTO NORMAL ANATOMY AND PHYSIOLOGY
The mammary gland is an excellent model organ for the study of how ECM remodeling contributes to tissue morphogenesis and functional differentiation [37, 38]. The gland's function is regulated by reproductive hormones during pubertal development and again in gestation and parturition. Then during lactation and involution, the mammary gland undergoes cycles of branching and formation of acini (also called alveoli). In addition, the surrounding epithelium and myoepithelium ECM is subject to continual assembly and degradation. A cell's cytoskeleton mediates a dynamic and reciprocal integration of tissue architecture and function as well as directs mammary gland development, tissue polarity, and tissue-specific gene expression. Pathology and abnormal development occurs when this interaction is dysregulated [19].
The mammary gland is a complex organ with many cell types from fibroblasts to adipocytes to epithelial cells. The epithelial cells are characterized as being of two types: luminal epithelial cells and myoepithelial cells, both embedded in an interstitial collagen network [38]. These epithelial cells form a branching network of ducts terminating in many spherical small lobules called acini. Polarized luminal epithelial cells generate a continuous layer lining each duct and acini and will eventually make and secrete milk proteins. The basal layer of luminal epithelium is composed of a discontinuous layer of myoepithelial cells and a thin basement membrane consisting of laminin [38], structural proteins that connect to cytoskeletal filaments and the nucleus through intermediate filaments and microfilaments [25].
During mammary gland development, ECM proteins are tightly regulated and expressed [39, 40]. In addition to laminin, collagen, entactin, and proteoglycans constitute the basal lamina and establish cell polarity in acini differentiation [41]. Collagen type I is observed along mammary ducts while collagen type IV and the laminins type I and type V are expressed around acini [42, 43]. Upon weaning, involution results in degradation and remodeling of these proteins and leads to a decrease in milk protein production [44]. Fibronectin content increases during ductal morphogenesis as well as expression of the fibronectin binding α5β1-integrin in mammary epithelial cells [45, 46]. Loss of fibronectin expression prevents proper gland development [47]. Fiber alignment is also important for appropriate development. Spatially aligned collagen fibers are observed in the terminal end buds prior to fat pad invasion, and a recent study using live cell imaging demonstrated how fiber alignment can affect epithelial cell morphology [48–50].
Remodeling of the external environment requires proper spatiotemporal expression of matrix metalloproteinases (MMPs) [51]. During development, MMP-2 plays a role in the initial invasion of epithelial cells into the stromal fat pad and suppresses lateral ductal branching [52]. In contrast, MMP-3 promotes branching and MMP-3 knockout mice revealed a diminished branching pattern compared to wild type [52]. MMP-14 is highly expressed in the terminal end bud and may assist MMP-2 in ductal invasion [53, 54]. Interestingly, in three-dimensional (3D) culture, there was higher recruitment activity of MMP-14 in a stiffer collagen environment, demonstrating cell-matrix cross talk; furthermore, MMP-14 may activate MAPK signaling [53, 54]. In addition, MMP activity can directly affect intracellular signaling by producing degradation fragments of ECM proteins that act as growth modulators, for example, EGFR is activated by laminin-5 following cleavage by MMP-2 [55].
In the human breast, integrins play major roles in development and function as well as in cancer progression [17, 41, 56, 57]. The β1-integrin, in conjunction with prolactin signaling, is necessary for mammary cell differentiation [58, 59]. The α6β4-integrin associates with hemi-desmosomes linking the plasma membrane with intracellular intermediate filaments that form a network along the basolateral aspect of the cells, establishing cell polarity [60, 61]. The α6β4-integrin has further been demonstrated to affect matrix remodeling by promoting SPARC expression [62]. In addition, both β1 and α6β4 have also been linked to β-casein production [63]. Loss of β4 signaling has been shown to lead to an increase in apoptosis, demonstrating a role in cell survival. In support of this, certain integrins, including α2, may also serve as tumor suppressors, and levels of the α2β1-integrin are reduced in aggressive breast cancers [64].
Obviously, estrogen and progesterone are key players in mammary gland development. Investigations in transgenic mice clearly show that the estrogen receptor (ER) is necessary for elongation of the mammary ducts during puberty [65]. Other studies demonstrate that the progesterone receptor (PR) is required for the growth of acini [66]. In the adult rodent and in human glands, the distribution of ER and PR fluctuates due to changes in estrogen and progesterone during reproductive cycles, pregnancy, lactation as well as age [67]. ER is present in the mammary gland in both isoforms, ERα and ERβ [68]. Basement membrane laminin type 1 and collagen type IV are involved in the maintenance of ERα expression, and in malignant breast cells this becomes disrupted, and the cells are no longer responsive to the ECM [69].
Over the last several decades, scientists have studied the interactions between the mammary cells and the ECM in 3D cultures that successfully mimic the in situ mammary gland [70–72]. When grown on 3D gels, murine and human epithelial cells are able to form aggregates and reorganize into structures of morphologically polarized cells that form acini-like hollow spheres surrounded by basal lamina [73, 74]. This apical-basal polarity is established by the ECM component laminin [75]. The presence of fibronectin can stimulate epithelial cell proliferation and increase acini size [76–78]. Strikingly, in the presence of lactogenic hormones, these mammary cells secrete caseins into the lumina [73, 74]. Furthermore, these interactions can occur in the absence of surrounding myoepithelial cells [70]. In contrast, cells grown in 2D monolayers or 3D collagen cultures without lactogenic hormones do not form acini-like structures and fail to secrete milk proteins [72, 79]. Interestingly, β-casein expression was also inhibited when cells were grown on stiffer gel substrates [75]. In addition, prolactin signaling is not enough to sustain milk protein production without the interaction of a laminin substrate binding the β1-integrin, allowing the necessary chromatin remodeling needed for tissue-specific gene expression [75, 80, 81]. Taken together, these studies validate that the ECM can direct tissue polarity and morphogenesis and even affect gene expression and nuclear remodeling. This provides unequivocal evidence that the ECM and the surrounding cells behave as a unit and firmly demonstrates the ECM and its cellular interactions are necessary for mammary gland development and function, substantiating a role for DR in the breast.
CONTRIBUTION OF DR TO MAMMARY TUMORIGENESIS
Dynamic reciprocity typically functions to maintain homeostasis in adult cells; however, an imbalance in the mechanochemical-signaling network can lead to tumorigenesis. Carcinogenesis in breast correlates with collagen cross-linking and ECM stiffening, creating a firm tumor. This creates mechanical force that the cell senses through integrin activity, leading to focal adhesion formation and activation of RhoA that ultimately alters gene expression patterns and can induce tumor invasion [82, 83]. Mammary cells grown in 3D culture on a stiff collagen matrix lose their normal cell polarity, increase proliferation, and adopt an invasive phenotype [77, 84]. Also, an increase in cellular tension by Rho-mediated cellular contractility leads to changes in matrix content and organization [85, 86]. Interestingly, inhibiting the β1-integrin reversed this phenotype [61, 87]. When lysyl oxidase, the collagen cross-linking enzyme is inhibited, metastatic potential of circulating breast cells was reduced [82]. Increased tumor stiffness led to activation of the micro-RNA miR-18a, which decreased levels of HOXA9-dependent PTEN transcription and promoted the malignant phenotype [88]. In breast cancer biopsies, miR-18a expression was correlated with increasing ECM tumor stiffness and inversely related to levels of PTEN and HOXA9 [88]. Therefore, the tumor matrix may have a profound effect on tumor cell behavior and provides an intriguing therapeutic target to prevent metastasis.
Remarkably, multicellular tissues are capable of biopolymer reorganization by mechanical signals and create very long, highly directional fibers, such as collagen lines, that may influence the location and time of tumor invasion [89–91]. Cultured mammary acini that have disrupted architecture will interconnect by forming these long collagen lines that somehow coordinate and even accelerate a transition to an invasive phenotype [92]. When investigators isolated these acini by laser cuts in the 3D culture, effectively disrupting the collagen lines, the acini reverted to a less invasive cell type [92]. Therefore, pairs and groups of acini can interact mechanically through the collagen matrix, and this matrix, in turn, can influence cell behavior.
MMP activity is linked with breast cancer invasion and metastasis and associated with a poor prognosis [93]. In dense matrices, MMPs will cleave fibers around integrin attachment sites allowing space for cell motility [94, 95]. Aberrant expression of MMP-3 prevented normal cell differentiation and led to adoption of an invasive phenotype [96, 97]. The integrin α3β1 has been linked to MMP-9 activity [98]. Induction of MMP-9 through increased activity of the Raf/MEK/ERK pathway led to targeted degradation of laminin type 1, destroying the basement membrane [99]. This resulted in altered tissue polarity and growth, leading the cells to exhibit a cancer phenotype. This phenotype was reversed by the inhibition of MMP-9 or MEK, and an increase in laminin type 1 was noted in a murine xenograft model [99]. Therefore, cell-generated destruction of the ECM, potentially through integrin activation by the matrix itself, leads to distorted tissue polarity and cell proliferation, mimicking an invasive cancer phenotype. When tissue architecture is continuously perturbed, mammary epithelial cells produce reactive oxygen species and ultimately undergo an epithelial-to-mesenchymal transition [100].
PTEN, a known tumor suppressor gene, colocalizes with the E-cadherin/β-catenin complex in 3D culture and supports acini formation [101]. E-cadherin-blocking antibodies reduce endogenous PTEN protein levels and inhibit cell-cell contact accumulation leading to a loss of cell polarity and growth control [101]. The addition of exogenous E-cadherin to cancer cells lacking the protein induced PTEN expression, supporting a role for cell-cell signaling in mammary gland homeostasis [101].
HoxA1 has been identified as a candidate gene that may serve as a possible driver of early breast cancer, confirmed by its overexpression in human breast lesions [102]. Delivery of lipidoid nanoparticles containing HoxA1 siRNA through the nipple to mice with breast tumors led to a decrease in tumor formation, and silencing HoxA1 within the mammary ducts in vivo led to a loss of hormonal expression and suppressed cell proliferation [102]. Strikingly, this phenomenon does not occur when the gene is injected directly into the tumor, suggesting that the dynamic interaction is locally and spatially mediated [102]. In summary, the studies highlighted here strongly demonstrate the role of DR in the mammary gland and that this interaction is responsible for maintaining development and function of the tissue. Also, when the DR-signaling exchange is altered, it can disrupt these homeostatic mechanisms and lead to a progression toward malignancy
DYNAMIC RECIPROCITY IN FOLLICULOGENESIS
There is increasing evidence that the ovarian ECM plays a critical role in follicle development. Primordial (dormant) follicles are localized to the collagen-rich ovarian cortex, which offers a rigid physical environment that supports follicular architecture and increases survival [103]. On the other hand, the rigidity of the cortical ECM limits expansion of the follicle and consequently oocyte maturation, maintaining the follicle in its quiescent state [104]. Throughout a woman's reproductive lifespan, a subset of follicles is recruited each cycle and enters the growing follicle pool. As a follicle migrates to the medulla of the ovary, it encounters a softer, more pliant ECM. This permits the follicle to expand and resume its development. Thus, changes in the stiffness of the ovarian ECM from cortex to medulla directly affect follicular cell behavior (Fig. 1B). The importance of ECM stiffness in folliculogenesis has been shown using in vitro models that recreate the complex ovarian microenvironment by using interpenetrating networks of fibrin and alginate with dynamic, cell-responsive mechanical properties [105]. Whereas older alginate-only hydrogels, which are nondegradable, became too rigid to support follicle growth as the follicle expanded (essentially sequestering the follicle in a cortex-like environment), the fibrin component in fibrin and alginate hydrogels degrades over time, softening the matrix and mimicking, in a temporal fashion, the spatial migration of a follicle from the stiff ovarian cortex to the soft medulla [106].
At the same time, these gels offer an excellent example of DR between matrix and cell. Follicular cells themselves produce their own ECM components, which are incorporated into the alginate scaffold. Additionally, the process of fibrin degradation is driven by soluble factors released by granulosa and thecal stromal cells, notably plasminogen activator [107, 108] and connective tissue growth factor (CTGF) [109]. Furthermore, physical fragmentation of ovaries from juvenile mice promoted follicle growth and led to the formation of mature oocytes through disruption of the Hippo-signaling pathway [110]. Remarkably, women with primary ovarian insufficiency who underwent ovarian fragmentation, Akt stimulation treatment, and autologous transplantation of the remnant tissue generated mature oocytes following in vitro fertilization methods [110]. In one patient, a live birth was achieved. It may be that fragmentation relieves the inhibition of the stiff matrix forces, allowing the residual follicles to develop. In agreement with this model, anovulatory women with polycystic ovary syndrome have increased number of follicles held quiescent in the ovarian cortex, which is stiffer and contains more collagen compared to normal ovaries [111].
There is also evidence that follicular fluid in follicles is accumulated by the osmotic forces of hyaluronan and versican, which are glycoproteins produced by granulosa cells [112]. Granulosa cells cultured in a 3D environment of collagen type I with leukemia-inhibiting factor were successfully transplanted back into the ovaries of immunodeficient mice and preferentially localized within antral follicles [113]. Thus, the growing understanding of the importance of DR in follicular development has already begun to be translated into advances in tissue bioengineering, with important implications for the field of fertility preservation.
DYNAMIC RECIPROCITY, CYTOKINES, AND OVULATION
Extensive matrix remodeling is paramount for the oocyte to proceed through folliculogenesis, ovulation, and development of a highly vascularized corpus luteum. Ovulation, the expulsion of an egg from a mature follicle, is triggered by the luteinizing hormone (LH) surge, which in turn stimulates morphological changes in the follicle that ultimately result in rupture. Follicular rupture is caused in part by decreased tensile strength at the follicular apex due to degradation of collagen fibers and in part by changes in intrafollicular pressure that facilitate rupture of the weakened follicular wall [114, 115].
The LH surge induces follicular cells to synthesize and secrete proteolytic enzymes, including MMPs, plasminogen activators and plasmin, and ADAMTS [116–120]. Under the influence of these mediators, the ECMs of the tunica albuginea and theca externa, as well as the basement membrane separating the granulosa and thecal cell layers, become fragmented and disorganized as collagen disintegrates. In mice, the process is similar with elevated levels of ECM components (laminin, collagen, perlecan, nidogens) in the basal lamina of developing follicles and corpora lutea, with collagen type IV being the predominant form at all stages of development [121]. Expression of matrix proteins, including HAPLN1, is driven by the LH surge, and deficiencies in key matrix proteins in the cumulus-oocyte complex reduce ovulation rates [122–126]. Mice lacking ADAMTS1 fail to cleave versican, an ECM proteoglycan, and demonstrate reduced rates of ovulation and fertilization as a result of impaired tissue remodeling [127]. Additionally, degradation of ECM by secreted plasmins and MMPs liberates ECM-bound proteins, including TNF-α and TIMP-3 [128]. The cytokine TNF-α resides at the interface of the cell and the ECM and may promote collagen fibril breakdown as well as apoptosis of ovarian superficial epithelial cells in the apical region of the preovulatory follicle [129]. Tissue inhibitors of metalloproteinase (TIMPs) counter the remodeling actions of MMPs and form a delicate balance of remodeling and maintenance [119]. This is a prime example of DR, whereby the cells orchestrate the disintegration of the matrix and in doing so, release cytokines and inhibitors that feed back to further modify cell behavior.
The final signal in ovulation may be endothelin-2 (EDN-2), which mediates smooth muscle cell (SMC) contraction. EDN-2 is transiently expressed in granulosa cells immediately prior to ovulation and is able to reach the SMC layer by diffusing across the thecal layer following carefully timed degradation of the thecal ECM [130, 131]. Contraction of individual SMCs results in follicular constriction, which increases follicular pressure and generates tension in the follicle wall. Eventually, the follicle ruptures at the apex where the tensile force is weakest due to the lack of SMCs and low structural integrity. The complex, back-and-forth choreography thus played out between soluble mediators, mechanical forces, matrix proteins, and shifting fluids is at the core of DR.
DYNAMIC RECIPROCITY IN UTERINE FIBROID GROWTH AND DEVELOPMENT
The hallmark of uterine fibroids is excessive ECM production and cell proliferation. The fibrotic matrix creates a stiff extracellular environment exerting mechanical force on surrounding cells. Transduction of this force ultimately results in a variety of cell responses, including cytoskeletal rearrangement, cell contraction, growth, and gene expression changes, including genes involved in ECM composition, all affecting how the fibroid cells interact with the extracellular environment. Therefore, as we begin to unravel the complexities of fibroid development and function, it has become clear that DR may underlie its growth and development.
The alteration of the ECM is altered compared to the surrounding myometrium is well described [22, 132]. Microarray analysis demonstrated that fibroids have elevated levels of genes involved in ECM formation, including collagen, proteoglycans, and elastin that are associated with growth [133, 134]. Furthermore, there is downregulation of other key ECM proteins, such as dermatopontin [135]. Electron microscopy analysis demonstrated that collagen fibrils are increased, loosely packed, and arranged in a nonparallel manner, not appreciated in nearby myometrium (Fig. 2A) [136]. The excessive matrix deposition and disorganization creates the fibrotic nature of this tissue, leading to an environment of increased mechanical force. TGFβ3, a known promoter of ECM production, is increased in fibroid tissue and also contributes to formation of its abnormal environment [137]. In addition, key proteins known to interact with TGFβ3, including dermatopontin and thrombospondin, have altered expression in fibroids compared to myometrium that may lead to increased TGFβ3 activity [135, 138].
FIG. 2.
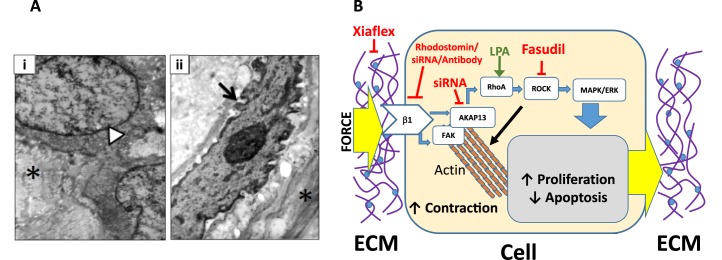
A) Electron microscopy images of myometrial and fibroid tissue. i) Normal myometrial SMC with a large, smooth nucleus (white arrow head), surrounded by tightly packed, organized collagen fibrils (asterisk) seen in cross-section. Original magnification ×11 500 (adjusted to magnification ×15 500 for image). ii) Image of a myofibroblast from a uterine fibroid. Note the angular and notched nucleus (black arrow) and condensed chromatin within the nucleus. Extracellular matrix features disordered collagen fibrils in the fibroid tissue (asterisk). Magnification ×15 500. B) A schematic representation of DR in fibroid cells. Mechanical stress is sensed by the fibroid cell that internalizes the signal and changes how the cell interacts with the ECM, altering the composition and organization of the external microenvironment. Integrin β1 signals the stiffness of the ECM, leading to activation of downstream-signaling events. FAK initiates actin polymerization resulting in cell contraction. AKAP13 activates RhoA, which in turn interacts with ROCK and activates the MAPK/ERK-signaling cascade, resulting in changes in cell proliferation, decreased apoptosis, and upregulation of genes involved in ECM composition and remodeling. Agents that perturb/stimulate the pathway are also listed (see text for explanation).
Expression patterns of integrins are critical to understanding how the cell responds to its altered environment. Integrin β1 is overexpressed in fibroid cells and plays a role in determining cell shape and proliferation [139, 140]. Decreased β1 activity is demonstrated to alter cytoskeletal integrity, inhibit cell spreading, and decrease growth. Also, disrupting β1 signaling leads to decreased activity of downstream proteins RhoA and ERK, demonstrating that integrin signals through the Rho family GTP-signaling proteins [140]. Integrin α11 is also elevated in fibroid cells and may play a role in myofibroblast differentiation [141]. Fibroid-derived myofibroblasts contribute to production of the excessive ECM microenvironment and stimulate leiomyoma cell proliferation [135, 142, 143].
Fibroid cells recognize their stiff microenvironment through the process of MT; however, compared to myometrial cell, fibroid cells have a defective perception of mechanical stress and are unable to respond to mechanical cues [144, 145]. The Rho-signaling cascade plays a role in the MT response. Levels of active RhoA and AKAP13, which activates RhoA, are increased in fibroid cells compared to myometrial cells. Inhibition of AKAP13 through siRNA inactivation while simultaneously treatment with lysophosphatidic acid, a known promoter of RhoA, was shown to lead to decreased levels of RhoA compared to myometrial cells [146]. Inhibition with Fasudil of Rho kinase (ROCK), a downstream target of RhoA, led to relaxed contraction of fibroid cells in 3D collagen gels [147]. In addition, fibroid cells plated on a stiff collagen substrate resembling the fibroid microenvironment demonstrated increased phosphorylation of FAK, decreased levels of p21, and activation of the MAPK-signaling pathway, leading to increased proliferation and altered cytoskeletal organization [148]. Disruption of the dense collagen matrix of fibroid cells with Xiaflex, a collagenase, results in relaxation of the cell and adoption of a phenotype similar to myometrial cells [149]. Furthermore, Fasudil treatment led to a decrease in ECM gene transcripts known to contribute to the fibroid fibrotic environment [147]. Thus, mechanical stress is sensed by the fibroid cell and leads to downstream activation of mechanical-signaling pathways that ultimately affect cell shape and contractility, promote growth, and direct changes in the ECM environment itself (Fig. 2B). This is a clear demonstration of the bidirectional interaction between the fibroid cell and its microenvironment that defines DR.
In addition to mechanical stress, fibroid cells are subjected to osmotic stress and have increased fluid content relative to the myometrium [150, 151]. The Rho-GEF Brx (AKAP13), previously described above in mechanical signaling, plays a key role in transducing the osmotic response through the transcription factor NFAT5, including upregulating osmolarity response genes [152]. Fibroid cells have increased NFAT5 expression compared to nearby myometrium and demonstrate increased expression of hyperosmolarity genes when exposed to osmotic stress [153]. The cellular osmotic response results in fluid exchange between the cell and ECM, thus affecting both cell shape and ECM composition, demonstrating another example of DR [154].
CONCLUSION
This minireview of DR and its impact on the physiologic functioning of female reproductive organs highlights the dynamic state between the cell and its surrounding ECM, leading to cyclic changes in tissue development that characterize the reproductive tract. The concept of DR allows us to avoid the popular emphasis of one molecule or one gene to explain these processes and utilizes a flexible approach that takes into account the signaling interactions that lead to changes in cell shape, tissue architecture, and the microenvironment. While DR in the mammary gland has been the most extensively explored, we provide direct evidence that similar dynamic interactions occur in other parts of the female reproductive tract as well. Our aim in writing this review was to emphasize the critical role of DR in reproduction and stimulate interest toward investigation of this concept in reproductive biology. Studies reviewed here demonstrate the exciting potential of this research to translate into the clinical realm, including fertility preservation in women with primary ovarian insufficiency or definitive management of uterine fibroids without relying on surgical intervention. Therefore, in addition to explaining normal physiological function, exploring DR may shed new light into the pathologic processes that occur in these tissues and provide an inspiring opportunity for novel therapeutic intervention. This pursuit may have powerful implications in the field of reproductive health.
ACKNOWLEDGMENT
The authors acknowledge Dr. Avner Hershlag for his support of T.R.S. and Drs. Amy Johnson and John Nulsen for their support of J.T.T. The authors further acknowledge the assistance of Dr. Mones Abu-Asab for work with electron microscopy
Footnotes
Supported by CRTP training NIH program (to S.C. and S.J.) and partial HD-008737-11 (to J.H.S.).
REFERENCES
- Leppert PC. Tissue remodeling in the female reproductive tract—a complex process becomes more complex: the role of Hox genes Biol Reprod 2012. 86 98. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Yu SY. Apoptosis in the cervix of pregnant rats in association with cervical softening. Gynecol Obstet Invest. 1994;37:150–154. doi: 10.1159/000292546. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Leppert PC, Yu SY. Cell death and proliferation and its relation to collagen degradation in uterine involution of rat. Connect Tissue Res. 1998;37:163–175. doi: 10.3109/03008209809002436. [DOI] [PubMed] [Google Scholar]
- Woessner JF, Brewer TH. Formation and breakdown of collagen and elastic in the human uterus during pregnancy and postpartum involution. Biochem J. 1963;89:75–82. doi: 10.1042/bj0890075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, Sage H. Synthesis and Secretion of Structural Macromolecules by Endothelial Cells in Culture. In: Nossel HL, Vogel HJ, editors. Pathobiology of the Endothelial Cell, vol. 6. New York: Academic Press; 1982. pp. 215–228. In: [Google Scholar]
- Sage H. Collagens of basement membranes J Invest Dermatol 1982. 79 (Suppl 1): 51s 59s [DOI] [PubMed] [Google Scholar]
- Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol. 2013;29:27–61. doi: 10.1146/annurev-cellbio-101512-122340. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125:3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. The riddle of morphogenesis: a question of solution chemistry or molecular cell engineering? Cell. 1993;75:1249–1252. doi: 10.1016/0092-8674(93)90612-t. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol. 1998;10:640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csapo A, Erdos T, De Mattos CR, Gramss E, Moscowitz C. Stretch-induced uterine growth, protein synthesis and function. Nature. 1965;207:1378–1379. doi: 10.1038/2071378a0. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Lloyd-Jacob MA. Effect of uterine volume on parturition. Am J Obstet Gynecol. 1963;85:806–812. doi: 10.1016/s0002-9378(16)35540-5. [DOI] [PubMed] [Google Scholar]
- de Mattos CE, Kempson RL, Erdos T, Csapo A. Stretch-induced myometrial hypertrophy. Fertil Steril. 1967;18:545–556. doi: 10.1016/s0015-0282(16)36373-7. [DOI] [PubMed] [Google Scholar]
- Gehler S, Ponik SM, Riching KM, Keely PJ. Bi-directional signaling: extracellular matrix and integrin regulation of breast tumor progression. Crit Rev Eukaryot Gene Expr. 2013;23:139–157. doi: 10.1615/critreveukargeneexpr.2013006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G, Davidson J, Kirsner R, Bornstein P, Herman I. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge S, Chang S, Barzilai JJ, Leppert P, Segars JH. Mechanical signaling in reproductive tissues: mechanisms and importance. Reprod Sci. 2014;21:1093–1107. doi: 10.1177/1933719114542023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids Obstet Gynecol Int 2014. 2014 783289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems Rep Prog Phys 2014. 77 046603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Bershadsky A, Henis YI, Geiger B. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci. 2011;124:1425–1432. doi: 10.1242/jcs.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Jeon YM, Lee HJ, Kim JG, Baek JA, Lee JC. Activation of RhoA and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol Cell Biochem. 2010;335:263–272. doi: 10.1007/s11010-009-0276-1. [DOI] [PubMed] [Google Scholar]
- Jeon YM, Kook SH, Son YO, Kim EM, Park SS, Kim JG, Lee JC. Role of MAPK in mechanical force-induced up-regulation of type I collagen and osteopontin in human gingival fibroblasts. Mol Cell Biochem. 2009;320:45–52. doi: 10.1007/s11010-008-9897-z. [DOI] [PubMed] [Google Scholar]
- Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC. Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J Cell Biochem. 2009;106:1060–1067. doi: 10.1002/jcb.22085. [DOI] [PubMed] [Google Scholar]
- Hitchcock JK, Katz AA, Schafer G. Dynamic reciprocity: the role of annexin A2 in tissue integrity. J Cell Commun Signal. 2014;8:125–133. doi: 10.1007/s12079-014-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel KL, Weaver VM, Bissell MJ. Structural cues from the tissue microenvironment are essential determinants of the human mammary epithelial cell phenotype. J Mammary Gland Biol Neoplasia. 1998;3:201–213. doi: 10.1023/a:1018751124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression Cold Spring Harb Perspect Biol 2011. 3 a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer VA, Xu R, Bissell MJ. Gene expression in the third dimension: the ECM-nucleus connection. J Mammary Gland Biol Neoplasia. 2010;15:65–71. doi: 10.1007/s10911-010-9163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely PJ. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. J Mammary Gland Biol Neoplasia. 2011;16:205–219. doi: 10.1007/s10911-011-9226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–225. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Woodward TL. Reciprocal regulation of extracellular matrix proteins and ovarian steroid activity in the mammary gland. Breast Cancer Res. 2001;3:365–372. doi: 10.1186/bcr324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TL, Mienaltowski AS, Modi RR, Bennett JM, Haslam SZ. Fibronectin and the alpha(5)beta(1) integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology. 2001;142:3214–3222. doi: 10.1210/endo.142.7.8273. [DOI] [PubMed] [Google Scholar]
- Liu K, Cheng L, Flesken-Nikitin A, Huang L, Nikitin AY, Pauli BU. Conditional knockout of fibronectin abrogates mouse mammary gland lobuloalveolar differentiation. Dev Biol. 2010;346:11–24. doi: 10.1016/j.ydbio.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher DA, Bissell MJ. Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr Biol. 2013;23:703–709. doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- Barnes C, Speroni L, Quinn KP, Montevil M, Saetzler K, Bode-Animashaun G, McKerr G, Georgakoudi I, Downes CS, Sonnenschein C, Howard CV, Soto AM. From single cells to tissues: interactions between the matrix and human breast cells in real time PLoS One 2014. 9 e93325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Mori H, Ghajar CM, Brownfield D, Galgoczy R, Bissell MJ. Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr Biol (Camb) 2011;3:1153–1166. doi: 10.1039/c1ib00073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD, Nelson CM, Chen CS, Zhang H, Bascom JL, Seiki M, Bissell MJ. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin beta1. Development. 2013;140:343–352. doi: 10.1242/dev.084236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan DS, DiPersio M. Integrin control of tumor invasion. Crit Rev Eukaryot Gene Expr. 2012;22:309–324. doi: 10.1615/critreveukargeneexpr.v22.i4.50. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Streuli CH. How integrins control breast biology. Curr Opin Cell Biol. 2013;25:633–641. doi: 10.1016/j.ceb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubiane GS, Valentijn A, Lowe ET, Akhtar N, Bagley S, Gilmore AP, Streuli CH. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci. 2004;117:271–280. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HHF, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha 6 beta 4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson KD, Shearstone JR, Maddula VS, Seligmann BE, Mercurio AM. Integrin beta4 regulates SPARC protein to promote invasion. J Biol Chem. 2012;287:9835–9844. doi: 10.1074/jbc.M111.317727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell. 1999;10:2817–2828. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O'Rear LD, Nashabi A, Li Z, Dupont WD, Zijlstra A, Zutter MM. The alpha(2)beta(1) integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest. 2011;121:226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80:137–148. doi: 10.1016/s0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci U S A. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaro V, Radisky DC, Ramos Castro NE, Weisz A, Bissell MJ. Malignant mammary cells acquire independence from extracellular context for regulation of estrogen receptor alpha. Clin Cancer Res. 2004;10:402s–409s. doi: 10.1158/1078-0432.ccr-031209. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Bilder D. Polarity determination in breast tissue: desmosomal adhesion, myoepithelial cells, and laminin 1. Breast Cancer Res. 2003;5:117–119. doi: 10.1186/bcr579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci U S A. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression BMC Med 2008. 6 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JP, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, Mossi R, Pujuguet P, Hager G, Bissell MJ. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol. 2009;184:57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen YY, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareva A, Downey CM, Ayres F, Liu W, Boyd SK, Hallgrimsson B, Jirik FR. The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells PLoS One 2009. 4 e5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CL, Ouyang M, Yu JY, Maslov J, Price A, Shen CY. Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc Natl Acad Sci U S A. 2012;109:5576–5582. doi: 10.1073/pnas.1114781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion BMC Med 2006. 4 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Ghosh RP, Engelke H, Rycroft CH, Cassereau L, Sethian JA, Weaver VM, Liphardt JT. Rapid disorganization of mechanically interacting systems of mammary acini. Proc Natl Acad Sci U S A. 2014;111:658–663. doi: 10.1073/pnas.1311312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev. 2009;28:129–135. doi: 10.1007/s10555-008-9174-3. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–342. [PubMed] [Google Scholar]
- Beliveau A, Mott JD, Lo A, Chen EI, Koller AA, Yaswen P, Muschler J, Bissell MJ. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 2010;24:2800–2811. doi: 10.1101/gad.1990410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier MV, Fata JE, Martin KJ, Yaswen P, Bissell MJ. Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res. 2009;69:4545–4552. doi: 10.1158/0008-5472.CAN-08-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock A, Krause S, Li H, Kowalski M, Goldberg MS, Collins JJ, Ingber DE. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice Sci Transl Med 2014. 6 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPolt PS, Yamoto M, Veljkovic M, Sincich C, Ny T, Tsafriri A, Hsueh AJ. Basic fibroblast growth factor induction of granulosa cell tissue-type plasminogen activator expression and oocyte maturation: potential role as a paracrine ovarian hormone. Endocrinology. 1990;127:2357–2363. doi: 10.1210/endo-127-5-2357. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Lu JK. Effects of aging on luteinizing hormone secretion, ovulation, and ovarian tissue-type plasminogen activator expression. Exp Biol Med (Maywood) 2001;226:127–132. doi: 10.1177/153537020122600210. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Hillier SG. Connective tissue growth factor in the ovarian paracrine system. Mol Cell Endocrinol. 2002;187:23–27. doi: 10.1016/s0303-7207(01)00702-x. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.”. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Irving-Rodgers HF. The roles of the ovarian extracellular matrix in fertility. Soc Reprod Fertil Suppl. 2010;67:217–230. doi: 10.7313/upo9781907284991.019. [DOI] [PubMed] [Google Scholar]
- Kossowska-Tomaszczuk K, Pelczar P, Guven S, Kowalski J, Volpi E, De Geyter C, Scherberich AA. Novel three-dimensional culture system allows prolonged culture of functional human granulosa cells and mimics the ovarian environment. Tissue Eng Part A. 2010;16:2063–2073. doi: 10.1089/ten.TEA.2009.0684. [DOI] [PubMed] [Google Scholar]
- Matousek M, Carati C, Gannon B, Brännström M. Novel method for intrafollicular pressure measurements in the rat ovary: increased intrafollicular pressure after hCG stimulation. Reproduction. 2001;121:307–314. doi: 10.1530/rep.0.1210307. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Woessner JF, Jr, Koos RD, Sear CH, LeMaire WJ. Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology. 1988;122:1715–1721. doi: 10.1210/endo-122-5-1715. [DOI] [PubMed] [Google Scholar]
- Curry TE, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24:228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- Rae MT, Price D, Harlow CR, Critchley HO, Hillier SG. Glucocorticoid receptor-mediated regulation of MMP9 gene expression in human ovarian surface epithelial cells. Fertil Steril. 2009;92:703–708. doi: 10.1016/j.fertnstert.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Cooke R, Nothnick W, Komar C, Burns P, Curry TE., Jr. Collagenase and gelatinase messenger ribonucleic acid expression and activity during follicular development in the rat ovary. Biol Reprod. 1999;61:1309–1316. doi: 10.1095/biolreprod61.5.1309. [DOI] [PubMed] [Google Scholar]
- Curry T, Osteen K. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Liu YX, Liu XM, Nin LF, Shi L, Chen SR. Serine protease and ovarian paracrine factors in regulation of ovulation. Front Biosci (Landmark Ed) 2013;18:650–664. doi: 10.2741/4128. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Hummitzsch K, Murdiyarso LS, Bonner WM, Sado Y, Ninomiya Y, Couchman JR, Sorokin LM, Rodgers RJ. Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice. Cell Tissue Res. 2010;339:613–624. doi: 10.1007/s00441-009-0905-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- Liu J, Park ES, Curry TE, Jr, Jo M. Periovulatory expression of hyaluronan and proteoglycan link protein 1 (Hapln1) in the rat ovary: hormonal regulation and potential function. Mol Endocrinol. 2010;24:1203–1217. doi: 10.1210/me.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H, Miyazaki J. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–1160. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276:7693–7696. doi: 10.1074/jbc.C000899200. [DOI] [PubMed] [Google Scholar]
- Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83:549–557. doi: 10.1095/biolreprod.110.084434. [DOI] [PubMed] [Google Scholar]
- Rosewell KL, Li F, Puttabyatappa M, Akin JW, Brannstrom M, Curry TE., Jr. Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle Biol Reprod 2013. 89 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res. 2003;44:50–57. [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, Jeoung M, Ko C. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010;22:780–787. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Risinger JI, Chandramouli GV, Bushel PR, Baird DD, Peddada SD. Gene expression in uterine leiomyoma from tumors likely to be growing (from black women over 35) and tumors likely to be non-growing (from white women over 35) PLoS One 2013. 8 e63909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium Fertil Steril 2004. 82 (Suppl 3): 1182 1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera MA, Feng L, Yonish B, Catherino W, Jung SH, Leppert P. Thrombospondin-1 and thrombospondin-2 mRNA and TSP-1 and TSP-2 protein expression in uterine fibroids and correlation to the genes COL1A1 and COL3A1 and to the collagen cross-link hydroxyproline. Reprod Sci. 2007;14:63–76. doi: 10.1177/1933719107309591. [DOI] [PubMed] [Google Scholar]
- Chen HM, Lin YH, Cheng YM, Wing LY, Tsai SJ. Overexpression of integrin-beta1 in leiomyoma promotes cell spreading and proliferation. J Clin Endocrinol Metab. 2013;98:E837–E846. doi: 10.1210/jc.2012-3647. [DOI] [PubMed] [Google Scholar]
- Malik M, Segars J, Catherino WH. Integrin beta1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012;31:389–397. doi: 10.1016/j.matbio.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey M, Salleh N, Leppert P. Collagen-binding alpha11 integrin expression in human myometrium and fibroids utilizing a novel RNA in situ probe. Reprod Sci. 2014;21:1139–1144. doi: 10.1177/1933719114522548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, Catherino WH, Segars JH. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, Korecki C, Iatridis J, Catherino WH, Tuan RS, Dhillon N, Leppert P, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:e471–e411. doi: 10.1016/j.ajog.2007.11.057. 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CM, Norian J, Korecki C, Taboas J, Tuan RS, Segars JH. Leiomyoma cells do not properly sense or respond to mechanical cues Fertil Steril 2009. 92 (suppl): Abstract O-155. [Google Scholar]
- Malik M, Britten J, Segars J, Catherino WH. Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fasudil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci. 2014;21:1126–1138. doi: 10.1177/1933719114545240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohestani F, Braundmeier AG, Mahdian A, Seo J, Bi J, Nowak RA. Extracellular matrix collagen alters cell proliferation and cell cycle progression of human uterine leiomyoma smooth muscle cells PLoS One 2013. 8 e75844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunengraber LN, Jayes FL, Leppert PC. Injectable clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Repro Sci. 2014;21:1452–1459. doi: 10.1177/1933719114553449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem FA, Predanic M. The hemodynamic effect of GnRH agonist therapy on uterine leiomyoma vascularity: a prospective study using transvaginal color Doppler sonography. Gynecol Endocrinol. 1995;9:253–258. doi: 10.3109/09513599509160454. [DOI] [PubMed] [Google Scholar]
- Okuda S, Oshio K, Shinmoto H, Tanimoto A, Asada H, Fujii T, Yoshimura Y, Kuribayashi S. Semiquantitative assessment of MR imaging in prediction of efficacy of gonadotropin-releasing hormone agonist for volume reduction of uterine leiomyoma: initial experience. Radiology. 2008;248:917–924. doi: 10.1148/radiol.2483071288. [DOI] [PubMed] [Google Scholar]
- Kino T, Takatori H, Manoli I, Wang Y, Tiulpakov A, Blackman MR, Su YA, Chrousos GP, DeCherney AH, Segars JH. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5 Sci Signal 2009. 2 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Keith DM, Malik M, Britten J, Segars J, Catherino WH. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril. 2011;95:2383–2387. doi: 10.1016/j.fertnstert.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Mechanotransduction of fluid stresses governs 3D cell migration. Proc Natl Acad Sci U S A. 2014;111:2447–2452. doi: 10.1073/pnas.1316848111. [DOI] [PMC free article] [PubMed] [Google Scholar]