ABSTRACT
The oocyte-to-zygote transition entails transforming a highly differentiated oocyte into totipotent blastomeres and represents one of the earliest obstacles that must be successfully hurdled for continued development. Degradation of maternal mRNAs, which likely lies at the heart of this transition, is characterized by a transition from mRNA stability to instability during oocyte maturation. Although phosphorylation of the oocyte-specific RNA-binding protein MSY2 during maturation is implicated in making maternal mRNAs more susceptible to degradation, mechanisms underlying mRNA degradation during oocyte maturation remain poorly understood. We report that DCP1A and DCP2, proteins responsible for decapping mRNA, are encoded by maternal mRNAs recruited for translation during maturation via cytoplasmic polyadenylation elements located in their 3′ untranslated regions. Both DCP1A and DCP2 are phosphorylated during maturation, with CDC2A being the kinase likely responsible for both, although MAPK may be involved in DCP1A phosphorylation. Inhibiting accumulation of DCP1A and DCP2 by RNA interference or morpholinos decreases not only degradation of mRNAs during meiotic maturation but also transcription of the zygotic genome. The results indicate that maternally recruited DCP1A and DCP2 are critical players in the transition from mRNA stability to instability during meiotic maturation and that proper maternal mRNA degradation must be successful to execute the oocyte-to-zygote transition.
Keywords: gamete biology, maternal mRNA degradation, meiotic maturation, mRNA decapping, oocyte maturation
INTRODUCTION
The balance between mRNA synthesis and mRNA turnover determines steady-state mRNA levels [1]. Regulation of mRNA degradation is gaining increased attention, because the process is highly regulated and therefore has significant impact on the overall pattern of gene expression [1]. In eukaryotic cells, functional mRNAs have a 5′ cap structure and a 3′ poly(A) tail that control translation and mRNA stability. Messenger RNA degradation usually involves deadenylation of the 3′ poly(A) tail, in which the CCR4-NOT complex plays a central role [2]; mRNAs with very short poly(A) tails are degraded either from the 5′ or 3′ end [3].
Removal of the 5′-monomethyl guanosine cap (decapping) is the critical step in the 5′→3′ mRNA degradation pathway. Decapping exposes mRNAs to exonucleases (e.g., XRN1) that rapidly degrade the mRNA from the 5′ end. The decapping complex was first identified in yeast, in which Dcp2p possesses catalytic activity and Dcp1p is a regulatory unit that stimulates Dcp2p decapping activity [4–6]. The mammalian orthologs of Dcp2 and Dcp1 are Dcp2 and Dcp1a/Dcp1b [7–9]. Although mammalian DCP2 possesses decapping activity, the ability of mammalian DCP1A/B to stimulate directly the decapping activity of DCP2 has not been demonstrated [7, 8, 10]. Several other proteins in the decapping complex also modulate decapping activities and have mammalian orthologs, including the Lsm1-7 complex, Dhh1 (also known in mammals as Ddx6/Rck/p54), Edc3, and Edc4 [1, 11–13].
Stockpiles of mRNAs are synthesized and accumulated during oocyte growth and serve as the maternal contribution that supports early embryo development before zygotic genome activation. Maternal mRNAs in mouse oocytes are unusually stable during the growth phase, which takes approximately 2.5 wk, with an average half-life of approximately 10–14 days, as compared to hours or minutes in somatic cells [14–17]. The oocyte-to-zygote transition entails coordinate removal of the maternal transcriptome and its replacement with a zygotic transcriptome. Oocyte maturation (i.e., resumption of meiosis) triggers a transition from mRNA stability to instability, in which many maternal mRNAs are extensively degraded [14, 18]. Consensus is growing that degradation of maternal mRNA is essential for the oocyte-to-zygote transition [19, 20] that entails transforming a highly differentiated oocyte into totipotent blastomeres. Maternal mRNA degradation accelerates loss of oocyte identity and facilitates a totipotent identity of blastomeres.
The transition to mRNA instability is facilitated by MSY2, an abundant, germ cell-specific RNA-binding protein that mediates global mRNA stability in mouse oocytes [21–23]. During oocyte maturation, MSY2 is phosphorylated by CDC2A, a consequence being that maternal mRNAs become more accessible to the oocyte's mRNA decay machinery [22]. It is unclear, however, whether the mRNA decay machinery becomes activated during meiotic maturation and, if so, how it targets maternal transcripts.
While studying the role of P-bodies in mRNA degradation in mouse oocytes, we noted that the abundance of DCP1A protein substantially increases during oocyte maturation [24]. This finding prompted us to examine how posttranscriptional control of the mRNA decay machinery regulates the transition from maternal mRNA stability to instability. We then reported that Dcp1a and Dcp2 are maternal mRNAs that are recruited during maturation via cytoplasmic polyadenylation elements (CPEs) [25–27], thus providing a mechanism for induction of maternal mRNA degradation. Inhibiting the maturation-associated increase in DCP1A and DCP2 protein not only prevents degradation of a large population of maternal mRNAs but also affects activation of the embryonic genome during the 2-cell stage.
MATERIALS AND METHODS
Mouse Oocyte/Egg/Embryo Collection, Cell Culture, and Microinjection
Full-grown, germinal vesicle (GV)-intact oocytes as well as metaphase II (MII) eggs, fertilized eggs, and 2-cell embryos were collected as previously described [28]. GV oocytes were cultured in Chatot Ziomek Brinster (CZB) medium containing 2.5 μM milrinone (Sigma) to inhibit GV breakdown (GVBD) [29]; MII eggs were cultured in CZB medium [30] and fertilized eggs in KSOM medium [31]. Metaphase I (MI) eggs were collected 6 h after transferring oocytes to milrinone-free CZB medium, and 2-cell embryos were isolated by flushing oviducts of superovulated and mated mice 42–44 h post-human chorionic gonadotropin injection. All animal experiments were conducted at the University of Pennsylvania, were approved by the Institutional Animal Use and Care Committee, and were consistent with National Institutes of Health (NIH) guidelines.
The GV oocytes were microinjected with approximately 5 pl of either short interfering RNAs (siRNAs) or morpholinos in bicarbonate-free Whitten medium [32] supplemented with 10 mM Hepes, 0.01% polyvinyl alcohol, and 2.5 μM milrinone as previously described [33]. The cRNA for Flag-Dcp1a, Flag-Dcp2, or luciferase reporter cRNA were injected at 0.5 μg/μl. The Dcp1a siRNA was 25 μM, and that of Dcp2 was 50 μM. When morpholinos were injected, the concentration was 1 mM.
DNA Constructs
To generate a luciferase reporter with a Dcp1a or Dcp2 3′ untranslated region (UTR), firefly luciferase coding sequence was excised from pGL4.10 (Promega) by a XhoI and XbaI double-digestion and inserted into pIVT vector containing T7 and T3 promoters in tandem at the 5′ flanking region, followed by a Xenopus β-globin 5′ UTR and a multiple cloning site (plasmid map is available upon request) to generate pIVT-Luc. The last 0.5-kb Dcp1a 3′ UTR with a poly(A) site was amplified using the primers (forward primers are listed first and reverse primers are listed second throughout) 5′-TACTCTAGAAAGGCCACTCACGAGGAGAGTT-3′ and 5′-CGTGAATTCTAGGTGCTAAAACTTGGTT-3′, and Dcp2 3′ UTR (NM_027490.1) was amplified by PCR using the primers 5′-TACTCTAGATGCTTGGGCACAGTTACTGCT-3′ and 5′-CGTGAATTCTTTATTTGGTTGTCTTCACATACAGC-3′. Amplified Dcp1a or Dcp2 3′ UTRs were digested by XbaI and EcoRI and inserted downstream of the coding sequence of the pIVT-Luc vector. pIVT-Luc with mouse Ccnb1 3′ UTR was a kind gift from Dr. Shin Murai (Toho University, Japan).
To generate luciferase reporter/Dcp1a or Dcp2 3′ UTR with mutated CPEs, pairs of oligonucleotides were synthesized with the desired mutated sequences, annealed, extended by Klenow 3′→5′ exonuclease, digested by EcoRI and XbaI, and inserted into EcoRI/XbaI-digested pIVT-Luc.
The following oligonucleotides were used in the construction of these pIVT/Dcp1a 3′ UTR variants: 5′-GGCTCTAGAAGCATTTTTCATCCTAAATTTTATATGTTTGCAAATATATTTTTTTAA-3′ and 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTAAAAAAATATATTTGCAAACAT-3′ to generate pIVT-Luc with shortened version of wild-type Dcp1a 3′ UTR; 5′-GGCTCTAGAAGCATTTTTCATCCTAAATTTTATATGTTTGCAAATATATTTTTTTAA-3′ and 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTCTTAAAAAAATATATTTGCAAACAT-3′ to generate to generate pIVT-Luc/Dcp1a 3′ UTR/mutated poly(A) signal (AAUAAA-to-AAGAAA mutation); 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTAAAAAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTGGCATCCTAAATTTTATATGTTTGCAAATATATTTTTTTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE1 (UUUUUCAU-to-UUUGGCAU mutation); 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTAAAAAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTTTCATCCTAAATTGGATATGTTTGCAAATATATTTTTTTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE2 (UUUUUAA-to-UUGGAUA mutation); 5′-GCTCTAGAAGCATTTTTCATCCTAAATTTTATATGTTTGCAAATATATTTGGGTAA-3′ and 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTACCCAAATATATTTGCAAACAT-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE3 (UUUUUUUAAU-to-UUUGGGUAAU mutation); 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTAAAAAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTGGCATCCTAAATTGGATATGTTTGCAAATATATTTTTTTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE1+2; 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTACCCAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTTTCATCCTAAATTGGATATGTTTGCAAATATATTTGGGTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE2+3; 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTACCCAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTGGCATCCTAAATTTTATATGTTTGCAAATATATTTGGGTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE1+3; and 5′-ACTGAATTCCTAAAACTTGGTTTTGAAATTTTATTACCCAAATATATTTGCAAACAT-3′ and 5′-GGCTCTAGAAGCATTTGGCATCCTAAATTGGATATGTTTGCAAATATATTTGGGTAA-3′ to generate pIVT-Luc/Dcp1a 3′ UTR/mutated CPE1+2+3.
The following oligonucleotides were used in the construction of these pIVT/Dcp2 3′ UTR variants: 5′-AGCTCTAGAGTTTAGACTTCTTGAAACTTTTTTTCCTGTTTTCAGTAGAGAAACTTCTGTTTATGCTGTA-3′ and 5′-TCCGAATTCTTCTCATTTATCATCAATTTTTATTTGGTTGTCTTCACATACAGCATAAACAGAAGTTT-3′ to generate pIVT-Luc with shortened version of wild-type Dcp2 3′ UTR; 5′-AGCTCTAGAGTTTAGACTTCTTGAAACTTTTTTTCCTGTTTTCAGTAGAGAAACTTCTGTTTATGCTGTA-3′ and 5′-TCCGAATTCTTCTCATTTATCATCAATTTTTCTTTGGTTGTCTTCACATACAGCATAAACAGAAGTTT-3′ to generate pIVT-Luc/Dcp2 3′ UTR/mutated poly(A) signal (AAUAAA-to-AAGAAA mutation); 5′-TCCGAATTCTTCTCATTTATCATCAATTTTTATTTGGTTGTCTTCACATACAGCATAAACAGAAGTTT-3′ and 5′-AGCTCTAGAGTTTAGACTTCTTGAAACTTGGGTTCCTGTTTTCAGTAGAGAAACTTCTGTTTATGCTGTA-3′ to generate pIVT-Luc/Dcp2 3′ UTR/mutated CPE1 (UUUUUUU-to-UUGGGUU mutation); 5′-AGCTCTAGAGTTTAGACTTCTTGAAACTTTTTTTCCTGTTTTCAGTAGAGAAACTTCTGTGGATGCTGTA-3′ and 5′-TCCGAATTCTTCTCATTTATCATCAATTTTTATTTGGTTGTCTTCACATACAGCATCCACAGAAGTTT-3′ to generate pIVT-Luc/Dcp2 3′ UTR/mutated CPE2 (UUUAU-to-UGGAU mutation); and 5′-TCCGAATTCTTCTCATTTATCATCAATTTTTATTTGGTTGTCTTCACATACAGCATCCACAGAAGTTT-3′ and 5′-AGCTCTAGAGTTTAGACTTCTTGAAACTTGGGTTCCTGTTTTCAGTAGAGAAACTTCTGTGGATGCTGTA-3′ to generate pIVT-Luc/Dcp2 3′ UTR/mutated CPE1+2.
To generate a Flag-tagged Dcp1a cRNA, mouse Dcp1a coding region was amplified from mouse cDNA clone MC201914 (OriGene) by PCR using the primers 5′- GAATGCGGCCGCGATGGCACTTTCCTGTTCCACAGT-3′ and 5′-GATCGTCGACGTCATAGGTTGTGGTTGTCT-3′. After enzymatic digestion by NotI and SalI, the amplified Dcp1a coding sequence was fused to pIVT-3xflag vector to make pIVT-3xflag-Dcp1a. Flag-tagged Dcp2 cRNA was generated by same approach.
In Vitro Transcription
The constructs (Flag-Dcp1a, Flag-Dcp2, and pIVT-Luc/Dcp1a or pIVT-Luc/Dcp2 3′ UTR) were verified by DNA sequencing and then linearized by EcoRI digestion. Capped cRNAs were made using in vitro transcription with T7 mMESSAGE mMachine (Ambion) according to the manufacturer's instruction. The 3xflag-tagged Dcp1a and Dcp2 cRNAs were polyadenylated using a Poly(A) Tailing Kit (Ambion) according to the manufacturer's instructions. Following in vitro transcription, template DNAs were digested by adding RNase-free DNase, and synthesized cRNA was purified by MEGAclear Kit (Ambion), precipitated, and redissolved in RNase-free water. A single mRNA band of the expected size was observed for each RNA sample on 1% formaldehyde denaturing agarose gel. Synthesized RNA was aliquoted and stored at −80°C.
For microinjection controls, Renilla luciferase based vector phRL-SV40 (Promega) was linearized by NotI, in vitro transcribed by T7, and polyadenylated by Poly(A) Tailing Kit following the manufacturer's instruction. A poly(A) tail (∼150 bp) was added to the 3′ terminal of synthesized Renilla Luc mRNA after polyadenylation as estimated by electrophoresis on 1% denaturing agarose gel.
Immunocytochemistry and Immunoblotting
Oocyte, egg, or embryo samples were fixed in 2.5% paraformaldehyde for 40 min at room temperature. The cells were then permeabilized for 15 min in PBS containing 0.2% Triton X-100, blocked in PBS containing 0.2% immunoglobulin G-free bovine serum albumin and 0.01% Tween-20 for 30 min (blocking solution), and then incubated with the primary antibody for 1 h at room temperature. The following antibodies/antisera were used at the following dilutions: rabbit anti-DCP1A antisera (a kind gift from Jens Lykke-Anderson, University of Colorado) at 1:150 dilution, rabbit anti-DCP2 (kindly provided by Dr. Megerditch Kiledjian, Rutgers University) at 1:150 dilution, and rabbit anti-trimethyl-Histone H3 (lys4) (H3K4me3; Millipore) at 1:300 dilution. After four 15-min washes in blocking medium, samples were incubated for 1 h with the appropriate cy5-conjugated secondary antibody (Jackson ImmunoResearch) diluted 1:100 in blocking solution. After an additional three 15-min washes in blocking solution, the samples were mounted in the VECTASHIELD solution containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories). Images were captured by a Leica TCS SP laser-scanning confocal microscope. For each experiment, all samples were processed in parallel, and the intensity of fluorescence was quantified using ImageJ software (NIH). Antibody specificity for DCP1A and DCP2 was verified by immunofluorescence (see Fig. 5A).
For immunoblotting, equal numbers of GV oocytes, MI eggs, MII eggs, fertilized eggs, and 2-cell embryos were lysed in SDS loading buffer [34], run in a 10% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane (Amersham). The following antibodies were used: rabbit anti-DCP1A antisera at 1:3000 dilution and rabbit anti-DCP2 antisera at 1:2000 dilution. Immunodetection was performed using horseradish peroxidase-conjugated secondary antibodies and ECL Advance reagents (Amersham) according to the manufacturer's instructions. As a loading control, membranes were stripped and reprobed with either a mouse anti-β-tubulin (TUBB) antibody (Sigma) at 1:20 000 dilution or a mouse anti-β-actin (ACTB) antibody (Sigma) at 1:10 000. Mobility shift detection of phosphorylated DCP2 was performed by phosphate affinity SDS-PAGE using Acrylamide-pendant Phos-tag (http://www.phos-tag.com/english/shouh/pt_ms_e_ver2.pdf).
Protein Kinase Assays
The CDC2A and mitogen-activated protein kinase (MAPK) activities were measured using histone H1 and myelin basic protein, respectively, as substrates as previously described [35].
Treatment of MII Protein Extract with Lambda Phosphatase
The MII-arrested eggs or oocytes injected with Flag-Dcp2 cRNA and then eggs matured in vitro to MII were subjected to two rounds of rapid freezing and thawing. The lysate was then treated by Lambda Protein Phosphatase (New England Biolabs) following the manufacturer's instructions.
Treatment of Oocytes and MII Eggs with Roscovitine or U0126
To identify the kinases involved in mouse DCP1A and DCP2 phosphorylation, oocytes were first injected with either a Flag-Dcp1a or Flag-Dcp2 cRNA and then matured in vitro in CZM medium until they reached MII, at which time the MII eggs were transferred to CZB medium containing 60 μM roscovitine or 10 μM U0126. The MII eggs were then cultured without an oil overlay at 37°C in an atmosphere of 5% CO2 in air for 6 h before being collected for immunoblot analysis.
RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated from 20–50 oocytes/eggs/embryos using an RNAqueous-Micro Kit (Ambion) and reverse transcribed with Superscript II Reverse Transcriptase (Invitrogen) using random hexamers as primers. The cDNA was quantified by quantitative real-time PCR (qPCR) using an ABI Prism 7000 thermocycler (Applied Biosystems). The ABI TaqMan Assay-on-Demand probe/primer sets used were Mm00460131_m1 for Dcp1a, Mm01264059_m1 for Dcp2, Mm00510343_m1* for Ppil3, Mm00457212_m1* for Upp1, Mm01300991_g1 for Rpl10a, Mm02601635_g1* for Rpl28, Mm04213524_gH for Rpl18a, Mm01198491_g1* for Rpl6, Mm00523063_m1* for Ndufs8, and Mm00838506_g1* for Mrpl22. PCR conditions were 40 cycles of 95°C for 15 sec and 60°C for 60 sec. Each transcript was analyzed in three independent samples except for the experiments assaying for Ppil3 and Upp1 transcripts, which were performed on two independent samples. Before RNA isolation, each sample was spiked with 2.5 ng of Gfp cRNA, and quantification was normalized to that of the spiked Gfp cRNA using the comparative cycle threshold method (ABI Prism 7700 Sequence Detection System).
Luciferase Reporter Assay
Full-grown GV oocytes were microinjected with approximately 5 pl of the mixture of pIVT-firefly Luc cRNA with Dcp1a or Dcp2 3′ UTR variants (0.5 μg/μl) and control Renilla Luc cRNA (0.075 μg/μl). Injected oocytes were transferred to milrinone-free medium and matured in vitro for 18 h. Controls were injected GV oocytes cultured for 18 h in CZB containing 2.5 μM milrinone. Luciferase activity was assayed by lysing oocytes/eggs in 1× passive lysis buffer and analyzed using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. For signal normalization, that firefly/Renilla luciferase activity readout from noninjected oocytes/eggs was subtracted as background, and firefly luciferase activity was then normalized to that of the coinjected Renilla luciferase reporter.
Small Interfering RNA-Mediated Knockdown of Dcp1a and Dcp2 and Microarray Analysis
Full-grown GV oocytes were injected with Dcp1a siRNA (On-TARGETplus SMARTpool L-065144-01; Dharmacon) or Dcp2 siRNA (On-TARGETplus SMARTpool L-040353-01; Dharmacon) as described above. Dcp1a and Dcp2 double-knockdown was achieved by injecting a mixture of the above siRNAs. Microinjected oocytes were cultured in CZB with milrinone for 20 h and then transferred to milrinone-free medium and allowed to mature to MII for 18 h, and total RNA was extracted from 30 eggs using a Pico-Pure RNA Isolation Kit (Applied Biosystems) and amplified for microarray analysis as previously described [36]. Microarray analyses were performed on three independent samples obtained from Dcp1a siRNA-injected, Dcp2 siRNA-injected, Dcp1a and Dcp2 siRNA-injected, and scrambled siRNA-injected control samples. Each sample was generated from 20 injected oocytes. Samples were hybridized to Affymetrix MOE430 2.0 GeneChips and processed according to Affymetrix instructions. Raw microarray data were analyzed as previously described [36] and were deposited in the Gene Expression Omnibus database (GSE27049).
Inhibition of Maturation-Associated Increase in DCP1A and DCP2 by Morpholinos
Full-grown GV oocytes were microinjected with approximately 5 pl of 1 mM Dcp1a morpholino or Dcp2 morpholino (GeneTools) or a mixture of Dcp1a and Dcp2 morpholino in bicarbonate-free Whitten medium supplemented with 10 mM Hepes, 0.01% polyvinyl alcohol, and 2.5 μM milrinone. A morpholino with a scrambled sequence served as the control. Following microinjection, oocytes were cultured in CZB medium with 2.5 μM milrinone for 3 h before in vitro maturation to MII in CZB medium without milrinone. The MII eggs were activated with 5 mM SrCl2 in modified CZB containing 2 mM ethyleneglycoltetra-acetic acid and 5 μg/ml of cytochalasin B for 6 h [37] and then cultured in KSOM at 37°C in 5% CO2 in air.
Global Transcription Assay
Global transcription was assayed by Click-iT RNA Imaging Kit (Invitrogen) according to the manufacturer's instruction. Briefly, 2-cell embryos were cultured with 2 mM 5-ethynyl uridine (EU) in CZB medium for 1 h before fixation in 3.7% paraformaldehyde for 1 h at room temperature. After washing and membrane permeabilization, incorporated EU was detected using the Click-iT detection molecule (Invitrogen) and visualized by confocal microscopy.
Statistical Analysis
One-way ANOVA were used to evaluate the differences between groups using Microsoft Excel software. A level of P < 0.05 was considered to be significant.
RESULTS
DCP1A and DCP2 Are Encoded by Dormant Maternal mRNAs and Phosphorylated During Maturation
Consistent with our previous finding that the amount of DCP1A increases during oocyte maturation [24], we found that although DCP1A and DCP2 were barely detectable in full-grown oocytes, their amounts increased dramatically during meiotic maturation (Fig. 1, A and B) as detected by either immunocytochemistry or immunoblotting. Oocytes apparently do not express Dcp1b [36, 38]. Interestingly, an increase in the amount of DCP2 was observed before DPC1A, with DCP2 being readily detected by MI whereas the bulk of DCP1A accumulation occurred after meiosis I.
FIG. 1.
Maturation-associated increase of DCP1A and DCP2 protein and relative transcript abundance. A) Immunofluorescent analysis of decapping complex components DCP1A and DCP2 in full-grown GV oocytes (GV), MI eggs, and MII eggs. Bar = 25 μm. B) Immunoblot analysis of decapping complex components DCP1A and DCP2 during oocyte maturation and early embryonic development. The blot was stripped and reprobed with TUBB antibody as a loading control. The experiment was conducted two times, and similar results were obtained in each case. C) Temporal expression profile of Dcp1a and Dcp2 transcripts during oocyte maturation and preimplantation embryogenesis. Relative abundance of Dcp1a and Dcp2 transcripts was determined by qPCR. 1C, 1-cell embryo; 2C, 2-cell embryo; 8C, 8-cell embryo. The experiment was performed three times, and data are expressed as the mean ± SEM.
In contrast to the protein levels, the relative amounts of Dcp1a and Dcp2 transcripts were little changed during the transition of full-grown oocytes to 1-cell embryos but were negligible in 2-cell and 8-cell embryos (Fig. 1C). This expression pattern was also observed in our microarray experiments, which showed that neither Dcp1a nor Dcp2 is expressed during zygotic genome activation [38, 39]. These data imply that maternally recruited DCP1A and DCP2 function not only during meiotic maturation but are also the source of decapping activity in the preimplantation embryo, because the maternal mRNAs are degraded and not replaced by zygotic transcripts during this period of time.
It should be noted that both DCP1A and DCP2 showed a reduced electrophoretic mobility in MII when compared to GV-intact oocytes and 1-cell and 2-cell embryos (Fig. 2). The shift in electrophoretic mobility was readily detected for DCP1A using normal conditions for gel electrophoresis, whereas including Phospho-tag in the gel greatly enhanced the ability to detect the shift for DCP2. In addition, a cRNA encoding Flag-Dcp2 was injected to study DCP2 phosphorylation, because at least 400 oocytes were required to detect endogenous DCP2.
FIG. 2.
Maturation-associated phosphorylation of DCP1A and DCP2. A) Immunoblot analysis of changes in electrophoretic mobility of DCP1A and DCP2 during oocyte maturation and early embryonic development. For DCP1A, 50 oocytes/eggs/embryos were loaded for each lane, and the blot was stripped and reprobed with TUBB antibody as a loading control. For DCP2, Flag-DCP2 was analyzed instead of endogenous DCP2, because large numbers of oocytes/eggs (n = 400) were required to detect endogenous DCP2. Fifty oocytes/eggs/embryos were loaded for each lane, and the blot was stripped and reprobed with TUBB antibody as a loading control. The experiment was conducted three times, and representative immunoblots are shown. GV, GV-intact oocyte; MI, MI egg; MII, MII egg; 1C, 1-cell embryo; 2C, 2-cell embryo. B) Phosphatase treatment of DCP1A and Flag-DCP2 in MII eggs. An MII egg extract was incubated with lambda protein phosphatase (+) before probing for DCP1A or Flag-DCP2, respectively. As a control, an MII egg lysate without phosphatase treatment (−) was run in parallel. TUBB served as loading control. The experiment was conducted two times, and representative immunoblots are shown. C) Time course of DCP1A and DCP2 phosphorylation during oocyte maturation. GV oocytes were injected with either Flag-Dcp1a or Flag-Dcp2 cRNA, then cultured overnight before allowing them to mature in vitro for the indicated times, at which point oocytes were removed for either immunoblot or kinase assays. CDC2A and MAPK activities were measured using histone H1 and myelin basic protein (MBP), respectively. The experiment was conducted two times, and representative immunoblots are shown. TUBB or ACTB served as loading controls. D) Effect of CDC2A and MAPK inhibitors on phosphorylation of DCP1A and DCP2. GV oocytes were injected with either Flag-Dcp1a or Flag-Dcp2 cRNA, allowed to in vitro mature for 16 h, then transferred to CZB medium with roscovitine or U0126 for additional 6 h before harvesting for immunoblot analysis. TUBB or ACTB served as loading controls. In parallel, dual kinase assays were performed to demonstrate CDC2A and MAPK activities were inhibited by adding the inhibitor or inhibitors. The decrease in MAPK activity following addition of roscovitine is a consequence of the decrease in CDC2A activity that is required to maintain elevated MAPK activity.
In contrast to a previous report of a maturation-associated electrophoretic shift in DCP1A following injection of a cRNA encoding Egfp-hDcp1a that did not attribute the shift to phosphorylation [40], we found that phosphatase treatment converted the slower-migrating form to the faster-migrating form (Fig. 2B). In addition, DCP2 was also phosphorylated, because phosphatase treatment also increased its electrophoretic mobility (Fig. 2B). Thus, both DCP1A and DCP2 are phosphorylated during maturation.
To identify the protein kinase that likely is responsible for the electrophoretic shift in DCP1A and DCP2, we examined when the electrophoretic shift occurred and the activity of two kinases that drive many of the events of oocyte maturation—namely, CDC2A and MAPK. During maturation, a dramatic increase in CDC2A activity occurs concomitant with GVBD, followed by activation of MAPK within an hour [41]. To facilitate detecting changes in electrophoretic mobility of either DCP1A or DCP2 during the early phases of maturation, when very little endogenous DPC1A or DCP2 is found, oocytes were first injected with a cRNA encoding either Flag-Dcp1a or Flag-Dcp2 cRNA. Following a 16-h overnight incubation in milrinone-containing medium to prevent maturation and permit synthesis of readily detectable amounts of DCP1A and DCP2, the oocytes were transferred to milrinone-free medium to initiate maturation.
The time course of DCP1A phosphorylation and activation of CDC2A and MAPK revealed that phosphorylation, as detected by the appearance of species of slower electrophoretic mobility, was initially correlated with CDC2A activation (Fig. 2C). MAPK may be the major kinase responsible for DCP1A phosphorylation at later times (i.e., between MI and MII), because the signal intensity of the phosphorylated form was dramatically increased relative to that of earlier times. Consistent with the earlier phosphorylation of DCP2 when compared to DCP1A, phosphorylated DCP2 was readily detected within 1.5 h of initiation of maturation, and its phosphorylation correlated with CDC2A activation, with no obvious role for MAPK (Fig. 2C).
To provide additional evidence for a role of both CDC2A and MAPK in DCP1A phosphorylation, MII eggs were treated either with roscovitine, which inhibits CDC2A; U0126, which inhibits MAPK activation; or both. DCP1A phosphorylation was assessed after 6 h of additional culture (Fig. 2D). Roscovitine treatment substantially inhibited DCP1A phosphorylation, whereas U0126 had little, if any, effect. Addition of both inhibitors, however, essentially abolished DCP1A phosphorylation. Similar results were also seen when the inhibitors were added shortly after GVBD and DCP1A phosphorylation was assessed by immunoblotting when controls had reached MII (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). These results suggest that CDC2A-mediated phosphorylation of DCP1A may be a prerequisite for MAPK-mediated phosphorylation. Consistent with the results of the time course of DCP2 phosphorylation implicating CDC2A, but not MAPK, as being responsible for DCP2 phosphorylation was that treatment with roscovitine, but not with U0126, effectively abolished the appearance of the phosphorylated form. The presence of species for both DCP1A and DCP2 with intermediate electrophoretic mobility likely indicates that each protein is phosphorylated on multiple sites.
3′ UTR Elements Drive Dcp1a and Dcp2 mRNA Recruitment
Recruitment of dormant maternal mRNAs is typically driven by 3′ UTR cis elements near the AAUAAA polyadenylation signal sequence [25, 26]. To confirm that Dcp1a and Dcp2 encode classic dormant maternal mRNAs, we first verified that 3′ UTRs of Dcp1a and Dcp2 transcripts regulate recruitment during maturation by injecting oocytes with a cRNA composed of the terminal 0.5 kb of the Dcp1a or Dcp2 3′ UTR fused downstream of the firefly luciferase coding sequence (Fig. 3A). Injected oocytes were then matured in vitro and the MII eggs assayed for luciferase activity. Control oocytes were injected with the cRNA but cultured in the presence of milrinone, a phosphodiesterase inhibitor, to prevent spontaneous maturation [29]. Each cRNA was recruited during maturation, as evidenced by the large increase in luciferase activity following maturation (Fig. 3A). This increase was significantly higher than even that directed by the cyclin B1 (Ccnb1) 3′ UTR, a maternal mRNA known to be recruited during maturation (Fig. 3A) [42].
FIG. 3.

Functional analysis of 3′ UTR of Dcp1a and Dcp2 mRNA. A) Schematic representation of Dcp1a and Dcp2 3′ UTR and luciferase reporter construct (top). Luc reporter cRNAs with the terminal 0.5 kb of Dcp1a or Dcp2 3′ UTRs were injected into GV oocytes. Following maturation, luciferase activity was analyzed in individual eggs. Injected oocytes cultured in milrinone-containing medium to inhibit maturation served as controls. The experiment was performed three times, and data are expressed as the mean ± SEM. B) Schematic of the Luc reporter cRNA used to identify functional CPEs in Dcp1a 3′ UTR. Three putative CPEs and one HEX were mutated. C) Schematic of the Luc reporter cRNA used to identify functional CPEs in Dcp2 3′ UTR. Two putative CPEs and one HEX were mutated. For these experiments, firefly luciferase reporter activities were normalized to a coinjected Renilla luciferase control and are expressed relative to the activity in oocytes injected with the same nonmutated reporter cRNA. The experiment was conducted three times, and data are presented as the mean ± SEM.
Different 3′ UTR sequence motifs, including CPEs, Musashi/polyadenylation response elements, Pumilio-binding elements, and DAZL-binding sequences regulate translation of mRNAs [43–46]. The 3′ terminal 0.5 kb of Dcp1a and Dcp2 3′ UTR contains three putative CPEs for Dcp1a and two putative CPEs for Dcp2 (Fig. 3, B and C). To identify which putative CPE or CPEs are functional in vivo, all putative CPEs were mutated, and Luc reporter cRNAs containing mutated CPE or CPEs were injected into oocytes and tested for luciferase activity following maturation.
Mutating CPE1 or CPE2, but not CPE3, significantly reduced Dcp1a reporter activity, with the double-mutation of CPE1 and CPE2 further reducing luciferase activity (Fig. 3B). These results suggest both CPE1 and CPE2 are required for recruitment of Dcp1a translation during oocyte maturation. As expected, mutating the polyadenylation hexanucleotide (HEX) AAUAAA substantially reduced luciferase activity, a finding consistent with a requirement for CPEs and HEX to initiate cytoplasmic polyadenylation [26]. Mutating CPE1, but not CPE2, significantly reduced Dcp2 reporter activity to a level similar to that for the mutated HEX reporter (Fig. 3C), suggesting that CPE1, the CPE closer to HEX, is the functional cis element largely responsible for recruitment of Dcp2 mRNA. Thus, Dcp1a and Dcp2 are canonical dormant maternal mRNAs recruited during oocyte maturation.
Elimination of Dcp1a and Dcp2 Transcripts Reduces the Extent of Degradation of Maternal mRNAs During Oocyte Maturation
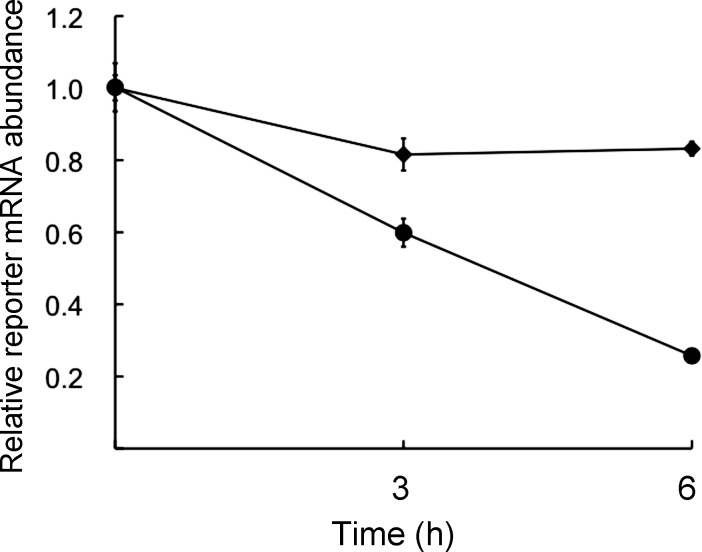
Oocyte maturation initiates a transition from mRNA stability to instability, which is clearly reflected in the loss of some 50 pg of poly(A)-containing RNA of the initial 80 pg present in GV-intact oocytes [15]. To verify that mRNAs are less stable in MII eggs than in GV-intact oocytes, oocytes or MII eggs were injected with a capped and polyadenylated Luc cRNA, and the amount of the RNA was measured as a function of time following injection (Fig. 4). Oocytes were incubated in milrinone-containing medium to inhibit maturation. As anticipated, the reporter cRNA was quite stable in oocytes but significantly degraded in the MII egg.
FIG. 4.
Luc cRNA is less stable in MII eggs than in GV-intact oocytes. GV-intact oocytes or MII eggs were injected with Luc cRNA, and one portion of each sample was immediately frozen (t = 0). The remaining portion of each group was then cultured for 3 or 6 h, at which times a portion was removed for qPCR; GV-intact oocytes were cultured in milrinone-containing medium to inhibit maturation. Data are expressed relative to the amount of Luc cRNA present at t = 0 and represent the mean ± range (n = 2). GV-intact oocytes, solid diamonds; MII-arrested eggs, solid circles.
We used RNA interference (RNAi) to address the role of maturation-associated accumulation of DCP1A and DCP2 in the transition from mRNA stability to instability. RNAi efficiently prevented the maturation-associated increase of DCP1A or DCP2 without affecting the meiotic maturation or MII arrest (Fig. 5A), although targeting Dcp2 was more effective than targeting Dcp1a. We then assessed the effect of inhibiting the maturation-associated increase in DCP1A and DCP2 on the stability of the report Luc cRNA. Accordingly, oocytes were injected with siRNAs targeting Dcp1a and Dcp2 and matured in vitro to MII, at which point the eggs were injected with the Luc cRNA; controls were injected with nontargeting, scrambled siRNAs. The amount of Luc cRNA was then assayed after 6 h and compared to that present immediately after injecting the MII eggs. We found that whereas only 45% ± 6% (n = 2) of the initial amount of Luc cRNA was present in controls, virtually all of it was present when the increase in DCP1A and DCP2 was inhibited (96% ± 8%, n = 2).
FIG. 5.
Effects of Dcp1a and Dcp2 knockdown on maternal mRNA stability. A) The effect of siRNA treatment on inhibiting the maturation-associated increase in DCP1A and DCP2 as assessed by immunocytochemistry and immunoblotting. GV, control uninjected oocytes; MII egg, oocytes injected with scrambled siRNA or with Dcp1a and Dcp2 targeting siRNAs and matured to MII. Bar = 25 μm. B) Analysis by qPCR of selected transcripts to assess effect of Dcp1a and Dcp2 knockdown. GV oocytes were microinjected with siRNAs targeting Dcp1a and Dcp2, cultured for 20 h in milrinone-containing medium, and then transferred to milrinone-free medium to initiate maturation; MII eggs were then collected for qPCR analysis. Transcript abundance is expressed relative to that of uninjected GV-intact oocytes. The experiment was performed three times, and data are expressed as the mean ± SEM. Gray bars, GV-intact oocytes; open bars, oocytes injected with scrambled siRNAs and matured to MII; black bars, oocytes injected with siRNAs targeting Dcp1a and Dcp2 mRNAs and matured to MII. *P < 0.05, **P < 0.01.
To extend these studies to endogenous mRNAs, we examined the effect of inhibiting the maturation-associated increase in DCP1A and DCP2 on degradation of a panel of mRNAs that are degraded during maturation [18] (Fig. 5B). Consistent with previous results [18], each transcript was degraded during maturation. Moreover, inhibiting the maturation-associated increase in DCP1A and DCP2 reduced the extent of degradation. The differences in the magnitude may reflect intrinsic differences in mRNA stability as well as the susceptibility of the transcripts to degradation from the 3′ end.
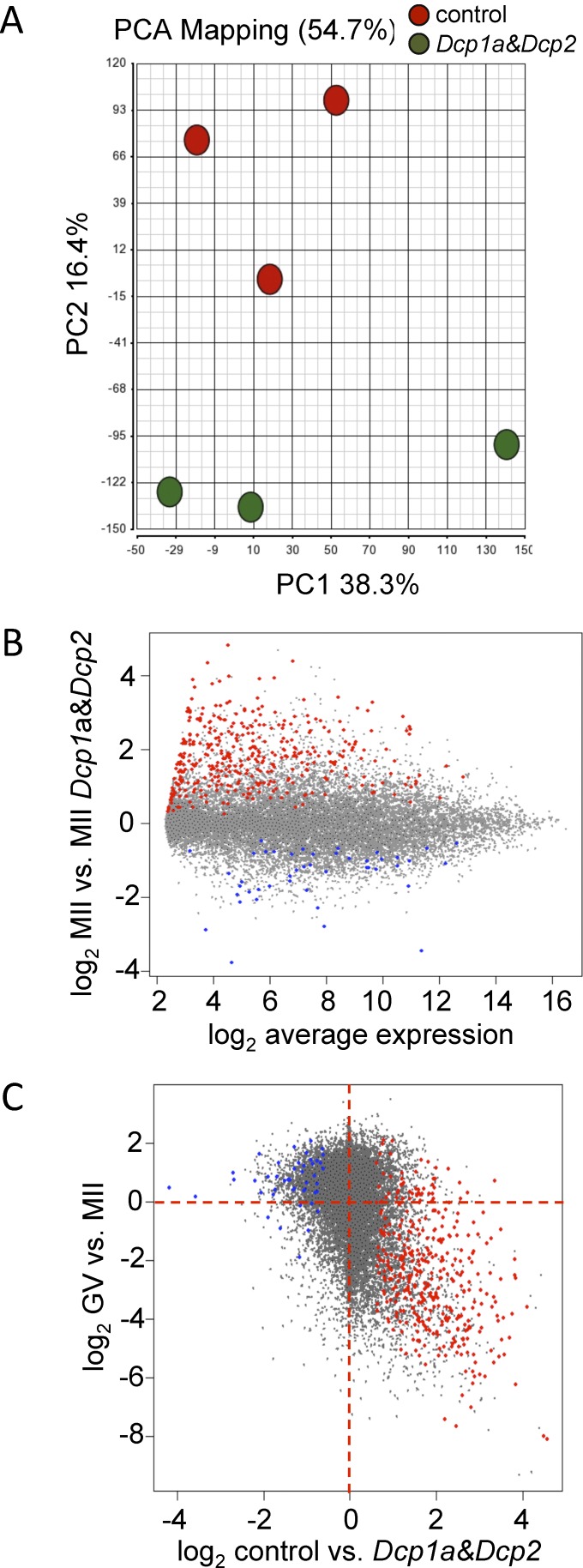
Last, we analyzed transcriptome changes associated with the loss of maturation-associated increase of DCP1A and DCP2. Full-grown oocytes were microinjected with Dcp1a and Dcp2 siRNAs; controls were injected with nontargeting, scrambled siRNAs. The oocytes were then cultured for 20 h in the presence of milrinone to permit extensive degradation of targeted mRNAs before maturation for additional 18 h, when the MII eggs were collected for microarray analysis. Analysis of differentially expressed transcripts, including principal component analysis of the microarray data, revealed a substantial perturbation of the transcriptome (Fig. 6A).
FIG. 6.
A) Principal component analysis of microarray profiles of MII eggs depleted of Dcp1a and Dcp2 transcripts. Each color-coded circle represents a projection of a complete microarray data set in a two-dimensional space formed by the top two principal components. The amount of variation covered by the top two principal components is indicated along each corresponding axis. Double-knockdown samples cluster apart from the controls, suggesting that knockdown of both Dcp1a and Dcp2 transcripts cause significantly distinct transcriptome changes. B) MII egg transcriptome changes upon Dcp1a and Dcp2 knockdown. The x-axis shows the average probe set hybridization signal, and the y-axis shows the relative expression change upon inhibition of decapping. Probe sets for which the relative signal was significantly increased or decreased (>1.5-fold; FDR < 0.05) in MII eggs following knockdown of Dcp1a and Dcp2 are shown as red and blue points, respectively. C) Extensive Dcp1a and Dcp2-dependent degradation of maternal mRNAs during maturation. The graph shows the relationship between transcriptome changes during oocyte maturation (shown on the y-axis) and changes following Dcp1a and Dcp2 double-knockdown (shown on the x-axis). Each point displays relative behavior of one Affymetrix probe set. Microarray data for oocyte maturation were taken from the literature [18]. The contribution of DCP1A and DCP2 to the maturation-associated mRNA degradation is clearly visible in the lower right quadrant. Transcripts for which degradation is independent of DCP1A and DCP2 are in the lower left quadrant. Probe sets for which the relative signal was increased or decreased in MII eggs following knockdown of Dcp1a and Dcp2 (see also B) are shown as red and blue points, respectively.
The perturbation of the transcriptome when both Dcp1a and Dcp2 were targeted was unlikely to be the result of off-targeting effects. First, ON-TARGET SMARTpool siRNAs have minimized off-targeting effects because of the covalent siRNA modifications and their pooling [47–49]. Second, the miRNA pathway, which forms the basis of off-targeting [50–52], is suppressed in oocytes [53, 54]. Third, the transcriptome was not significantly perturbed when Dcp1a and Dcp2 siRNA pools were used individually (data not shown). Fourth, bioinformatic analysis of the oligonucleotide motifs associated with transcriptome changes [55, 56] did not provide any evidence for off-targeting (data not shown).
The ANOVA of differentially expressed transcripts revealed a predominant increase in the relative abundance of transcripts when Dcp1a and Dcp2 mRNAs were targeted (Fig. 6B). Whereas the relative abundance of 45 transcripts (probe sets) was significantly decreased more than 1.5-fold (false-discovery rate [FDR] < 0.05), the relative abundance of 380 transcripts was significantly increased more than 1.5-fold (FDR < 0.05), suggesting that following Dcp1a and Dcp2 double-knockdown, these transcripts were degraded to a lesser extent than in controls (Supplemental Table S1). Transcripts for which the relative abundance significantly decreased following Dcp1a and Dcp2 knockdown were typically stable during meiotic maturation (Fig. 6C, blue points). The degradation of these transcripts following Dcp1a and Dcp2 knockdown could reflect a secondary effect induced by stabilization of other mRNAs or an accelerated, selective, decapping-independent mRNA degradation. Of particular interest is that the majority (70.3%) of the 380 transcripts that were degraded to a lesser extent in Dcp1a and Dcp2 double-knockdown oocytes are common to those that are normally degraded during maturation [18], as shown by a combined display of relative transcriptome changes during and following maturation in Dcp1a and Dcp2 knockdown oocytes (Fig. 6C, red points).
Inhibition of Decapping Affects Zygotic Genome Activation
To ascertain whether degradation of maternal mRNAs is essential for the oocyte-to-zygote transition, the maturation-associated increase in both DCP1A and DCP2 was inhibited by microinjecting full-grown oocytes with Dcp1a and Dcp2 morpholinos, and after maturation in vitro, the MII eggs were activated by SrCl2 and diploidized by treatment with cytochalasin B [57]. Activated MII eggs normally undergo zygotic genome activation, as shown by microarray profiling [58]. We took a morpholino approach rather than an siRNA approach to shorten the time before egg activation, because we have noted that extended periods of culture before egg activation compromises development.
Inhibiting the maturation-associated increase in DCP1A and DCP2 should cause, in 2-cell embryos, elevated levels of transcripts normally degraded during maturation. We tested this hypothesis by analyzing expression of Ppil3 and Upp1, which are two examples of maternal mRNAs that are extensively degraded during oocyte maturation in a decapping-dependent manner (Supplemental Table S1). Consistent with the expected extended survival of maternal mRNAs, Ppil3 and Upp1 transcript abundance was approximately 2-fold greater in 2-cell embryos relative to controls (Fig. 7A). Furthermore, microinjection of Dcp1a and Dcp2 morpholinos also inhibited genome activation, as assessed by EU incorporation, by approximately 50% (Fig. 7B). The reduced EU incorporation also correlated with a similar reduction in the amount of H3K4me3 methylation, which marks active promoters [59] (Fig. 7C).
FIG. 7.
Effect of inhibiting maturation-associated increase in DCP1A and DCP2 on genome activation. A) Increased levels of maternal mRNAs Ppil3 and Upp1 following inhibition of the maturation-associated increase in DCP1A and DCP2. The qPCR experiment was performed two times, and data are presented as the mean ± range. B) Global transcription in 2-cell embryos following inhibiting the maturation-associated increase in DCP1A and DCP2 using morpholinos. Representative staining images of control and experimental 2-cell embryos and quantification of the data are shown. The experiment was performed four times. The difference between the two groups is significant (P < 0.001). Bar = 25 μm. C) H3K4me3 methylation is reduced following inhibition of the maturation-associated increase in DCP1A and DCP2. Representative staining images of control and experimental 2-cell embryos and quantification of the data are shown. The experiment was performed two times. The difference between the two groups is significant (P < 0.001). Bar = 25 μm.
DISCUSSION
The present results implicate recruitment of Dcp1a and Dcp2 transcripts in the transition from mRNA stability to instability during oocyte maturation and shed more light on the role of dormant maternal mRNAs in driving the egg-to-embryo transition. The transition from mRNA stability to instability occurs in the absence of transcription and therefore must be driven by one or more posttranscriptional mechanisms (e.g., recruitment of maternal mRNAs). Likewise, posttranscriptional mechanisms could contribute to reprogramming gene expression—for example, by recruiting transcription factors and chromatin remodelers. A “thought experiment” would suggest that maternally recruited mRNAs would encode for functions that should not operate in oocytes but that need to be in place by the 1-cell stage. For example, oocytes should lack the capacity for DNA replication, but 1-cell embryos should have this capacity. And indeed, both CDC6 and ORC6L, critical components for assembly of a functional origin of replication, are encoded by maternally recruited mRNAs [60, 61]. A maturation-associated increase in the amount of inositol 1,4,5-triphosphate (IP3) receptor increases sensitivity to IP3-mediated release of intracellular Ca2+ [62], which drives the early events of egg activation [63]. In fact, a recent analysis of the composition of polysomes in oocytes and eggs is consistent with this thought experiment [43].
A regulatory subunit, DCP1A stimulates the catalytic activity of DCP2 by approximately 10-fold through stabilizing the catalytically active closed conformation of DCP2 [6]. In yeast, Dcp1p is a phosphoprotein [5], and in the mouse, DCP1A is phosphorylated during brain development, neural differentiation, and cellular stress [64]. In yeast, Dcp2 is phosphorylated by Ste20 in response to stress, but phosphorylation does not alter decapping activity even though mRNA degradation is perturbed [65]. We find that both DCP1A and DCP2 are phosphorylated during maturation, with CDC2A implicated in phosphorylating both DCP1A and DCP2, and MAPK is possibly involved in phosphorylating DCP1A. Both DCP1A and DCP2 have S/TP consensus sequences that could be phosphorylated by these proline-directed protein kinases. A recent study [66] demonstrated that JNK phosphorylates DCP1A; phosphorylation affects P-body formation. Adding the JNK inhibitor SP600125 at a concentration 100-fold greater than the median inhibition concentration after GVBD does not inhibit the maturation-associated electrophoretic shift in DCP1A (Supplemental Fig. S2)—that is, this MAPK family member is unlikely to be involved. The significance of the maturation-associated phosphorylation of DCP1A and DCP2 is not known. We have no explanation for why a previous report did not attribute the change in electrophoretic mobility to phosphorylation [40]. Current studies identifying the phosphorylation sites in DCP1A and DCP2 will permit functional studies similar to those conducted for MSY2 [22] to assess the role of DCP1A and DCP2 phosphorylation in mRNA degradation during maturation.
Recruitment of Dcp1a and Dcp2 mRNA is clearly implicated in the transition from mRNA stability to instability during the course of oocyte maturation. Inhibiting the increase in DCP1A and DCP2 inhibits not only degradation of an injected Luc mRNA in MII eggs but also that of many mRNAs that are normally degraded during the course of maturation. Noteworthy is that mRNAs for which degradation is perturbed following inhibition of decapping are stable and highly abundant in full-grown GV oocytes [67] (Supplemental Fig. S3). Minimal overlap exists between transcripts degraded during meiotic maturation and mRNAs that are intrinsically unstable in full-grown oocytes [18, 67]. This observation highlights the contribution of recruiting dormant maternal Dcp1a and Dcp2 mRNAs to the first wave of induced maternal mRNA degradation during meiosis.
We also performed a bioinformatic analysis of the 3′ UTRs of maternal transcripts. Although we failed to identify specific 3′ UTR sequence motifs associated with mRNA degradation, we did note that transcripts for which degradation is reduced in MII eggs when the increase in DCP1A and DCP2 is inhibited or are normally degraded during maturation have shorter 3′ UTRs (data not shown). These results raise the question of to what extent the selectivity of maternal mRNA degradation during oocyte maturation is driven by sequence-specific recruitment of the degradation machinery and by the loss of protection of a large portion of the transcriptome against degradation (e.g., protection conferred by RNA-binding proteins, such as MSY2).
In yeast, deadenylation is the major locus of control that regulates mRNA degradation and is linked to decapping [68]. A similar situation appears to exist in mammalian cells [69], but the molecular basis for the linkage is not well understood. Oocytes may differ, however, in the role that deadenylation serves in triggering decapping. In Xenopus oocytes, deadenylation is uncoupled from decapping [70]. A similar situation may exist in mouse oocytes. If deadenylation is the primary trigger for meiotic mRNA degradation, it is hard to envision why inhibiting the increase in DCP1A and DCP2 would inhibit degradation of mRNAs as seen by microarrays, because our transcriptome profiling relies on the presence of a poly(A) tail. This finding also suggests that degradation from the 5′ end of transcripts is the dominant pathway for mRNA degradation during oocyte maturation. Nevertheless, recruitment of critical components of the 3′→5′ degradation machinery are also likely to be involved, because PAN2 and CNOT7, key deadenylases of the 3′→5′ degradation machinery [3], are recruited during maturation (data not shown). The increase in activity of the 3′→5′ degradation machinery may account for why substantial amounts of mRNA degradation are observed for many transcripts even when the maturation-associated increase in DCP1A and DCP2 is inhibited.
Degradation of maternal mRNAs appears to be critical for genome activation, because inhibiting the maturation-associated increase in DCP1A and DCP2 inhibits EU incorporation by 2-cell embryos. The molecular basis for the linkage between inhibiting maternal mRNA degradation and genome activation remains unclear. In addition, whether this global inhibition of transcription effects all genes or only a subset of genes is not known and is the subject of ongoing studies.
The following model emerges for the transition from mRNA stability to instability that occurs during oocyte maturation: During oocyte growth, mRNAs are relatively stable, because binding of MSY2 confers mRNA stability, miRNA activity is suppressed, and the activity of the mRNA degradation machinery, although present—mRNAs do turn over—is low. Maturation is associated with phosphorylation of MSY2, which makes mRNAs more susceptible to degradation, and recruitment of maternal mRNAs encoding DCP1A and DCP2 increases the mRNA degradation capacity of the maturing oocyte. That most of the increase in DCP1A occurs between MI and MII further suggests that degradation of the bulk of maternal mRNAs occurs late in maturation. The net result is the transition from mRNA stability to instability and degradation of maternal mRNAs. This transcriptome remodeling subsequently enables the full extent of the zygotic genome activation during the 2-cell stage.
Supplementary Material
ACKNOWLEDGMENT
J.M. thanks Shin Murai (Toho University School of Medicine) for the luciferase reporter plasmid.
Footnotes
Supported by an NIH grant HD022681 to R.M.S. and the EMBO SDIG program, ME09039 grant, Czech Science Foundation Centrum of Excellence (P305/12/G034), and a Purkynje Fellowship to P.S.
REFERENCES
- Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, Parker R. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell. 2001;104:377–386. doi: 10.1016/s0092-8674(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Coller J, Parker R. Eukaryotic mRNA decapping. Annu Rev Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- Beelman CA, Stevens A, Caponigro G, LaGrandeur TE, Hatfield L, Fortner DM, Parker R. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature. 1996;382:642–646. doi: 10.1038/382642a0. [DOI] [PubMed] [Google Scholar]
- LaGrandeur TE, Isolation Parker R. and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor SN, Jones BN, Hernandez GA, Gross JD. A split active site couples cap recognition by Dcp2 to activation. Nat Struct Mol Biol. 2010;17:1096–1101. doi: 10.1038/nsmb.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol Cell Biol. 2002;22:8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E, Cougot N, Meyer S, Babajko S, Wahle E, Seraphin B. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc Natl Acad Sci U S A. 2002;99:12663–12668. doi: 10.1073/pnas.192445599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N, Babajko S, Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger-Gron M, Fillman C, Norrild B, Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell. 2005;20:905–915. doi: 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Ingelfinger D, Arndt-Jovin DJ, Luhrmann R, Achsel T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA. 2002;8:1489–1501. [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar M, Parker R. Identification of Edc3p as an enhancer of mRNA decapping in Saccharomyces cerevisiae. Genetics. 2004;166:729–739. doi: 10.1534/genetics.166.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarova R, De Leon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev Biol. 1980;74:1–8. doi: 10.1016/0012-1606(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Bachvarova R, De Leon V, Johnson A, Kaplan G, Paynton BV. Changes in total RNA, polyadenylated RNA, and actin mRNA during meiotic maturation of mouse oocytes. Dev Biol. 1985;108:325–331. doi: 10.1016/0012-1606(85)90036-3. [DOI] [PubMed] [Google Scholar]
- Brower PT, Gizang E, Boreen SM, Schultz RM. Biochemical studies of mammalian oogenesis: synthesis and stability of various classes of RNA during growth of the mouse oocyte in vitro. Dev Biol. 1981;86:373–383. doi: 10.1016/0012-1606(81)90195-0. [DOI] [PubMed] [Google Scholar]
- Jahn CL, Baran MM, Bachvarova R. Stability of RNA synthesized by the mouse oocyte during its major growth phase. J Exp Zool. 1976;197:161–171. doi: 10.1002/jez.1401970202. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRenzo C, Seydoux G. A clean start: degradation of maternal proteins at the oocyte-to-embryo transition. Trends Cell Biol. 2004;14:420–426. doi: 10.1016/j.tcb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Medvedev S, Pan H, Schultz RM. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol Reprod. 2011;85:575–583. doi: 10.1095/biolreprod.111.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev S, Yang J, Hecht NB, Schultz RM. CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers maternal mRNA degradation during mouse oocyte maturation. Dev Biol. 2008;321:205–215. doi: 10.1016/j.ydbio.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Deng M, Medvedev S, Yang J, Hecht NB, Schultz RM. Transgenic RNAi-mediated reduction of MSY2 in mouse oocytes results in reduced fertility. Dev Biol. 2004;268:195–206. doi: 10.1016/j.ydbio.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Flemr M, Ma J, Schultz RM, Svoboda P. P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol Reprod. 2010;82:1008–1017. doi: 10.1095/biolreprod.109.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nature reviews. Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Pique M, Lopez JM, Foissac S, Guigo R, Mendez R. A combinatorial code for CPE-mediated translational control. Cell. 2008;132:434–448. doi: 10.1016/j.cell.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–229. doi: 10.1016/j.bbagrm.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Erbach GT, Lawitts JA, Papaioannou VE, Biggers JD. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol Reprod. 1994;50:1027–1033. doi: 10.1095/biolreprod50.5.1027. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Nutrient requirements for the culture of preimplantation mouse embryo in vitro. Adv. Biosci. 1971;6:129–139. [Google Scholar]
- Kurasawa S, Schultz RM, Kopf GS. Egg-induced modifications of the zona pellucida of mouse eggs: effects of microinjected inositol 1,4,5-trisphosphate. Dev Biol. 1989;133:295–304. doi: 10.1016/0012-1606(89)90320-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 2000;127:4147–4156. doi: 10.1242/dev.127.19.4147. [DOI] [PubMed] [Google Scholar]
- Pan H, O'Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol. 2005;286:493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Wakayama T. Efficient strontium-induced activation of mouse oocytes in standard culture media by chelating calcium. J Reprod Dev. 2007;53:1207–1215. doi: 10.1262/jrd.19067. [DOI] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005;283:40–57. doi: 10.1016/j.ydbio.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Swetloff A, Conne B, Huarte J, Pitetti JL, Nef S, Vassalli JD. Dcp1-bodies in mouse oocytes. Mol Biol Cell. 2009;20:4951–4961. doi: 10.1091/mbc.E09-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Tay J, Hodgman R, Richter JD. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev Biol. 2000;221:1–9. doi: 10.1006/dbio.2000.9669. [DOI] [PubMed] [Google Scholar]
- Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata S, Kotani T, Mita K, Kawasaki T, Katsu Y, Nagahama Y, Yamashita M. Involvement of Xenopus Pumilio in the translational regulation that is specific to cyclin B1 mRNA during oocyte maturation. Mech Dev. 2003;120:865–880. doi: 10.1016/s0925-4773(03)00160-6. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 2006;20:199–209. doi: 10.1101/gad.1383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MD, Fox CA, Hunt T, Vande Woude G, Wickens M. The 3′-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994;8:926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JW, Chi JT, Gong D, Schaner ME, Brown PO, Ferrell JE. Minimizing off-target effects by using diced siRNAs for RNA interference. J RNAi Gene Silencing. 2006;2:181–194. [PMC free article] [PubMed] [Google Scholar]
- Parsons BD, Schindler A, Evans DH, Foley E. A direct phenotypic comparison of siRNA pools and multiple individual duplexes in a functional assay. PLoS One. 2009;4:e8471. doi: 10.1371/journal.pone.0008471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EM, Birmingham A, Baskerville S, Reynolds A, Maksimova E, Leake D, Fedorov Y, Karpilow J, Khvorova A. Experimental validation of the importance of seed complement frequency to siRNA specificity. RNA. 2008;14:853–861. doi: 10.1261/rna.704708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, Baskerville S, Maksimova E, Robinson K, Karpilow J, Marshall WS, Khvorova A. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J Biol Chem. 2003;278:44312–44319. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- Ma J, Flemr M, Stein P, Berninger P, Malik R, Zavolan M, Svoboda P, Schultz RM. MicroRNA activity is suppressed in mouse oocytes. Curr Biol. 2010;20:265–270. doi: 10.1016/j.cub.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, Blelloch R. MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr Biol. 2010;20:271–277. doi: 10.1016/j.cub.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ, Zavolan M, Svoboda P, Filipowicz W. Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Nucleic Acids Res. 2006;34:4801–4815. doi: 10.1093/nar/gkl646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008;5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak J, Paldi A, Weber M, Maro B. Genetically identical parthenogenetic mouse embryos produced by inhibition of the first meiotic cleavage with cytochalasin D. Development. 1991;111:763–769. doi: 10.1242/dev.111.3.763. [DOI] [PubMed] [Google Scholar]
- Vassena R, Han Z, Gao S, Baldwin DA, Schultz RM, Latham KE. Tough beginnings: alterations in the transcriptome of cloned embryos during the first two cell cycles. Dev Biol. 2007;304:75–89. doi: 10.1016/j.ydbio.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Anger M, Stein P, Schultz RM. CDC6 requirement for spindle formation during maturation of mouse oocytes. Biol Reprod. 2005;72:188–194. doi: 10.1095/biolreprod.104.035451. [DOI] [PubMed] [Google Scholar]
- Murai S, Stein P, Buffone MG, Yamashita S, Schultz RM. Recruitment of Orc6l, a dormant maternal mRNA in mouse oocytes, is essential for DNA replication in 1-cell embryos. Dev Biol. 2010;341:205–212. doi: 10.1016/j.ydbio.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-associated increase in IP3 receptor type 1: role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev Biol. 2003;254:163–171. doi: 10.1016/s0012-1606(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Blumenthal J, Behar L, Elliott E, Ginzburg I. Dcp1a phosphorylation along neuronal development and stress. FEBS Lett. 2009;583:197–201. doi: 10.1016/j.febslet.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Choi EJ, Parker R. Dcp2 phosphorylation by Ste20 modulates stress granule assembly and mRNA decay in Saccharomyces cerevisiae. J Cell Biol. 2010;189:813–827. doi: 10.1083/jcb.200912019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzeczkowski K, Beuerlein K, Muller H, Dittrich-Breiholz O, Schneider H, Kettner-Buhrow D, Holtmann H. Kracht M. c-Jun N-terminal kinase phosphorylates DCP1a to control formation of P bodies. J Cell Biol. 2011;194:581–596. doi: 10.1083/jcb.201006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschendorf M, Stein P, Oakeley EJ, Schultz RM, Peters AH, Svoboda P. Abundant transcripts from retrotransposons are unstable in fully grown mouse oocytes. Biochem Biophys Res Commun. 2006;347:36–43. doi: 10.1016/j.bbrc.2006.06.106. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Decker CJ, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Chang TC, Yamashita Y, Zhu W, Zhong Z, Chen CY, Shyu AB. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–1063. doi: 10.1038/nsmb1016. [DOI] [PubMed] [Google Scholar]
- Gillian-Daniel DL, Gray NK, Astrom J, Barkoff A, Wickens M. Modifications of the 5′ cap of mRNAs during Xenopus oocyte maturation: independence from changes in poly(A) length and impact on translation. Mol Cell Biol. 1998;18:6152–6163. doi: 10.1128/mcb.18.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.