ABSTRACT
Genetic modification of germline stem cells (GSCs) is an alternative approach to generate large transgenic animals where transgenic GSCs are transplanted into a recipient testis to generate donor-derived transgenic sperm. The objective of the present study was to explore the application of viral vectors in delivering an enhanced green fluorescent protein (EGFP) transgene into GSCs for production of transgenic gametes through germ cell transplantation. Both adeno-associated virus (AAV)- and lentivirus (LV)-based vectors were effective in transducing pig GSCs, resulting in the production of transgenic sperm in recipient boars. Twenty-one boars treated with busulfan to deplete endogenous GSCs and nine nontreated boars received germ cell transplantation at 12 wk of age. Semen was collected from recipient boars from 5 to 7 mo posttransplantation when boars became sexually mature, and semen collection continued for as long as 5 yr for some boars. The percentage of ejaculates that were positive for the EGFP transgene ranged from 0% to 54.8% for recipients of AAV vector-transduced germ cells (n = 17) and from 0% to 25% for recipients of LV vector-transduced germ cells (n = 5). When semen from two AAV recipients was used for in vitro fertilization (IVF), 9.09% and 64.3% of embryos were transgenic. Semen collected from two LV-vector recipients produced 7.7% and 26.3% transgenic IVF embryos. Here, we not only demonstrated AAV-mediated GSC transduction in another large animal model (pigs) but also showed, to our knowledge for the first time, that LV-mediated GSC transduction resulted in transgene transmission in pigs.
Keywords: germ cell transplantation, germline stem cells, spermatogenesis, spermatogonial stem cells, transgenesis, transgenic animal, transplantation, viral vectors
INTRODUCTION
Large transgenic animals have been generated for a variety of purposes, such as to produce large quantities of biopharmaceutical proteins, to provide tissues compatible for transplantation in humans, and to generate animal models for biomedical research. In this context, pigs are a popular large animal model because of their similarities to humans in both physiology and diseases, such as diabetes and cystic fibrosis [1].
For large animals in which embryonic stem (ES) cells are not yet widely available, the most common approach for generating transgenic animals is somatic cell nuclear transfer (SCNT) [2–4]. Although SCNT has been used to generate transgenic farm animals, such as goats [5], pigs [6], sheep [7], and cattle [8], the method is inefficient, costly, and time-consuming. Moreover, animals resulting from SCNT frequently suffer from developmental abnormalities associated with nuclear reprogramming [9–11].
An approach currently being considered to complement SCNT for producing transgenic animals is germ cell transplantation using genetically modified male germline stem cells (GSCs). GSCs are unipotent stem cells in the testis that self-renew and undergo differentiation to form sperm [12]. When exogenous GSCs are transplanted into the seminiferous tubules of a recipient testis, they are able to colonize the recipient testis and establish donor-derived spermatogenesis [13, 14]. This characteristic of GSCs makes them an attractive vehicle for transgene transmission. Previous reports demonstrated that rodent GSCs can be genetically manipulated in vitro by a variety of methods and transplanted into recipients to produce transgenic gametes [15–19]. Those studies laid the foundation for the potential application of GSCs in producing large transgenic animals.
So far, long-term culture of GSCs has been achieved only in mice and rats. Primary GSCs are quiescent or, at best, proliferate very slowly in culture, making the use of standard transfection methods difficult. Currently, the prevailing method used to deliver a transgene into GSCs is viral vector-mediated transduction. Transduction of rodent GSCs by lentiviral (LV) or retroviral (RV) vectors proved to be successful and resulted in transgenic mouse and rat offspring [15–18].
In our previous study, we investigated the application of adeno-associated virus (AAV) in GSC transduction [20]. AAV is a small, nonpathogenic, dependent parvovirus with a 4.7-kb, single-stranded linear genome [21]. AAVs are capable of infecting nonreplicating cells, which makes them an attractive delivery system for quiescent GSCs. Because AAV is a dependent virus, it does not carry the same biosafety restrictions as RV and LV vectors. Animals exposed to AAV can be maintained under standard husbandry conditions. We previously demonstrated that AAV is capable of stably transducing both mouse and goat GSCs in vitro, resulting in transgene transmission through the male germline [20]. That research confirmed that germ cell transplantation-mediated transgenesis through GSCs provides a feasible and important alternative for generating large transgenic animals.
In the present study, we extended the use of AAV and LV vectors to pigs and showed that viral vector-transduced pig GSCs were able to colonize the recipient testis and generate transgenic sperm. The transgene was detected in embryos produced by in vitro fertilization (IVF) using transgenic sperm.
MATERIALS AND METHODS
Viral Vectors
The EGFP (enhanced green fluorescent protein) vectors used in the present study (AAV2-CMV-EGFP, AAV2-PGK2-EGFP, AAV2-ACR3-EGFP, VSV-G.HIV.PGK1.EGFP, and VSV-G.HIV.PGK2.EGFP) express the EGFP transgene under the control of either a ubiquitous promoter, CMV (cytomegalovirus) or PGK1 (phosphoglycerate kinase 1) or a cell type-specific promoter (PGK2 or ACR3). PGK2 encodes the testis-specific phospho-glycerate kinase 2 and is only expressed in meiotic spermatocytes and in postmeiotic male germ cells [22]. The ACR3 promoter drives the expression of Acrosin in spermatids and spermatozoa [23]. All the vectors were obtained from the Vector Core of the University of Pennsylvania. The ACR3 promoter was derived from mouse sequence, and the PGK1 and PGK2 promoters were derived from human sequences. The PGK2 promoter was a gift from Dr. John McCarrey (University of Texas, San Antonio), and the ACR3 promoter was a gift from Dr. George Gerton (University of Pennsylvania).
Viral Transduction of GSCs
For GSC transduction in vitro, donor cells were collected from the testes of 10- to 12-wk-old piglets. Single-cell suspensions were prepared using a sequential enzymatic digestion protocol as described previously [24] with minor changes. Briefly, the tunica albuginea and visible connective tissue were removed. The exposed seminiferous tubules were dissociated with collagenase (2 mg/ml, Type IV; Sigma) in Dulbecco modified Eagle medium (DMEM) at 37°C for 20–40 min with occasional agitation, followed by the addition of hyaluronidase (1 mg/ml; Sigma) for 15–20 min. The tissue was then rinsed twice in Dulbecco phosphate-buffered saline without Ca2+ and further digested with 0.25% (w/v) trypsin and 1 mM ethylenediaminetetra-acetic acid at 37°C for 5–10 min. DNase I (7 mg/ml; Sigma) in DMEM was added as needed. Fetal bovine serum was added to stop enzymatic digestion. The resulting cell suspension was filtered through 100-, 70-, and 40-μm cell strainers sequentially (BD Biosciences). The single cells were then collected by centrifugation at 500 × g for 5 min at 16°C, and the pellet was resuspended in DMEM. For AAV vector transduction, 0.2–1.1 × 109 testicular cells were exposed to AAV2-CMV-EGFP, AAV2-PGK2-EGFP, or AAV2-ACR3-EGFP at a multiplicity of infection (MOI) of 0.3–8.8 × 103 genome copies/cell. Germ cells were transduced for 3 h in vitro, washed twice in DMEM for 5 min each, and transplanted. Because a high titer of LV is required for transduction of the large number of cells, spermatogonia (including GSCs) were enriched from the initial testicular cell suspension before LV transduction. The testicular cell suspension (1.1–1.2 × 109 cells) was subjected to Staput velocity sedimentation as previously described [25]. Briefly, testicular cells were loaded and subjected to sedimentation in a 2%–4% bovine serum albumin (BSA) gradient in 25 mM Hepes-buffered DMEM for 2.5 h, followed by collection of eighty 12-ml fractions. Fractions were examined under a phase-contrast microscope to identify those enriched for spermatogonia. Cell suspensions (1.2–1.6 × 108 cells) that were enriched for spermatogonia were collected and assessed for cell viability and used for transduction. For LV vector transduction, 0.2–0.4 × 108 cells were infected by VSV-G.HIV.PGK1.EGFP or VSV-G.HIV.PGK2.EGFP at an MOI of 140–154 overnight, and transduced cells were washed twice in DMEM for 5 min each and transplanted.
Preparation of Recipient Animals
Pregnant sows were treated twice with busulfan (7.5 mg/kg) at Days 98 and 108 of gestation to deplete endogenous germ cells in fetuses [26]. Treated piglets were used for germ cell transplantation at 12 wk of age. Age-matched normal boars were used as nontreated recipients. We have previously shown that germ cell transplantation between unrelated pigs does not result in immune rejection of transplanted cells; therefore, recipient pigs do not need to be genetically matched to the donor or immune-suppressed [24, 27].
Germ Cell Transplantation and Semen Collection
Transplantation was performed by ultrasound-guided cannulation of the rete testis with delivery of cells by gravity flow as described previously [20, 24]. Briefly, transplantation was performed under general anesthesia and aseptic surgical conditions. A linear incision was made lateral and parallel to the median raphe, and the testis enclosed in the parietal vaginal tunic was exposed. An i.v. catheter (20-gauge × 1-1/4 inch length; Surflo; Terumo Medical Co.) was inserted through the cauda epididymis and testis into the rete testis using ultrasound scanning. The position of the catheter was monitored from longitudinal and cross-sectional planes, and its direction was adjusted to ensure positioning in the center of the rete testis. Once the catheter was inserted, a small drop of a tissue adhesive solution (Close Liquid Suture; Braun Veterinary Care) was applied to anchor the catheter to the testis. After removing the steel needle, an infusion set containing the cell suspension was connected to the catheter. The average volume of the cell suspension injected into each testis was 3–5 ml, depending on testis size, with a flow rate of approximately 0.5–1 ml/min. For boars in which only one testis was injected, the contralateral testis was removed. Semen was collected from recipients by manual stimulation. Semen collection started at approximately 5–7 mo posttransplantation when pigs became sexually mature and continued for more than 5 yr for some boars. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and performed in accordance with relevant guidelines and regulations.
Genotyping Semen Samples
For the isolation of sperm DNA, 107 sperm/ejaculate were incubated for 1 h at 37°C in the presence of 100 mM dithiothreitol in lysis buffer before DNA extraction. Genomic DNA was extracted from sperm using a commercially available kit (QIAmp DNA Mini Kit; Qiagen). All DNA samples were analyzed by PCR in triplicate. For PCR detection of the EGFP transgene, the following primers were used: 5′-TCA CCT TGA TGC CGT TCT TCT-3′ and 5′-GCA AGC TGA CCC TGA AGT TCA-3′, resulting in a 372-bp fragment, or 5′-CGG CCA CAA GTT CAG CGT GTC CGG CG-3′ and 5′-CCA TGT GAT CGC GCT TCT CGT TGG GG-3′, resulting in a 586-bp fragment. Amplification of a 274-bp fragment of the beta-actin gene served as the internal control, for which the following primers were used: 5′-TGTGCTGTCCCTGTACG CCTCTG-3′ and 5′-CAGTGGCCATCTCCTGCTCGAAGT-3′. Positive-control DNA was from EGFP-transgenic mice (Tg(ACTB-EGFP)D4Nagy/J; The Jackson Laboratory).
In Vitro Fertilization
Boar semen was processed and shipped in X-Cell extender (IMV Corporation) at a concentration of 50 × 106 sperm/ml to the University of Illinois, where IVF was performed. Porcine blastocysts were produced using in vitro maturation, IVF, and embryo culture as previously described [28–31]. Briefly, cumulus-oocyte complexes (COCs) were vacuum-aspirated from 3- to 8-mm follicles. Selected COCs were matured in vitro at a density of 50 COCs/well in a four-well plate for 42–44 h at 38.7°C in 7% CO2 in humidified air. After maturation, oocytes were denuded of the surrounding cumulus cells, washed three times in modified Tris-bufferred medium (mTBM) supplemented with 2 mM caffeine, 0.2% (w/v) fraction V BSA, and 1× PSA (100 U penicillin, 100 μg streptomycin, 0.25 μg amphotercin; Invitrogen). Oocytes were placed into 50-μl drops of mTBM [32] at a density of 20 oocytes/drop under 10 ml of mineral oil. Sperm were prepared by density separation using a gradient of 45%:90% Percoll (GE Healthcare Life Sciences). After centrifugation (20 min at 700 × g, room temperature), the sperm pellet was washed twice in 5 ml DPBS (Invitrogen), diluted in mTBM, and added to drops containing oocytes for a final sperm concentration of 250 000 sperm/ml. Gametes were coincubated for 5 h in 6% CO2 in humidified air. After coincubation, presumptive zygotes were washed three times and cultured in 50 μl of NCSU-23 medium (10 zygotes/drop) [33] containing 0.4% crystallized BSA under 10 ml of mineral oil in 6% CO2, 10% O2, balance N2 for 6 days, when embryonic blastocyst development was determined. Unless specified otherwise, all chemicals were from Sigma-Aldrich.
Genotyping of Porcine IVF Embryos
Porcine embryos were generated by IVF using semen from four boars (Tables 1 and 2). Embryos were analyzed at Day 6 of development. Negative-control embryos were obtained using sperm from control boars. The genomic DNA from an EGFP-transgenic mouse served as positive control for EGFP (a gift from Dr Yuko Fujiwara, Children's Hospital, Boston, MA). Embryos were processed for genomic DNA extraction as previously described [20]. Briefly, the embryos were treated with acid Tyrode solution and Pronase (Sigma) to remove the zona pellucida and washed three times for 5 min each in Hanks balanced salt solution containing 0.66 mg/ml of polyvinylpyrrolidone-10 (Sigma). Embryos were lysed by the freeze-thaw method. Mouse genomic DNA samples (positive control) were serially diluted in dH2O to 10-, 5-, and 2-cell equivalents of DNA per microliter. To increase the amount of DNA available for genotyping, the genomic DNA present in the lysed embryos and controls was subjected to primer extension preamplification (PEP) PCR using AmpliTag DNA Polymerase (PerkinElmer) and random primers (pd(N)6 random hexamer; Amersham). After PEP, the PCR products were used as templates for nested PCR using primers specific to EGFP and beta-actin. Two sets of EGFP primers were used, with one set being closer to the 5′ of the coding region (GFP5′) and the other closer to the 3′ (GFP3′). The EGFP primers used were as follows: for primer set GFP5′, 5′-CGGCCACAAGTTCAGCGTGTC-3′ and 5′-TCACCTTGATGCCGTTCTTCTGC-3′ (first round with an expected size of 419 bp), 5′-GCAAGCTGACCCTGAAGTTCA-3′ and 5′-TTGTCGGCCATGATATAGACGTT-3′ (second round with an expected size of 349 bp); for primer set GFP3′: 5′-GCAAGCTGACCCTGAAGTTCA-3′ and 5′-TATTACTTGTACAGCTCGTCCATGCC-3′ (first round with an expected size of 602 bp), 5′-CACATGAAGCAGCACGACTTCT-3′ and 5′-TCACCTTGATGCCGTTCTTCTGC-3′ (second round with an expected size of 230 bp). The primers used for beta-actin were as follows: 5′- GTTTGAGACCTTCAACACGCCGG-3′ and 5′-CGTTGCCGATGGTGATGACCTG-3′ (first round with an expected size of 398 bp), 5′-TGTGCTGTCCCTGTACGCCTCTG-3′ and 5′-CAGTGGCCATCTCCTGCTCGAAGT-3′ (second round with an expected size of 274 bp). Selective PCR products were sequenced to confirm the identity of amplicons to be the EGFP gene. Most of the embryos were positive for both primer sets; however, a few embryos were positive for only one of the primer sets. Embryos were scored as positive so long as one of two paired sets yielded positive result and the sequencing result was confirmative.
TABLE 1.
Summary of 17 recipients transplanted with AAV vector.
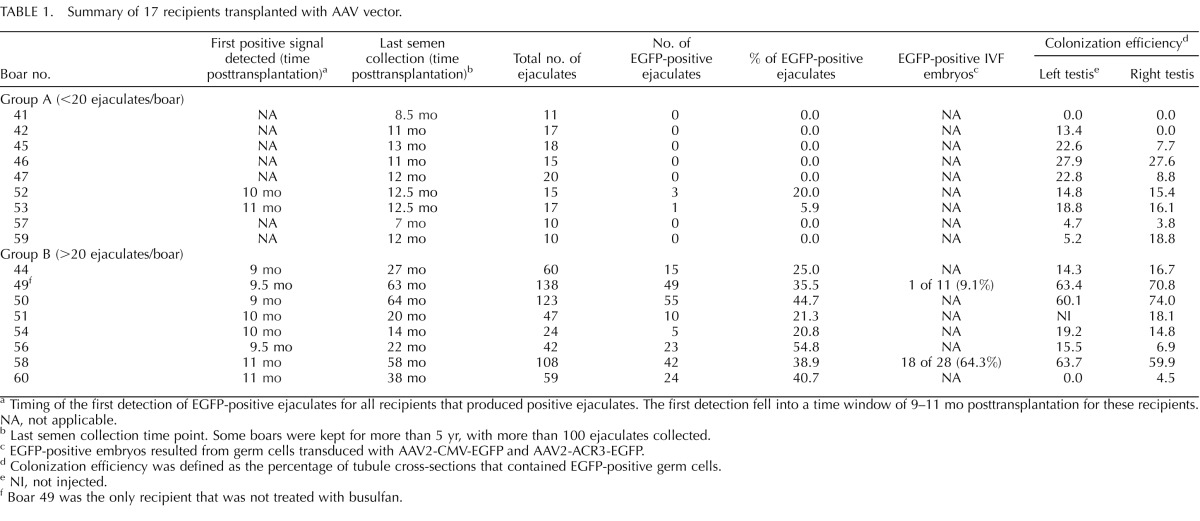
TABLE 2.
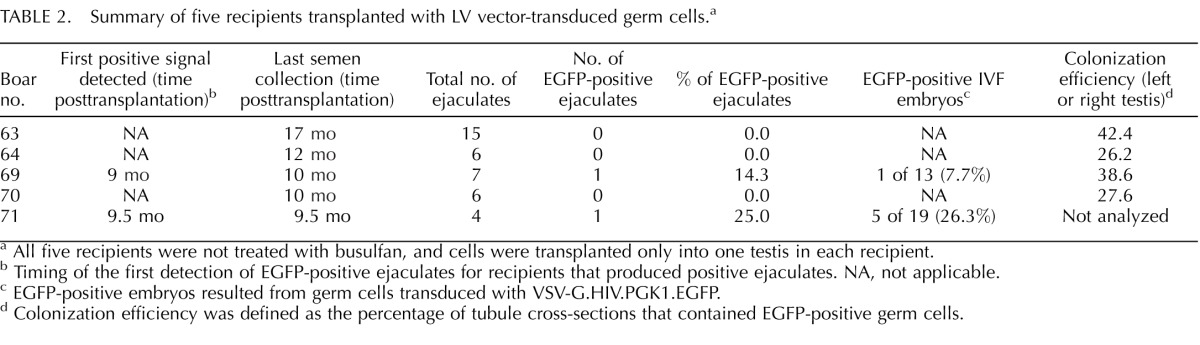
Summary of five recipients transplanted with LV vector-transduced germ cells.a
Immunohistochemistry
Germ cells positive for EGFP were detected on cross-sections of testicular tissue by immunohistochemistry. Paraffin sections were dewaxed and dehydrated through xylene followed by a graded series of graded ethanol washes (100%, 95%, 70%, and 50% ethanol, 5 min each). Antigen retrieval was performed by boiling samples in citrate buffer in a microwave for 1 min and cooling for 30 min. Endogenous peroxidase activity was eliminated by treating samples with 3% H2O2 for 5 min. After three washes with PBS buffer (5 min each), samples were blocked using 5% goat serum in PBS for 1 h before addition of primary antibodies. Primary antibody incubation was done at 4°C overnight using rabbit polyclonal anti-EGFP antibodies (ab 6556; Abcam). After primary antibody incubation, samples were washed three times in PBS for 5 min each and then incubated with biotinylated goat anti-rabbit IgG antibodies (PK-4001, VECTASTAIN; Vector Laboratories) at a dilution of 1:300 for 30 min at room temperature. Samples then were subject to incubation with VECTASTAIN ABC reagent for 30 min at room temperature. After PBS washes, samples were incubated with peroxidase substrate (VECTOR NovaRED, SK-4800; Vector Laboratories) for 1–5 min. After rinsing with PBS, sections were counterstained with Harris hematoxylin, rehydrated with ethanol, mounted with mounting media, and analyzed by bright-field microscopy.
RESULTS
AAV Vector-Mediated GSC Transduction and Transplantation
For AAV-transduced GSCs, 21 busulfan-treated and 4 nontreated boars (n = 25 in total) were used as recipients for transplantation. Two boars died unexpectedly at a young age, and six boars were killed young for analysis of the effect of busulfan on colonization of transplanted GSCs. Therefore, only 17 boars produced ejaculates for transgene analysis (summarized in Table 1). Among these 17 boars, 16 were treated with busulfan, and 1 was nontreated. We divided these 17 recipients into two groups (group A or group B) based on the number of ejaculates collected. Group A consisted of nine boars that were killed relatively young (between 10 and 16 mo of age). For those boars, semen collection terminated at 7- to 12-mo posttransplantation, and less than 20 ejaculates/boar were collected and evaluated. Out of nine boars, the EGFP transgene was detected in ejaculates from two boars (20% and 5.9% of ejaculates from boars 52 and 53, respectively). Group B consisted of eight boars that were kept from 18 to 72 mo of age. For those boars, semen collection went beyond 14 mo posttransplantation (ranging from 14 to 64 mo), and more than 20 ejaculates/boar were collected and evaluated. In particular, three boars (boars 49, 50, and 58) had more than 100 ejaculates collected. All the boars in group B produced EGFP-positive ejaculates. The percentage of positive ejaculates ranged from 20.8% to 54.8% (mean ± SD, 35.3% ± 12%, n = 8) (Table 1).
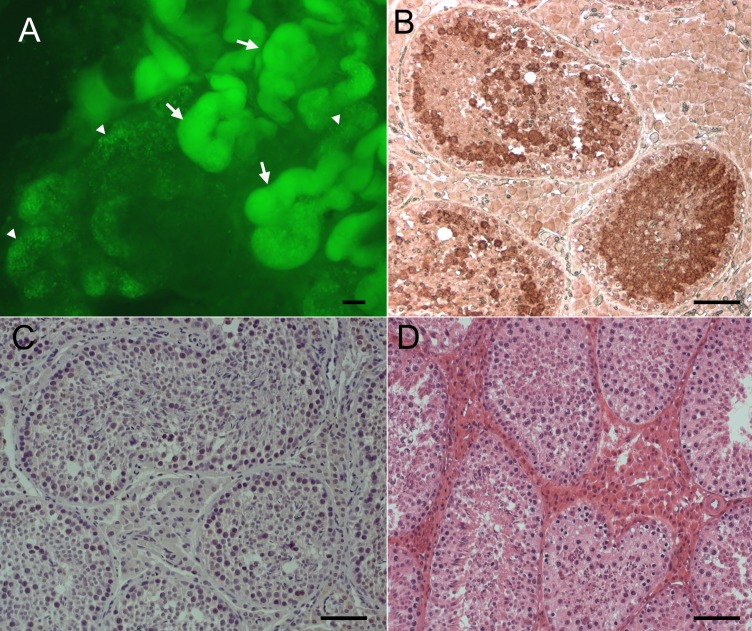
In addition to analyzing ejaculates, we also examined the testes from 17 boars after death. When the whole-mounted seminiferous tubules were analyzed under a dissecting microscope equipped with epifluorescence, EGFP-positive cells were detected in the seminiferous tubules (Fig. 1A). Because only GSCs are capable of colonizing the seminiferous tubules, EGFP fluorescence originated from stably transduced GSCs. We also noticed that EGFP-positive tubules demonstrated the classic pattern of well-established colonies, with long stretches of completely filled segments as well as a web of small groups of cells (Fig. 1A). When testis cross-sections were stained with anti-EGFP antibodies, EGFP was specifically detected in spermatocytes and spermatids in the testis that received transplantation from AAV2-PGK2-EGFP-transduced germ cells (Fig. 1, B and C). When testis sections of recipients were compared to those of an age-matched, nontreated adult boar, no gross developmental or anatomical difference was observed (Fig. 1, B and D). For analysis of colonization of transduced GSCs, colonization efficiency was determined for each transplanted testis from recipients (Table 1). Colonization efficiency was defined as the percentage of tubules that contained EGFP-positive germ cells. Approximately 150–300 tubule cross-sections were scored for each testis. Of seven boars that did not produce EGFP-positive ejaculates (group A), six had some degree of EGFP-positive tubules (Table 1). For example, boar 46 had 27.9% and 27.6% colonization efficiency in the left and right testis, respectively; however, EGFP was not detected in the 15 ejaculates analyzed.
FIG. 1.
EGFP transgenic donor cells colonized the seminiferous tubules and established donor-derived spermatogenesis. A) Fluorescent images of whole-mounted seminiferous tubules from a recipient 8 mo posttransplantation. Fluorescent segments are tubules colonized by EGFP-positive donor germ cells. Arrows indicate long stretches of segments with multiple layers of EGFP-positive germ cells, and arrowheads indicate a web of EGFP-positive germ cells. B) EGFP expression was detected in the seminiferous tubules of boar 60 at 3 yr posttransplantation. Boar 60 was a busulfan-treated recipient that received transplantation of germ cells transduced with AAV2-PGK2-EGFP. EGFP driven by the germ cell-specific PGK2 promoter is specifically expressed in spermatocytes and spermatids. C) Control staining without the primary antibody for B. D) Hematoxylin-and-eosin staining of a section from an age-matched, nontreated adult boar testis. Bar = 200 μm (A) and 50 μm (B–D).
Among 25 boars used in the present study, four pairs of boars were set up to compare the effect of busulfan treatment on colonization of transplanted GSCs. For each pair, one recipient was treated with busulfan, and the other was not. Two boars in each pair received the same number of donor cells from the same cell preparation for transplantation, and the animals were killed at the same time for analysis. Three pairs were killed young for analysis of colonization efficiency of transduced germ cells in the testis; therefore, no ejaculates were collected from those three young pairs. When colonization efficiency was compared within the pairs, we did not observe a significant difference between the busulfan-treated and nontreated recipients (data not shown). The fourth pair (boars 49 and 50) was kept beyond sexual maturity for semen collection. Genotyping results indicated that 35.5% of ejaculates (49/138) were EGFP-positive for boar 49 (nontreated) and that 44.7% of ejaculates (55/123) were EGFP-positive for boar 50 (busulfan-treated). Although we saw a slightly higher percentage of positive ejaculates in the busulfan-treated boar (boar 50), we cannot conclude from this limited data set that the testis depleted of endogenous germ cells using the current protocol offers a better environment for transduced GSCs.
LV Vector-Mediated GSC Transduction and Transplantation
Because we did not observe a significant advantage of depleting endogenous germ cells in recipients of busulfan treatment, we used nontreated recipients for LV-transduced GSCs. Five prepubertal recipients received transplantation of LV-transduced GSCs at 12 wk of age, and all were killed at 12 mo posttransplantation. Only a few ejaculates were collected from each recipient (Table 2). Out of five boars, two (boars 69 and 71) produced positive ejaculates, with one positive ejaculate out of seven from boar 69 and one positive ejaculate out of four from boar 71. When we examined the testes from three recipients that failed to produce positive ejaculates, we observed colonization of EGFP-positive germ cells in the tubules (Table 2). This was similar to what we had observed in the testes of AAV-transduced recipients.
Transgenic Embryos Produced by IVF
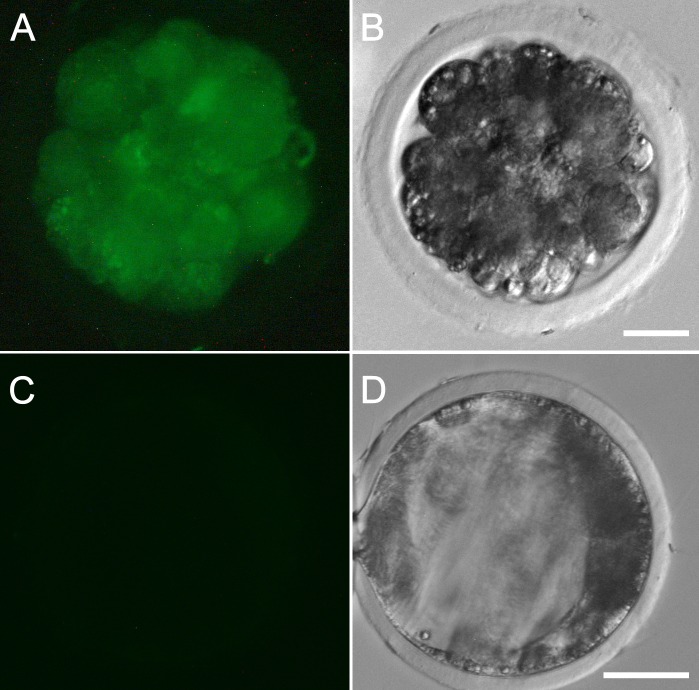
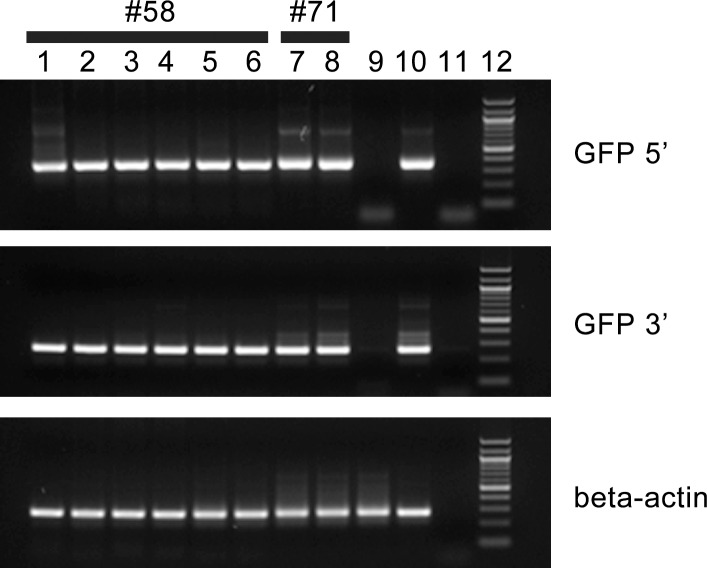
Sperm collected from two recipients of AAV vector-transduced germ cells (boars 49 and 58) and two recipients of LV vector-transduced germ cells (boars 69 and 71) were used for IVF. Sperm from a control boar were used to produce negative-control embryos. The preimplantation development of IVF embryos was monitored for 6 days, and the most advanced embryos observed were at the blastocyst stage. The percentage of IVF embryos that developed to the blastocyst stage (10.6%) was comparable to what has been reported previously (12.9% ± 1.5% [28]). A comparable number of blastocysts (∼15%) was observed in the IVF control group using control sperm. Boars 50 and 58 produced 9.1% (1/11) and 64.3% (18/28) transgenic embryos, respectively. Boars 69 and 71 produced 7.7% (1/13) and 26.3% (5/19) transgenic embryos, respectively. Representative fluorescent images of embryos are shown in Figure 2. As reported previously [34–36], we detected EGFP expression in embryos that carry the CMV-EGFP transgene, indicating that the CMV promoter is active in porcine preimplantation embryos. IVF embryos were genotyped using EGFP-specific primers, and the results are presented in Figure 3.
FIG. 2.
EGFP transgene is expressed in IVF embryos produced by sperm from recipient boars. Fluorescent (A) and bright-field (B) images of an embryo generated by IVF using sperm from one of the recipients that produced positive ejaculates are shown. The control embryo (C and D) was generated by using sperm from a control boar. Bar = 25 μm (A and B) and 50 μm (C and D).
FIG. 3.
EGFP transgene is present in IVF embryos produced by sperm from recipient boars. Representative genotyping results from embryos from two recipients (boars 58 and 71) are shown. Two sets of EGFP primers, GFP5′ and GFP3′, were used, resulting in a product of 349 and 230 bp, respectively. Amplification of beta-actin was used as internal control, and the product size was 274 bp. Lanes 1–6: representative embryo samples from recipient boar 58; lanes 7 and 8: representative embryo samples from recipient boar 71; lane 9: embryo from control boar; lane 10: EGFP-positive mouse embryo (positive control); lane 11: no-DNA control; lane 12: 100-bp markers. Boar 58 was a busulfan-treated recipient that received AAV vector-transduced germ cells. Boar 71 was a nontreated recipient that received LV vector-transduced germ cells.
DISCUSSION
Transgenesis Through In Vitro Transduction of GSCs by Viral Vectors
In the present study, we demonstrated that pig GSCs could be transduced in vitro by AAV vectors. Transduced GSCs were able to colonize the recipient testis, initiate donor-derived spermatogenesis, and produce transgenic sperm. To prove the transmission of the transgene, we generated transgenic embryos by IVF using transgenic sperm. Lentiviral transduction of porcine germ cells in vitro has been described previously [37]. However, the transduced cells were not transplanted, and transmission of the transgene has not been reported. Here, we showed that LV vector-transduced GSCs colonized the recipient testis and produced transgenic sperm. We also showed that transgenic sperm were capable of passing the haplotype to embryos through IVF.
After germ cell transplantation, we screened ejaculates from recipients starting from 5 to 7 mo posttransplantation for the presence of transgene. We noticed that we were able to detect the EGFP transgene by PCR starting at 9–11 mo posttransplantation for all recipients that produced positive ejaculates (Tables 1 and 2). We propose that this time window (9–11 mo) does not indicate a developmental delay in spermatogenesis from transduced GSCs; instead, it likely represents a threshold effect on the time that is required for transplanted transgenic GSCs to home in on the niche and proliferate to reach a certain population size, therefore producing an amount of transgenic sperm that is detectable by PCR. We also were able to detect the transgene in ejaculates from some 5-yr-old recipient boars. This indicated that the transgene was stably integrated into the genome of porcine GSCs.
Among all 23 recipients used for ejaculate analysis (n = 17 boars from the AAV experiment and 5 boars from the LV experiment) (Tables 1 and 2), we did not detect EGFP-positive ejaculates from 10 (n = 7 boars from the AAV experiment and 3 boars from the LV experiment). All of these 10 boars had less than 20 ejaculates/boar collected, because semen collection terminated at 7–12 mo posttransplantation (Tables 1 and 2). The last collection time points of those animals overlapped with the time window during which the transgene was first detectable in other recipients (9–11 mo). When we examined colonization of transplanted GSCs in the testis of those 10 recipients, we detected colonization in nine of them (Tables 1 and 2). Therefore, we suspected that either viral transduction and/or colonization of GSCs were less efficient in those nine recipients, resulting in a lesser number of positive sperm, which likely was beyond the detection sensitivity of PCR at the time of screening. In other words, the time required by a small number of transgenic GSCs to reach the proposed threshold would be longer. Because those recipients were killed right after semen collection terminated, we could not investigate the possibility of detecting EGFP-positive ejaculates later on, when the proposed threshold was reached. Alternatively, the small number of transgenic GSCs colonized could not produce enough positive sperm to be detected by PCR during the life span of the animals.
Given the size of a mature pig testis, we did not determine the total number of colonies in each testis. We analyzed the number of tubule cross-sections containing EGFP-positive germ cells on randomly chosen tissue sections. When we aligned the percentage of positive ejaculates with colonization efficiency, we found that a high percentage of positive ejaculates did not always correlate with a high percentage of colonization efficiency (Tables 1 and 2). Because colonization is not homogenous throughout the testis, sampling may not have accurately reflected overall colonization of transplanted GSCs. However, the analysis documented colonization and spermatogenesis from transplanted GSCs and provided an estimate of colonization efficiency expressed as the number of EGFP-positive tubules on representative cross-sections.
The depletion of endogenous germ cells in recipients has been a standard practice for germ cell transplantation ever since the seminal studies in rodents that showed its beneficial effects on colonization of exogenous donor germ cells and donor-derived spermatogenesis [13, 14]. In the present study, we used a total of six non-busulfan-treated recipients. One received AAV vector-transduced GSCs; the other five were recipients for LV-transduced GSCs. Within the scope of the present study, we did not see a clear advantage to busulfan treatment using the current protocol for prepubertal recipients in improving colonization of GSCs and production of transgenic sperm. Although busulfan treatment does not seem to be essential and the presence of endogenous germ cells in prepubertal recipients may not interfere with colonization of exogenous transgenic germ cells, wild-type sperm produced from endogenous germ cells can greatly dilute transgenic sperm in ejaculates.
Large Animal Transgenesis Through GSCs
Transgenesis through male GSCs has tremendous potential in species such as goats and pigs in which ES cell-based transgenic technology is not yet available and currently practiced methods for producing transgenic animals generally are inefficient. Introduction of genetic modifications in the germline can circumvent problems associated with manipulation of early embryos and developmental abnormalities associated with SCNT and reprogramming [9–11, 38]. Once transgenic GSCs colonize the seminiferous tubules of the recipient testis, they can continuously produce transgenic sperm over the life of the recipient. Moreover, by transplanting transduced GSCs into prepubertal recipients, the time to production of transgenic sperm is shortened compared to production of a transgenic founder animal by SCNT. In addition, in transgenic disease models where mutant males cannot be maintained to breeding age, the number of affected offspring generated by mating can be increased by transplanting GSCs from prepubertal affected males to healthy recipients.
It remains a challenge to culture male GSCs from large animals for extended periods of time. So far, with the exception of some mouse and rat strains, protocols for long-term culture of GSCs have not been established. This makes viral transduction the most common approach for delivering transgenes into GSCs for genetic manipulation. Recently, we showed that nucleofection could be used as an alternative to viral transduction in transfecting goat GSCs, resulting in production of transgenic sperm [39]. This extends the utility of the germ cell transplantation approach to transgenes that cannot be easily packaged into viral vectors.
Recent advances in transgenic technology greatly enhance the utility of GSCs in making genetically engineered animal models. Knockout rats were produced through Sleeping Beauty transposon-mediated mutagenesis in rat GSCs [40, 41]. Genetic modification of chicken primordial germ cells by piggyBac and Tol2 transposons led to germline transmission of the transgene and production of transgenic offspring [42]. Knockout pigs have been generated by using a combination of zinc-finger nuclease (ZFN)-mediated, targeted mutagenesis in pig somatic cells and SCNT [43–46]. More recently, development of transcription activator-like effector nucleases (TALENs) has opened another avenue for targeted gene modification [47, 48]. TALENs have been used to create site-specific modifications in zebrafish [49, 50], rats [51], as well as cattle and pigs[52] at levels equivalent to those achieved with ZFNs. The potential of GSCs in making large knockout animal models will be maximized if transposon-mediated and nuclease-mediated mutagenesis are achieved in GSCs.
Footnotes
Current address: College of Animal Science and Technology, Northwest A&F University, 22 Xi-Nong Road, Yangling, Shaanxi 712100, China.
Current address: Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada.
Current address: Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Tennessee, Knoxville, TN 37996.
Current address: National Foundation for Fertility Research, Lone Tree, CO 80124.
Supported by National Institutes of Health, National Center for Research Resources (NCRR), Office of Research Infrastructure Programs (ORIP) grant RR17359.
These authors contributed equally to this work.
REFERENCES
- Whyte JJ, Prather RS. Genetic modifications of pigs for medicine and agriculture. Mol Reprod Dev. 2011;78:879–891. doi: 10.1002/mrd.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KH. Nuclear transfer in farm animal species. Semin Cell Dev Biol. 1999;10:245–252. doi: 10.1006/scdb.1999.0310. [DOI] [PubMed] [Google Scholar]
- Gama Sosa MA, De Gasperi R, Elder GA. Animal transgenesis: an overview. Brain Struct Funct. 2010;214:91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Olby N. Perspectives on transgenic livestock in agriculture and biomedicine: an update. Reprod Fertil Dev. 2011;23:56–63. doi: 10.1071/RD10246. [DOI] [PubMed] [Google Scholar]
- Behboodi E, Ayres SL, Memili E, O'Coin M, Chen LH, Reggio BC, Landry AM, Gavin WG, Meade HM, Godke RA, Echelard Y. Health and reproductive profiles of malaria antigen-producing transgenic goats derived by somatic cell nuclear transfer. Cloning Stem Cells. 2005;7:107–118. doi: 10.1089/clo.2005.7.107. [DOI] [PubMed] [Google Scholar]
- Lai L, Prather RS. Progress in producing knockout models for xenotransplantation by nuclear transfer. Ann Med. 2002;34:501–506. doi: 10.1080/078538902321117706. [DOI] [PubMed] [Google Scholar]
- Schnieke AE, Kind AJ, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM. Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nat Biotechnol. 1998;16:642–646. doi: 10.1038/nbt0798-642. [DOI] [PubMed] [Google Scholar]
- Arnold DR, Fortier AL, Lefebvre R, Miglino MA, Pfarrer C, Smith LC. Placental insufficiencies in cloned animals—a workshop report. Placenta. 2008;29((suppl A)):S108–S110. doi: 10.1016/j.placenta.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Bacci ML. A brief overview of transgenic farm animals. Vet Res Commun. 2007;31((suppl 1)):9–14. doi: 10.1007/s11259-007-0001-z. [DOI] [PubMed] [Google Scholar]
- Dinnyes A, Tian XC, Yang X. Epigenetic regulation of foetal development in nuclear transfer animal models. Reprod Domest Anim. 2008;43((suppl 2)):302–309. doi: 10.1111/j.1439-0531.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci U S A. 1994;91:11287–11289. doi: 10.1073/pnas.91.24.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, Garbers DL. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2002;99:14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Shinohara T, Avarbock MR, Brinster RL. Retrovirus-mediated gene delivery into male germ line stem cells. FEBS Lett. 2000;475:7–10. doi: 10.1016/s0014-5793(00)01606-9. [DOI] [PubMed] [Google Scholar]
- Nagano M, Watson DJ, Ryu BY, Wolfe JH, Brinster RL. Lentiviral vector transduction of male germ line stem cells in mice. FEBS Lett. 2002;524:111–115. doi: 10.1016/s0014-5793(02)03010-7. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl. 2007;28:353–360. doi: 10.2164/jandrol.106.001511. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ogura A, Shinohara T. Adenovirus-mediated gene delivery into mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2007;104:2596–2601. doi: 10.1073/pnas.0609282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Megee S, Zeng W, Destrempes MM, Overton SA, Luo J, Galantino-Homer H, Modelski M, Chen F, Blash S, Melican DT, Gavin WG, et al. Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB J. 2008;22:374–382. doi: 10.1096/fj.07-8935com. [DOI] [PubMed] [Google Scholar]
- Russell DW, Kay MA. Adeno-associated virus vectors and hematology. Blood. 1999;94:864–874. [PMC free article] [PubMed] [Google Scholar]
- Bhullar B, Schmidt JV, Truong T, Rancourt D, van der Hoorn FA. Germ cell specific promoter drives ectopic transgene expression during embryogenesis. Mol Reprod Dev. 2001;59:25–32. doi: 10.1002/mrd.1003. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nishimune Y, Okabe M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–283. doi: 10.1016/s0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–28. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–1540. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005;26:698–705. doi: 10.2164/jandrol.05032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I. Germ cell transplantation in pigs–advances and applications. Soc Reprod Fertil Suppl. 2006;62:331–339. [PubMed] [Google Scholar]
- Paczkowski M, Yuan Y, Fleming-Waddell J, Bidwell CA, Spurlock D, Krisher RL. Alterations in the transcriptome of porcine oocytes derived from prepubertal and cyclic females is associated with developmental potential. J Anim Sci. 2011;89:3561–3571. doi: 10.2527/jas.2011-4193. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Ida JM, Paczkowski M, Krisher RL. Identification of developmental competence-related genes in mature porcine oocytes. Mol Reprod Dev. 2011;78:565–575. doi: 10.1002/mrd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Paczkowski M, Krisher RL. The effect of leptin on maturing porcine oocytes is dependent on glucose concentration. Mol Reprod Dev. 2012;79:296–307. doi: 10.1002/mrd.22029. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Krisher RL. In vitro maturation (IVM) of porcine oocytes. Methods Mol Biol. 2012;825:183–198. doi: 10.1007/978-1-61779-436-0_14. [DOI] [PubMed] [Google Scholar]
- Abeydeera LR, Day BN. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol Reprod. 1997;57:729–734. doi: 10.1095/biolreprod57.4.729. [DOI] [PubMed] [Google Scholar]
- Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl. 1993;48:61–73. [PubMed] [Google Scholar]
- Park KW, Lai L, Cheong HT, Im GS, Sun QY, Wu G, Day BN, Prather RS. Developmental potential of porcine nuclear transfer embryos derived from transgenic fetal fibroblasts infected with the gene for the green fluorescent protein: comparison of different fusion/activation conditions. Biol Reprod. 2001;65:1681–1685. doi: 10.1095/biolreprod65.6.1681. [DOI] [PubMed] [Google Scholar]
- Lai L, Park KW, Cheong HT, Kuhholzer B, Samuel M, Bonk A, Im GS, Rieke A, Day BN, Murphy CN, Carter DB, Prather RS. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol Reprod Dev. 2002;62:300–306. doi: 10.1002/mrd.10146. [DOI] [PubMed] [Google Scholar]
- Park KW, Lai L, Cheong HT, Cabot R, Sun QY, Wu G, Rucker EB, Durtschi D, Bonk A, Samuel M, Rieke A, Day BN et al. Mosaic gene expression in nuclear transfer-derived embryos and the production of cloned transgenic pigs from ear-derived fibroblasts. Biol Reprod. 2002;66:1001–1005. doi: 10.1095/biolreprod66.4.1001. [DOI] [PubMed] [Google Scholar]
- Kim BG, Cho CM, Lee YA, Kim BJ, Kim KJ, Kim YH, Min KS, Kim CG, Ryu BY. Enrichment of testicular gonocytes and genetic modification using lentiviral transduction in pigs. Biol Reprod. 2010;82:1162–1169. doi: 10.1095/biolreprod.109.079558. [DOI] [PubMed] [Google Scholar]
- Niemann H, Kues W, Carnwath JW. Transgenic farm animals: present and future. Rev Sci Tech. 2005;24:285–298. [PubMed] [Google Scholar]
- Zeng W, Tang L, Bondareva A, Luo J, Megee SO, Modelski M, Blash S, Melican DT, Destrempes MM, Overton SA, Gavin WG, Ayres S, et al. Nonviral transfection of goat germline stem cells by nucleofection results in production of transgenic sperm after germ cell transplantation. Mol Reprod Dev. 2012;79:255–261. doi: 10.1002/mrd.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izsvak Z, Frohlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Methods. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z, Izsvak Z, Medrano G, Chapman KM, Hamra FK. Sleeping Beauty transposon mutagenesis in rat spermatogonial stem cells. Nat Protoc. 2011;6:1521–1535. doi: 10.1038/nprot.2011.378. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Taylor L, Sherman A, Kawakami K, Takahashi Y, Sang HM, McGrew MJ. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons Proc Natl Acad Sci U S A 2012. 109 E1466E 1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, Cost GJ, Niemann H. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Umeyama K, Matsunari H, Takayanagi S, Haruyama E, Nakano K, Fujiwara T, Ikezawa Y, Nakauchi H, Nagashima H. Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochem Biophys Res Commun. 2010;402:14–18. doi: 10.1016/j.bbrc.2010.09.092. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Zhao J, Wells KD, Samuel MS, Whitworth KM, Walters EM, Laughlin MH, Prather RS. Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs. Mol Reprod Dev. 2011;78:2. doi: 10.1002/mrd.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Yang H, Li W, Zhao B, Ouyang Z, Liu Z, Zhao Y, Fan N, Song J, Tian J, Li F, Zhang J et al. Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res. 2011;21:979–982. doi: 10.1038/cr.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Lo TW, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, Zhang L, Rebar EJ, et al. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011;29:699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesson L, Usal C, Menoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng X, Zhang L, Gregory PD, Anegon I et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]