Abstract
Colorectal cancer (CRC) screening uptake among minorities and those with lower incomes is suboptimal. Behavioral interventions specifically tailored to these populations can increase screening rates and save lives. The Precaution Adoption Process Model (PAPM) allows assignment of a decisional stage for adoption of a behavior such as CRC screening. Here, we characterize the PAPM decisional stage distribution among 470 low income, racially and ethnically diverse study participants at intake into a behavioral intervention study designed to increase CRC screening uptake. We staged participants for stool blood test (SBT) and colonoscopy separately and used the highest stage for the two tests as the ‘overall’ stage for CRC screening. For SBT, sex, language (English versus Spanish) and doctor recommendation were significantly related to PAPM stage for CRC screening. For colonoscopy, language, education level, doctor recommendation and self-efficacy were related to stage. For overall CRC screening stage, all the variables associated with either SBT or colonoscopy, with the exception of language were significant. This study suggests attending to these key variables in designing interventions to promote CRC screening, particularly with respect to medically underserved populations.
Introduction
Colorectal cancer (CRC), the second most common cause of cancer death in the United States for cancer affecting both men and women, is preventable and curable [1]. Minorities and those with low incomes have lower rates of screening and higher rates of CRC mortality than the majority population [2]. Many factors contribute to suboptimal uptake of cancer screening within the nation’s underserved subgroups: inadequate access to screening services, limited awareness of screening guidelines and requirements and aversion to features of screening procedures may all play important roles [3–5]. Less studied is the decision-making process that screening eligible adults go through as they consider getting tests within the health care system. Although research has shown a strong relationship between provider recommendations and CRC screening completion, there are many individuals who receive a provider recommendation, yet do not complete testing [6–8]. In other situations, screening eligible individuals may receive a recommendation, decide to move forward with testing but have delays of months or even years between the recommendation, the decision and eventual screening completion.
For those receiving a physician CRC screening recommendation, lack of awareness of CRC risk, preventability and screening options may lead to low personal relevance, low motivation and low perceived behavioral control [9]. Even with awareness and education, beliefs, such as cancer fatalism and barriers associated with poverty may affect CRC screening completion [10, 11]. Physician or healthcare system programs to promote CRC screening are challenged by the need to increase awareness, educate, address specific beliefs and barriers and provide access to screening.
The concept of tailoring information to patients’ stage of readiness and decision making is receiving increasing attention [12]. A useful model for evaluating people’s stage of readiness for adopting preventive behaviors (precautions) like CRC screening is the Precaution Adoption Process Model (PAPM) [13, 14]. The PAPM is a stage theory of health behavior, which describes the process of behavior change as a series of seven steps or stages (Fig. 1) [13]. We chose the PAPM because of our prior qualitative studies and because this model allowed us to differentiate between participants who are unaware of recommendations (Stage 1) and those who are simply unengaged with the issue of CRC screening (Stage 2). In addition, unlike other behavioral theories, the PAPM framework allows us to identify those who are aware of the screening methods and their CRC risk yet have decided not to be screened (Stage 4) [15].
Fig. 1.
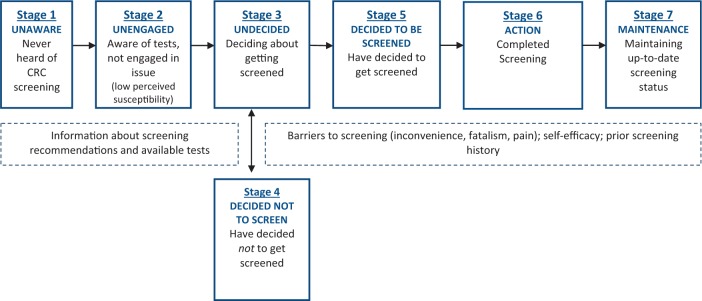
Description of the stages of the PAPM that were used to stage participants for CRC screening in this study.
The PAPM has been previously used to evaluate decisional stage for CRC screening in primary care patients in Massachusetts, in participants completing the National Cancer Institute’s Health Information National Trends Survey and in an intervention in Adelaide, Australia [16–18]. The studies by Ferrer et al. and Costanza et al. were undertaken in the United States. Both studies used information from participants who were primarily white and non-Hispanic (85% and 94%, respectively). Trauth et al. also explored CRC screening stage in a low-income population in the United States (also predominantly white, 90%) using a different stage model, the Transtheoretical Model (TTM), from which the PAPM was developed [19]. In the TTM, people who are unaware of an issue/risk and those who are aware but not considering making a change in their behavior are grouped into the precontemplation stage and cannot be distinguished from one another. However, Trauth et al. subdivided their precontemplation participants into unaware (comparable to PAPM Stage 1) and precontemplation (comparable to PAPM Stage 2). Participants in all three of the US studies were found to be distributed throughout the stages with numerous factors relating to stage, such as worry/perceived risk, fatalism, prior screening history for other cancers and some demographic factors (sex, financial situation, education level, marital status, etc.).
Here, we describe analysis of data on PAPM staging collected at enrollment in a large CRC screening study comprised entirely of screening eligible low-income patients recruited from urban safety-net clinics. This project provided a unique opportunity to explore the baseline decisional stage distribution in low-income population that was less than one-third white. Prior to participation in a motivational intervention to improve CRC screening uptake, study participants were staged for CRC screening decision making using PAPM questions. PAPM staging questions were asked for both colonoscopy and ‘home stool blood tests’ [SBT: refers to fecal occult blood testing (FOBT) or fecal immunochemical testing (FIT), kits performed at home; for the sake of consistency, we use SBT hereafter to refer to FIT and FOBT]. Because all participants were age 50 or over (one participant was 49 at enrollment) but not current with CRC screening, we anticipated that the majority of participants would fall within Stages 1–4, recognizing that some would have already decided to be screened by a particular method (Stage 5, Fig. 1) but would have not yet completed screening (FIT or colonoscopy was offered at no cost to participants in the study). Here, we describe PAPM decisional stage distribution prior to beginning the intervention and discuss the variables associated with decisional stage at intake in this racially and ethnically diverse, low-income sample. We are not aware of other studies describing CRC decisional stage distribution or the correlates of PAPM stage among such a diverse group of underserved patients or as part of efforts to reduce CRC screening disparities for minorities.
Methods
This study was approved by the Institutional Review Board of the University of Kansas Medical Center. We recruited a convenience sample of 470 participants from the waiting rooms of nine safety-net clinics in a large metropolitan area between 2008 and 2010. Study staff approached potential participants and asked them to complete a questionnaire to determine eligibility (i.e. age 50 or over, not up-to-date with CRC screening). The eligibility form also assessed participants’ awareness of CRC screening options and PAPM decisional stage for CRC screening. We assessed PAPM decisional stage for SBT, colonoscopy, and created an ‘overall’ CRC screening decisional stage by combining the answers for both SBT and colonoscopy. PAPM stages were determined to be ‘unaware’, ‘unengaged’, ‘deciding’, ‘decided not to screen’ or ‘decided to be screened’ (PAPM Stages 1–5; see Fig. 1) based on questions asked for each test modality: i.e. ‘Have you thought about doing/having a stool blood test/colonoscopy in the future?’ and ‘Which of the following statements best describes your thoughts about doing an at-home stool blood test in the future?’ Items were adapted from the work of Weinstein, Costanza and Myers [14, 16, 20]. This work is based on data from the participants (n = 470) who were eligible for and consented to participate in the study; no data were retained for those who were ineligible or who declined to participate.
Upon consent and enrollment into the study (after completion of the eligibility questionnaire), participants completed additional surveys on touchscreen computers. The touchscreen survey collected demographic information, and all variables were measured by self-report from participants. Cancer fatalism items assessed attitudes toward getting cancer and the effectiveness of treatments. We adapted items from the Powe Cancer Fatalism Inventory with all 10 cancer fatalism items measured by Likert scale [11, 21]. These included: ‘I think if someone has cancer, it is already too late to get treated’ and ‘I think many people who have cancer treatment get better and go on with their lives’. The responses to the fatalism questions were reverse coded as necessary and summed; higher scores indicated more fatalistic cancer beliefs. Perceived self-efficacy refers to participants’ self-confidence in their ability to complete a test. Self-efficacy was assessed with one question: ‘If you decided you wanted to get colon cancer screening, how likely is it that you could do a test?’ adapted from prior multi-item self-efficacy measures [22–24]. For CRC perceived risk, we utilized a three-item scale developed by Vernon et al. [25]. Example items included ‘How likely do you think it is that you will develop colon cancer in the future?’ and ‘How often do you worry about getting colon cancer?’
Theoretical framework
The PAPM suggests that adopting new precautions, such as CRC screening, requires conscious awareness, decision and deliberate action (Fig. 1) [13, 14]. The PAPM also suggests that people in different decisional stages may benefit from different kinds of interventions. Initially (Stage 1), people need general information about the existence of CRC and screening. As they gain awareness (Stage 2), they may be more receptive to information about screening guidelines, risk factors for CRC and mortality associated with CRC. Engagement with relevant information should motivate people to decide whether or not to screen (Stage 3). In this stage, most people will evaluate the pros and cons of screening; elaboration of the potential risks and benefits of action may be helpful in motivating people to decide to be screened (Stage 5), or may lead some people to decide not to be screened (Stage 4). Once people have decided to be screened (Stage 5), they must overcome any barriers to screening and take whatever action is necessary to be screened (Stage 6). Finally, after having overcome the barriers and implemented a behavior, people will need to face various challenges to maintaining the behavior (Stage 7).
PAPM staging
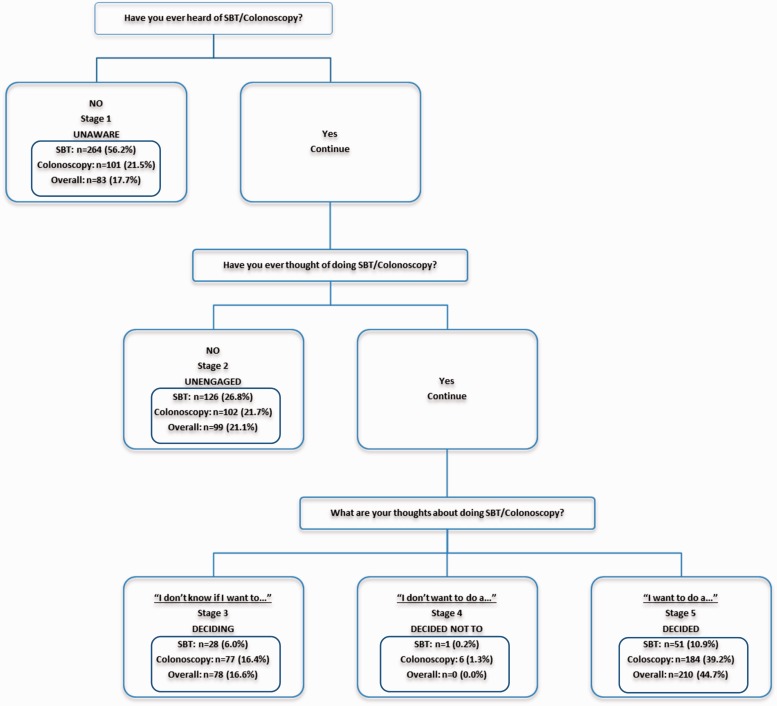
The staging schema is depicted in Fig. 2. Based on their answers to the questions on the screening form, participants could be placed in any of the PAPM stages from 1 to 5 (Fig. 1). Participants were staged for SBT, colonoscopy and overall CRC screening. Overall screening stage represented the most advanced stage of screening adoption reported by each participant, whether that was for colonoscopy or SBT. Participants who were in Stage 4 (decided not to be screened) would have been considered Stage 4 for overall screening if they were in Stage 4 for both SBT and colonoscopy (n = 0; Fig. 3A).
Fig. 2.
PAPM staging schema including the number (and percent) of participants at each stage for each testing modality and overall.
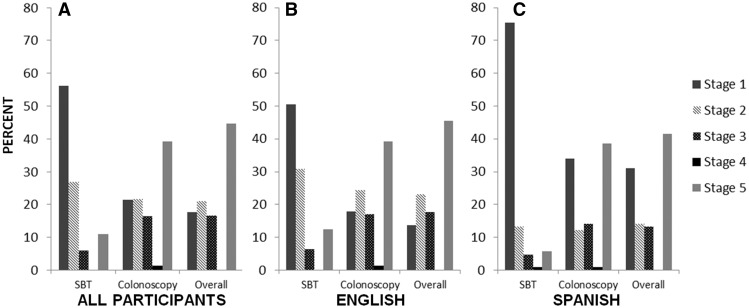
Fig. 3.
PAPM stage distribution of participants at baseline. (A) Stage distribution for all participants. (B) Stage distribution for English-speaking participants. (C) Stage distribution for Spanish-speaking participants.
Analyses
We applied polytomous logistic regression with baseline logit models to look for variables associated with decisional stage for adoption of CRC screening at intake. Stage 1 (unaware) was chosen as the reference category. Twelve explanatory variables were included in the models: clinic where the participant was recruited; age; sex; marital status; education; employment status; insured status; language (English or Spanish); report of a prior doctor’s recommendation to be screened for CRC; self-efficacy for CRC screening; perceived risk of CRC and cancer fatalism score (see Table I). Note that we combined race and ethnicity information into a single race/ethnicity variable for analysis. Race/ethnicity was highly overlapping with language, requiring that we include only one of these variables in our analyses to avoid the issue of co-linearity. We chose to use language rather than race/ethnicity because of predictive power and sparseness (there were 14 participants in the ‘other’ race/ethnicity group that would have been discarded if race/ethnicity was used as a predictor).
Table I.
Participant characteristics
| Characteristic | N (%) |
|---|---|
| Age | |
| Mean age in years (±SD) | 56.5 (±5.8) |
| Sex | |
| Female | 299 (63.6%) |
| Male | 171 (36.4%) |
| Ethnicity/race | |
| Hispanic | 126 (27%) |
| Non-Hispanic white | 132 (28%) |
| Non-Hispanic African American | 198 (42%) |
| Non-Hispanic other | 14 (3%) |
| Language | |
| English | 364 (77.4%) |
| Spanish | 106 (22.6%) |
| Marital status | |
| Married or living with partner | 155 (33%) |
| Divorced or separated | 171 (36%) |
| Widowed or never married | 144 (31%) |
| Education | |
| High school or below | 281 (60%) |
| Some college or above | 189 (40%) |
| Employment | |
| Full-time/part-time/seasonal | 139 (29.6%) |
| Looking/homemaker/student/retired | 247 (52.6%) |
| Disability | 84 (17.9%) |
| Insured | |
| Yes | 110 (23.4%) |
| No | 360 (76.6%) |
| Doctor recommendation | |
| Yes | 243 (51.7%) |
| No | 227 (48.3%) |
| Prior screening | |
| SBT | 70 (14.9%) |
| Colonoscopy | 46 (9.8%) |
| Either SBT or colonoscopy | 105 (22.3%) |
| Self-efficacy (very high) | |
| Yes | 264 (56.2%) |
| No | 206 (43.8%) |
| Perceived risk (range: 3–9) | |
| Mean perceived risk (±SD) | 4.9 (1.5) |
| Fatalism score (range: 10–30) | |
| Mean fatalism score (±SD) | 24.1 (3.4) |
Results
Participant characteristics
Participant characteristics (n = 470) are listed in Table I. The study sample was racially and ethnically diverse (note that race/ethnicity information was combined into one race/ethnicity variable for analysis, see ‘Methods’ section). Most of the participants spoke English as their primary language (n = 364, 77.4%), whereas 22.6% (n = 106) spoke Spanish. The majority of the participants rated themselves as having ‘very high’ self-efficacy (being very likely to complete a CRC screening test upon deciding to do so; n = 264, 56.2%). On a scale ranging from 3 to 9, with 9 meaning the highest perceived susceptibility to CRC, the mean perceived risk in the study sample was 4.9 (±1.5). The mean fatalism score was 24.1 (±3.4) on a scale ranging from 10 to 30, with higher numbers indicating more fatalistic beliefs.
Stage distributions
The PAPM stage distributions of the 470 study participants for SBT, colonoscopy and overall screening are depicted in Fig. 3, and the numbers of participants in each stage for each test are shown in Fig. 2. All participants were staged for both tests and therefore had a separate stage for SBT, colonoscopy and overall. Strikingly, 56.2% (n = 264) of participants had not heard of SBT at intake (Stage 1), whereas only 21.5% (n = 101) of participants had not heard of colonoscopy. A sizeable minority of participants (17.7%; n = 83) were unaware of either screening alternative, placing them in Stage 1 overall. Interestingly, approximately one-third of Spanish-speaking participants were in Stage 1 for overall screening, with three quarters being in Stage 1 for SBT (Fig. 3C). Participants who were aware but unengaged were more evenly distributed across test type (Stage 2). Fewer participants were undecided for SBT (Stage 3) than for colonoscopy and overall. Very few participants had decided against screening (Stage 4): 0.2% (n = 1) for SBT and 1.3% (n = 6) for colonoscopy. None of these participants had decided against screening using both tests (overall), meaning that those who had decided against colonoscopy had not decided against SBT and vice versa. Among participants who had already decided to screen (Stage 5), 10.9% (n = 51) had decided to be screened by SBT and 39.2% (n = 184) by colonoscopy. (Note that participants could indicate that they wanted both SBT and colonoscopy; they were not forced to choose one test modality or the other at this point in time.) Overall, 44.7% (n = 210) expressed an intention to be screened by one or both methods at intake (Stage 5).
SBT decisional stage
At intake, decisional stage for SBT was significantly associated with sex [Wald , P = 0.003], language [Wald , P = 0.009] and doctor’s recommendation [Wald , P < 0.0001]. Baseline logit models were adjusted for appropriate variables (Table II). After adjustment, women were more likely than men to be in Stages 2 or 3 as opposed to Stage 1. Compared to Spanish-speaking participants, English-speaking participants were less likely to be in Stage 1 and more likely to be in Stages 2 or 5 (Table II; Fig. 3B and C). Participants whose doctors had recommended CRC screening in the past were more likely to be in Stages 2, 3 and 5 versus Stage 1 than their counterparts without a doctor’s recommendation.
Table II.
Odds ratios, 95% confidence intervals and P-values obtained from the baseline logit models
| CRC screening method | Stage 2 | Stage 3 | Stage 5 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| SBTa | ||||||
| Female sex | 2.40 (1.43, 4.03) | 0.0009* | 3.00 (1.10, 8.17) | 0.031* | 1.84 (0.90, 3.77) | 0.10 |
| English language | 2.80 (1.22, 6.39) | 0.015* | 4.58 (1.24, 17.0) | 0.023* | 3.72 (1.14, 12.2) | 0.030* |
| Doctor recommendation | 3.19 (1.96, 5.19) | <0.0001* | 3.28 (1.33, 8.13) | 0.010* | 4.40 (2.12, 9.10) | <0.0001* |
| Colonoscopyb | ||||||
| English language | 3.68 (1.40, 9.68) | 0.008* | 1.15 (0.42, 3.17) | 0.79 | 0.97 (0.43, 2.19) | 0.93 |
| College education or higher | 2.00 (0.96, 4.17) | 0.07 | 3.16 (1.44, 6.94) | 0.004* | 3.23 (1.62, 6.43) | 0.0008* |
| Doctor recommendation | 2.05 (1.09, 3.86) | 0.025* | 4.78 (2.41, 9.49) | <0.0001* | 3.03 (1.71, 5.36) | 0.0001* |
| Self-efficacy | 0.79 (0.43, 1.45) | 0.44 | 1.30 (0.67, 2.53) | 0.43 | 2.06 (1.18, 3.60) | 0.011* |
| Overallc | ||||||
| Female sex | 3.43 (1.72, 6.83) | 0.0005* | 3.22 (1.54, 6.74) | 0.002* | 2.73 (1.46, 5.11) | 0.002* |
| College education or higher | 1.75 (0.76, 4.04) | 0.19 | 3.32 (1.38, 7.96) | 0.007* | 3.98 (1.82, 8.69) | 0.0006* |
| Doctor recommendation | 2.71 (1.33, 5.52) | 0.006* | 7.55 (3.52, 16.2) | <0.0001* | 5.61 (2.91, 10.8) | <0.0001* |
| Self-efficacy | 1.14 (0.59, 2.20) | 0.69 | 1.81 (0.89, 3.69) | 0.10 | 2.17 (1.18, 3.98) | 0.013* |
*Indicates statistical significance. aAdjusted for clinic, age, marital status, education, employment status, insurance status, self-efficacy, perceived risk and fatalism. bAdjusted for clinic, age, gender, marital status, employment status, insurance status, perceived risk and fatalism. cAdjusted for clinic, age, preferred language, marital status, employment status, insurance status, perceived risk and fatalism. Variance inflation factor <2 for all explanatory variables.
Colonoscopy decisional stage
At intake, decisional stage for colonoscopy was significantly associated with language [Wald , P = 0.024], education [Wald , P = 0.006], doctor’s recommendation [Wald , P < 0.0001] and self-efficacy [Wald , P = 0.003]. Baseline logit models were adjusted for appropriate variables (Table II). After adjustment, English-speaking participants were less likely than Spanish-speaking participants to be in Stage 1 and more likely to be in Stage 2, for colonoscopy (Table II; Fig. 3B and C). Compared to participants with high school diploma/General Educational Development (GED) attainment or lower education levels, participants who completed college or received even higher education levels were more likely to be in Stage 3 and Stage 5 versus Stage 1 for colonoscopy but were not significantly more likely to be in Stage 2 than in Stage 1. Participants whose doctors had recommended CRC screening in the past were more likely to be in Stages 2, 3 and 5, respectively, than their counterparts without a doctor’s recommendation. Finally, participants who ranked themselves at the highest level of self-efficacy for CRC screening were more likely to be in Stage 5 (decided to screen by colonoscopy) than in Stage 1 (unaware of colonoscopy screening).
Overall CRC screening decisional stage
At intake, overall decisional stage for either CRC screening test was significantly associated with sex [Wald , P = 0.002], education [Wald , P = 0.001], doctor’s recommendation [Wald , P < 0.0001] and self-efficacy [Wald , P = 0.029]. Baseline logit models were adjusted for appropriate variables (Table II). After adjustment, women were more likely than men to be in Stages 2, 3 or 5 as opposed to Stage 1 (Table II). As in the colonoscopy staging model, participants who completed college or attained even higher education levels were more likely to be in Stage 3 and Stage 5 versus Stage 1 as compared to participants with high school diploma/GED or lower education levels but were not significantly more likely to be in Stage 2. Participants whose doctors had recommended CRC screening in the past were more likely to be in Stages 2, 3 and 5 for overall stage than their counterparts without a doctor’s recommendation after adjusting the other variables. Participants who ranked themselves as having very high self-efficacy for CRC screening were more likely to be in Stage 5 than in Stage 1 for overall stage compared to participants who placed their self-efficacy below very high (Table II). The odds in favor of being in Stages 2 or 3 against Stage 1 for overall screening were not significantly associated with self-efficacy.
Discussion
Stage models assume that people go through a progression of thoughts or stages when considering a health behavior and ultimately taking action. Weinstein and Sandman’s revised PAPM outlines several possible reasons that an individual may not have adopted a given preventive health behavior [26]. All people who have not acted are not the same, and determining where in the decision-making process they are should allow researchers to design more effective interventions. Recognizing that not all people start at the same decisional stage and thus might not have the same interventional needs could help improve the efficacy of intervention efforts [12]. In this analysis of baseline data from racially and ethnically diverse (see Table I), low-income participants enrolled in a CRC screening intervention, we wanted to characterize and better understand the decisional diversity in participants pre-intervention to distinguish among the following: (i) people who were completely unaware of CRC screening methods (Stage 1); (ii) people who did not feel that CRC screening was relevant to them (i.e. not engaged; Stage 2); (iii) people who were aware, engaged and trying to decide whether or not to screen for CRC, and if so, what screening method to use (Stage 3) and (iv) people who had already thought about screening and actively decided against it (Stage 4). From a preventive care perspective, those who have already decided to screen and have chosen their preferred screening method (Stage 5) may be in need of screening opportunities and assistance overcoming barriers to screening, rather than intensive education. Those who have already adopted a screening behavior (Stage 6) and remain up to date with screening over time (Stage 7) were not eligible for this study and are unlikely to need intervention.
Information about decisional stage distribution for CRC screening among the underserved, and not necessarily their ultimate screening completion, is crucial for better targeting efforts to increase suboptimal screening rates in these populations. We found a large percentage (82.3%, Fig. 3A) of our study population had heard of at least one CRC screening method; however, nearly 18% of our participants were unaware of CRC screening options. While we would expect that those in Stages 3 (deciding) and 5 (decided to) are likely to eventually get screened, those in Stages 1 (unaware) and 2 (unengaged) are at most risk of not making a final decision about being screened. Our findings indicate that interventions tailored to underserved populations in Stages 1 and 2 that are designed to move people forward in the decision-making process by providing information necessary to raise awareness and perceived susceptibility would be important in this population.
Minority participants are well represented in our sample (27% Hispanic, 42% non-Hispanic African American, 28% non-Hispanic white and 3% non-Hispanic other; Table I). African Americans and Hispanics, especially Hispanics who speak primarily Spanish, have not been well represented in studies on CRC screening decision making to date, and members of these racial/ethnic groups have different perceptions, barriers and influences on their decision-making process with regard to CRC screening than whites [27–29].
It is important to note that we considered another potential factor that could presumably influence decisional stage for CRC: prior CRC screening experience. First, English-speaking participants were more likely to have prior CRC screening experience than Spanish-speaking participants, which could explain why more Spanish-speaking participants were in Stage 1 (unaware) than English-speaking participants (Fig. 3). Because prior experience of CRC screening was used to define PAPM staging (participants are above Stage 1 if they have had prior screening), participants with prior screening were all in Stages 2–5, and none were in Stage 1. As a result of this, completion of prior screening and its effect on stage could not be included in the full analysis model but were explored herein by polytomous logistic regression, although the data from these analyses are not shown. A subset analyses using participants in Stages 2, 3 and 5 (n = 205 for SBT, n = 363 for colonoscopy and n = 387 for overall) revealed that prior screening was not significantly associated with PAPM staging (results not shown) for any screening test.
Women were more likely than men to be unengaged (Stage 2) or deciding (Stage 3) for SBT and Stage 2, 3 or 5 (decided) for overall screening. In 2010, women and men had approximately equal rates of CRC screening (58.8% for women, 58.5% for men); however, in some studies of smaller groups, women were less likely than men to be up-to-date with CRC screening [30–32]. Other reports have shown that men were more likely to be screened by sigmoidoscopy or colonoscopy (by self-report or medical data), whereas women were more likely to be screened by SBT (self-report) [33]. Our data suggest that women may be more likely to be aware of and considering CRC screening than men, but progress through the stages differently than men, with men potentially moving from deciding to act (Stage 5) to action (Stage 6) more quickly than women.
A physician’s recommendation can be powerful for promoting CRC screening. Multiple studies have identified this association [34, 35]. In our study, across screening methods, people with a doctor’s recommendation were nearly eight times more likely to be in Stage 3 (deciding whether or not to screen) and nearly six times more likely to be in Stage 5 (having decided in favor of screening) versus Stage 1 (unaware of screening) than those without such recommendation (Table II). Interestingly, although Spanish-speaking participants were just as likely to report a doctor’s recommendation to screen for CRC, they were less likely than English-speaking participants to be aware of specific screening methods (Fig. 3). This may be due to language barriers with physicians who are primarily English speaking. In addition, health literacy could play an important role, as studies have linked low English proficiency with poor health and low screening rates [36–38]. Discussion of screening may not be as detailed when there is a language barrier or the patient may not understand the explanation of tests provided.
Education is often a factor in screening participation. Our findings that participants with college education or higher were more likely to be deciding (Stage 3) or decided (Stage 5) than unaware (Stage 1) for colonoscopy and overall screening are consistent with other studies where higher education is associated with CRC screening uptake [39–41]. Despite low incomes, we see here that the relationship between education and screening uptake persists with decisional stage for CRC screening.
The highest level of self-efficacy was associated only with decisional stage for colonoscopy, and the association was strong enough to be a factor in overall decisional stage. It may be that the requirements of colonoscopy preparation are high enough that having very high self-efficacy for screening is essential for those deciding to screen using this method, but less important in deciding to screen using SBT. Future studies that address specific barriers for each screening method may be able to address this finding more fully.
Our data are useful for researchers and providers alike because we report staging for SBT and colonoscopy independently, in addition to staging for overall CRC screening. Knowing decisional stage distribution by test type is important because colonoscopy may not a viable option for many people, particularly those with low incomes, even when colonoscopy is preferred. SBT is simple and inexpensive with far fewer logistical barriers to completion than colonoscopy (i.e. no transportation required, no laxative, no adherence to clinic schedules, etc.), although social and psychological barriers can hinder SBT completion. We found a disproportionate number of participants who were unaware of SBT as a screening method compared to colonoscopy (56.2% of participants were in Stage 1 for SBT as opposed to 21.5% in Stage 1 for colonoscopy; Fig. 3A), with a striking lack of awareness of SBT among Spanish speakers (Fig. 3C). Some studies have shown that SBT is still preferred by some patients over colonoscopy, even when out of pocket costs are equivalent [42]. For many low-income patients, having a low-cost, low-risk, time-effective screening method may make the difference between getting screened and not getting screened for CRC; it is important to improve education and communication about this alternative.
The purpose of this initial study was to examine decisional stage at study intake for a racially and ethnically diverse group of low-income adults who were not up to date with CRC screening. Decisional stage information is useful for understanding and identifying where patients are in considering screening and for better characterizing the interventional needs of the group. The inherent diversity of individuals makes a tailored approach critical for maximum success in an intervention targeted at individuals. Tailored interventions may be ideal for overcoming obstacles to care and advancing a patient’s decisional stage. Tailoring on appropriate variables can truly meet the patient’s decisional stage and psychosocial needs, diverting the limited time available for intervention in clinics to where it is most needed. Awareness of decisional stage for this population should allow for the design of more effective interventions that address the needs of the patient and facilitate progression to the next decisional stage. We gathered this baseline information about decisional stage to inform a tailored CRC screening intervention designed to walk people through subsequent decisional stages by heightening awareness of screening methods, increasing the personal relevance of CRC risk, asking questions that encourage engagement, elaboration and self-efficacy and encouraging personal choice and commitment to undergoing CRC screening.
Acknowledgements
A strong partnership with the Wyandotte Country Kansas Safety Net Clinic Coalition was critical for the success of this study. The authors would like to thank study staff in the Family Medicine Research Division, particularly Angela Watson, M.B.A., Marina Carrizosa-Ramos, Heraclio Perez, Megan Eckles, M.P.H., Andrew Witt, Kris Neuhaus, M.D., M.P.H., Crystal Lumpkins, Ph.D. and Aaron Epp, for help with recruitment, survey administration and data collection.
Funding
The National Cancer Institute at the National Institutes of Health [R01 CA123245 (PI: Greiner) and U54 CA154253 (PI: Greiner) to C.M.H., C.M.D. and K.A.G., U54-CA153460 (PI: Colditz) to A.S.J.]; and the Barnes-Jewish Hospital Foundation [to A.S.J.].
Conflict of interest statement
None declared.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro JA, Klabunde CN, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene P, Mehta P, Yeary KH, et al. Using population data to reduce disparities in colorectal cancer screening, arkansas, 2006. Prev Chronic Dis. 2012;9:E138. doi: 10.5888/pcd9.110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis TC, Dolan NC, Ferreira MR, et al. The role of inadequate health literacy skills in colorectal cancer screening. Cancer Invest. 2001;19:193–200. doi: 10.1081/cnv-100000154. [DOI] [PubMed] [Google Scholar]
- 5.Ghevariya V, Duddempudi S, Ghevariya N, et al. Barriers to screening colonoscopy in an urban population: a study to help focus further efforts to attain full compliance. Int J Colorectal Dis. 2013;28:1497–503. doi: 10.1007/s00384-013-1708-7. [DOI] [PubMed] [Google Scholar]
- 6.Wolf MS, Satterlee M, Calhoun EA, et al. Colorectal cancer screening among the medically underserved. J Health Care Poor Underserved. 2006;17:47–54. doi: 10.1353/hpu.2006.0037. [DOI] [PubMed] [Google Scholar]
- 7.Hudson SV, Ferrante JM, Ohman-Strickland P, et al. Physician recommendation and patient adherence for colorectal cancer screening. J Am Board Fam Med. 2012;25:782–91. doi: 10.3122/jabfm.2012.06.110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernon SW, Bartholomew LK, McQueen A, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011;41:284–99. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 10.Born W, Engelman K, Greiner KA, et al. Colorectal cancer screening, perceived discrimination, and low-income and trust in doctors: a survey of minority patients. BMC Public Health. 2009;9:363. doi: 10.1186/1471-2458-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greiner KA, James AS, Born W, et al. Predictors of fecal occult blood test (FOBT) completion among low-income adults. Prev Med. 2005;41:676–84. doi: 10.1016/j.ypmed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Longo DR, Woolf SH. Rethinking the information priorities of patients. JAMA. 2014;311:1857–8. doi: 10.1001/jama.2014.3038. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–86. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein ND, Lyon JE, Sandman PM, et al. Experimental evidence for stages of health behavior change: the precaution adoption process model applied to home radon testing. Health Psychol. 1998;17:445–53. doi: 10.1037//0278-6133.17.5.445. [DOI] [PubMed] [Google Scholar]
- 15.Greiner KA, Born W, Nollen N, et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005;20:977–83. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costanza ME, Luckmann R, Stoddard AM, et al. Applying a stage model of behavior change to colon cancer screening. Prev Med. 2005;41:707–19. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Flight IH, Wilson CJ, Zajac IT, et al. Decision support and the effectiveness of web-based delivery and information tailoring for bowel cancer screening: an exploratory study. JMIR Res Protoc. 2012;1:e12. doi: 10.2196/resprot.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer RA, Hall KL, Portnoy DB, et al. Relationships among health perceptions vary depending on stage of readiness for colorectal cancer screening. Health Psychol. 2011;30:525–35. doi: 10.1037/a0023583. [DOI] [PubMed] [Google Scholar]
- 19.Trauth JM, Ling BS, Weissfeld JL, et al. Using the transtheoretical model to stage screening behavior for colorectal cancer. Health Educ Behav. 2003;30:322–36. doi: 10.1177/1090198103030003007. [DOI] [PubMed] [Google Scholar]
- 20.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 21.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–92. [PubMed] [Google Scholar]
- 22.Myers RE, Vernon SW, Tilley BC, et al. Intention to screen for colorectal cancer among white male employees. Prev Med. 1998;27:279–87. doi: 10.1006/pmed.1998.0264. [DOI] [PubMed] [Google Scholar]
- 23.McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiol Biomarkers Prev. 2008;17:2231–7. doi: 10.1158/1055-9965.EPI-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012;118:2726–34. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–32. [PubMed] [Google Scholar]
- 26.Weinstein ND, Sandman PM. A model of the precaution adoption process: evidence from home radon testing. Health Psychol. 1992;11:170–80. doi: 10.1037//0278-6133.11.3.170. [DOI] [PubMed] [Google Scholar]
- 27.Brittain K, Murphy VP. Sociocultural and health correlates related to colorectal cancer screening adherence among urban African Americans. Cancer Nurs. 2014;00:1–7. doi: 10.1097/NCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46:228–36. doi: 10.1016/j.amepre.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Moehring J, Stuhr S, et al. Barriers to colorectal cancer screening in Hispanics in the United States: an integrative review. Appl Nurs Res. 2013;26:218–24. doi: 10.1016/j.apnr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–5. [PubMed] [Google Scholar]
- 31.Yager SS, Chen L, Cheung WY. Sex-based disparities in colorectal cancer screening. Am J Clin Oncol. 2014;37:555–60. doi: 10.1097/COC.0b013e318282a830. [DOI] [PubMed] [Google Scholar]
- 32.Gancayco J, Soulos PR, Khiani V, et al. Age-based and sex-based disparities in screening colonoscopy use among medicare beneficiaries. J Clin Gastroenterol. 2013;47:630–6. doi: 10.1097/MCG.0b013e31828345c8. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan S, Wolf JL. Colorectal cancer screening and prevention in women. Women's Health (Lond Engl) 2011;7:213–26. doi: 10.2217/whe.11.7. [DOI] [PubMed] [Google Scholar]
- 34.Guerra CE, Schwartz JS, Armstrong K, et al. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22:1681–8. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert A, Kanarek N. Colorectal cancer screening: physician recommendation is influential advice to Marylanders. Prev Med. 2005;41:367–79. doi: 10.1016/j.ypmed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Sentell T, Braun KL. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17(Suppl. 3):82–99. doi: 10.1080/10810730.2012.712621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garbers S, Chiasson MA. Inadequate functional health literacy in Spanish as a barrier to cervical cancer screening among immigrant Latinas in New York City. Prev Chronic Dis. 2004;1:A07. [PMC free article] [PubMed] [Google Scholar]
- 38.Guerra CE, Krumholz M, Shea JA. Literacy and knowledge, attitudes and behavior about mammography in Latinas. J Health Care Poor Underserved. 2005;16:152–66. doi: 10.1353/hpu.2005.0012. [DOI] [PubMed] [Google Scholar]
- 39.Power E, Miles A, von Wagner C, et al. Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncol. 2009;5:1371–88. doi: 10.2217/fon.09.134. [DOI] [PubMed] [Google Scholar]
- 40.Guessous I, Dash C, Lapin P, et al. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Holden DJ, Jonas DE, Porterfield DS, et al. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–76. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 42.Quick BW, Hester CM, Young KL, et al. Self-reported barriers to colorectal cancer screening in a racially diverse, low-income study population. J Commun Health. 2013;38:285–92. doi: 10.1007/s10900-012-9612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]