Abstract
This pilot study evaluated an innovative diabetes symptom awareness and self-management educational program for Mexican Americans, a fast growing minority population experiencing a diabetes epidemic. Patients with diabetes need assistance interpreting and managing symptoms, which are often annoying and potentially life-threatening. A repeated measures randomized controlled trial was conducted with 72 Mexican Americans aged 25–75 years with type 2 diabetes. Experimental condition participants received eight weekly, in-home, one-on-one educational and behavior modification sessions with a registered nurse focusing on symptom awareness, glucose self-testing and appropriate treatments, followed by eight biweekly support telephone sessions. Wait-listed control condition participants served as comparisons at three time points. Hierarchical linear modeling was used to evaluate the effects of the intervention between- and within groups on psychosocial, behavioral and clinical outcomes. Participants were predominantly female, middle-aged, moderately acculturated and in poor glycemic control. Experimental group participants (n = 39) significantly improved glycemic control, blood pressure, symptoms, knowledge, self-efficacy, empowerment and quality of life. Post intervention focus groups reported satisfaction with the symptom focus. Addressing symptoms led to clinical and psychosocial improvements. Symptoms seem to be an important motivator and a useful prompt to engage patients in diabetes self-management behaviors to relieve symptoms and prevent complications.
Most patients with diabetes experience symptoms such as headaches, increased thirst and urination, numbness and tingling of extremities, blurry vision, irritability, dizziness and fatigue that could be caused by hyper- or hypoglycemia, side effects of medications, diabetes complications or other conditions such as migraines, allergies and menopause [1–9]. Although a variety of symptoms have been reported by Mexican Americans, African Americans, American Indians and White Americans with type 2 diabetes mellitus (T2DM), studies that compare the symptom experiences between Mexican Americans and other racial or ethnic groups are lacking. In two studies that surveyed Mexican Americans about their diabetes symptoms, 96–98% of respondents reported having an average of 5–10 symptoms in the previous month that they attributed to diabetes [1, 10].
Patients frequently rely on their symptoms to indicate disease status [11, 12] such as blood glucose levels [13, 14], and to direct their diabetes self-management activities [5, 15]. People who report having more symptoms have lower quality of life [1, 2, 16], lower self-efficacy [17] and higher glycosylated hemoglobin (HbA1c) levels [1, 2]. Patients’ desire for symptom relief prompts them to adjust their diet or activity or take medications [3]. Because diabetes symptoms are most effectively ameliorated by restoring blood glucose levels to the normal range, having symptoms can be an important motivator for patients to take the necessary steps that result in glycemic control.
Mexican Americans are two to three times as likely as non-Hispanic Whites to develop T2DM, and Mexican Americans tend to develop T2DM at a younger age, show poorer glycemic control and suffer higher rates of diabetes-related complications [18]. Mexican Americans face significant challenges to managing their T2DM because they are less likely than non-Hispanic Whites to have health insurance or access to medical care or receive standard recommended treatments [18–20]. As a result, many Mexican Americans have depended on self-evaluation and self-management of diabetes symptoms as the foundation for their day-to-day diabetes care [21]. However, few Mexican American study participants (about 8%) checked their blood glucose levels before treating symptoms [1, 10]. As a result, patients’ actions to relieve their symptoms might not target the underlying cause of the symptom and might lead to inadequate symptom relief, and in the long-term might accelerate diabetes complications [3].
We designed and pilot-tested a new approach to diabetes self-management education in which symptom identification and treatment were foundational components. The curriculum teaches three important steps to diabetes symptom self-management. First, patients must recognize that their symptoms may be related to their blood glucose levels. Second, patients must act on these symptoms by checking their blood glucose levels. Third, consistent with national guidelines they must take appropriate action based on their blood glucose levels. Because symptoms can be similar for high and low glucose levels, it is crucial that patients check their glucose levels; otherwise patients may misinterpret the meaning of their symptoms and apply ineffective strategies, which may lead them to suffer from unrelieved symptoms and diabetes-related complications.
This article reports on the efficacy, feasibility and patient satisfaction with the diabetes symptom educational intervention designed for Mexican Americans. The study was guided by the following questions: (i) What was the efficacy of the intervention? Specifically, what is the difference in HbA1c, body mass index (BMI), diabetes-related knowledge, self-efficacy to manage diabetes, empowerment, number of symptoms and quality of life between experimental and control groups over time? (ii) Was the intervention feasible? and (iii) What were the participants’ perceptions of the intervention?
Methods
Design, sample, setting and culturally tailored intervention
The study used a two group (experimental and control) by three time points (baseline, 2 and 6 months) repeated measures randomized controlled trial design. The study was set in urban and rural communities in Central Texas where Hispanics comprise 34% of the population [22]. The target population was Mexican American adults aged 25–75 years with T2DM.
A power analysis was conducted to calculate the size of sample needed to detect differences in the primary variable (HbA1c) that were expected to range from 13 to 18% based on preliminary studies [2, 23] with a two-tailed test and 80% power. A sample of 30 in each group was a conservative estimate since repeated measures analysis to be performed with planned contrasts would have a more precise error term and more degrees of freedom than the t-test on which the calculation was based [24]. From previous work with this population, we expected 20% attrition. Therefore, to compensate for the expected loss, we enrolled 72 participants (36 per group) to ensure a final sample of 60.
The convenience sample of participants was recruited from an email listserve, health fairs, community gathering places such as public libraries and grocery stores and the waiting rooms of several clinics serving low-income Mexican Americans. Women who were currently or recently pregnant and people currently or recently treated for cancer or on renal dialysis were excluded. Institutional review board approval was obtained before the study began.
Participants were randomly assigned to the experimental (symptom-based diabetes self-management education [DSME] program) or the wait-listed control group (WLC) at the baseline home visit when written consent was obtained and baseline data were collected. Participants in the WLC condition served as a comparison to the intervention group. WLC participants received the usual health care, counseling and education available from their regular health care providers and had their data collected at three time points (baseline, 2 and 6 months), after which they were offered the home-based portion of the intervention.
Participants in the experimental condition received eight weekly in-home, interactive, one-on-one educational and behavior modification sessions with a bilingual registered nurse (RN). The intervention components were informed by findings from focus groups and survey studies with members of the target population [1, 3, first author, unpublished data] and were designed to be congruent with common Mexican American cultural preferences. Besides offering the intervention instruction and materials in both English and Spanish, food demonstrations included foods common to local Mexican Americans (e.g. corn and flour tortillas, enchiladas, tacos, quesadillas, beans and rice, empanadas and pan dulce). In addition, project staff strove to have polite and pleasant social interactions with participants (simpático) and demonstrated respect and caring for participants [personalismo] in conversations and by making home visits. Furthermore, the intervention incorporated the importance of family identity, unity, decision-making and support (e.g. family members were invited to join the educational sessions, and were acknowledged as an important motivator for health) [25, 26].
The DSME sessions focused on symptom awareness, glucose self-testing and appropriate treatments. The symptom-focused approach addressed standard American Diabetes Association (ADA) recommended curricular elements [27] and used participants’ symptoms as a key motivator for patients to control blood glucose levels and to prioritize behavioral changes needed to control blood glucose. The intervention curriculum included 15 lessons, designed to be interactive and tailored to the participants’ particular symptoms and previous knowledge. Four of the lessons (Overview, Eating, Physical Activity and Managing Emotions) were provided to every intervention participant and each participant selected four additional lessons according to their priorities and adapted by the RN to the participant’s preferred learning style. RNs provided nurse case management as needed. For example, participants were assisted to register for pharmacy programs to purchase medicines at reduced costs and received referrals for family members who were ill.
The home visit sessions were followed by eight biweekly support telephone sessions in which the RN interventionist offered encouragement and facilitated problem solving to promote healthy diabetes symptom and self-management behaviors. Project staff referred participants to a clinic to serve as a medical home at the participant’s request. Elements of the intervention are listed in Table I.
Table I.
Diabetes symptom-based self-management intervention components
| In-home sessions | Eight one-on-one tailored educational sessions with the assigned RN, each session lasting 30–60 min. Family members were encouraged to attend the sessions |
| Curriculum | Based on focus group and survey study findings, guided by research literature and Leventhal’s Self-Regulatory Model |
| Topics | All intervention participants received Topic No. 1 during the first session. For sessions No. 2–8, all participants selected from the remaining topics depending on their priority symptoms. All participants received Topics No. 2–4, but could choose to have them during any visit. The topics addressed immediate and long-term symptom relief through diabetes self-management and specific behavioral strategies. |
| |
| Glucose monitoring | Participants received a glucose meter, strips to test up to three times/day for 6 months and lancets. RNs reviewed glucose meter results and patterns at each visit in conjunction with symptoms and self-management strategies |
| Educational materials | All materials and interactions were delivered in the participant’s preferred language (English or Spanish or both). RNs reviewed relevant patient handouts with participants at each session |
| Individualized approach | Participants were assisted to access resources as needed, e.g. completing forms to receive generic medications at reduced cost, or referrals to family members to accessible clinics |
| Goal setting | RNs assisted participants to set realistic personal goals for symptom relief and diabetes control and problem-solving strategies to meet those goals |
| Telephone follow-up | Participants received eight biweekly follow-up sessions via phone to clarify educational materials and assist with problem-solving and motivation |
Data collection and instrumentation
Data were collected on personal measures (demographic and disease characteristics and acculturation level), clinical measures (HbA1c, total symptoms, symptom severity, lipid levels, blood pressure, BMI) and psychosocial variables (diabetes knowledge, self-efficacy, empowerment, readiness to change self-management behaviors and quality of life. All measures were made at all three data collection points with the exception of demographic information, acculturation and height, which were measured at baseline only. All questionnaires were translated and back-translated into regional Spanish by native bilingual speakers. Due to potentially low literacy levels and high prevalence of vision impairments in this population, all questionnaires were read aloud to participants in Spanish or English, according to their preferences, and their responses recorded on the form.
Participant descriptors included participants’ age, sex, education, marital status, length of time with diabetes, diabetes treatment and concurrent comorbid conditions were recorded on a form developed for this study. Acculturation level was assessed via the 4-item Short Acculturation Scale for Hispanics [24, 28], which in this study achieved a Cronbach’s alpha of 0.95.
To evaluate metabolic control venous blood samples were collected and analysed for HbA1c, triglycerides, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol. All blood samples were analyzed in the same certified, licensed and accredited commercial clinical laboratory. Height and weight were measured with a portable, accurate scale and stadiometer and used to calculate BMI as kg/m2. Blood pressure was measured according to American Heart Association guidelines [29]. The average of three readings, each 1 min apart, was used.
Diabetes symptoms frequency was measured by the Diabetes Symptom Self-Care Inventory’s (DSSCI) 38-symptom subscale (with two additional options for ‘other’ symptoms). Study participants indicated the frequency of each symptom in the prior week (on a scale from 0 = never to 4 = constantly) and the subjective importance of the symptom to the participant (0 = not at all important to 4 = very important). Each participant’s total number of symptoms in the previous week was obtained by summing all symptoms with frequency codes from 1 to 4. A measure of severity was obtained by multiplying the symptom frequency and importance responses; higher scores indicate higher severity of symptoms. Higher total number of symptoms has correlated with the Center for Epidemiology Studies—Depression scale scores (r = 0.65, P < 0.001), diabetes illness identification (r = 0.57, P < 0.001) and with worse quality of life (r = −0.42, P < 0.001), indicating the DSSCI is a valid measure of diabetes symptoms [3].
Participants’ knowledge of diabetes was measured using the Spoken Knowledge in Low Literacy in Diabetes (SKILLD) Scale. The SKILLD measures core knowledge needed for T2DM self-management including glucose self-monitoring, recognizing and treating acute complications and activities to prevent long-term complications. The SKILLD consists of 10 open-ended questions and secondary questions or prompts used as needed. Scores represent the number of correct responses [30, 31].
The Diabetes-39 is a tool designed to capture aspects of quality of life relevant to diabetes [32]. Respondents are asked to indicate the impact of each item on the quality of life. The questionnaire’s 39 items address diabetes control (12 items), anxiety and worry (4 items), energy and mobility (15 items), social and peer burden (5 items) and sexual functioning (3 items). The response scale ranges from 1 = ‘not affected at all’ to 7 = ‘extremely affected.’ Items are summed and a weighted mean is obtained for each subscale and a total standardized summated score is obtained. Total scores can range from 0 to 100 with higher scores indicating that diabetes had a significant negative impact on quality of life, i.e. higher scores equate to worse quality of life [32]. The total Diabetes-39 achieved a Cronbach’s alpha of 0.94 and subscale alphas ranged from 0.73 to 0.96 in this study.
The Perceived Diabetes Self-Management Scale (PDSMS) is a measure of self-efficacy that asks patients to rate their agreement with eight statements about the difficulty of diabetes self-management. The response choices anchor points are labeled, 1 = ‘Strongly disagree’ and 5 = ‘Strongly agree.’ Four items are reverse coded before scoring. Scores range from 8 to 40, higher scores indicated more confidence in diabetes self-management [33]. The PDSMS achieved a Cronbach’s alpha of 0.70 in this study.
The Diabetes Empowerment Scale (DES) also measures diabetes-related psychosocial self-efficacy [34]. We used the 8-item short form version that uses a 5-point Likert response scale (1 = strongly disagree, 5 = strongly agree). The overall score is a summated mean scale score [35] with higher scores indicating a greater sense of psychosocial self-efficacy to manage diabetes. In this study, the Cronbach’s alpha was 0.80.
Stages of Readiness to Change Diabetes-Related Behaviors was measured with a 4-item questionnaire adapted for this study from a survey based on the Transtheoretical Model [36]. Scores did not demonstrate adequate internal consistency and were therefore not used in these analyses.
All participants were given $40 for completing data collections at 2 months (Time 2) and 6 months (Time 3) and they were sent a copy of their lab test results after each data collection point. During the home visits they received a glucose meter and an allotment of testing strips to allow for three tests per day for 6 months.
Data analysis strategy
To evaluate effects of the intervention, we used linear mixed model techniques to perform repeated measures analyses of the data [37]. The fixed effects were group (Intervention or WLC), time (baseline, 2 and 6 months), the interaction (group and time) and covariates of age, gender and time since diagnosis to adjust for possible confounding effects. The random effect was subject-within group, and a heterogeneous auto-correlated covariance matrix was used to represent the correlated data structure. Planned contrasts were made between groups at each time and times within each group. SAS 9.3 was used for all analyses. The 0.05 level was used to determine statistical significance. All analyses were conducted using the ‘intention to treat’ principle.
Feasibility, fidelity and participant satisfaction
Process measures were incorporated to determine intervention feasibility, fidelity and participant satisfaction. Feasibility was determined by staff logs of home visits attempted and completed. Fidelity was evaluated by investigators’ review of 25% of the audio recordings made of all the intervention home visit sessions. Satisfaction was evaluated by focus groups held with a subsample of individuals who agreed to participate after completing the intervention. A trained bilingual and bicultural moderator led the focus groups assisted by a bilingual note taker. After obtaining written consent to participate and explaining guidelines for the group discussion, we audio-recorded the focus groups. We later reviewed the recordings for content analysis. Focus group participants received an additional $25.
Findings
Characteristics of the study participants
Participants (N = 72) were predominantly female (67%), middle-aged, of a moderate acculturation level and well educated. Almost half (45%) were Spanish-speaking. They had been diagnosed with T2DM for a median of 3 years. Most (53%) treated their diabetes with oral hypoglycemic agents; 28% were prescribed insulin alone or with oral agents. Almost all (90%) reported having at least one comorbid condition, most commonly hypertension. At baseline HbA1c was 8.5% (69 mmol/mol), diabetes knowledge was low (score of 50%), and on average they reported 14 symptoms in the previous week. The participants in both groups were similar in gender, age, acculturation, diabetes duration and treatment and comorbid conditions. However, despite random assignment to groups, participants in the control condition had significantly higher baseline HbA1c and fasting blood glucose levels (see Table II).
Table II.
Participants’ profile at baseline on key study variables, mean ± standard deviation or frequency (%)
| Characteristic | Experimental n = 39 | Control n = 33 | Total N = 72 |
|---|---|---|---|
| Women | 24 (61.5) | 24 (72.7) | 48 (66.7) |
| Age (in years; range 29–68) | 50 ± 8.7 | 49.1 ± 9.7 | 49.6 ± 9.1 |
| Acculturation (range 4–20, α = .95) | 11.2 ± 6.5 | 11.9 ± 5.7 | 11.6 ± 6.1 |
| Education (years) | 12.3 ± 4.4 | 12.2 ± 4.1 | 12.3 ± 4.3 |
| Diabetes duration (in years; range 0.03–30) | 6.2 ± 7.1 | 7.2 ± 7.4 | 6.7 ± 7.2 |
| Diabetes treatment modalities | |||
| No medication | 3 (7.7) | 1 (3.0) | 4 (5.6) |
| Oral agents | 24 (61.5) | 17 (51.5) | 41 (56.9) |
| Byetta | 0 | 1 (3.0) | 1 (1.4) |
| Insulin | 4 (10.3) | 3 (9.1) | 7 (9.7) |
| Oral and insulin | 4 (10.3) | 9 (27.3) | 13 (18.1) |
| Oral and Byetta | 4 (10.3) | 2 (6.1) | 6 (8.3) |
| With any co-morbid condition | 36 (92.3) | 29 (87.9) | 65 (90.3) |
| With hypertension | 22 (56.4) | 16 (48.5) | 38 (52.8) |
| Number of comorbidities (range 0–5) | 1.7 ± 1.0 | 1.9 ± 1.3 | 1.8 ± 1.2 |
| Number of symptoms (α = .88) | 13.9 ± 8.0 | 15.1 ± 6.2 | 14.4 ± 7.2 |
| Symptom severity (α = 0.97) | 119.3 ± 16.7 | 136.4 ± 14.6 | 127.3 ± 94.4 |
| Body mass index (kg/m2) | 35.7 ± 8.8 | 36.3 ± 7.3 | 36.0 ± 8.0 |
| Fasting plasma glucose (mg/dl)* | 140.0 ± 53.8 | 189.9 ± 91.5 | 163.8 ± 77.3 |
| Total cholesterol (mg/dl) | 188.2 ± 42.1 | 180.8 ± 49.5 | 184.8 ± 45.5 |
| HDL cholesterol (mg/dl) | 46.4 ± 10.5 | 45.2 ± 15.0 | 45.9 ± 12.8 |
| LDL cholesterol (mg/dl) | 107.6 ± 32.4 | 98.3 ± 45.4 | 103.4 ± 38.7 |
| Triglycerides (mg/dl) | 170.8 ± 113.2 | 194.5 ± 150.0 | 181.8 ± 131.2 |
| Systolic BP (mmHg) | 124.5 ± 13.2 | 126.5 ± 15.1 | 125.4 ± 14.0 |
| Diastolic BP (mmHg) | 78.4 ± 10.6 | 78.0 ± 7.9 | 78.2 ± 9.4 |
| HbA1c (% then mmol/mol)* | 7.8 (62) ± 2.0 | 9.3 (78) ± 2.4 | 8.5 (69) ± 2.3 |
| Diabetes knowledge (range 0–10; α = 0.64) | 5.3 ± 1.9 | 5.0 ± 2.5 | 5.2 ± 2.2 |
| Self-efficacy (range 8–40, α = 0.70) | 20.8 ± 5.9 | 20.8 ± 6.4 | 20.8 ± 6.1 |
| Diabetes empowerment (range 8–40, α = 0.80) | 31.0 ± 6.7 | 32.5 ± 5.4 | 31 7 ± 6.2 |
| Diabetes quality of life (range 0–100, α = 0.94) | 62.7 ± 25.8 | 68.2 ± 22.6 | 65.3 ± 24.4 |
*t-Test for differences between experimental and control group significant at P < 0.05.
Intervention effects
The experimental group (n = 39) demonstrated a significant decrease in HbA1c from baseline to Time 2 (of about 0.7 percentage points) when baseline HbA1c was added as a covariate (F[2, 43] = 10.237, P < 0.001), a clinically meaningful improvement [38]. Participants in the wait-listed control group demonstrated smaller improvements in HbA1c (of about 0.2 percentage points). The experimental group (n = 39) showed significant group-by-time interaction effects on improvements in number of symptoms, symptom severity, diabetes knowledge, self-efficacy, empowerment, the energy and mobility domain of quality of life and total and LDL cholesterol levels (Table III). There were significant within experimental group improvements in diastolic blood pressure, HDL and total quality of life. There were no significant changes in systolic blood pressure, triglycerides, or BMI (some effects not shown in Table III).
Table III.
Means ± standard errors and P values for selected between, within and interaction effects
| Group | Baseline | Time 2 | Time 3 | BL versus T2 | BL versus T3 |
|---|---|---|---|---|---|
| HbA1c %(mmol/mol)a | |||||
| Experimental | 8.6 (70) ± 0.0 | 7.8 (62) ± 0.2 | 7.9 (63) ± 0.3 | 0.001 | 0.065 |
| WLC | 8.6 (70) ± 0.0 | 8.3 (67) ± 0.3 | 8.5 (69) ± 0.3 | 0.609 | 0.842 |
| WLC versus Exp. | 1.00 | 0.097 | 0.171 | 0.097 | 0.171 |
| Number of symptoms (range 0–38) | |||||
| Experimental | 14.3 ± 1.4 | 10.5 ± 1.2 | 9.2 ± 1.4 | 0.007 | 0.002 |
| WLC | 15.2 ± 1.5 | 11.6 ± 1.3 | 12.6 ± 1.6 | 0.051 | 0.163 |
| WLC versus Exp. | 0.710 | 0.485 | 0.042 | 0.075 | 0.158 |
| Symptom frequency and severity (range 0–152) | |||||
| Experimental | 222.3 ± 85.8 | 70.1 ± 10.1 | 61.9 ± 12.5 | 0.069 | 0.061 |
| WLC | 127.9 ± 93.5 | 79.2 ± 11.2 | 102.5 ± 14.0 | 0.590 | 0.784 |
| WLC versus Exp. | 0.458 | 0.550 | 0.033 | 0.399 | 0.284 |
| Systolic BP | |||||
| Experimental | 124.7 ± 2.3 | 127.2 ± 3.1 | 127.0 ± 2.8 | 0.420 | 0.472 |
| WLC | 127.2 ± 2.5 | 128.4 ± 3.4 | 125.4 ± 3.0 | 0.725 | 0.618 |
| WLC versus Exp. | 0.450 | 0.799 | 0.693 | 0.767 | 0.393 |
| Diastolic BP | |||||
| Experimental | 78.5 ± 1.5 | 73.2 ± 1.5 | 77.1 ± 1.5 | 0.004 | 0.472 |
| WLC | 78.7 ± 1.7 | 75.7 ± 1.6 | 75.9 ± 1.6 | 0.128 | 0.201 |
| WLC versus Exp. | 0.943 | 0.261 | 0.590 | 0.386 | 0.653 |
| Total cholesterol | |||||
| Experimental | 188.32 ± 7.7 | 179.14 ± 7.3 | 164.1 ± 8.7 | 0.174 | 0.009 |
| WLC | 178.48 ± 8.4 | 185.93 ± 8.0 | 195.6 ± 9.6 | 0.300 | 0.086 |
| WLC versus Exp. | 0.384 | 0.526 | 0.016 | 0.093 | 0.003 |
| HDL cholesterol | |||||
| Experimental | 46.3 ± 1.9 | 44.2 ± 1.9 | 43.9 ± 2.0 | 0.184 | 0.137 |
| WLC | 43.4 ± 2.1 | 44.0 ± 2.1 | 44.4 ± 2.2 | 0.613 | 0.548 |
| WLC versus Exp. | 0.309 | 0.805 | 0.864 | 0.202 | 0.147 |
| LDL cholesterol | |||||
| Experimental | 107.7 ± 6.3 | 99.4 ± 5.6 | 89.95 ± 6.0 | 0.150 | 0.015 |
| WLC | 97.7 ± 7.0 | 105.5 ± 6.1 | 106.88 ± 6.9 | 0.234 | 0.258 |
| WLC versus Exp. | 0.283 | 0.471 | 0.063 | 0.066 | 0.014 |
| Triglycerides | |||||
| Experimental | 173.8 ± 22.3 | 184.3 ± 24.6 | 166.3 ± 29.1 | 0.483 | 0.736 |
| WLC | 198.5 ± 24.5 | 200.8 ± 27.0 | 224.2 ± 31.9 | 0.885 | 0.286 |
| WLC versus Exp. | 0.449 | 0.648 | 0.179 | 0.709 | 0.311 |
| Self-efficacy | |||||
| Experimental | 31.0 ± 1.0 | 34.7 ± 1.0 | 35.5 ± 1.0 | 0.001 | 0.001 |
| WLC | 32.6 ± 1.1 | 32.3 ± 1.1 | 32.0 ± 1.1 | 0.743 | 0.652 |
| WLC versus Exp. | 0.278 | 0.094 | 0.017 | 0.016 | 0.008 |
| Quality of life energy and mobility subscale (range 0–100)b | |||||
| Experimental | 24.3 ± 3.7 | 19.0 ± 2.7 | 17.7 ± 2.6 | 0.044 | 0.033 |
| WLC | 22.8 ± 3.0 | 20.1 ± 3.0 | 21.3 ± 2.9 | 0.342 | 0.649 |
| WLC versus Exp. | 0.945 | 0.020 | 0.053 | 0.502 | 0.262 |
| Diabetes knowledge (range 0–10) | |||||
| Experimental | 5.4 ± 0.4 | 6.6 ± 0.34 | 5.8 ± 0.3 | 0.001 | 0.015 |
| WLC | 4.9 ± 0.4 | 2.6 ± 0.67 | 5.0 ± 0.4 | 0.871 | 0.754 |
| WLC versus Exp. | 0.335 | 0.001 | 0.137 | 0.024 | 0.059 |
Planned comparison results from linear mixed model with fixed effects for group, time, group × time, and covariates of age, gender and time from diagnosis and a random effect for subject nested within groups. BL, Baseline; Exp., experimental condition; Mo., months; WLC, wait-listed control group.
aBaseline HbA1c added as a covariate.
bLower scores equal better quality of life.
Feasibility, fidelity and satisfaction
Thirty-seven percent of pilot participants completed all eight sessions, 62.5% completed seven visits and only 9% completed fewer than half; the mean number of visits was 6.5 (SD 1.7), demonstrating good retention, as well as the feasibility and acceptability of providing the intervention in participants’ homes. Follow-up telephone sessions were also well attended (67% completed 6 or more follow-up telephone sessions). Analysis of audio-recordings of 25% of intervention sessions and of the RNs’ written documentation of the follow-up telephone sessions confirmed that RNs delivered the intervention according to the protocol.
Of the 30 participants who agreed to join in the focus groups to discuss their experiences with the intervention, 11 participants attended one of the two focus groups conducted in English or the one in Spanish; each group met for approximately 90 min. Overall, the focus group participants’ comments about the intervention were positive. In particular, they praised the support of the RN, the convenience of the in-home sessions, the RN’s non-judgmental approach, the pace of learning, the flexible schedule, the tailored content and the inclusion of family members. The lesson on Eating with Diabetes was most popular but participants reported that they also benefitted from the other topics. Of the symptom focus, a participant stated, ‘We learned that the top end and the low end [of glucose levels] have the same symptoms…Through checking my blood, I learned.’ A sample of participants’ comments about what they gained from the intervention follows:
‘The nurse said, ‘you have to monitor what’s going on with your body.’ I was trying different things, different foods just to get through the nausea but I started checking [my glucose levels] and that helped.’
‘For me the visit was always personal. [The RN] would ask me which symptom worried me the most. Depending on my answer, I would note that she would get ready with the information, personal not general, personal to me, depending on what worried me the most.’
‘I hadn’t really thought about what the symptoms were. I just looked at the machine. But [the RN] helped me figure out what was what. OK, when I feel like this, my blood sugar is low. That’s a signal. We got a list. I fall asleep, that’s one of my reactions to high sugar. At least I know what’s going on.’
‘For me I have changed everything. All of my life. Because one feels the symptoms but you don’t know why. You feel tired or anxious, irritated and you feel it in your life, with your family. But managing your symptoms, well the anxiety then goes away, not completely but it gets better. You feel less irritable. And you feel better and it makes everything feel better, your relationships, your work and your family. That was from the program [intervention] because one knows what is harmful but you don’t understand it until someone explains it. So it’s for yourself, not from watching commercials or programs. You don’t feel it’s for yourself. But when it’s for you, you know it is and your life changes.’
Comments indicated that participants felt the home visits were beneficial and the diabetes self-management topics were relevant, particularly those on eating and symptoms. They reported the visits and the follow-up phone calls motivated them to keep glucose levels under control.
Discussion
This intervention supports the importance of focusing on patients’ diabetes symptoms. Approaching DSME from the perspective of patients’ recent symptoms helps patients understand the immediate effects of their behaviors on symptoms and diabetes status. This pilot test of a symptom-focused DSME program showed statistically significant and meaningful clinical and psychosocial benefits to participants. Intervention participants showed statistically and clinically significant improvements in HbA1c at 2 months although those improvements were not sustained at 6 months. The intervention also improved number of symptoms, and symptom severity, DBP, total and LDL cholesterol levels, diabetes knowledge, self-efficacy, empowerment and quality of life.
The improvement in HbA1c at Time 2 was followed by a reduction in the improvement of HbA1c at Time 3. The experimental group’s HbA1c increased from Time 2 to Time 3 yet remained lower than at baseline. The analysis was inadequately powered to detect a change from baseline to Time 3 due to larger than expected attrition. Although we oversampled to account for attrition of 20%, at 6 months we had 36% attrition, possibly a result of the duration of the study or participants’ preference for an intervention instead of a wait-listed condition. The smaller sample at 6 months reduced the power to detect a change in HbA1c from baseline to Time 3. The observed power from baseline to Time 2 was 0.995 but from Time 2 to Time 3 was 0.463. It is also possible that other factors affected the changes in HbA1c, such as seasonal illnesses that might lead to increases in HbA1c despite the patient’s best efforts. In future studies, enrolling a larger sample to account for higher attrition, offering two or three comparison interventions instead of a wait-listed control condition, and assessing HbA1c over a longer period of time (e.g. 9 or 12 months) would help determine whether the intervention has a sustainable effect on patients’ HbA1c over time.
Over half the sample began the study with HbA1c levels <8.0% (57.7% total; 71.8% of the experimental group and 39.4% of the control group). Participants with higher HbA1c at baseline have a higher potential for significant improvements in HbA1c. In this sample, participants with HbA1c of 8% (64 mmol/mol) or more (n = 24) demonstrated a decrease in HbA1c from baseline to time 3 of 1.3 percentage points compared to participants with HbA1c less than 8% (64 mmol/mol; n = 31) who increased HbA1c on average by 0.2 percentage points (t = −2.294, df = 25.393, P = 0.03). It is possible that the intervention effect is different for participants of high versus low HbA1c level but a larger sample would be needed to conduct analyses for differential effects between experimental and control groups using the HbA1c scores at all three time points and controlling for the covariates used in the analyses presented in this article.
Participants regarded the intervention favorably and completed most of the educational and follow-up telephone sessions. In the Starr County Diabetes Education studies, attendance was the best predictor of improvement in HbA1c [23]. Thus, the patients’ satisfaction with the intervention may have contributed to the symptom-focused intervention’s effectiveness and shows potential for success when tested in larger studies.
Delivering the intervention in participants’ homes allowed RNs to tailor the information to address patients’ own concerns. Many participants find it difficult to make clinic appointments due to work commitments, lack of transportation or child care or lack of available appointments (first author, unpublished data). The home visits allowed for tailored professional-level education and referrals to health care providers, discount medication programs and community services. Further research is needed to explore the effectiveness of the home-based intervention in comparison to effective interventions offered in community or clinic settings, particularly for Mexican Americans with T2DM [23, 39, 40].
Participants’ comments indicated that the symptom-focused approach was effective in increasing their awareness of the connection between their bodily sensations, their diabetes status and their behaviors. Using the symptom-focused curriculum, the RNs helped the intervention participants recognize symptoms that might relate to their diabetes, test their glucose levels, and take appropriate actions that included changes in foods eaten, quantities, timing of meals, physical activity and stress management. Addressing patients’ symptoms is a meaningful way to engage patients in their disease management.
The study’s voluntary and convenience sample is likely not representative of all Mexican Americans with T2DM; participants were likely more motivated to change behaviors than patients who declined to enroll or did not contact the investigative team to learn more about the study. Therefore, the study results should be interpreted in this context. Four additional limitations should be considered. First, the study’s power and generalizability are limited by the small sample size, which might have inflated the influence of individual values in the data set. Although a power analysis recommended a sample size of 60, the effect of the intervention on the main outcome at Time 3 and the sample size at Time 3 were lower than anticipated. Therefore, although adequately powered to detect improvements in diabetes knowledge, self-efficacy, quality of life and blood pressure, the study was not powered sufficiently to detect changes in HbA1c at 6 months. Second, although a significant effect was seen at Time 2, when the data were collected 2 months after baseline to measure effects after completion of the home visits, that point in time likely did not capture maximum changes in HbA1c because red blood cells are replaced every 120 days. Replication studies should be conducted with larger sample sizes, choose a measurement point (e.g. 4 months) to capture the biggest change in HbA1c, and target patients with higher HbA1c levels. Third, although random assignment to group produced statistically equal groups in most of our outcome variables, at baseline, the experimental and control groups’ HbA1c levels were significantly different; the control group’s mean was almost 1.5 points higher than the experimental group’s mean. Because of this difference, we used the baseline HbA1c as a covariate in the analyses. Finally, it is likely we did not capture all relevant experiences that may have impacted outcomes in this study. For instance, this study did not measure participants’ literacy levels, previous diabetes education or the quality of usual care. Although the randomization process should have distributed these unmeasured characteristics evenly between the two groups, it would be useful to consider their effect on diabetes outcomes.
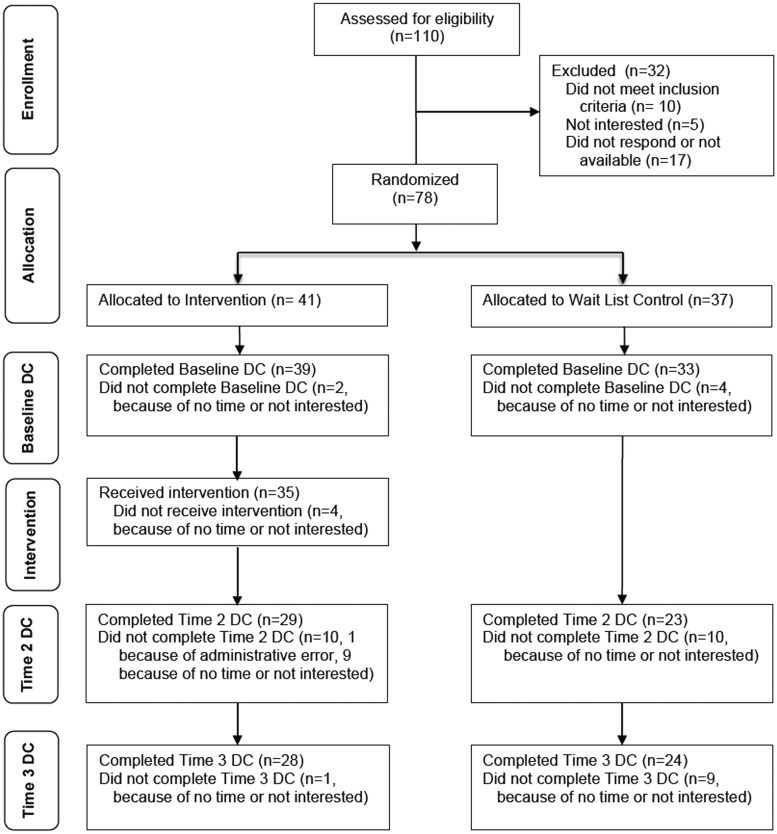
Fig. 1.
CONSORT diagram of each step of the Study. DC = Data Collection.
Conclusion
This study tested the feasibility and effectiveness of an RN-delivered symptom-focused diabetes self-management intervention in participants’ home. The symptom-focused one-on-one sessions allowed for individualized education that addressed the patients’ symptom concerns and helped them understand diabetes self-management in the context of symptom relief. Participants were satisfied with the sessions and the method of delivery. Diabetes symptom self-management education is a new way to motivate patients to take steps necessary to achieve and maintain glucose levels within a normal range.
Acknowledgements
We gratefully acknowledge assistance from research assistants, data collectors, RN interventionists, consultants, community partners and the participants.
Funding
National Institute of Diabetes and Kidney and Digestive Diseases at the National Institutes of Health (R21DK076705).
Conflict of interest statement
None declared.
References
- 1.García AA. Symptom prevalence and treatments among Mexican Americans with type 2 diabetes. Diab Educ. 2005;31:543–54. doi: 10.1177/0145721705278801. [DOI] [PubMed] [Google Scholar]
- 2.Garcia AA. Clinical and life quality differences between Mexican American diabetic patients at a free clinic and a hospital-affiliated clinic in Texas. Public Health Nurs. 2008;25:149–58. doi: 10.1111/j.1525-1446.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 3.García AA. The Diabetes Symptom Self-Care Inventory for Mexican Americans: development and pilot testing. J Pain Symptom Manag. 2011;41:715–27. doi: 10.1016/j.jpainsymman.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman MJ, Norton CK, Beene L. Diabetes symptoms, health literacy, and health care use in adult Latinos with diabetes risk factors. J Cult Divers. 2012;19:4–9. [PubMed] [Google Scholar]
- 5.Kirk JK, Arcury TA, Ip E, et al. Diabetes symptoms and self-management behaviors in rural older adults. Diabetes Res Clin Pract. 2014;107:54–60. doi: 10.1016/j.diabres.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcury TA, Skelly AH, Gesler WM, et al. Diabetes meanings among those without diabetes: explanatory models of immigrant Latinos in rural North Carolina. Soc Sci Med. 2001;59:2183–93. doi: 10.1016/j.socscimed.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen AB, Gannik D, Siersma V, et al. The relationship between HbA1c level, symptoms and self-rated health in type 2 diabetic patients. Scand J Prim Health Care. 2011;29:157–64. doi: 10.3109/02813432.2011.585542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mier N, Bocanegra-Alonso A, Zhan D, et al. Clinical depressive symptoms and diabetes in a binational border population. J Am Board Fam Med. 2008;21:223–33. doi: 10.3122/jabfm.2008.03.070255. [DOI] [PubMed] [Google Scholar]
- 9.Skelly AH, Dougherty M, Gesler WM, et al. African American beliefs about diabetes. West J Nurs Res. 2006;28:9–29. doi: 10.1177/0193945905280298. [DOI] [PubMed] [Google Scholar]
- 10.Brown SA, Upchurch SL, García AA, et al. Symptom-related self-care of Mexican-Americans with NIDDM: preliminary findings of the Starr County diabetes education study. Diab Educ. 1998;24:331–9. doi: 10.1177/014572179802400308. [DOI] [PubMed] [Google Scholar]
- 11.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:686–76. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 12.Teel CS, Meek P, McNamara AM, et al. Perspectives unifying symptom interpretation. Image J Nurs Sch. 1997;29:175–81. doi: 10.1111/j.1547-5069.1997.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 13.Cox DJ, Gonder-Frederick L, Polonsky W, et al. Blood glucose awareness training (BGAT-2): long-term benefits. Diab Care. 2001;24:637–42. doi: 10.2337/diacare.24.4.637. [DOI] [PubMed] [Google Scholar]
- 14.Cox DJ, Kovatchev B, Koev D, et al. Hypoglycemia anticipation, awareness and treatment training (HAATT) reduces occurrence of severe hypoglycemia among adults with type 1 diabetes mellitus. Int J Behav Med. 2004;11:212–8. doi: 10.1207/s15327558ijbm1104_4. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez CA, Bradish GI, Rodger NW, et al. Self-awareness in diabetes: using body cues, circumstances, and strategies. Diab Educ. 1999;25:576–84. doi: 10.1177/014572179902500410. [DOI] [PubMed] [Google Scholar]
- 16.Chesla CA, Chun KM, Kwan CML. Cultural and family challenges to managing type 2 diabetes in immigrant Chinese Americans. Diab Care. 2009;32:1812–6. doi: 10.2337/dc09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco WP, Wells KJ, Friedman A, et al. Adherence, body mass index, and depression in adults with type 2 diabetes: the meditational role of diabetes symptoms and self-efficacy. Health Psychol. 2007;26:693–700. doi: 10.1037/0278-6133.26.6.693. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160:517–25. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National Diabetes Surveillance System: Prevalence of Diabetes. Data and Trends: Data from the National Health Interview Survey. National Center for Health Statistics. 2013. Available at: http://www.cdc.gov/diabetes/statistics/meduse/fig5.htm. Accessed: 18 May 2014. [Google Scholar]
- 20.De Heer HD, Balcázar HG, Morera OF, et al. Barriers to care and comorbidities along the U.S.-Mexico border. Public Health Rep. 2013;128:480–8. doi: 10.1177/003335491312800607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diab Care. 1998;21:2180–4. doi: 10.2337/diacare.21.12.2180. [DOI] [PubMed] [Google Scholar]
- 22.United States Census Bureau State and County Quick Facts: Travis County. 2014. http://quickfacts.census.gov/qfd/states/48/48453.html. Accessed: 3 August 2014. [Google Scholar]
- 23.Brown SA, Blozis SA, Kouzekanani K, et al. Dosage effects of diabetes self-management education for Mexican Americans: the Starr County Border Health Initiative. Diab Care. 2005;3:527–32. doi: 10.2337/diacare.28.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Marín G, Marín BV. Research with Hispanic Populations. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 26.Chong N. The Latino Patient: A Cultural Guide for Health Care Providers. Yarmouth, ME: Intercultural Press; 2002. [Google Scholar]
- 27.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diab Care. 2012;35(Suppl. 1):S101–S108. doi: 10.2337/dc12-s101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marín G, Sabogal F, Marín BV, et al. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183–205. [Google Scholar]
- 29.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professional from the subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 30.Rothman RL, Malone R, Bryant B, et al. Spoken Knowledge in Low Literacy in Diabetes Scale: a diabetes knowledge scale for vulnerable patients. Diab Educ. 2005;31:215–24. doi: 10.1177/0145721705275002. [DOI] [PubMed] [Google Scholar]
- 31.Garcia AA, Zuñiga J, Reynolds R, et al. Evaluation of the Spoken Knowledge in Low Literacy in Diabetes Scale (SKILLD) for use with Mexican Americans. J Transcult Nurs. 2014;26:279–86. doi: 10.1177/1043659614524246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer JG, Earp JL. Development of an instrument for assessing the quality of life of people with diabetes: Diabetes-39. Med Care. 1997;35:440–53. doi: 10.1097/00005650-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS) J Behav Med. 2007;30:395–401. doi: 10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 34.Anderson RM, Funnell MM, Fitzgerald JT, et al. The Diabetes Empowerment Scale. Diab Care. 2000;23:739–43. doi: 10.2337/diacare.23.6.739. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RM, Funnell MM, Nwankwo R, et al. Evaluating a problem-based empowerment program for African-Americans with diabetes: results of a randomized controlled trial. Ethn Dis. 2005;15:671–78. [PubMed] [Google Scholar]
- 36.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Health Education. San Francisco, CA: Jossey-Bass; 1997. pp. 60–84. [Google Scholar]
- 37.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd edn. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 38.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 39.Brown SA, Garcia AA, Kouzekanani K, et al. Culturally competent diabetes self-management education for Mexican Americans: The Starr County Border Health Initiative. Diab Care. 2002;25:259–68. doi: 10.2337/diacare.25.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorig K, Ritter PL, Villa F, et al. Spanish diabetes self-management with and without automated telephone reinforcement: Two randomized trials. Diab Care. 2008;31:408–14. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]