ABSTRACT
Environmental contamination of drinking water with chromium (Cr) has been increasing in more than 30 cities in the United States. Previous studies from our group have shown that Cr affects reproductive functions in female Sprague Dawley rats. Although it is impossible to completely remove Cr from the drinking water, it is imperative to develop effective intervention strategies to inhibit Cr-induced deleterious health effects. Edaravone (EDA), a potential inhibitor of free radicals, has been clinically used to treat cancer and cardiac ischemia. This study evaluated the efficacy of EDA against Cr-induced ovarian toxicity. Results showed that maternal exposure to CrVI in rats increased follicular atresia, decreased steroidogenesis, and delayed puberty in F1 offspring. CrVI increased oxidative stress and decreased antioxidant (AOX) enzyme levels in the ovary. CrVI increased follicle atresia by increased expression of cleaved caspase 3, and decreased expression of Bcl2 and Bcl2l1 in the ovary. EDA mitigated or inhibited the effects of CrVI on follicle atresia, pubertal onset, steroid hormone levels, and AOX enzyme activity, as well as the expression of Bcl2 and Bcl2l1 in the ovary. In a second study, CrVI treatment was withdrawn, and F1 rats were injected with estradiol (E2) (10 μg in PBS/ethanol per 100 g body weight) for a period of 2 wk to evaluate whether E2 treatment will restore Cr-induced depletion of AOX enzymes. E2 restored CrVI-induced depletion of glutathione peroxidase 1, catalase, thioredoxin 2, and peroxiredoxin 3 in the ovary. This is the first study to demonstrate the protective effects of EDA against any toxicant in the ovary.
Keywords: chromium, edaravone, ovary, oxidative stress
INTRODUCTION
Hexavalent chromium (CrVI) has been used in various industries such as leather and textiles, metallurgical, chemical, and automobile [1–3]. Because of increased use and improper disposal of CrVI, its levels in the water, soil, and air continue to increase [4–6]. Significant contamination with CrVI has been found in the drinking water sources of more than 30 cities in the United States, including cities in Oklahoma and California [7]. According to the Environmental Protection Agency (EPA) report in February 2014, well water from Midland, Texas, contains 5.28 ppm chromium (Cr) [8]. The California EPA has set maximum contamination level for Cr as 100 ppb or 0.1 ppm [9]. Occupational exposure to CrVI is found among approximately one-half million industrial workers in the United States and several million worldwide [10]. CrVI is known to cause various health problems, including infertility in men and women and developmental problems in children [11]. Women working in dichromate manufacturing industries and tanneries and living around Cr-contaminated areas have high levels of Cr in blood and urine and encounter gynecological illness, abortion, postnatal hemorrhage, and birth complications [12–15]. Our recent study demonstrated that maternal exposure to trivalent Cr (CrIII) accelerates follicle atresia through elevated oxidative stress [16]. Although Cr is known to adversely affect reproductive health in women [17], the specific mechanisms of reproductive toxicity are not clearly understood.
At physiological pH, CrVI exists in the form of an oxyanion with an overall 2− charge that resembles sulfate and phosphate, and CrVI is taken up by cells via the anionic transport system [18, 19]. This transport system, along with intracellular reduction reactions, allows Cr to accumulate in cells at concentrations that are much higher than extracellular levels [20, 21]. Once CrVI enters the cell, it is reduced to CrIII by various intracellular reductants [22, 23]. During the intracellular reduction process of CrVI to CrIII, a spectrum of reactive oxygen species (ROS) (i.e., superoxide [O2•−], hydrogen peroxide [H2O2], and hydroxyl radical [•OH]) is produced [24], with the depletion of antioxidant (AOX) enzymes such as superoxide dismutase (Sod1), glutathione peroxidase (Gpx1), and catalase (Cat) resulting in oxidative stress [25]. Oxidative stress has been proposed as a major pathway of Cr toxicity [24]. Thus, reduction of ROS generation within cells is expected to reduce or prevent Cr toxicity.
Given the fact that usage of Cr has been exponentially increasing both in the United States and in developing countries, and that environmental Cr contamination is one of the greatest threats to human health because of difficulties in removing Cr from the soil and water, it is imperative to develop potential intervention strategies. We previously reported that vitamin C protects F1 offspring subjected to CrIII exposure through lactation during Postnatal Days (PND) 1–21 [16, 26, 27]. Follicular atresia and granulosa cell (GC) apoptosis in offspring were blocked by inhibiting cell death pathways [27] and promoting cell survival and/or cell growth pathways [16]. In furthering improved intervention strategies designed to protect the ovary against Cr toxicity, we tested the hypothesis that cotreatment of edaravone (EDA) with CrVI or posttreatment of estradiol (E2) after postnatal exposure to CrIII protects the ovary against CrIII toxicity in F1 offspring.
EDA (3-methyl-1-phenyl-2-pyrazolin-5-one; MCI-186) is a potent, low-molecular-weight, free-radical scavenger that has been clinically used to reduce the neuronal damage following ischemic stroke [28]. It has been shown to quench •OH, peroxynitrite (ONOO−), and both water-soluble and lipid-soluble peroxyl radical (LOO•) [28, 29]. EDA is widely distributed in tissues and readily crosses the blood-brain barrier [30]. In addition to its effects on •OH removal, EDA modulates inflammatory processes, matrix metalloproteinase levels, nitric oxide production, and both apoptotic and necrotic cell death [31]. EDA provides neuroprotection from traumatic brain injury [32] and cerebral infarction [33] and ameliorates several neurological diseases such as Parkinson [34] and Alzheimer diseases [35]. It has also been approved as a treatment for acute ischemic stroke [28, 36]. In a rat coronary occlusion model, EDA reduced the myocardial infarction area, maintained myocardial ATP content, decreased mitochondrial swelling, reduced cytochrome c release, increased the expression of Bcl2, and reduced the number of apoptotic cells and DNA fragmentation [37]. To the best of our knowledge, the protective effects of EDA have not been studied in the ovary. Moreover, our recent [16] and current studies suggest CrIII induces a decrease in E2 levels that accompany depletion of AOX enzymes. Therefore, the purpose of the current study was twofold: first, to evaluate efficacy of EDA against the adverse effects of CrIII in F1 offspring, and second, to evaluate the ability of E2 to restore the CrIII-induced decrease in AOX enzymes.
MATERIALS AND METHODS
Chemicals
Reagents, supplies, and primary antibodies used in this study are listed in Supplemental Tables S1 and S2 (Supplemental Data are available online at www.biolreprod.org).
Animals
Timed pregnant Sprague-Dawley rats were purchased from Charles River Laboratories and maintained in Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facilities with a 12L:12D regime at 23–25°C and provided with Teklad 6% mouse/rat diet and water ad libitum. Animal use protocols were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, were in accordance with the standards established by Guiding Principles in the Use of Animals in Toxicology and specific guidelines and standards of the Society for the Study of Reproduction, and were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
In Vivo Dosing and Experimental Design
Dose-Response Studies.
Lactating rats were divided into the following seven groups and treated with various doses of CrVI in drinking water as follows: Rats from group 1 received regular drinking water, and rats from groups 2, 3, 4, 5, 6, and 7 received 5, 10, 25, 50, 100, and 200 ppm potassium dichromate (CrVI) in drinking water, respectively. On the day of birth, male pups were removed and the litters were culled to 4 female pups per dam; thus, each treatment group consisted of 5 dams (F0) and 20 F1 female pups. In cases where fewer than four female pups were delivered, additional pups were fostered from other litters. In all experimental groups, the lactating dams received CrVI treatment in drinking water from the day of parturition to Day 21 postpartum. During this period, the F1 offspring received Cr through the dam's milk. Because CrVI is rapidly converted into CrIII in cells, tissues, and biological fluids, the Cr found in dam's milk is predominantly CrIII. On PND 25, female pups from four dams (n = 20; 4 pups/dam) from each group were euthanized under CO2 anesthesia followed by cervical dislocation, and blood and ovaries were collected. Effects of Cr on follicle atresia and apoptosis of oocytes and GCs were determined. Based on the effects of Cr on follicle atresia and the intensity of TUNEL-positive staining, a CrVI dose of 50 ppm was chosen to determine the ability of EDA to mitigate toxicity and the ability of E2 to restore the Cr-induced decrease in AOX enzymes in the ovary.
Effects of EDA on CrVI Toxicity.
Lactating dams were divided into the following three groups: 1) control: dams (n = 5) received regular drinking water; 2) CrVI treatment: dams (n = 5) received 50 ppm potassium dichromate dissolved in drinking water, 3) CrVI + EDA treatment: dams (n = 5) received 50 ppm CrVI with EDA (15 mg/kg body wt., i.p., daily injections). On the day of birth the litters were culled to 4 female pups per dam rat; thus, each treatment group consisted of 5 dams (F0) and 20 F1 female pups. CrVI doses were chosen based on dose-response studies as described above and drinking water levels of total Cr in the most polluted places in the United States and developing countries. Drinking water levels of Cr in Midland, TX, have been reported as 5.28 ppm [8], and levels have been reported as 19–31 ppm in India [2, 38]. The EDA dose was chosen based on dose-response studies and literature using rodent models [39–41]. After weaning on PND 22, F1 female pups were fed with regular drinking water and diet. One group was euthanized on PND 25 and ovaries were collected and fixed in 4% buffered formaldehyde. Another group was maintained separately to observe pubertal onset by monitoring the vaginal opening [26].
Effects of E2 on CrVI-Induced Depletion of AOX Enzymes in the Ovary.
In order to determine if E2 could restore the ovary from Cr-induced oxidative damage, and depletion of AOX enzymes, the following experiments was conducted. Lactating dams were divided into three groups: 1) control: rats (n = 5) received regular drinking water; 2) CrVI treatment: rats (n = 5) received 50 ppm potassium dichromate (CrVI) dissolved in drinking water from the day of parturition until Day 21 postpartum; and 3) CrVI + E2 treatment: rats (n = 5) received 50 ppm potassium dichromate (CrVI) dissolved in drinking water from the day of parturition until Day 21 postpartum. On the day of birth the litters were culled to 4 female pups per dam; thus, each treatment group consisted of 5 dams (F0) and 20 F1 female pups. On PND 22, F1 female pups were weaned from their dams, caged separately, provided with regular drinking water and diet, and divided into the following three groups: 1) control: on PND 25, F1 female rats (n = 20) from control dams received only vehicle; 2) CrVI: F1 female rats (n = 20) from CrVI-treated dams received only vehicle; and 3) CrVI + E2 treatment: F1 female rats (n = 20) from CrVI-treated dams received E2 for 2 wk from PND 25 through PND 39. Stock solution of E2 was prepared in ethanol and diluted with sterile 1× PBS to prepare stock concentrations of 10 μg E2/100 μl PBS. Rats received i.p. injections of either vehicle (approximately 1 μl ethanol in 100 μl PBS) or E2 (10 μg in PBS/ethanol per 100 g body weight) using a syringe equipped with a 28-gauge needle, and were euthanized on PND 40 for further analyses. At the end of the treatment, the rats were euthanized under CO2 anesthesia followed by cervical dislocation, and blood and ovaries were collected.
Histological Evaluation of Follicular Atresia
Ovaries were fixed in Bouin solution for 24 h and transferred to 70% ethanol. After fixation, the tissues were dehydrated, embedded, serially sectioned (5 μm), mounted on glass slides, and stained with hematoxylin and eosin. Every 12th section was used to count atretic follicle numbers to avoid duplicate counting [42, 43]. Moreover, those follicles with intact oocytes were included in the counting. Ovaries from at least 10 or 12 pups were included in the study. Atresia of primordial, primary, secondary, and antral follicles was counted separately from that of both the ovaries. Because CrVI induced atresia of follicles in all developmental stages, the numbers of different classes of atretic follicles were added together and expressed as percentages. The criteria to identify atretic follicles was according to Osman [44] and Borgeest et al., [45] as follows: degenerative changes in the GC layer(s), which shows cell shrinkage, pyknosis, and karyorrhexis, and/or degenerative changes in the oocyte such as the breakdown of the nuclear membrane with oocyte fragmentation. Antral follicles were considered atretic if they contained disorganized GCs with at least 20 apoptotic bodies in the GC layer(s).
TUNEL Assay on Paraffin-Embedded Tissue Sections
Paraffin-embedded tissue sections were deparaffinized in xylene and used for TUNEL assay using a TUNEL assay kit (Roche Applied Science) as described previously [16].
Immunohistochemistry
Paraffin sections from the ovary were fixed in 4% buffered paraformaldehyde (PFA) (6 g PFA, 325 mg NaOH, 15 ml 10× PBS in 100 ml diethyl pyrocarbonate-treated double-distilled H2O, pH 7.2) for 4 h at 4°C and processed using Vectastain Elite ABC kit (Vector Laboratories). An avidin/biotin-based peroxidase system was used to detect the biotinylated secondary antibody and the sections were developed using the chromogen 3-amino-9-ethylcarbazole, a peroxidase substrate that produces an insoluble end product that is red in color and observed visually, and quantified using a semiquantitative method (Image ProPlus software; Media Cybernetics, Inc.) [46]. The intensity of staining for each protein was quantified on the oocytes, GCs, and theca cells (TCs) from 10–12 sections; sections were at least 100 μm apart from each other. The average staining intensity of multiple follicles was calculated. Details of primary antibodies, secondary antibodies, dilutions, host species, immunogen, and percentage of homology with rats and mice are given in Supplemental Table S1.
Measurement of Oxidative Damage in the Ovary
Oxidative damage was measured by estimating the levels of H2O2 and lipid peroxide (LPO) in the ovary as described [16]. Briefly, ovaries were dissected out and homogenized in 50 mM Tris-HCl buffer, pH 7.5, and centrifuged at 10 000 × g for 15 min. The supernatants were used for the assay. Details of all the assay kits used in the current study are given in Supplemental Table S2.
Measurement of AOX Enzymes
Activities of Sod1, Cat, Gpx1, and glutathione reductase (Gsr) were estimated in the ovaries of F1 offspring using commercial kits (Cayman Chemical). Ovaries were dissected out and homogenized in 50 mM Tris-HCl buffer, pH 7.5, and centrifuged at 10 000 × g for 15 min. The supernatants were used for the assay as we previously described in detail [16].
Measurement of Steroid Hormones
Serum levels of steroid hormones E2, testosterone (T), and progesterone (P4) were estimated in F1 female pups on PND 25 using ELISA kits (DRG Diagnostics) according to the manufacturer's instructions. The sensitivities of the assays for E2, T, and P4 were 9.0 pg/ml, 0.06 ng/ml, and 0.04 ng/ml, respectively. The intra-assay and interassay coefficients of variation ranged from 4.0% to 7.3%.
Statistical Analyses
Dose-response relationships were analyzed by repeated measures of multivariate ANOVA. Least-squares regression analysis was used to determine effects of treatment, dose, and treatment × dose interactions. All other numerical data were subjected to one-way ANOVA to detect the effects of treatment and dose interactions. The Tukey-Kramer HSD test was used to adjust for multiple pair-wise comparisons of means. We took the nested structure into account in the design. Mixed-models analysis was used to account for any correlation between the results of pups from the same dam. Mixed models were used to model both fixed effects (in this case treatment) and random effects (in this case dams and pups) [47]. Statistical analyses were performed using general linear models of Statistical Analysis Systems (SAS) and P < 0.05 was considered significant.
RESULTS
In Vivo Studies
Cr Increased Follicular Atresia and Apoptosis of Follicular Cells and Decreased Steroid Hormones and EDA Inhibited Cr Effects.
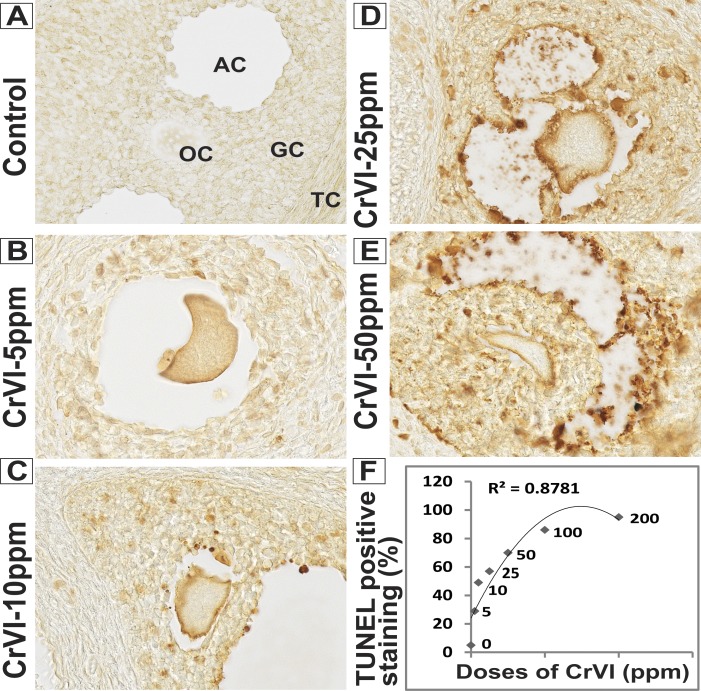
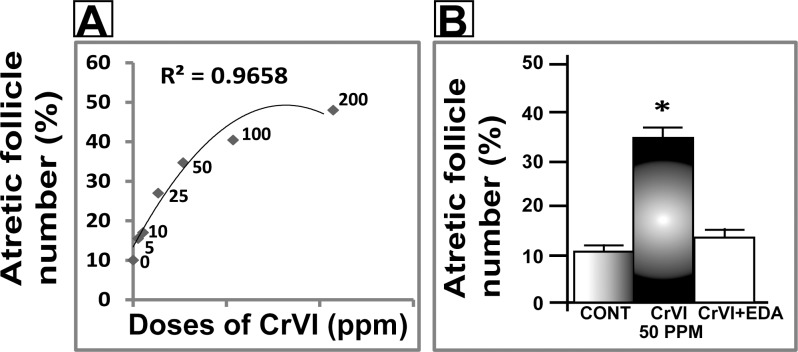
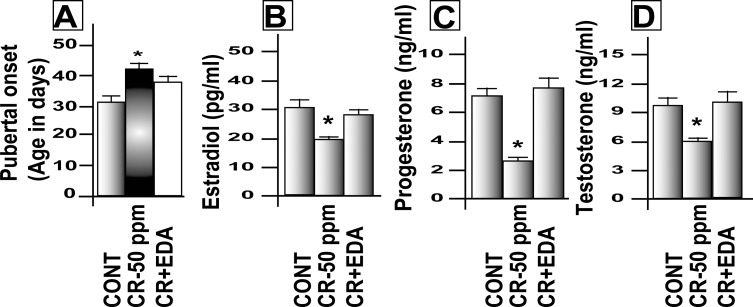
A dose-response study was performed with CrVI doses of 5, 10, 25, 50, 100, and 200 ppm. CrVI significantly (P < 0.05) increased the percentage of atretic follicles with degenerated oocytes and GCs with pyknotic nuclei. CrVI increased apoptosis of oocytes and GCs in a dose-dependent manner based on TUNEL staining (Fig. 1, A–F; TUNEL staining for 100- and 200-ppm CrVI doses is not shown). Moreover, CrVI increased follicle atresia in a dose-dependent manner (Fig. 2A). CrVI significantly (P < 0.05) delayed the onset of puberty and decreased E2, T, and P4, and EDA inhibited the effects of CrVI on E2, T, and P4 (Fig. 3, A–D).
FIG. 1.
Dose-response of maternal exposure to CrVI on oocyte and GC apoptosis in F1 offspring. Lactating dams (n = 5) received different doses of CrVI (5–200 ppm) in drinking water with or without EDA, as described in Materials and Methods. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, TUNEL assay was performed in paraffin-embedded sections of the ovary. Intensity of TUNEL staining was measured in oocytes (OC), GCs, and TCs as described. Representative images of control (A), CrVI-5 ppm (B), CrVI-10 ppm (C), CrVI-25 ppm (D), and CrVI-50 ppm (E) and dose-response curve of CrVI on apoptosis (F) based on the intensity of TUNEL staining are shown. TUNEL images for CrVI-100 ppm and −200 ppm are not shown. Data are presented as the mean ± SEM of the (average) percentage of oocytes, GCs, and TCs with TUNEL-positive staining from 15–20 ovaries. Original magnification ×400 (A–E). AC, antral cavity.
FIG. 2.
Dose-response of maternal exposure to CrVI on follicular atresia in F1 offspring. Lactating dams (n = 5) received different doses of CrVI (5–200 ppm) in drinking water with or without EDA, as described in Materials and Methods. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested, paraffin-embedded sections were stained with H&E, and atretic (primordial, primary, secondary, and antral) follicle numbers were counted. Dose-response curve of CrVI on percentage of atretic follicles (A) and mitigative effect of EDA on CrVI (50 ppm)-induced decrease in follicle atresia (B) are shown. Data are presented as mean ± SEM. *Control versus CrVI, P < 0.05.
FIG. 3.
Effects of maternal exposure to CrVI on pubertal onset and serum steroid hormone levels. Lactating dams were divided into three groups: control, rats (n = 5) received regular drinking water; CrVI, rats (n = 5) received CrVI (50 ppm) in drinking water; and CrVI + EDA, rats (n = 5) received CrVI (50 ppm) in drinking water and EDA (15 mg/kg body wt., i.p., daily injections). Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested, paraffin-embedded sections were stained with H&E, and atretic (primordial, primary, secondary, and antral) follicle numbers were counted. Serum levels of steroid hormones E2, P4, and T were measured on PND 25 in F1 female pups. Another set of the rats from the above groups was maintained in regular drinking water and diet until pubertal onset. Mitigative effect of EDA on CrVI-induced delay in pubertal onset (A) and decrease in E2 (B), P4 (C), and T (D) are shown. Data are presented as mean ± SEM. *Control versus CrVI, P < 0.05.
EDA Mitigated CrVI-Induced Increase in Cleaved Caspase-3 and Decrease in Bcl2 and Bcl2l1 in the Ovary.
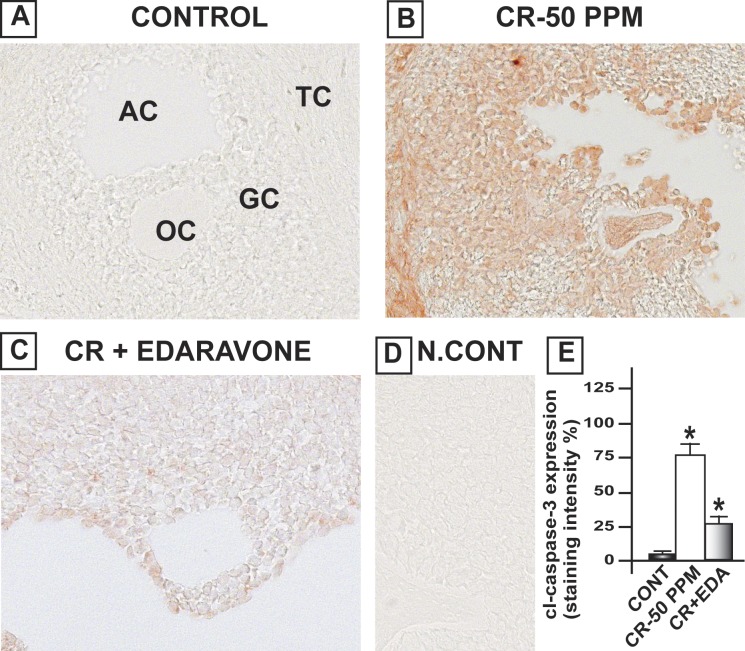
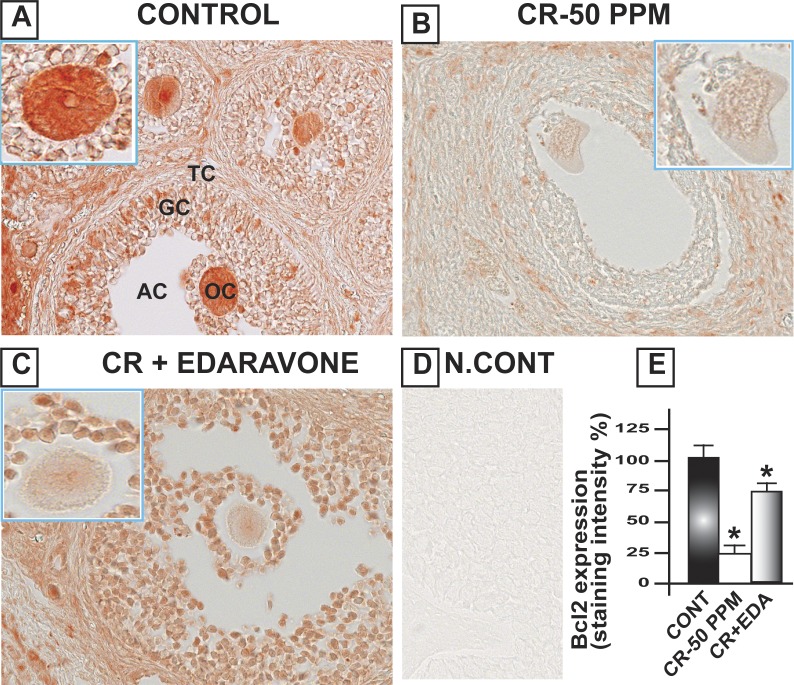
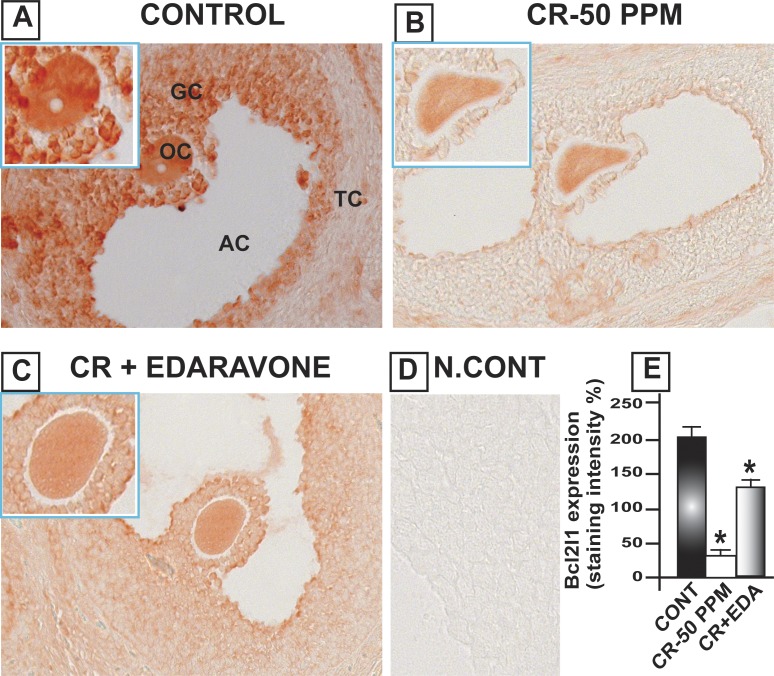
CrVI increased the expression of cleaved caspase-3 (Casp3) in the oocytes, GCs, and TCs of the ovary, compared to control. Casp3 was barely detected in the control ovary, and CrVI upregulated cleaved Casp3 expression. Casp3 expression was highest in TCs and moderate in oocytes and GCs. EDA mitigated CrVI-induced increase in cleaved Casp3 expression (Fig. 4). In control rats, Bcl2 was abundantly expressed with the following order of intensity in different cell types: oocytes > GCs and > TCs. CrVI significantly (P < 0.05) decreased expression of Bcl2 compared to control. EDA mitigated the CrVI-induced decrease in Bcl2 (Fig. 5). Bcl2l1 was very abundantly expressed in the oocytes and GCs and moderately expressed in TCs. CrVI significantly decreased expression of Bcl2l1 compared to control. EDA mitigated the CrVI-induced decrease in Bcl2l1 (Fig. 6).
FIG. 4.
EDA mitigates CrVI-induced increase in cleaved (cl) Casp3 expression in the ovary of F1 rats. Lactating dams (n = 5 in each group) were exposed to CrVI-50 ppm in drinking water with or without EDA treatment. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested from F1 rats and immunohistochemistry (IHC) of Casp3 was performed in paraffin-embedded tissue sections. Staining intensity (integrated optical density) in oocytes (OC), GCs, and TCs and average intensity were calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + EDA (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + EDA, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity.
FIG. 5.
EDA mitigates CrVI-induced decrease in Bcl2 expression in the ovary of F1 rats. Lactating dams (n = 5 in each group) were exposed to CrVI-50 ppm in drinking water with or without EDA treatment. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested and immunohistochemistry (IHC) of Bcl2 was performed in paraffin-embedded tissue sections. Intensity of staining was measured in oocytes (OC), GCs, and TCs, and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + EDA (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Oocytes are magnified in the insets for A, B, and C. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + EDA, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity.
FIG. 6.
EDA mitigates CrVI-induced decrease in Bcl2l1 expression in the ovary of F1 rats. Lactating dams (n = 5 in each group) were exposed to CrVI-50 ppm in drinking water with or without EDA treatment. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested and immunohistochemistry (IHC) of Bcl2l1 was performed in paraffin-embedded tissue sections. Staining intensity was measured in oocytes (OC), GCs, and TCs, and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + EDA (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Oocytes are magnified in the insets for A, B, and C. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + EDA, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity.
EDA Mitigated Cr-Induced Increase in Oxidative Stress and Decrease in AOX Enzymes in the Ovary.
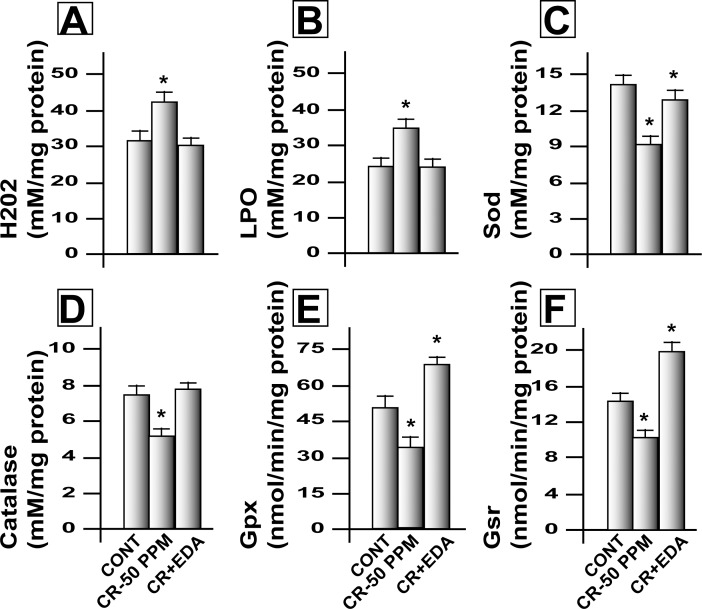
CrVI significantly (P < 0.05) increased LPO and H2O2 levels in the ovary and EDA inhibited CrVI-induced oxidative stress (Fig. 7, A and B). CrVI decreased activity of AOX enzymes Sod1, Cat, Gpx1, and Gsr in the ovary of F1 rats, and EDA inhibited the adverse effects of CrVI on all of these enzymes (Fig. 7, C–F).
FIG. 7.
EDA mitigates CrVI-induced increase in free radicals and decrease in antioxidant enzyme activity in the ovary of F1 rats. Lactating dams (n = 5) were exposed to CrVI-50 ppm in drinking water with or without EDA treatment. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, ovaries were harvested and levels of free radicals (H2O2 and LPO) and antioxidant enzymes (Gpx, Sod, Cat, and Gsr) were estimated in the homogenates of whole ovaries. Histograms of mitigative effect of EDA on CrIII-induced increase in H2O2 (A) and LPO (B) and decrease in Sod (C), Cat (D), Gpx (E), and Gsr (F) are shown. Data are presented as the mean ± SEM of 10–12 F1 rats. *Control versus CrVI-50 ppm or CrVI-50 ppm + EDA, P < 0.05.
E2 Treatment Restored CrVI-Induced Decrease in the Expression of AOX Enzymes in the Ovary.
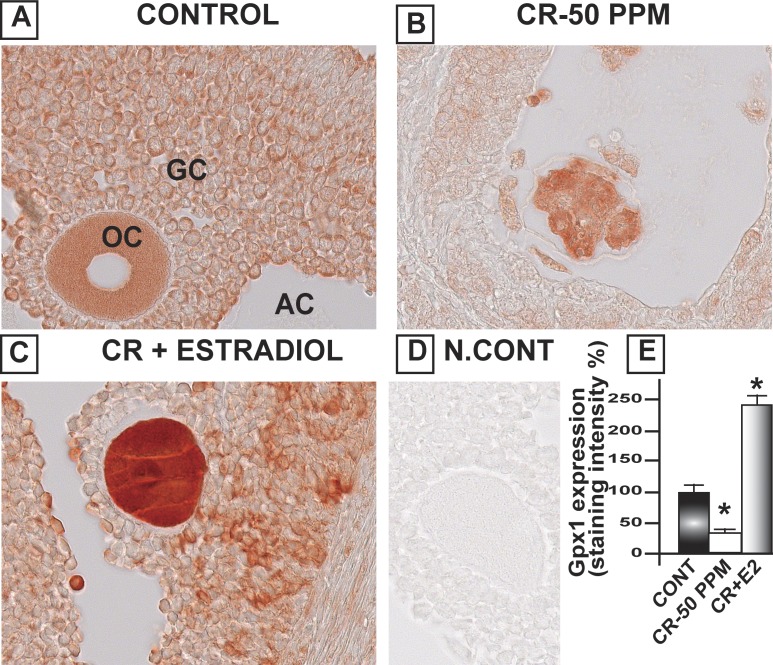
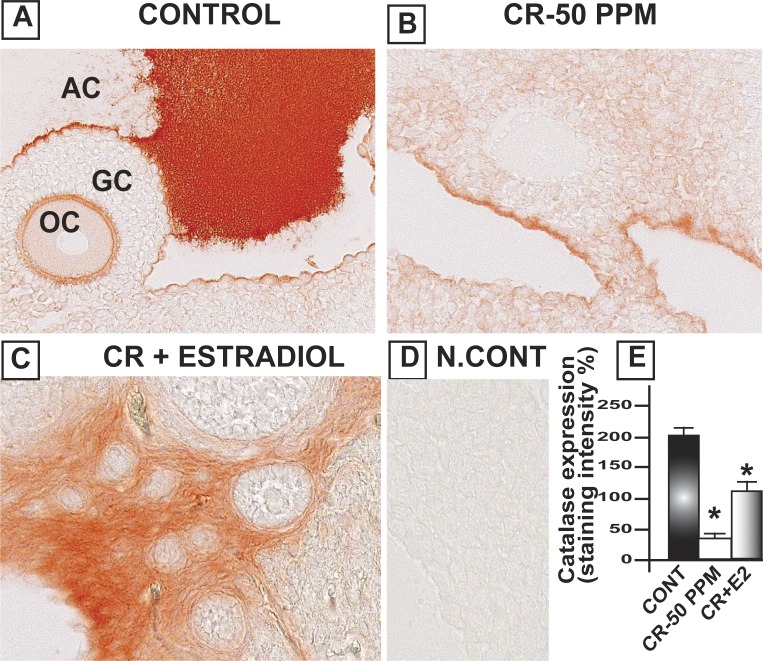
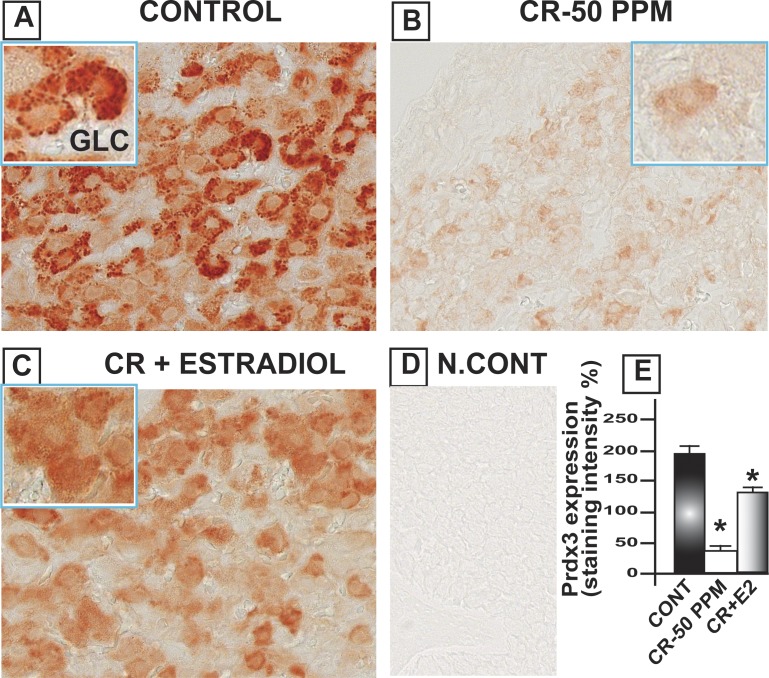
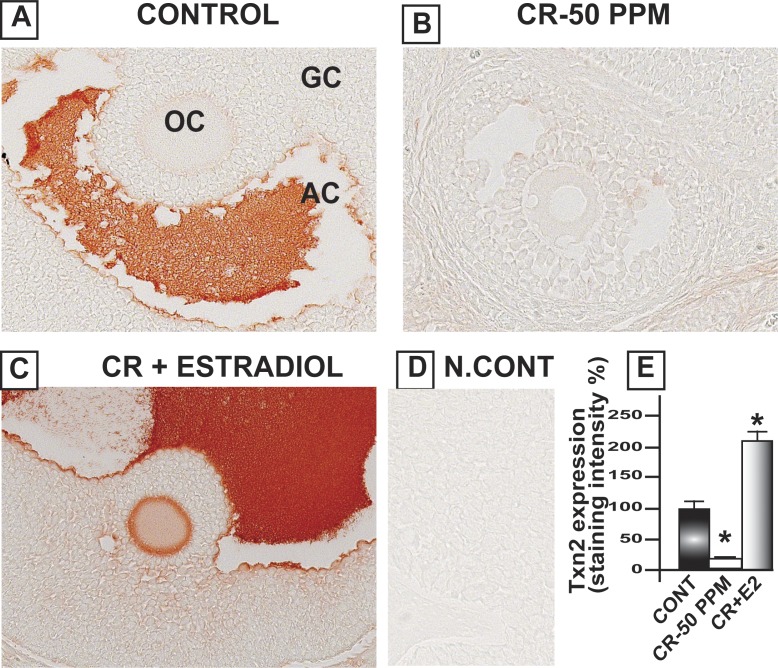
CrVI significantly decreased Gpx1 in the oocytes, GCs, and TCs. E2 upregulated Gpx1 in the oocytes compared to both control and CrVI treatment (Fig. 8). Interestingly, Cat was highly expressed in the follicular fluid and moderately expressed in the basal membrane of the oocytes as well as GCs. CrVI significantly decreased Cat in the oocytes and GCs. Moreover, E2 caused a shift in the expression pattern of Cat in the ovary. E2 increased the expression of Cat in the theca interstitial cell compartments in the ovary compared to both control and Cr groups (Fig. 9). In control ovaries, Prdx3 was very abundantly expressed in the granulosa lutein cells of the corpus luteum (CL), and very minimally expressed in the follicular compartments. CrVI downregulated Prdx3 expression in the CL, and E2 mitigated the effects of CrIII (Fig. 10). In the control ovary, Txn2 was abundantly expressed in the follicular fluid. CrVI significantly (P < 0.01) decreased Txn2. E2 significantly upregulated Txn2 in the follicular fluid, GCs, and basal membrane of the oocyte compared to both control and CrVI (Fig. 11).
FIG. 8.
Estradiol restores CrVI-induced decrease in the expression of Gpx1 in the ovary of F1 rats. Lactating dams (n = 5) were given regular drinking water (control) or water containing CrVI-50 ppm. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, Cr-exposed rats were treated with either vehicle or E2. Immunohistochemistry (IHC) of Gpx1 was performed in paraffin-embedded sections of the ovary. Staining intensity was measured in oocytes (OC), GCs, and TCs, and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + E2 (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + E2, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity.
FIG. 9.
Estradiol restores CrVI-induced decrease in the expression of Cat in the ovary of F1 rats. Lactating dams (n = 5) were given regular drinking water (control) or water containing CrVI-50 ppm. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, CrVI-exposed rats were treated with either vehicle or E2. Immunohistochemistry (IHC) of Cat was performed in paraffin-embedded sections of the ovary. Staining intensity was measured in oocytes (OC), GCs, and TCs, and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + E2 (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + E2, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity.
FIG. 10.
Estradiol restores CrVI-induced decrease in the expression of Prdx3 in the ovary of F1 rats. Lactating dams (n = 5) were given regular drinking water (control) or water containing CrVI-50 ppm. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, CrIII-exposed rats were treated with either vehicle or E2. Immunohistochemistry (IHC) of Prdx3 was performed in paraffin-embedded tissue sections of the ovary. Staining intensity was measured and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + E2 (C), and negative control (D) and histogram of average intensity of IHC staining in the CL (E) are shown. Oocytes are magnified in the insets for A, B, and C. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + E2, P < 0.05. Original magnification ×400 (A–D). GCL, granulosa-lutein cells.
FIG. 11.
Estradiol restores CrVI-induced decrease in the expression of Txn2 in the ovary of F1 rats. Lactating dams (n = 5) were given regular drinking water (control) or water containing CrVI-50 ppm. Suckling F1 female offspring (n = 20) received Cr through dam's milk during PND 1–21. On PND 25, Cr-exposed rats were treated with either vehicle or E2. Immunohistochemistry (IHC) of Txn2 was performed in paraffin-embedded sections of the ovary. Staining intensity was measured and average intensity was calculated. Representative images of control (A), CrVI-50 ppm (B), CrVI-50 ppm + E2 (C), and negative control (D) and histogram of average intensity of IHC staining (E) are shown. Data are presented as mean ± SEM. *Control versus CrVI-50 ppm or CrVI-50 ppm + E2, P < 0.05. Original magnification ×400 (A–D). AC, antral cavity; OC, oocytes.
DISCUSSION
The current studies clearly indicate that maternal exposure to CrVI accelerated follicular atresia in PND 25 F1 offspring in a dose-dependent manner. Our previous study indicated that Cr decreased the number of healthy primordial, preantral, and antral follicles [26]. EDA effectively protected the ovaries of the F1 offspring from CrVI-induced increase in follicle atresia. CrVI increased cleaved Casp3 in the ovary. Our recent report also indicated that prenatal exposure to 25 ppm CrVI during the organogenesis window (Gestational Day 9.5–14.5) significantly upregulated cleaved Casp3 and apoptosis of germ cells and somatic cells on PND 1, whereas it was not detectable in the control ovary [48]. Therefore, Casp3-mediated cell death may be one of the key mechanisms for CrVI-induced increase in apoptosis in the ovarian cells followed by follicle atresia. Interestingly, EDA mitigated CrVI-induced increase in Casp3 expression. Earlier findings indicate that elevation in blood levels of E2, T, and P4 precede pubertal onset monitored by vaginal opening [49]. Endocrine disruptors such as lead [50] and atrazine [51] are known to delay pubertal onset through a decrease in E2 levels. In accordance with these studies, the current study also indicates that CrVI decreases levels of E2, T, and P4, accompanied by a delay in pubertal onset.
Our previous study indicated that CrVI induced selective subcellular translocation of Bcl2 and Bcl2l1 from mitochondria to cytoplasm in primary cultures of GCs. In contrast, CrVI increased translocation of Bad from cytoplasm to mitochondria, induced apoptosis, and accelerated follicular atresia [27]. Bcl2 has been associated with resistance to apoptosis in a variety of mammalian systems [52–54]. Deletion of antiapoptotic Bcl2 results in fewer oocytes and primordial follicles at 6 wk of age [55]. Bcl2 family members Bcl2, Bcl2l1, Bax, and Bad proteins are the key mediators of the intrinsic apoptotic pathway in the ovary [56, 57]. Therefore, the current study suggests that CrIII-induced reproductive failure may be due to increased cell death and follicular atresia mediated through Casp3, as well as a decrease in cell survival/antiapoptotic machinery such as Bcl2 and Bcl2l1.
In the cellular reduction of CrVI, a spectrum of ROS (O2•−, H2O2, and •OH) is produced. Once inside the cells or in body fluids, most CrVI is converted to CrIII [22]. Enormous amounts of free radicals are produced during this reduction/detoxification process that alter redox balance and increase oxidative stress. Whereas CrVI enters cells by facilitated transport, CrIII enters cells by passive diffusion or phagocytosis of precipitates, resulting in much lower uptake [58, 59]. However, Sayato et al. [60] demonstrated the comparative metabolic fate of labeled Cr chloride (CrIII) and sodium chromate (CrVI) and interaction of these compounds in rat liver and blood after their oral and intravenous administration. Gastrointestinal absorption of both the compounds was below 1% of the oral dose. Studies in mice and yeast indicate that CrIII causes more damage to the DNA than CrVI. Once inside the cells or in body fluids, most CrVI will be converted to CrIII. Therefore in the current study, even though dams received CrVI, F1 pups received Cr through dam's milk in the form of CrIII. CrIII can be reduced to CrII by biological reductants, and CrII reacts with H2O2 to generate •OH, resulting in oxidative stress [61]. Thus, the current study clearly demonstrates a Cr-induced increase in follicular atresia in F1 offspring, mainly through increased oxidative stress and concomitant decrease in AOX enzymes and steroid hormone levels. EDA, being a potent scavenger of free radicals, mitigated Cr-induced follicular atresia, an increase in oxidative stress, and prevented Cr-induced decrease in AOX enzymes.
E2 plays important roles in preventing oxidative stress [62]. E2 depletion leads to apoptosis and DNA damage as well as elevated oxidative stress in the lymphocytes of rats, which is reversed by supplementing E2 [63]. E2 supplementation reverses the age-related depletion of AOX enzymes and increase in free radicals in rat liver [64]. Therefore, the current study suggests that the decrease in steroidogenesis due to increased follicular atresia may predispose the ovary to decreased AOX enzyme gene transcription and activity, resulting in oxidative stress. On the other hand, decreased AOX enzyme levels may also have indirectly contributed to the decrease in steroidogenic machinery and diminished steroidogenesis. In order to evaluate whether decreased E2 levels contributed to the depletion of AOX enzymes and to investigate whether CrVI-induced AOX enzyme depletion could be restored by supplementing E2, Cr-exposed rats were treated with E2 for a period of 2 wk. Protein expression of key cytosolic AOX enzymes Gpx1 and Cat along with the mitochondrial AOX enzymes Txn2 and Prdx3 was detected in control ovaries, and Cr significantly decreased the expression of Gpx1, Cat, Txn2 and Prdx3, whereas treatment with E2 restored Cr-induced depletion of these enzymes. Gpx1 and Txn2 levels were higher than the control in E2-treated groups. Mitochondria-specific Txn2 and Prdx3 are the major regulators of mitochondrial H2O2 concentration and apoptosis, and Gpx1 is a major cytosolic isoform in mammalian cells that plays a critical role in the protection of cells against oxidative damage by H2O2. It has been shown that aging decreased mRNA expressions of mitochondrial AOX genes Prdx3 and Txn2 and increased oxidative stress markers in mouse ovary, indirectly indicating an association between ovarian steroids and redox balance [65]. E2 increased glutathione content in human erythrocytes treated with CrVI [66]. E2 supplementation increased AOX enzymes in the liver and brain of ovariectomized rats [67]. E2 at nM and μM concentrations decreased lipid peroxidation caused by sodium fluoride in mitochondria isolated from human placenta [68]. The oocyte is one of the cells that express the highest levels of glutathione (∼10 mM) [69]. Particularly, the current study showed that E2 increased Gpx1 in the oocyte and Txn2 in the oocyte and follicular fluid surrounding the oocyte, suggesting an important role of these enzymes in oocyte survival against Cr toxicity.
Prdx3 plays a very important role in the elimination of H2O2 in luteal cells [70]. Prdx3 eliminates H2O2 and reduces the number of •OH produced by the Fenton reaction and the subsequent tissue injury [71]. Interestingly, Prdx2− knockout mice were shown to have more severe tissue damage and higher mortality rates than controls, even when Cat and Gpx proteins were normally expressed [71]. E2 inhibits peroxidation of low-density lipoprotein in postmenopausal women [72]. We have found that Prdx3 protein levels were highly expressed in granulosa lutein cells, but not in the other cell types in the follicular compartment, suggesting an important role of Prdx3 in particular in CL survival and function. Thus, the current data indicate that Cr-induced decrease in E2 may be one of the mechanisms for the depletion of AOX enzymes and increased ROS, and E2 plays an AOX role in the ovary to protect against Cr toxicity.
EDA is a potent free radical scavenger [31, 37, 73]. Its protective effects have been studied in several pathological conditions [28, 31, 32, 34, 36, 37, 41, 74, 75]. For example, EDA reduces the steroid-induced osteonecrosis in rabbits by suppressing the accumulation of lipid peroxidative products and oxidative DNA damage [76]; EDA has preventive and curative effects on diabetic neuropathy [77]; and EDA reduces cytochrome c release and increases the expression of Bcl2 in myocardial infarction [74]. It protects hepatocytes from Fas-induced mitochondrial-dependent apoptosis by regulating mitochondrial Bcl2l1 and Bax in mice with fulminant hepatic failure [75]. EDA attenuates cisplatin-induced renal dysfunction, mitochondrial damage, real protein oxidation, and tubular apoptosis in rats [41]. In spite of its importance, only two studies have been conducted so far to study the effect of EDA on the ovary: 1) EDA protects the ovary from experimentally induced ovarian torsion/detorsion ischemia/reperfusion injury [78]; and 2) prophylactic treatment with EDA prevents ischemia/reperfusion-induced ovarian damage during pneumoperitoneum in an experimental rat model [79]. This is the first study on the protective effect of EDA against any toxicant.
Footnotes
Supported by National Institutes of Health (NIH)/National Institute of Environmental Health Sciences (NIEHS) grant ES020561-01 to S.K.B.
REFERENCES
- Jacobs J, Testa SM. Overview of chromium(VI) in the environment: background and history In: Guertin J, Jacobs JA, Avakian CP. (eds.), Chromium(VI) Handbook. Boca Raton, FL: CRC Press; 2005: 1 20 [Google Scholar]
- Von Burg R, Liu D. Chromium and hexavalent chromium. J Appl Toxicol. 1993;13:225–230. doi: 10.1002/jat.2550130315. [DOI] [PubMed] [Google Scholar]
- Papp JF, Lipin BR. Chromite In: Kogel JE, Trivedi NC, Barker JM, Krukowski ST. (eds.), Industrial Minerals and Rocks, 7th ed. Englewood, CO: Society for Mining, Metallurgy, and Exploration; 2006: 309 334 [Google Scholar]
- OSHA. Occupational exposure to hexavalent chromium; final rule In: Labor DO (ed.), Federal Register, vol. 71. Washington, DC: Occupational Safety and Health Administration; 2006: 63238 63245 [PubMed] [Google Scholar]
- Shanker AK, Venkateswarlu B. Chromium: environmental pollution, health effects and mode of action In: Nriagu JO. (ed.), Encyclopedia of Environmental Health. Burlington: Elsevier Publishers; 2011: 650 659 [Google Scholar]
- Pellerin C, Booker SM. Reflections on hexavalent chromium: health hazards of an industrial heavyweight. Environ Health Perspect. 2000;108:A402–A407. doi: 10.1289/ehp.108-a402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. Chromium-6 in U.S. tap water In: Houlihan J, Sharp R, Bruzelius N. (eds.), Chromium-6 Is Widespread in US Tap Water. Washington, DC: Environmental Working Group; 2010: 1 23 [Google Scholar]
- Malott V. West County Road 112 ground water plume In: EPA (ed.), EPA Region 6. Dallas, TX: Environmental Protection Agency; 2014: 1 7 [Google Scholar]
- CDPH. Chromium-6 in Drinking Water: MCL Update In: EPA (ed.), California Department of Public Health, California; 2014. [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP. The risk of male subfecundity attributable to welding of metals. Studies of semen quality, infertility, fertility, adverse pregnancy outcome and childhood malignancy. Int J Androl. 1993;16((suppl 1)):1–29. doi: 10.1111/j.1365-2605.1993.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Shmitova LA. Content of hexavalent chromium in the biological substrates of pregnant women and puerperae engaged in the manufacture of chromium compounds. Gig Tr Prof Zabol. 1980:33–35. [in Russian] [PubMed] [Google Scholar]
- Jendryczko A, Drozdz M, Magner K. Preliminary studies of chromium concentration in the myometrium in the third trimester of pregnancy, in chorionic tissue in the first trimester and in the blood of pregnant women [in Polish] Ginekol Pol. 1984;55:691–694. [PubMed] [Google Scholar]
- Greene LE, Riederer AM, Marcus M, Lkhasuren O. Associations of fertility and pregnancy outcomes with leather tannery work in Mongolia: a pilot study. Int J Occup Environ Health. 2010;16:60–68. doi: 10.1179/107735210800546100. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cai WW, Lee DJ. Occupational hazards and pregnancy outcomes. Am J Ind Med. 1992;21:397–408. doi: 10.1002/ajim.4700210312. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Sivakumar KK, Nithy TK, Arosh JA, Hoyer PB, Burghardt RC, Banu SK. Postnatal exposure to chromium through mother's milk accelerates follicular atresia in F1 offspring through increased oxidative stress and depletion of antioxidant enzymes. Free Radic Biol Med. 2013;61C:179–196. doi: 10.1016/j.freeradbiomed.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostoli P, Catalani S. Metal ions affecting reproduction and development. Met Ions Life Sci. 2011;8:263–303. [PubMed] [Google Scholar]
- Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 1995;120:931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- DeFlora S, Serra D, Basso C, Zanacchi P. Mechanistic aspects of chromium carcinogenicity. Arch Toxicol. 1989;13:28–39. doi: 10.1007/978-3-642-74117-3_3. [DOI] [PubMed] [Google Scholar]
- Wetterhahn KE, Hamilton JW. Molecular basis of hexavalent chromium carcinogenicity: effect on gene expression. Sci Total Environ. 1989;86:113–129. doi: 10.1016/0048-9697(89)90199-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kluz T, Salnikow K, Costa M. Comparison of the cytotoxicity, cellular uptake, and DNA-protein crosslinks induced by potassium chromate in lymphoblast cell lines derived from three different individuals. Biol Trace Elem Res. 2002;86:11–22. doi: 10.1385/BTER:86:1:11. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Chiu A, Katz AJ, Beaubier J, Chiu N, Shi X. Genetic and cellular mechanisms in chromium and nickel carcinogenesis considering epidemiologic findings. Mol Cell Biochem. 2004;255:181–194. doi: 10.1023/b:mcbi.0000007274.25052.82. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Banu SK, Samuel JB, Arosh JA, Burghardt RC, Aruldhas MM. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis and pituitary hormone synthesis in developing Wistar rats. Toxicol Appl Pharmacol. 2008;232:180–189. doi: 10.1016/j.taap.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, Burghardt RC. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol. 2011;251:253–266. doi: 10.1016/j.taap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Yanai H, Namiki Y. Fukatsu Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tanaka M, Watanabe K, Takamatsu Y, Tobe A. Research and development of the free radical scavenger edaravone as a neuroprotectant. Yakugaku Zasshi. 2004;124:99–111. doi: 10.1248/yakushi.124.99. [DOI] [PubMed] [Google Scholar]
- Lapchak P, Zivin J. The lipophilic multifunctional antioxidant edaravone (Radicut) improves behavior following embolic strokes in rabbits: a combination therapy study with tissue plasminogen activator. Exp Neurol. 2009;215:95–100. doi: 10.1016/j.expneurol.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Uchikado H, Miyagi N, Morimoto Y, Ito T, Tancharoen S, Miura N, Miyata K, Sakamoto R, Kikuchi C, Iida N, Shiomi N, et al. Beyond neurological disease: new targets for edaravone [review] Int J Mol Med. 2011;28:899–906. doi: 10.3892/ijmm.2011.795. [DOI] [PubMed] [Google Scholar]
- Wang G-H, Jiang Z-L, Li Y-C, Li X, Shi H, Gao Y-Q, Vosler P, Chen J. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28:2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Kawahara K-i, Tancharoen S, Matsuda F, Morimoto Y, Ito T, Biswas KK, Takenouchi K, Miura N, Oyama Y, Nawa Y, Arimura N et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther. 2009;329:865–874. doi: 10.1124/jpet.108.149484. [DOI] [PubMed] [Google Scholar]
- Xiong N, Xiong J, Khare G, Chen C, Huang J, Zhao Y, Zhang Z, Qiao X, Feng Y, Reesaul H, Zhang Y, Sun S et al. Edaravone guards dopamine neurons in a rotenone model for Parkinson's disease. PLoS ONE. 2011;6:e20677–e20677. doi: 10.1371/journal.pone.0020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Gong K, Ma T, Zhang L, Zhao N, Zhang X, Tang P, Gong Y. Protective effect of edaravone against Alzheimer's disease-relevant insults in neuroblastoma N2a cells. Neurosci Lett. 2012;531:160–165. doi: 10.1016/j.neulet.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Feng S, Yang Q, Liu M, Li W, Yuan W, Zhang S, Wu B, Li J. Edaravone for acute ischaemic stroke. Cochrane Database Syst Rev. 2011;12 doi: 10.1002/14651858.CD007230.pub2. CD007230. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Takeshige N, Miura N, Morimoto Y, Ito T, Tancharoen S, Miyata K, Kikuchi C, Iida N, Uchikado H, Miyagi N, Shiomi N, et al. Beyond free radical scavenging: beneficial effects of edaravone (Radicut) in various diseases [review] Exp Ther Med. 2012;3:3–8. doi: 10.3892/etm.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Bihari V, Agarwal SK, Verma V, Kesavachandran CN, Pangtey BS, Mathur N, Singh KP, Srivastava M, Goel SK. Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India) PLoS ONE 2012. 7 e47877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp RH. Altered chromium excretion in pregnancy: a physiological change? Am J Clin Nutr. 1982;35:776–778. doi: 10.1093/ajcn/35.4.776. [DOI] [PubMed] [Google Scholar]
- Saner G. Urinary chromium excretion during pregnancy and its relationship with intravenous glucose loading. Am J Clin Nutr. 1981;34:1676–1679. doi: 10.1093/ajcn/34.9.1676. [DOI] [PubMed] [Google Scholar]
- Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia//reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Osman P. Rate and course of atresia during follicular development in the adult cyclic rat. J Reprod Fertil. 1985;73:261–270. doi: 10.1530/jrf.0.0730261. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- Kratzer DD, Littell RC. Appropriate statistical methods to compare dose responses of methionine sources. Poult Sci. 2006;85:947–954. doi: 10.1093/ps/85.5.947. [DOI] [PubMed] [Google Scholar]
- Sivakumar KK, Stanley JA, Arosh JA, Pepling ME, Burghardt RC, Banu SK. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring. Dev Biol. 2014;388:22–34. doi: 10.1016/j.ydbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CR, Jr, , Mahesh VB. Hormonal events surrounding the natural onset of puberty in female rats. Biol Reprod. 1976;14:347–353. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Shaikh F. Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol Appl Pharmacol. 1996;136:361–371. doi: 10.1006/taap.1996.0044. [DOI] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE, Schmid J, Cooper RL. The effects of atrazine on female Wistar rats: an evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol Sci. 2000;58:366–376. doi: 10.1093/toxsci/58.2.366. [DOI] [PubMed] [Google Scholar]
- Douarre C, Gomez D, Morjani H, Zahm J-M, O'Donohue M-F, Eddabra L, Mailliet P, Riou J-F, Trentesaux C. Overexpression of Bcl-2 is associated with apoptotic resistance to the G-quadruplex ligand 12459 but is not sufficient to confer resistance to long-term senescence. Nucleic Acids Res. 2005;33:2192–2203. doi: 10.1093/nar/gki514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T-M, Barbone D, Fennell D, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson M, Nagaraja A, Matzuk M. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipia A, Hsu SY, Hsueh AJ. Expression and function of a proapoptotic Bcl-2 family member Bcl-XL/Bcl-2-associated death promoter (BAD) in rat ovary. Endocrinology. 1997;138:5497–5504. doi: 10.1210/endo.138.12.5588. [DOI] [PubMed] [Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile of chromium In: Portier CJ (ed.), Chromium. Atlanta, GA: U.S. Department of Health and Human Services; 2012: 49 102 [Google Scholar]
- Bucher J. NTP toxicity studies of sodium dichromate dihydrate (CAS No. 7789-12-0) administered in drinking water to male and female F344/N rats and B6C3F1 mice and male BALB/c and am3-C57BL/6 mice. Toxic Rep Ser. 2007;72:1–4. [PubMed] [Google Scholar]
- Sayato Y, Nakamuro K, Matsui S, Ando M. Metabolic fate of chromium compounds. I. Comparative behavior of chromium in rat administered with Na251CrO4 and 51CrCl3. J Pharmacobiodyn. 1980;3:17–23. doi: 10.1248/bpb1978.3.17. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Hanaki A. Spin-trapping studies on the reactions of Cr(III) with hydrogen peroxide in the presence of biological reductants: is Cr(III) non-toxic? Biochem Int. 1990;22:343–352. [PubMed] [Google Scholar]
- Ayres S, Tang M, Subbiah MTR. Estradiol-17β as an antioxidant: some distinct features when compared with common fat-soluble antioxidants J Lab Clin Med 1996. 128 A1. [DOI] [PubMed] [Google Scholar]
- Tang XL, Liu XJ, Tian Q, Zhang W. Dynamic oxidative Stress and DNA damage induced by oestrogen deficiency and protective effects of puerarin and 17β-Oestradiol in ovariectomized rats. Basic Clin Pharmacol Toxicol. 2012;111:87–91. doi: 10.1111/j.1742-7843.2012.00864.x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kale RK, Baquer NZ. Estradiol modulates membrane-linked ATPases, antioxidant enzymes, membrane fluidity, lipid peroxidation, and lipofuscin in aged rat liver J Aging Res 2011. 2011 580245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembacz KP, Sawicka E, Dlugosz A. Role of estradiol in chromium-induced oxidative stress. Acta Pol Pharm. 2012;69:1372–1379. [PubMed] [Google Scholar]
- Abbas AM, Elsamanoudy AZ. Effects of 17beta-estradiol and antioxidant administration on oxidative stress and insulin resistance in ovariectomized rats. Can J Physiol Pharmacol. 2011;89:497–504. doi: 10.1139/y11-053. [DOI] [PubMed] [Google Scholar]
- Dlugosz A, Roszkowska A, Zimmer M. Oestradiol protects against the harmful effects of fluoride more by increasing thiol group levels than scavenging hydroxyl radicals. Basic Clin Pharmacol Toxicol. 2009;105:366–373. doi: 10.1111/j.1742-7843.2009.00411.x. [DOI] [PubMed] [Google Scholar]
- Gardiner CS, Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod. 1994;51:1307–1314. doi: 10.1095/biolreprod51.6.1307. [DOI] [PubMed] [Google Scholar]
- Foyouzi N, Cai Z, Sugimoto Y, Stocco C. Changes in the expression of steroidogenic and antioxidant genes in the mouse corpus luteum during luteolysis. Biol Reprod. 2005;72:1134–1141. doi: 10.1095/biolreprod.104.037598. [DOI] [PubMed] [Google Scholar]
- Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- Bednarek-Tupikowska G, Tupikowski K, Bidzinska B, Bohdanowicz-Pawlak A, Antonowicz-Juchniewicz J, Kosowska B, Milewicz A. Serum lipid peroxides and total antioxidant status in postmenopausal women on hormone replacement therapy. Gynecol Endocrinol. 2004;19:57–63. doi: 10.1080/09513590412331272328. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26:101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, Tanaka E. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013;14:13909–13930. doi: 10.3390/ijms140713909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasou T, Kwon AH, Tsuji K, Qiu Z, Okumura T, Kamiyama Y. Edaravone prevents Fas-induced fulminant hepatic failure in mice by regulating mitochondrial Bcl-xL and Bax. Shock. 2008;30:212–216. doi: 10.1097/shk.0b013e31816171f4. [DOI] [PubMed] [Google Scholar]
- Li G-Y, Feng Y, Cheng T, Yin J-M, Zhang C-Q. Edaravone, a novel free radical scavenger, prevents steroid-induced osteonecrosis in rabbits. Rheumatology. 2013;52:438–447. doi: 10.1093/rheumatology/kes313. [DOI] [PubMed] [Google Scholar]
- Saini A, Kumar HSA, Sharma S. Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur J Pharmacol. 2007;568:164–172. doi: 10.1016/j.ejphar.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Kara M, Daglioglu Y, Kuyucu Y, Tuli A, Tap O. The effect of edaravone on ischemia-reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2012;162:197–202. doi: 10.1016/j.ejogrb.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Ergenoglu M, Erbas O, Akdemir A, Yeniel A, Yildirim N, Oltulu F, Aktug HS, Taskiran D. Attenuation of ischemia/reperfusion-induced ovarian damage in rats: does edaravone offer protection? Eur Surg Res. 2013;51:21–32. doi: 10.1159/000353403. [DOI] [PubMed] [Google Scholar]