ABSTRACT
Over the past decade, engineered nanomaterials (ENMs) have garnered great attention for their potentially beneficial applications in medicine, industry, and consumer products due to their advantageous physicochemical properties and inherent size. However, studies have shown that these sophisticated molecules can initiate toxicity at the subcellular, cellular, and/or tissue/organ level in diverse experimental models. Investigators have also demonstrated that, upon exposure to ENMs, the physicochemical properties that are exploited for public benefit may mediate adverse endocrine-disrupting effects on several endpoints of mammalian reproductive physiology (e.g., steroidogenesis, spermatogenesis, pregnancy). Elucidating these complex interactions within reproductive cells and tissues will significantly advance our understanding of ENMs as an emerging class of novel endocrine disruptors and reproductive toxicants. Herein we reviewed the recent developments in reproductive nanotoxicology and identified the gaps in our knowledge that may serve as future research directions to foster continued advancement in this evolving field of study.
Keywords: endocrine disruptors, engineered nanomaterials, nanotoxicology, reproduction, steroidogenesis
INTRODUCTION
In recent years, the emerging advancement of nanotechnologies for potential therapeutic and consumer benefit has elevated concern regarding the possible toxicological consequences of such molecules in biological systems and the environment [1–3], both with respect to physiologic effects generally and reproductive function and fertility in particular [4]. Nanotechnology, as defined by the United States Nanotechnology Initiative, is “the understanding and control of matter at dimensions of roughly 1–100 nanometers, where unique phenomena enable novel applications” [5]. Intriguingly, it has been estimated that nanotechnology may exceed the impact of the Industrial Revolution and is projected to become a $1 trillion market by the year 2015 [6]. Engineered nanomaterials (ENMs) such as quantum dots (QDs), dendrimers, silica, carbon nanotubes and fullerenes, oxides (e.g., titanium dioxide, iron oxide), metals (e.g., gold, silver, and aluminum), and liposomes have been exploited and utilized for their unique physicochemical properties (e.g., enhanced surface area, surface reactivity and functionalization, solubility, shape, and aggregative potential) due to their inherent size [7]. Note that this is not an exhaustive list; ENMs come in vastly different sizes, shapes, and compositions (Fig. 1). Size-related properties (i.e., surface area and size distribution), chemical composition (i.e., purity, crystallinity, electronic properties), surface structure (i.e., surface reactivity, surface groups, inorganic/organic conjugates, morphology), solubility, shape, and aggregative potential have been exploited for consumer products, such as sporting goods, tires, stain-resistant clothing, sunscreens, cosmetics, and electronics [8, 9]. Gold nanoparticles (GNPs) have also been investigated for many beneficial applications that include novel uses in targeted drug delivery [10], electronics [11], bioimaging [12], and industry [13, 14]. For example, CYT-6091, a novel nanomedicine composed of recombinant human tumor necrosis factor alpha conjugated to 27-nm GNPs, has shown promise in targeting malignant tumors in vivo and has exhibited less toxicity than recombinant human tumor necrosis factor alpha alone [10]. However, other studies have shown that in vivo exposure to GNPs can induce pulmonary toxicity [15], neurotoxicity [16], cardiotoxicity [17], nephrotoxicity [18], and hepatotoxicity [19] in rodents. Hence, exposure to ENMs may yield health effects that justify the current public health concern. The elevated current and future uses of ENMs augment the potential risk for environmental and occupational exposures to ENMs that might ultimately cause detrimental health effects to workers and the general population. With that said, it is reasonable to postulate that the same aforementioned physicochemical properties that are exploited for medical and consumer applications may indeed be the initiators of unforeseen toxicological effects that result in harmful cytotoxicity, which upon biodistribution and cell entry [20, 21], could ultimately alter gene [22] and protein [23] expression; induce cellular states of oxidative stress, inflammation, and cell death [24, 25]; and/or modulate enzyme activity [26]. Such evidence suggests that the potentially deleterious consequences of ENM exposure include disruption of complex biochemical and physiologic processes involved in mammalian reproduction (e.g., gonadal steroidogenesis). The purposes of this minireview were (1) to briefly explore our current understanding of the possible health effects of ENM exposure on mammalian reproductive physiology; (2) to gain an appreciation for the potential safety risks associated with the consumer and therapeutic medical applications of ENMs, and; (3) to highlight future research directions that will enhance our understanding of the potential impacts of ENMs on the mammalian reproductive system.
FIG. 1.
Examples of incidental and engineered nanomaterials. (Adapted with permission from Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicol Sci 2008; 101:4–21; Oxford University Press on behalf of the Society of Toxicology).
GONADAL STEROIDOGENESIS: AN EMERGING TARGET OF ENGINEERED NANOMATERIALS
Brief Overview of Steroidogenesis
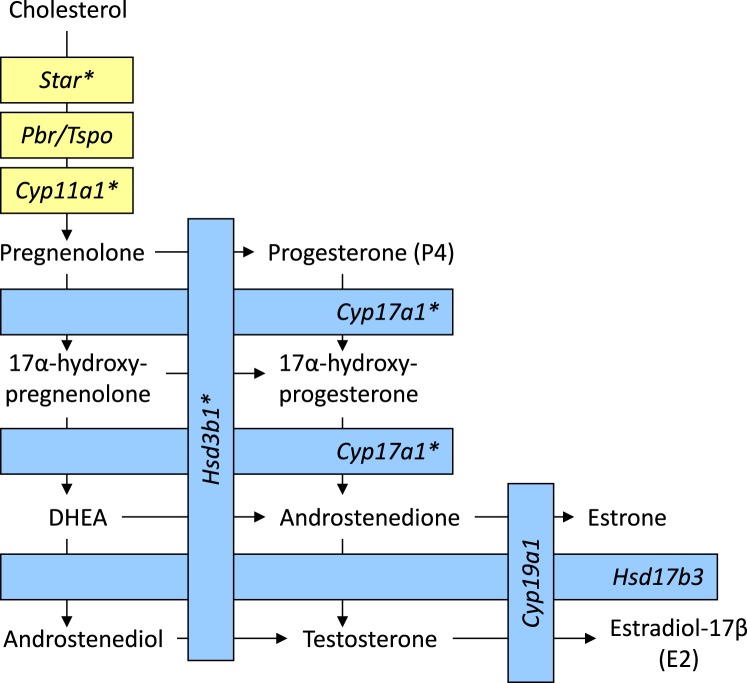
Steroid biosynthesis is a biochemical pathway that is critical to gonadal (i.e., ovarian [theca and granulosa cell] and testicular [Leydig and Sertoli cell]) function [27, 28]. Herein, we will briefly review the steps of steroidogenesis prior to our discussion of ENMs as potential endocrine disruptors (Fig. 2). The molecular precursor for steroid biosynthesis is cholesterol. Cholesterol arrives at the gonad via low-density lipoproteins in the blood stream [27]. Upon binding of the cholesterol to low-density lipoprotein receptors, the resultant complexes are internalized and hydrolyzed via lysosomes that release cholesterol for steroid biosynthesis [29]. This is the predominant mechanism of cellular cholesterol acquisition, in addition to liberation of cholesterol from intracellular lipid droplets; de novo local synthesis of cholesterol occurs but does not serve as a major contributing source in vivo [30]. In order to be used as a substrate for steroid biosynthesis, cholesterol must translocate from the thecal or Leydig cytosol into the mitochondria via the steroidogenic acute regulatory (STAR) protein of the outer mitochondrial membrane [31]. STAR acts to transport cholesterol from the thecal or Leydig outer mitochondrial membrane to the inner mitochondrial membrane. Hence, STAR's action is the rate-limiting step in acute steroid biosynthesis. Additionally, recent evidence has shown that STAR's action requires the presence and coordination of the peripheral-type benzodiazepine receptor/translocator protein (PBR/TSPO); thus, the cooperative action of several proteins may be necessary for trans- and intramitochondrial transport of cholesterol in steroid synthesis [32]. Upon reaching the inner mitochondrial membrane, cholesterol is made available as a substrate for the side-chain cleavage enzyme (CYP11A1), which catalyzes the conversion of cholesterol to pregnenolone [33]. Thereafter, pregnenolone diffuses to the thecal or Leydig smooth endoplasmic reticulum and is converted into progesterone (P4) via the action of 3β-hydroxysteroid dehydrogenase enzyme (HSD3B1) or into 17α-hydroxypregnenolone via 17α-hydroxylase (CYP17A1) [33, 34]. Additionally, within thecal or Leydig smooth endoplasmic reticulum, P4 and 17α-hydroxypregnenolone may be converted to 17α-hydroxyprogesterone via CYP17A1 and HSD3B1, respectively. 17α-Hydroxyprogesterone is then metabolized to androstenedione by the cleavage of the C-20, 21 side chain via the enzyme 17,20 lyase (CYP17A1). Likewise, 17α-hydroxyprogesterone can be directly converted to androstenedione or may be converted to dehydroepiandrosterone (DHEA; a weak androgen) via the enzyme 17,20 lyase (CYP17A1) and thereafter catalyzed to androstenedione by HSD3B1 [34]. Then androstenedione, a weak androgen, may be converted to testosterone, a potent androgen, via 17β-hydroxysteroid dehydrogenase (HSD17B3) but may alternatively be converted to the estrogen estrone via the enzyme aromatase (CYP19A1). Testosterone can also be converted to estradiol-17β via CYP19A1, and estrone to estradiol-17β via HSD17B3 [34]. Thus, both androstenedione and testosterone can serve as precursors for estrogen production. The smooth endoplasmic reticulum of ovarian granulosa and testicular Sertoli cells constitutes the major site of estrogen synthesis via CYP19A1. Several enzymes and other factors in the ovarian steroidogenic pathway (e.g., CYP11A1, STAR, CYP19A1) are regulated by the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland [35], which are themselves controlled in part via the complex feedback actions of E2 and P4 [36]. In summary, steroidogenesis involves the intricate coordination of several proteins and enzymes that ultimately yield the sex hormones required for optimal fertility. Hence, disruption of gonadal cells and tissues and/or steroidogenesis via exogenous molecules and particles such as ENMs would affect steroidogenic output and ultimately perturb mammalian reproductive function.
FIG. 2.
A simplified representation of gonadal steroidogenesis. Yellow and blue represent the biochemical steps that occur within the mitochondria or smooth endoplasmic reticulum, respectively. *Steroidogenic gene that has been shown to be dysregulated upon exposure (in vitro, ex vivo, and/or in vivo) to a particular type of nanoparticle. Star = steroidogenic acute regulatory protein; Pbr/Tspo = peripheral-type benzodiazepine receptor/translocator protein; Cyp11a1 = side-chain cleavage enzyme; Cyp17a1 = 17-alpha-hydroxylase/17,20 lyase; Hsd3b1 = 3β-hydroxysteroid dehydrogenase; Hsd17b3 = 17β-hydroxysteroid dehydrogenase; Cyp19a1 = aromatase.
ENMs as Novel Endocrine Disruptors: Effects on Steroidogenesis
Recent research suggests that ENMs represent an evolving class of novel endocrine disruptors that may cause reproductive toxicity [4, 37, 38]. Many of the clues pertaining to the steroidogenic effects of particulate matter have been gleaned from studies of ambient carbon nanoparticles (e.g., diesel exhaust and carbon black) [39–44]. For instance, Komatsu et al. [43] evaluated the direct effect of diesel exhaust particles, titanium dioxide, and carbon black nanoparticles on mouse Leydig TM3 cells. Quantitative real-time reverse-transcription PCR (Q-RT-PCR) revealed that cells treated with 100 μg/ml diesel exhaust particles for 16 h significantly increased their expression of the inducible form of heme oxygenase-1 (Hmox1). Hmox1 is known to exert antioxidant properties and is a sensitive marker of oxidative stress. A similar effect was not observed in cells treated with titanium dioxide or carbon black. Thus, diesel exhaust particles may indeed induce oxidative stress in mouse Leydig cells. Additionally, a significant effect on Star expression was not observed after 16 h under any treatment condition; however, significantly enhanced mRNA expression of Star was observed in cells treated with diesel exhaust particles or carbon black after a 48-h period of incubation. Other studies have demonstrated that low exposure (15.4 ± 1.0 μg/m3) to nanoparticle-rich diesel exhaust particles can elevate plasma testosterone concentrations in male rats via increased expression of associated steroidogenic genes (i.e., Star, Cyp11a1, and Cyp17a1) [42]. Moreover, Li et al. [39] demonstrated that exposing pregnant rats to either nanoparticle-rich or filtered diesel exhaust for 19 days of gestation yielded a significantly decreased serum concentration of maternal progesterone, concomitant with a significant decrease in the expression of P450 side-chain cleavage enzyme (Cyp11a1), 3-beta-hydroxysteroid dehydrogenase (Hsd3b1), and LH receptor (Lhcgr) in the corpus luteum compared to that in controls (i.e., exposed to clean air). As one might expect, these experimental groups also yielded a significant increase in serum concentrations of LH compared to that in controls. These observations of hormonal changes suggest the possible suppression of corpora lutea upon exposure to nanoparticle-rich or filtered diesel exhaust, which could ultimately result in pregnancy loss. The knowledge gained from these studies serves as a foundation for investigations focused on evaluating ENMs as potential endocrine-disrupting toxicants.
Our laboratory has shown that 10-nm GNPs (2.85 × 1010 particles/ml) are able to enter rat ovarian granulosa cells, translocate into lipid droplets, alter mitochondrial morphology, and subsequently modulate estrogen production [45]. Specifically, transmission electron microscopy studies showed GNPs within the cytoplasm (at 1 and 3 h of culture), vacuoles (at 1 h), and lipid droplets (at 5 h) after in vitro coculture of granulosa cells and GNPs. Interestingly, 10-nm GNPs were found to be juxtaposed with the outer mitochondrial membrane of healthy mitochondria (at 24 h) and inside apparently damaged, swollen mitochondria (at 5 and 24 h). Furthermore, granulosa cells incubated with 10-nm GNPs for 1, 3, and 5 h precipitated an increased accumulation/efflux of E2. However, after 24 h, there was a decrease in E2 accumulation by granulosa cells. Based on our results, it is reasonable to rationalize that GNPs enter ovaries, perturb the mitochondrial membrane(s), and significantly affect steroidogenesis and gene expression via an oxidative stress-mediated mechanism.
Oxidative stress is a well-known inhibitor of ovarian sex steroid production [46]. For instance, it is reasonable to postulate that ENMs such as GNPs may impact sex steroid hormone production via altered expression of steroidogenesis-related and/or oxidative stress-related enzymes or STAR, as observed with ambient particulates. However, we also have demonstrated that low-dose GNPs (∼7 nm) can subtly modulate steroidogenic gene expression, independent of oxidative stress or inhibin, in an ex-vivo ovarian culture model [22]. Regression analyses showed a positive relationship between both Star (P < 0.05, r2 = 0.278) and Cyp11a1 (P < 0.001, r2 = 0.366) expression and P4 accumulation upon exposure to 1.43 × 106 particles/ml. Additional analyses showed that estradiol accumulation was positively associated with Hsd3b1 (P < 0.05, r2 = 0.181) and Cyp17a1 (P < 0.01, r2 = 0.301) expression upon exposure to 1.43 × 103 and 1.43 × 109 particles/ml, respectively. To our knowledge, the lowest experimental dose included in this study (i.e., 1.43 × 103 particles/ml) is the lowest effective exposure dose of GNPs to be reported to date. Interestingly, we estimated this experimental dose to be approximately 200,000-fold less than the amount of GNPs administered in a therapeutic dose of CYT-6091 [22]. The results of this study suggest that low-dose GNPs are capable of eliciting endocrine-modulating effects via unique nonmonotonic mechanisms, similar to those used by other endocrine disruptors such as bisphenol A, rather than a traditional dose-dependent (i.e., monotonic) response [47]. Li et al. [48] have also shown that in-vivo exposure of mice to ω-aminoethyl polyethylene glycol (PEG-NH2)-modified GNPs (45 mg/kg) elevated plasma testosterone concentrations after all experimental time periods (i.e., 7, 14, 21, and 30 days) without a concurrent impact on plasma LH or FSH or on fertility. However, exposure of mice to ω-methoxy polyethylene glycol (mPEG)-modified GNPs (45 mg/kg or 225 mg/kg) in the same study had no effect on plasma testosterone concentrations compared to that in controls or other treatment groups. This evidence suggests that the surface functionality of a particular ENM may mediate a biological response(s). Moreover, titanium dioxide (TiO2) nanoparticles have been shown to modulate serum concentrations of sex-steroid hormones in female rodents [49, 50]. For example, Gao et al. [50] demonstrated that TiO2 nanoparticles (∼6 nm; 10 mg/kg) elevated serum concentrations of E2, P4, and FSH and reduced serum concentrations of LH and testosterone in treated mice compared to that in controls. DNA microarray analysis also revealed that ∼228 genes of known ovarian function (e.g., Star, Cyplla1, and Cyp17a1) were upregulated in treated mice (compared those in controls). Conversely, zinc oxide [51] and calcium phosphate [52] nanoparticles have been shown to lack any effect on steroidogenesis in vivo and in vitro, respectively. Therefore, further research is required to identify the specific factors (e.g., sex) that drive nanotoxicologic effects on reproductive physiology.
EFFECTS OF ENMS ON OTHER ENDPOINTS OF MAMMALIAN REPRODUCTION
Males
In general, studies pertaining to the effects of ENMs on mammalian reproduction in males have focused on testicular steroidogenesis, in-vivo biodistribution to testis and/or epididymis (e.g., gold [20, 48, 53], silver [54–56], titanium [57, 58], ceria [59], and cobalt-chromium [60] nanoparticles), spermatogenesis [61–64], and sperm viability [65–68]. For example, Balasubramanian et al. [53] demonstrated that 20-nm GNPs were still present in rat testis (at a concentration of 0.6 [0.01] ng/mg tissue; mean ± SD) 1 month after intravenous injection (15.1 μg gold/ml). Lee et al. [56] demonstrated that after a 4-month recovery period, the presence of silver in testis was persistent compared to that in ovary after daily exposures (100 mg/kg/day and 500 mg/kg/day) of rats to silver nanoparticles (10 and 25 nm, respectively) via oral gavage for 4 weeks. This evidence supports the notion that biological barriers (e.g., blood–testis barrier) play a role in the particodynamics of ENMs and ultimately mediate the potential for bioaccumulation and/or compartmentalization of nanoparticles in vivo. Braydich-Stolle et al. [69, 70] reported that silver nanoparticles significantly inhibited proliferation of mouse spermatogonial stem cells (compared to that in controls) in vitro and suggested that the molecular mechanism of this toxic effect may involve disruption of the GDNF/FYN kinase signaling pathway. Recently, Gromadzka-Ostrowska et al. [64] reported that silver nanoparticles exert a decrease in rat epididymal sperm count that is dependent on particle size (20 and 200 nm), dose (5 and 10 mg/kg body mass), and time (1, 7, or 28 days) compared to that in controls in vivo. Similarly, in vivo exposure of mice to zinc oxide nanoparticles (ZnO-NP; diameter not reported; 50 and 300 mg/kg) for 35 consecutive days has been shown to significantly reduce the number of testicular and epididymal sperm compared to that in controls [71]. In this study, ZnO-NP-treated epididymal sperm also exhibited a decrease in motility and an increase in the presence of morphological abnormalities compared to that in controls. It has also been demonstrated that weekly intra-articular injections of cobalt-chromium nanoparticles (500 μg/kg) into adult male rats (1 injection/week for a total of 10 weeks) yielded a significant reduction in epididymal sperm motility, viability, and concentration with a concurrently elevated presence of sperm abnormalities and testicular damage (compared to that in controls) [60]. Moreover, Gao et al. [58] reported that TiO2 nanoparticles (5–6 nm) induced testicular damage and reduced spermatogenesis and sperm viability (i.e., decreased motility and increased abnormalities) in a dose-dependent (2.5, 5, and 10 mg/kg) manner in vivo (intragastric administration each day for a total of 90 days) compared to that in controls. The results of that study also revealed a statistically significant dose-dependent increase in serum concentrations of E2 and P4, with a concurrent decrease in the serum concentrations of LH, FSH, and testosterone compared to those in controls. Gene expression studies showed significant dysregulation of genes related to spermatogenesis (e.g., Adam3, Ly6e), apoptosis/oxidative stress (e.g., Gpx5), and signal transduction (i.e., Cfd and Gyk1) in high-dose-treated mice compared to those in controls. Interestingly, Bai et al. [72] reported that repeated intravenous injections of water soluble carbon nanotubes caused reversible testicular damage with no significant effect on fertility. Therefore, further research is necessary to comprehensively delineate the mechanistic effects of ENMs on the male reproductive system.
Females
Research evaluating the impacts of ENMs on endpoints of female reproduction (other than steroidogenesis) are severely lacking in both scope and number. To date, the vast majority of such studies are focused on pregnancy [63, 73–80] and in vitro/ex vivo models of placental transfer [81–83], with few studies addressing the potential effects of ENMs on oogenesis [84, 85], oocyte maturation and follicular development [86–89], or the estrogen-receptor signaling pathway [90]. For example, Blum et al. [73] showed that daily inhalation of 230 μg/m3 cadmium oxide nanoparticles decreased the incidence of pregnancy by 23%, delayed maternal weight gain, altered placental weight, decreased fetal length, and delayed neonatal growth in a CD-1 mouse model. In addition, Tian et al. [76] demonstrated that intrauterine inflammation can enhance the maternofetal transfer of GNPs (3 and 13 nm) in a size-dependent manner in the late gestational stages of mouse pregnancy (compared to those in healthy controls); however, 32-nm GNPs were not shown to cross the placenta of either healthy controls or mice with intrauterine inflammation. Other studies have shown that exposure of pregnant dams to TiO2 nanoparticles induces reproductive toxicity (in male offspring [63]), neurotoxicity [78, 79], or hepatic toxicity [80] in offspring. With respect to oogenesis, Boisen et al. [84] reported that daily inhalation exposure to TiO2 nanoparticles (i.e., ∼42.4 mg/m3 from gestation days [GD] 8–18) by pregnant C57Bl/6J mice did not increase the rate of extended simple tandem repeat (ESTR) mutations in the female germ line of second generation (F2) offspring compared to those in control mice exposed to filtered clean air. Similarly, Boisen et al. [85] also reported that in vivo exposure of pregnant C57Bl/6J mice to carbon black nanoparticles via intratracheal instillation (67 μg/animal; GD 7, 10, 15, and 18) did not affect the rate of ESTR mutations in F2 females compared to that in controls. Exceedingly few studies have evaluated the effects of ENMs on oocyte maturation and follicle development. For instance, TiO2 nanoparticles (25 nm, 12.5–50 μg/ml) have been shown to inhibit rat follicle development and oocyte maturation in vitro [86]. That study also revealed a dose-dependent decrease in the survival rate of follicles, the formation rate of antral follicles, and the release rate of the cumulus-oocyte complex. Similar studies using QDs (e.g., CdSe-cored and ZnS-coated CdSe [87]; Cdse/CdS/ZnS [88]; and CdTe/ZnTe QD-transferrin bioconjugates [89]) suggest that these extremely diverse and complex ENMs significantly disrupt oocyte maturation and/or follicular development in vitro (compared to that in controls). Moreover, Jain et al. [90] reported that CdTe QDs induce, via both genomic and nongenomic estrogen-signaling pathways, estrogenic effects (e.g., cell proliferation) in vitro (using the human breast cancer cell line MCF-7), comparable to estradiol-17β treatment (as a positive control). Interestingly, pretreatment with ICI 182,780, a selective estrogen receptor antagonist, abolished the estrogenic effects observed in the MCF-7 cells, thus further supporting the potential interaction of CdTe QDs and estrogen receptors. In the same study, additional experiments demonstrated that QDs can mediate estrogenic effects on the murine uterus in vivo. For instance, chronic exposure (i.e., alternate day dosing [25 mg/kg] for 2 weeks via intraperitoneal injection) of prepubertal mice to QDs yielded an approximately 2-fold increase (P < 0.001) in uterine weight compared to that in both positive (estradiol-17β) and negative (sterile double-distilled water) controls. Moreover, using the identical chronic exposure regimen with an adult ovariectomized mouse model, it was shown that QDs (25 mg/kg) induced a ∼2.5-fold increase in uterine weight (compared that in negative controls, P < 0.001) to an extent that was comparable to estradiol-17β (∼2.0-fold increase in uterine weight compared with that in negative control, P < 0.001). Hence, CdTe QDs are potential endocrine-disrupting molecules that necessitate further research.
CLOSING REMARKS
ENMs have garnered immense consideration for potentially beneficial applications in medicine/science, consumer products, and technology; yet toxicological investigation of the possible adverse effects of ENMs on mammalian reproduction and fertility remains in its nascency. Research has shown that the advantageous physicochemical properties of these molecules, which are exploited for commercially beneficial applications, may dictate in vivo biodistribution and ultimately mediate mechanisms of cellular toxicity, for example, oxidative stress, altered gene expression, and cell death via necrosis, apoptosis, or autophagy. In recent years, our laboratory has explored GNPs as potentially novel ovarian endocrine disruptors by using both in vitro and ex-vivo experimental approaches. Although such models may significantly contribute to our knowledge of gonadal nanotoxicity, they may not adequately predict potential effects of ENMs under physiological conditions in situ. Recent evidence has illustrated the apparent lack of correlation between in vitro and in vivo effects of ENMs on pulmonary function [91], tumor growth [92], and human integument [93]. For instance, Zogovic et al. [92] demonstrated that nano-C60 (buckminsterfullerenes or “buckyballs”) exhibit potent anticancer activity in vitro but may actually potentiate tumor growth in vivo. Conversely, other investigators have demonstrated that in vivo ENM studies may indeed support in vitro findings [94–96]. In lieu of such variability, the extremely complex interactions between physiological condition (e.g., pH, temperature, metabolic state, hormonal status) and unique physicochemical properties (e.g., size, surface chemistry, surface area) exhibited by ENMs support the possibility of a diverse, yet specific, in vivo response upon exposure, different from that in vitro, thereby making such effects difficult to model. Such molecular complexities warrant the comprehensive study of the effects of ENMs on reproductive physiology in vivo. Furthermore, in vivo study permits (1) the systemic evaluation of pharmacokinetics/toxicokinetics/particokinetics and pharmacodynamics (i.e., absorption, distribution, metabolism, and excretion [ADME]) of nanoparticles under physiologic conditions); (2) the evaluation of potential long-term (i.e., months, years, or throughout a particular animal's lifespan) impacts of acute and chronic exposures of ENMs on mammalian reproduction (such evidence is currently absent from the literature); and (3) the assessment of critical mechanistic endpoints of subcellular function (e.g., cell death, gene and protein expression, and oxidative stress) in response to realistic and environmentally relevant routes of exposure to ENMs (e.g., dermal, inhalation, ingestion). All of these factors may contribute significantly to an observed toxicological response upon ENM exposure and therefore must be thoroughly investigated. In addition, it is important to note that the comparison of the experimental nanomaterial concentrations described here with those that are relevant to the environment and humans is very difficult due to the current challenges of predictive exposure modeling in the risk assessment of nanomaterials [97] and a lack of nanomaterial-specific analytical measurement techniques [98–99]. These challenges are perpetuated by the diverse biophysicochemical properties of ENMs that (1) are much more complex than those of more widely studied endocrine disruptors such as dioxin and bisphenol A; and that (2) ultimately dictate the interactions of ENMs within biologic and environmental matrices [100].
In summary, the long-term goal is to better understand, at molecular, cellular, and organ systems levels, the role of ENMs as potential endocrine disruptors and/or toxicants in mammalian reproductive tissues. The incipient and evolving field of reproductive nanotoxicology will advance and expedite the evaluation of the potential effects of ENMs on reproductive endpoints (e.g., steroid hormone production), which will significantly benefit and contribute to the development of risk assessments focused on ENMs and mammalian fertility. In turn, the knowledge gained will (1) foster the creation of interventions so as to more closely and meticulously monitor and then prevent excessive exposure to ENMs that can exert untoward effects on reproductive physiology; (2) serve to advance discovery and education within the emerging fields of nanotoxicology and reproductive nanomedicine [101–103]; and (3) most importantly, contribute to the knowledge required to further educate our society about the potentially harmful effects of ENMs.
Footnotes
Supported by National Institutes of Environmental Health Sciences grant P30 ES004184.
REFERENCES
- Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol Sci. 2011;120((suppl 1)):S109–129. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebounova LV, Morgan H, Grassian VH, Brenner S. Health and safety implications of occupational exposure to engineered nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:310–321. doi: 10.1002/wnan.174. [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Massimiani M, Magrini A, Camaioni A, Pietroiusti A. Physico-chemical properties mediating reproductive and developmental toxicity of engineered nanomaterials. Curr Med Chem. 2012;19:4488–4494. doi: 10.2174/092986712803251566. [DOI] [PubMed] [Google Scholar]
- Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre N, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicol Sci. 2008;101:4–21. doi: 10.1093/toxsci/kfm169. [DOI] [PubMed] [Google Scholar]
- Kessler R. Engineered nanoparticles in consumer products. Environ Health Perspect. 2011;119:a120–125. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow Wilson International Center for Scholars [ Internet]. A nanotechnology consumer products inventory http://www.nanotechproject.org/consumerproducts. Accessed 11 November 2013. [Google Scholar]
- Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, , Gannon WE, Walker M, Sqidel GD, Yuldasheva N, Tamakarin L. Phase 1 and pharmacokinetic studies of CYT-6091, a novel PEGlyated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombeger M, Simon U. On the application potential of gold nanoparticles in nanoelectronics and biomedicine. Philos Transact A Math Phys Eng Sci. 2010;368:1405–1453. doi: 10.1098/rsta.2009.0275. [DOI] [PubMed] [Google Scholar]
- Sun IC, Lee S, Koo H, Kwon IC, Choi K, Ahn CH, Kim H. Caspase sensitive gold nanoparticle for apoptosis imaging in live cells. Bioconjug Chem. 2010;21:1939–1942. doi: 10.1021/bc1003026. [DOI] [PubMed] [Google Scholar]
- Ngo YH, Li D, Simon GP, Garnier G. Paper surfaces functionalized by nanoparticles. Adv Colloid Interface Sci. 2011;163:23–38. doi: 10.1016/j.cis.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Hussain S, Vanoirbeek JA, Luyts K, De Vooght V, Verbeken E, Thomassen LC, Martens JA, Dinsdale D, Boland S, Marano F, Nemery B, Hoet PH. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37:299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- Chen YS, Hung YC, Lin LW, Liau I, Hong MY, Huang GS. Size-dependent impairment of cognition in mice caused by the injection of gold nanoparticles Nanotechnology 2010. 21 48 485102. [DOI] [PubMed] [Google Scholar]
- Abdelhalim MA. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure Lipids Health Dis 2011. 10 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim MA, Jarrar BM. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles Lipids Health Dis 2011. 10 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim MA, Jarrar BM. Histological alterations in the liver of rats induced by different gold nanoparticle sizes, doses and exposure duration J Nanobiotechnology 2012. 10 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2012;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9:1521–1532. doi: 10.1002/smll.201201390. [DOI] [PubMed] [Google Scholar]
- Larson JK, Carvan MJ, III , Teeguarden JG, Watanabe G, Taya K, Krystofiak E, Hutz RJ. Low-Dose gold nanoparticles exert subtle endocrine-modulating effects on the ovarian steroidogenic pathway ex vivo independent of oxidative stress. Nanotoxicology. 2014;8:856–866. doi: 10.3109/17435390.2013.837208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Lo SL. Ng CT, Gurung RL, Hartono D, Hande MP, Ong CN, Bay BH, Yung LY. Genomic instability of gold nanoparticles treated human lung fibroblast cells. Biomaterials. 2011;32:5515–5523. doi: 10.1016/j.biomaterials.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity Part Fibre Toxicol 2012. 9 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G. Brandau, Simon U, Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2012;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- Cho WS, Cho W, Jeong J, Choi M, Han BS. Shin Hs, Hong J, Chung BH, Jeong J, Cho MH. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2010;245:116–123. doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism, nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Izzo G, Landreh L, Weisser J, Söder O. Endocrine disruptors and Leydig cell function J Biomed Biotechnol 2010. 2010 684504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureck RW, Strauss JF., III. Progesterone synthesis by luteinized human granulosa cells in culture: the role of de novo sterol synthesis and lipoprotein-carried sterol. J Clin Endocrinol Metab. 1982;54:367–373. doi: 10.1210/jcem-54-2-367. [DOI] [PubMed] [Google Scholar]
- Jay RH, Sturley RH, Stirling C, McGarrigle HH, Katz M, Reckless JP, Betteridge DJ. Effects of pravastatin and cholestyramine on gonadal and adrenal steroid production in familial hypercholesterolaemia. Br J Clin Pharmacol. 1991;32:417–422. doi: 10.1111/j.1365-2125.1991.tb03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco D. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulus V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I. Steroidogenic enzymes: structure, function, and the role of regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- Penning T. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev. 1997;18:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- Lapointe E, Boerboom D. WNT signaling and the regulation of ovarian steroidogenesis. Front Biosci (Schol Ed) 2011;3:276–285. doi: 10.2741/s151. [DOI] [PubMed] [Google Scholar]
- Christensen A, Bentley GE, Cabrera R, Ortega HH, Perfito N, Wu TJ, Micevych P. Hormonal regulation of female reproduction. Horm Metab Res. 2012;44:587–591. doi: 10.1055/s-0032-1306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14:16732–16801. doi: 10.3390/ijms140816732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Kobayashi N, Naya M, Hanai S, Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. Reprod Toxicol. 2010;30:343–352. doi: 10.1016/j.reprotox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. Effects of exposure to nanoparticle-rich diesel exhaust on pregnancy in rats. J Reprod Dev. 2013;59:145–150. doi: 10.1262/jrd.2012-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li X, Jigami J, Hasegawa C, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhal Toxicol. 2012;24:599–608. doi: 10.3109/08958378.2012.702140. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Ito Y, Ramdhan DH, Yanagiba Y, Hayashi Y, Wang D, Li CM, Taneda S, Suzuki AK, Taya K, Watanabe G, Kamijima M et al. Effect of nanoparticle-rich diesel exhaust on testicular and hippocampus steroidogenesis in male rats. Inhal Toxicol. 2012;24:459–467. doi: 10.3109/08958378.2012.688225. [DOI] [PubMed] [Google Scholar]
- Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, Kamijima M, Nakajima T. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol Lett. 2009;191:103–108. doi: 10.1016/j.toxlet.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, Tekeda K. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol In Vitro. 2008;22:1825–1831. doi: 10.1016/j.tiv.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hiyoshi K, Ichinose T, Takano H, Oshio S, Sugawara I, Takeda K, Shibamoto T. Effect of nanoparticles on the male reproductive system in mice. Int J Androl. 2009;32:337–342. doi: 10.1111/j.1365-2605.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- Stelzer RV, Hutz RJ. Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in-vitro estrogen accumulation. J Reprod Develop. 2009;55:685–690. doi: 10.1262/jrd.20241. [DOI] [PubMed] [Google Scholar]
- Margolin Y, Aten RF, Behrman HR. Antigonadotropic and antisteroidogenic actions of peroxide in rat granulosa cells. Endocrinology. 1999;127:245–250. doi: 10.1210/endo-127-1-245. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, , Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and non-monotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Wang F, Liu ZM, Wang YC, Wang J, Sun F. Gold nanoparticles elevate plasma testosterone levels in male mice without affecting fertility. Small. 2013;9:1708–1714. doi: 10.1002/smll.201201079. [DOI] [PubMed] [Google Scholar]
- Tassinari R, Cubadda F, Moracci G, Aureli F, D'Amato M, Valeri M, De Baerardis B, Raggi A, Mantovani A, Passeri D, Rossi M, Maranghi F. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology. 2014;8:654–662. doi: 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- Gao G, Yuguan Z, Bing L, Zhao X, Zhang T, Sheng L, Hu R, Gui S, Sang X, Sun Q, Cheng J, Cheng Z et al. Ovarian dysfunction and gene-expression characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ Toxicol Pharmacol. 2013;35:67–71. doi: 10.1016/j.etap.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Lui X, Qin D, Cui Y, Chen L, Li H, Chen Z, Gao L, Li Y, Liu J. The effect of calcium phosphate nanoparticles on hormone production and apoptosis in human granulosa cells Reprod Biol Endocrinol 2010. 8 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian SK, Jiitwat J, Manikandan J, Ong C, Yu L, Ong W. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim P, Yoon J, Lee B, Choi K, Kil KH, Park K. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology. 2013;7:1120–1130. doi: 10.3109/17435390.2012.710660. [DOI] [PubMed] [Google Scholar]
- Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim YS, Song KS, Ryu HR, Sung JH, Park JD, Park HM, Song NW, Shin BS, Marshak D, Ahn K, Lee JEet al. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats Part Fibre Toxicol 2013. 10 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sycheva LP, Zhurkov VS, Iurchenko VV, Daugel-Dauge NO, Kovalenko MA, Krivtsova EK, Durnev AD. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat Res. 2011;726:8–14. doi: 10.1016/j.mrgentox.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Gao G, Ze Y, Zhao X, Sanh X, Zheng L, Ze X, Gui S, Sheng L, Sun Q, Hong J, Yu X, Wang L et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J Hazard Mater. 2013;258–259:133–143. doi: 10.1016/j.jhazmat.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Geraets L, Oomen AG, Schroester JD, Coleman VA, Cassee FR. Tissue distribution of inhaled micro- and nano-sized cerium dioxide particles in rats: results from a 28-day exposure study. Toxicol Sci. 2012;127:463–473. doi: 10.1093/toxsci/kfs113. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen Z, Zuo Q, Song F, Wu D, Cheng W, Fan W. Reproductive toxicity in adult male rats following intra-articular injection of cobalt-chromium nanoparticles. J Orthop Sci. 2013;18:1020–1026. doi: 10.1007/s00776-013-0458-2. [DOI] [PubMed] [Google Scholar]
- Lan Z, Yang WX. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood-testis barrier. Nanomedicine (Lond) 2012;7:579–596. doi: 10.2217/nnm.12.20. [DOI] [PubMed] [Google Scholar]
- Lucas B, Fields C, Hofmann MC. Signaling pathways in spermatogonial stem cells and their disruption by toxicants. Birth Defects Res C Embryo Today. 2009;87:35–42. doi: 10.1002/bdrc.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyjovska ZO, Boisen AM, Jackson P, Wallin H, Vogel U, Hougaard KS. Daily sperm production: application in studies of prenatal exposure to nanoparticles in mice. Reprod Toxicol. 2013;36:88–97. doi: 10.1016/j.reprotox.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Gromadzka-Ostrowska J, Dziendzikowska K, Lankoff A, Dobrzyńska M, Instanes C, Brunborg G, Gajowik A, Radzikowska J, Wojewódzka M, Kruszewski M. Silver nanoparticle effects on epididymal sperm in rats. Toxicol Lett. 2012;214:251–258. doi: 10.1016/j.toxlet.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor U, Barchanski A, Kues W, Barcikowski S, Rath D. Impact of metal nanoparticles on germ cell viability and functionality. Reprod Domest Anim. 2012;47:359–368. doi: 10.1111/j.1439-0531.2012.02099.x. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V, Sereemaspun A, Rojanathanes R. Effect of gold nanoparticles on spermatozoa: the first world report. Fertil Steril. 2009;91:e7–8. doi: 10.1016/j.fertnstert.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Moretti E, Terzuoli G, Renieri T, Iacoponi F, Castellini C, Giordano C, Collodel G. In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia. 2013;45:392–396. doi: 10.1111/and.12028. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxciol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, Hofmann MC. Silver nanoparticles disrupt GNDF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci. 2010;116:577–589. doi: 10.1093/toxsci/kfq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi AR, Khorsandi L, Moridian M. The effects of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet. 2013;30:1203–1209. doi: 10.1007/s10815-013-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Zhang Y, Zhang J, Mu Q, Zhang W, Butch ER, Snyder SE, Yan B. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat Nanotechnol. 2010;5:683–689. doi: 10.1038/nnano.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Xiong JQ, Hoffman C, Zelikoff JT. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol Sci. 2012;126:478–486. doi: 10.1093/toxsci/kfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, Yoshida T, Ogura T, Nabeshi H, Nagano K, Abe Y, Kamada H et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6:321–328. doi: 10.1038/nnano.2011.41. [DOI] [PubMed] [Google Scholar]
- Jo E, Seo G, Kwon JT, Lee M, Lee Bc, Eom I, Kim P, Choi K. Exposure to zinc oxide nanoparticles affects reproductive development and biodistribution in offspring rats. J Toxicol Sci. 2013;38:525–530. doi: 10.2131/jts.38.525. [DOI] [PubMed] [Google Scholar]
- Tian X, Zhu M, Du L, Wang J, Fan Z, Liu J, Zhao Y, Nie G. Intrauterine inflammation increases materno-fetal transfer of gold nanoparticles in a size-dependent manner in murine pregnancy. Small. 2013;9:2432–2439. doi: 10.1002/smll.201300817. [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Massimiani M, Palmieri G, Bernardini R, Sachetti C, Bergamaschi A, Vecchione L, Magrini A, Bottini M, Pietroiusti A. Biodistribution and toxicity of peglyated single wall carbon nanotubes in pregnant mice Part Fibre Toxicol 2013. 10 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa M, Tainaka H, Kawashima N, Shimizu M, Takeda K. Effect of fetal exposure to titanium dioxide nanoparticle on brain development—brain region information. J Toxicol Sci. 2012;37:1247–1252. doi: 10.2131/jts.37.1247. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Mizuo K, Shinkai Y, Oshio S, Takeda K. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J Toxicol Sci. 2010;35:479–756. doi: 10.2131/jts.35.749. [DOI] [PubMed] [Google Scholar]
- Jackson P, Halappanavar S, Hougaard KS, Williams A, Madsen AM, Lamson JS, Anderson O, Yauk C, Wallin H, Vogel U. Maternal inhalation of surface-coated nanosized titanium dioxide in C57BL/6 mice: effects in prenatally exposed offspring on hepatic DNA damage and gene expression. Nanotoxicology. 2013;7:85–96. doi: 10.3109/17435390.2011.633715. [DOI] [PubMed] [Google Scholar]
- Myllynen PK, Loughran MJ, Howard CV, Sormunen R, Walsh AA, Vähäkangas KH. Kinetics of gold nanoparticles in the human placenta. Reprod Toxicol. 2008;26:130–137. doi: 10.1016/j.reprotox.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Correia Carreira S, Walker L, Paul K, Saunders M. The toxicity, transport and uptake of nanoparticles in the in vitro BeWo b30 placental cell barrier model used within NanoTEST Nanotoxicology ( in press). Published ahead of print 3 September 2013; DOI:10.3109/17435390.2013.833317. [DOI] [PubMed] [Google Scholar]
- Sønnegaard Poulsen M, Mose T, Leth Maroun L, Mathiesen L, Ehlert Knudsen L, Rytting E. Kinetics of silica nanoparticles in the human placenta Nanotoxicology (in press). Published online ahead of print 1 July 2013; DOI:10.3109/17435390.2013.812259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen AM, Shopley T, Jackson P, Hougaard KS, Wallin H, Yauk CL, Vogel U. NanoTIO2 (UV-Titan) does not induce ESTR mutations in the germline of prenatally exposed female mice Part Fibre Toxicol 2012. 9 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen AM, Shipley T, Jackson P, Wallin H, Nellmann C, Vogel U, Yauk CL, Hougaard KS. In utero exposure to nanosized carbon black (Printex90) does not induce tandem repeat mutations in female murine germ cells. Reprod Toxicol. 2013;41:45–48. doi: 10.1016/j.reprotox.2013.06.068. [DOI] [PubMed] [Google Scholar]
- Hou J, Wan XY, Wang F, Xu GF, Liu Z. Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Academ J Second Milit Med Univ. 2009;29:869–873. [Google Scholar]
- Hsieh MS, Shiao NH, Chan WH. Cytotoxic effects of CdSe quantum dots on maturation of mouse oocytes, fertilization, and fetal development. Int J Mol Sci. 2009;10:2122–2135. doi: 10.3390/ijms10052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Yong KT, Xu GX, Lin XT, Zhou XQ, Qu JL, Chen S, Hanben C. Invasion of CdSe/CdS/ZnS quantum dots for oocytes in vitro maturation. Chin Laser. 2009;37:2730–2734. [Google Scholar]
- Xu G, Lin S, Law WC, Roy I, Lin X, Mei S, Ma H, Chen S, Niu H, Wang X. The invasion and reproductive toxicity of QDs-transferrin bioconjugates on preantral follicle in vitro. Theranostics. 2012;2:734–745. doi: 10.7150/thno.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain MP, Vaisheva F, Maysinger D. Metalloestrogenic effects of quantum dots. Nanomedicine (Lond) 2012;7:23–37. doi: 10.2217/nnm.11.102. [DOI] [PubMed] [Google Scholar]
- Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Zogovic NS, Nikolic NS, Vranjes-Djuric SD, Harhaji LM, Vucicevic LM, Janjetovic KD, Misirkic MS, Todorovic-Markovic BM, Markovic ZM, Milonjic SK, Trajkovic VS. Opposite effects of nanocrystalline fullerene (C60) on tumor cell growth in vitro and in vivo and a possible role of immunosuppression in the cancer-promoting activity of C60. Biomaterials. 2009;30:6940–6946. doi: 10.1016/j.biomaterials.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim JN, Jeong SH, Choi JE, Lee S, Choi BH, Lee JP, Sohn KH, Park KL, Kim MK, Son SW. Assessment of dermal toxicity of nanosilica using cultured keratinocytes, a human skin equivalent model and an in vivo model. Toxicology. 2010;267:178–181. doi: 10.1016/j.tox.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Simberg D, Zhang WM, Merkulov S, McCrae K, Park JH, Sailor MJ, Ruoslahti E. Contact activation of kallikrein-kinin system by supramagnetic iron oxide nanoparticles in vitro and in vivo. J Control Release. 2009;140:301–305. doi: 10.1016/j.jconrel.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184:18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Stoeger T, Takenaka S, Frankenberger B, Ritter B, Karg E, Maier K, Schulz H, Schmid O. Deducing in vivo toxicity of combustion-derived nanoparticles from a cell-free oxidative potency assay and metabolic activation of organic compounds. Environ Health Perspect. 2009;117:54–60. doi: 10.1289/ehp.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som C, Nowack B, Krug HF, Wick P. Toward the development of decision supporting tools that can be used for safe production and use of nanomaterials. Acc Chem Res. 2013;46:863–872. doi: 10.1021/ar3000458. [DOI] [PubMed] [Google Scholar]
- Szakal C, Roberts SM, Westerhoff P, Bartholomaeus A, Buck N, Illuminato I, Canady R, Rogers M. Measurement of nanomaterials in foods: integrative consideration of challenges and future prospects. ACS Nano. 2014;8:3128–3135. doi: 10.1021/nn501108g. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen J, Kang Y, Hong S, Zheng Y, Sun H, Xu C. Targeted paclitaxel nanoparticles modified with follicle-stimulating hormone β 81–95 peptide show effective antitumor activity against ovarian carcinoma. Int J Pharm. 2013;453:498–505. doi: 10.1016/j.ijpharm.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Pattison S, Ye L, Tuohey L, Sluka P, MacDiarmid J, Brahmbhatt H, Johns T, Horne AW, Brown J, Tong S. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology. 2013;154:911–919. doi: 10.1210/en.2012-1832. [DOI] [PubMed] [Google Scholar]
- Chaudhury K, Babu KN, Singh AK, Das S, Kumar A, Seal S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine. 2013;9:439–448. doi: 10.1016/j.nano.2012.08.001. [DOI] [PubMed] [Google Scholar]