ABSTRACT
Granulosa cell formation and subsequent follicular assembly are important for ovarian development and function. Two members of the GATA family of transcription factors, GATA4 and GATA6, are expressed in ovarian somatic cells early in development, and their importance in adult ovarian function has been recently highlighted. In this study, we demonstrated that the embryonic loss of Gata4 and Gata6 expression within the ovary results in a strong down-regulation of genes involved in the ovarian developmental pathway (Fst and Irx3) as well as diminished expression of the pregranulosa and granulosa cell markers SPRR2 and FOXL2, respectively. Postnatal ovaries deficient in both Gata genes show impaired somatic cell proliferation and arrested follicular development at the primordial stage, where oocytes are either enclosed by one layer of squamous granulosa cells or remain in germ cell nests/clusters. Furthermore, germ cell nests and primordial follicles are predominantly localized to the central region of the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries, where the boundary between the medulla and cortex is almost nonexistent. Lastly, most of the oocytes are lost early in development in conditional double mutant ovaries, which confirms the importance of normally differentiated granulosa cells as supporting cells for oocyte survival. Thus, both GATA4 and GATA6 proteins are fundamental regulators of granulosa cell differentiation and proliferation, and consequently of proper follicular assembly during normal ovarian development and function.
Keywords: differentiation, granulosa cells, ovarian development
INTRODUCTION
The members of the GATA family are transcriptional regulatory proteins that possess a conserved DNA-binding domain that binds to the GATA motif (WGATAR) found in the promoter region of numerous genes [1, 2]. Depending upon their pattern of expression, the vertebrate GATA proteins have been divided into two groups. GATA1, GATA2, and GATA3 are mostly found in hematopoietic cell linages, but they have also been detected in some organs such as the inner ear, brain, and spinal cord. On the other hand, GATA4, GATA5, and GATA6 are expressed in gonads, heart, liver, lung, and gut epithelium [2, 3]. Among the latter three GATA members, the mammalian ovary expresses GATA4 and GATA6 predominantly; however, the embryonic mouse ovary also expresses the transcription factor GATA2 only in germ cells for a short period of time, that is, from Embryonic Day 11.5 (E11.5) to E15.5 [4, 5].
During ovarian development in mice, both Gata4 and Gata6 transcripts are prominently expressed at E15 [6], but later studies have shown that GATA4 protein can be detected in the somatic cells of the ovary as early as E10.5 [7–10]. In the postnatal ovary, GATA4 and GATA6 are present in granulosa cells where they (directly or indirectly) regulate expression of numerous genes [2, 3]. In particular, GATA4 is abundantly expressed in granulosa cells of primordial follicles undergoing flattened-to-cuboidal transition, in primary, preantral and antral follicles, and in theca cells. In contrast, GATA4 expression is negligible in primordial follicles and luteal cells [3]. GATA6 is also found in granulosa and theca cells of large follicles and, unlike GATA4, is expressed in oocytes and luteal cells [3].
The transcription factors GATA4 and GATA6 are important regulators of genes involved in steroidogenesis. Experimental evidence demonstrated that both transcription factors are strong activators of the type 2 3β-hydroxysteroid dehydrogenase (3β-HSD) promoter in steroidogenic cells [11]. In addition, the nuclear receptor steroidogenic factor 1 (Nr5a1, also known as Sf1) and liver receptor homolog-1 gene products act synergistically with GATA4 and GATA6 in the activation of the type 2 3β-HSD promoter [11]. Other promoters such as anti-Müllerian hormone (Amh), Sf1, inhibinα, and steroidogenic acute regulatory protein (Star) also contain the consensus GATA-binding sequence and are activated by GATA4 [7, 12]
The importance of GATA4 in ovarian development and function has been highlighted recently. Depending upon the type of promoter driving the Cre-Lox recombination system (Amhr2, Cyp19, or Sf1-Cre), the loss of Gata4 expression within the ovary produced either subfertile or infertile animals [8, 13, 14]. The degree of disruption in follicular development and of formation of ovarian cystic structures varied depending on the Cre recombinase, but regardless of the system used, conditional mutant females showed an aberrant response to exogenous gonadotropins [8, 13, 14]. The differences found among these transgenic models can be explained by the timing of Cre-recombination (Sf1-Cre is activated embryonically while Cyp19- and Amhr2-Cre act postnatally). The timing of Cre-mediated deletion of Gata4 relative to the emerging ovarian expression of Gata6 could be very important because GATA6 could completely (or partially) support ovarian follicular development in the absence of GATA4 [8, 14].
Recently, Bennett et al. [14] deleted both Gata4 and Gata6 genes in the ovary by using the Cyp19-Cre line (Cre is driven by the cytochrome P450 [Cyp] 19 aromatase), which is mainly expressed in granulosa cells of the antral follicles. In this model, folliculogenesis initiates normally, and primary and multilayer follicles were observed. However, few follicles reached the antral stage, with no large preovulatory follicles or corpora lutea. The conditional double mutant females demonstrate irregular estrous cycles, have small ovaries, and are infertile [14].
Here, we show that the simultaneous deletion of Gata4 and Gata6 within the ovary by the Sf1-driven Cre (conditional double mutant) produced a more striking ovarian phenotype with a reduction in the embryonic expression of the pregranulosa and granulosa cell markers SPRR2 and FOXL2, as well as other genes involved in the ovarian developmental pathway. Furthermore, early postnatal ovaries lacking both genes develop poorly, with a disruption in somatic cell proliferation and an early block in follicular development where oocytes are either still forming ovigerous cords (clusters of germ cells) or are enclosed by flattened granulosa cells (primordial follicles). Finally, a dramatic loss of oocytes was observed in ovaries from conditional double mutant animals before reaching prepubertal age. Thus, GATA4 and GATA6 play a crucial role in granulosa cell development, and therefore folliculogenesis, proper ovarian growth, and function.
MATERIALS AND METHODS
Generation of Mouse Strains
The Gata4flox/flox [15] and Gata6flox/flox [16] mice were obtained from the Jackson Laboratory repository. The transgenic Sf1Cre mice (a gift from the late Dr. Keith Parker) possess an artificial chromosome (BAC) that contains Sf1 (Nr5a1) regulatory elements directing Cre expression to somatic cells of the gonads (as well as to select extragonadal tissues [17]). The experimental strains with Sf1Cre-mediated deletions were produced by crossing flox mice with Sf1Cre-containing animals and then backcrossing to generate homozygous deletions. Animals carrying homozygous deletions of Gata4 by Sf1Cre are sterile [8], while animals carrying homozygous deletion of Gata6 are fertile. Hence, Sf1Cre; Gata4flox/+ Gata6flox/flox males were backcrossed with Gata4flox/flox Gata6flox/flox females to generate double conditional mutants (Sf1Cre; Gata4flox/flox Gata6flox/flox). The Axin2LacZ; Sf1Cre; Gata4flox/flox Gata6flox/flox were obtained by crossing Sf1Cre; Gata4flox/+ Gata6flox/flox males with AxinLacZ Gata4flox/flox Gata6flox/flox females. All the animals were maintained on a mixed 129/C57BL/6 background. Primers utilized for genotyping are listed in the Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org). Experiments were performed under approved animal protocols in accordance with the guidelines established by the University of Florida, Institutional Animal Care and Use Committees (IACUC).
X-Gal Staining
Mouse embryos were isolated at E13.5, and most of the viscera were removed to facilitate gonadal exposure to the staining solution. Embryos were washed in Dulbecco phosphate-buffered saline (DPBS) (Sigma-Aldrich) and fixed for 1 h with a fixative solution: DPBS containing 1% (v/v) formaldehyde, 0.2% (v/v) glutaraldehyde, 2 mM MgCl2, 5 mM ethylenediaminetetraacetic acid, and 0.02% (v/v) NP-40. After three washes in DPBS containing 0.02% (v/v) NP-40 for 30 min each to remove the fixative, embryos were incubated at 37°C with a staining solution: DPBS containing 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.02% (v/v) NP-40, 0.01% (v/v) Na-deoxycholate, and 1 mg/ml of X-gal. Once an adequate color developed, the reaction was stopped by three brief washes in DPBS. Embryonic gonads were dissected out and fixed overnight in 4% (w/v) paraformaldehyde. Three independent litters (n = 3) containing Axin2LacZ; Sf1Cre; Gata4flox/flox Gata6flox/flox female embryos were collected for this experiment.
Whole Mount In-Situ Hybridization
Gonad-mesonephros complexes from wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox females at E15.5 were fixed in 4% (w/v) paraformaldehyde in DPBS overnight at 4°C and processed as previously described [18]. Tissues were hybridized with digoxigenin-labeled RNA probes for Fst, Bmp2, and Foxl2, and the riboprobes were detected using an alkaline phosphatase-conjugated antibody and the BM Purple chromogenic solution (Roche Diagnostics Corporation).
Immunofluorescence
Ovaries from wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox animals were collected and processed in optimal-cutting-temperature freezing media. Two (Postnatal Day 9 [PND 9]) or more independent samples were analyzed for each developmental stage tested. Immunofluorescence experiments were carried out on 5–7 μm cryosections as previously described [8], and the following primary antibodies were used: OCT3/4; GATA4, and AMH (1:300) from Santa Cruz Biotechnologies, MVH (1:300) from Abcam, FOXL2 (1:300; Everest Biotech), GATA6 (1:300; Cell Signaling), WT1 (1:500; Epitomics); SF1 (1:300; TransGenic, Inc.), phospho-Histone H3 (1:300; Millipore), SOHLH1 (1:300; a gift from Dr. Alex Rajkovic); SOX9 (1:300; Millipore); Laminin (1:300; MP Biomedicals), and SPRR2 (1:100; Enzo Life Sciences). The tyramide signal amplification system (TSA; PerkinElmer Inc.) was used to amplify the signal of the SPRR2 antibody. Ovarian sections were observed in an Olympus BX51 fluorescence microscope (Olympus America Inc.). Images were captured with an Olympus DP72 digital camera (Olympus) using 10, 20, and 40× objectives and 4′,6-diamidino-2-phenylindole (DAPI), fluorescein isothiocyanate, and rhodamine filters. Merged color overlay images were assembled with Adobe Photoshop CS5 Extended (version 12.0.4x32) (Adobe Systems Incorporated).
Bromodeoxyuridine Assay
Wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox females at PND 4 as well as pregnant mice were injected subcutaneously with bromodeoxyuridine (BrdU) (Sigma-Aldrich) at a dose of 0.1 mg/g of body weight for 1 h (PND 4) or 2 h (pregnant) before euthanasia. Ovaries were harvested and processed as described above. Ovarian sections were treated with 0.0625% (v/v) trypsin and 5% (v/v) heat-inactivated fetal bovine serum (Sigma-Aldrich) in DPBS for 5 min each followed by DNase I (50 units/ml; Roche Diagnostics Corporation) for 30 min at 37°C. BrdU-incorporation was detected by using a sheep anti-BrdU antibody (1:300; Abcam) to assess cell proliferation. Nuclei were counterstained with DAPI (Vector Laboratories Inc.). The percentage of proliferating cells (BrdU-positive cells) at PND 4 was determined relative to the number of DAPI-positive cells. Two ovarian sections from different females (n = 3) of each genotype were used for cell counting. Percentage data were subjected to arcsine transformation before statistical analysis (Student t test; two-tailed) and the significance considered at P < 0.05.
Cell Death Detection Assay
Cell death was assessed with the in situ cell death detection kit (i.e., TUNEL [terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling]) (Roche Diagnostics Corporation). Sections from wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries were permeabilized with DPBS containing 0.1% (v/v) Triton-X and 0.1% (w/v) sodium citrate for 30 min at room temperature and then incubated with TUNEL reaction mixture (TMR Red-conjugated dUTP and the enzyme terminal deoxynucleotidyl transferase) at 37°C for 1 h in a humidified box. Positive controls were incubated with DNase I (New England Biolabs) at 37°C for 30 min, and negative controls were incubated in the absence of terminal deoxynucleotidyl transferase. Nuclei were stained with DAPI (Vector).
RNA Extraction and cDNA Synthesis
The TRI reagent (Sigma-Aldrich) was employed for total RNA extraction from wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at different stages of development (E13.5, E15.5, E18.5, and PND 4) according to the vendor's specifications. RNA concentration was assessed spectrophotometrically with the NanoDrop Lite (Thermo Fisher Scientific Inc.) treated with DNase I (New England Biolabs) to eliminate genomic DNA contamination following the manufacturer's instructions. Oligo-dT primers (Integrated DNA Technologies) were utilized to synthesize cDNA from equal amounts of total RNA by using the M-MLV (Moloney Murine Leukemia Virus) Reverse Transcriptase kit (Invitrogen) according to the manufacturer's instructions.
Quantitative RT-PCR
The SYBR Green PCR master mix (Applied Biosystems) was utilized for quantitative RT-PCR (qPCR) reactions and transcript levels analyzed on an ABI 7500 real-time PCR system (Applied Biosystems) using 40 cycles of 95°C for 15 sec and 60°C for 1 min in a 2-step thermal cycle, preceded by two initial steps: 2 min at 50°C and 10 min at 95°C. The primers used are listed in Supplemental Table S2 and were obtained from the Integrated DNA Technologies. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) served as the normalizing gene. Samples were analyzed in duplicates from at least three biological replicates and the fold change was calculated by the ΔΔCt method. The Student t test (two-tailed) was utilized for statistical analysis, and significance was considered at P < 0.05. The data analyzed were ΔCt values, and the results were plotted as fold-change differences using the GraphPad Prism (6.02 version) software.
Hematoxylin and Eosin Staining
Ovaries from wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox animals were collected at PND 6 and 9 for histological analysis. Sections were cut at 5 μm of thickness, dewaxed, rehydrated, and stained with modified Harris hematoxylin (Thermo Fisher). Acid-alcohol—1% (v/v) HCl in 70% (v/v) ethanol—was employed for staining differentiation, Eosin-Y (Thermo Scientific) for counterstaining, and Permount (Fisher Scientific) as the mounting media.
RESULTS
The Pregranulosa Cell Marker SPRR2 Is Decreased by Simultaneous Deletion of Gata4 and Gata6 at E13.5
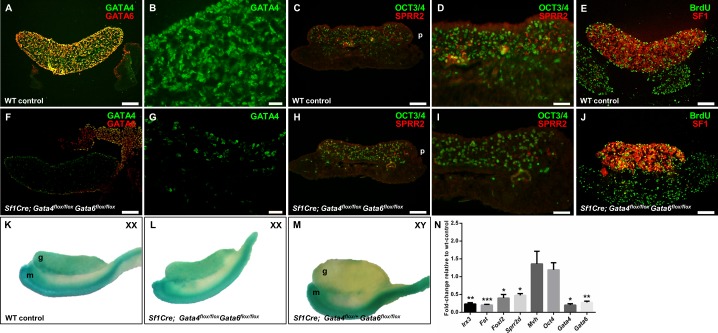
The transcription factor GATA4 is present in somatic cells of the ovary as early as E10.5 [7, 9, 10]. In contrast to earlier findings [6], we show here that GATA6 protein was detected in embryonic somatic cells of ovaries at E13.5 (Fig. 1A). Deletions of Gata4 and Gata6 using a Cre recombinase under control of the Sf1 promoter was shown to be highly efficient (compare Fig. 1, A and F, and Supplemental Fig. S1, A and D). In the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries, we observed a reduction in GATA4- and GATA6-positive cells as early as E13.5, where both GATA4 and GATA6 proteins were almost absent from the somatic cells of the gonad. Very few GATA4-positive cells were observed in the gonad (Fig. 1G) and were presumably interstitial cells. Coelomic epithelial cells in the conditional double mutant remained positive for GATA4, with some cells expressing both GATA4 and GATA6 proteins (Fig. 1F).
FIG. 1.
Gene expression analysis in E13.5 control and Sf1Cre; Gata4flox/flox Gata6flox/flox samples. A–J) Representative sections of wild-type control (A–E) and Sf1Cre; Gata4flox/flox Gata6flox/flox (F–J) ovaries at Embryonic Day 13.5 (E13.5). Ovarian sections were stained with antibodies against GATA4 (green) and GATA6 (red) (A and F); the small proline-rich protein 2 (SPRR2; red) and the pluripotent germ cell marker OCT3/4 (green) (C and H); and the cell proliferation marker bromodeoxyuridine (BrdU; green) and the nuclear receptor SF1 (red) (E and J). B, G, D, and I are higher magnifications of A, F, C, and H, respectively. B and G are visualized for GATA4 staining. Note a decrease in SPRR2 expression in the mutant ovary (H, I). Bars = 100 μm (A, C, E, F, H, J), 50 μm (D, I), and 20 μm (B, G). K–M) Gonad-mesonephros complexes from an XX wild-type control (K), XX Axin2LacZ; Sf1Cre; Gata4flox/flox Gata6flox/flox (L), and XY Axin2LacZ; Sf1Cre; Gata4flox/+ Gata6flox/flox (M) were stained with X-gal for LacZ expression. Note the comparable staining of the ovaries (K, L) and the lack of LacZ expression in the male gonad (M), as expected. Gonad (g); mesonephros (m); posterior side of the gonad (p). N) Gene expression analysis by qPCR of wild-type controls and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at E13.5. Genes examined included Irx3, Fst, Foxl2, Sprr2d, Mvh, Oct4, Gata4, and Gata6, and the results are shown as the means ± SEM of fold change relative to wild-type controls from at least three biological replicates with significance considered at *P < 0.05, **P < 0.01, and ***P < 0.001.
The small proline-rich protein 2 (SPRR2) is considered a pregranulosa cell marker [19]. Two Sprr2 genes, Sprr2α and Sprr2d, are preferentially expressed in pregranulosa cells from E11.5 to E13.5, with expression biased toward the posterior end of the gonad. SPRR2 colocalized with a subset of GATA4-positive cells in the embryonic ovary [19]. Our immunofluorescence experiments revealed that in both wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries, SPRR2 is mostly expressed toward the posterior end of the gonad (Fig. 1, C, D, H, and I). These experiments also demonstrated fewer SPRR2-positive cells in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (compare Fig. 1, C, D, H, and I). To provide a quantitative assessment for gene expression in the control and conditional double mutant ovaries, we performed qPCR analysis. While the granulosa cell markers Sprr2d and Foxl2 were significantly down-regulated (P < 0.05) in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries, both germ cell transcripts examined (Mvh and Oct4) were not different from wild-type controls (Fig. 1N). The Forkhead box L2 (FOXL2) transcription factor is one of the earliest markers of ovarian development in mammals. Foxl2 is expressed in granulosa and theca cells of all follicular stages and is absolutely required for normal ovarian differentiation and follicular development [20]. In addition, other genes involved in ovarian development such as Irx3 and Fst were also down-regulated (P < 0.01 and P < 0.001, respectively; Fig. 1N) in ovaries with simultaneous deletion of Gata4 and Gata6. A decrease in somatic cell proliferation in the conditional double mutant ovary could be responsible for the lower number of pregranulosa cells. However, BrdU-labeling experiments showed no major differences in cell proliferation between wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (compare Fig. 1, E and J).
The role of the canonical Wnt/β-catenin pathway in ovarian differentiation has been well documented (e.g., [21–24]). The Axin2 gene is commonly used as a reporter of canonical WNT/β-catenin (CTNNB1) pathway activity [25, 26]. Moreover, the expression of Axin2 is sexually dimorphic, with ovarian but not testicular-specific expression [21, 27]. We used an Axin2LacZ transgenic line to determine the effect of the simultaneous deletion of Gata4 and Gata6 within the gonad on the activity of the WNT/β-catenin pathway. X-Gal staining revealed no apparent difference in Axin2 expression between wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox embryonic ovaries (Fig. 1, K and L). In contrast and as expected, the male gonad was negative for X-gal staining at E13.5 (Fig. 1M).
FOXL2 Expression Is Reduced in Sf1Cre; Gata4flox/flox Gata6flox/flox Embryonic Ovaries
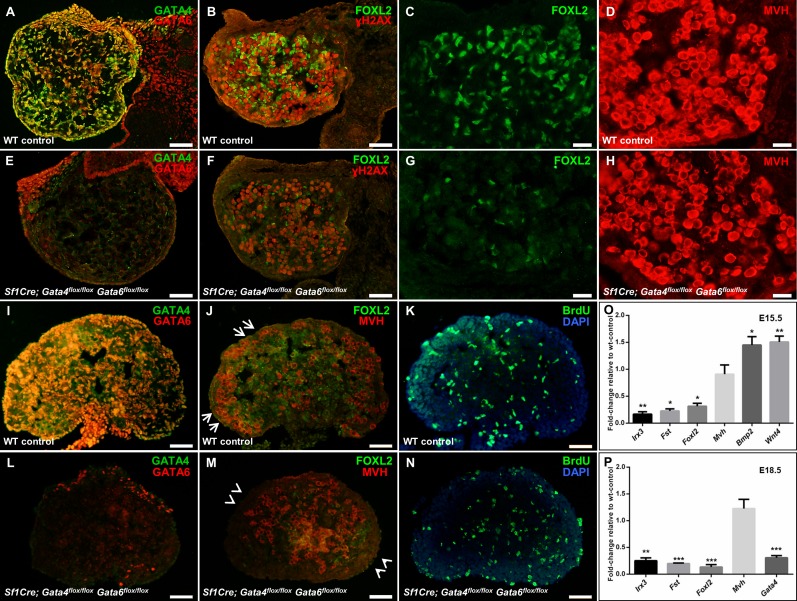
At E15.5 and E18.5, the expression of GATA4 and GATA6 proteins was not detectable in the majority of the somatic cells of Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries when compared with wild-type controls, where both proteins were abundantly expressed in somatic cells (compare Fig. 2, A, E, I, and L; and Supplemental Fig. S1, B, C, E, and F).
FIG. 2.
Gene expression analysis in E15.5 and E18.5 control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovarian samples. A–N) Representative images of wild-type control (A–D, I–K) and Sf1Cre; Gata4flox/flox Gata6flox/flox (E–H, L–N) ovarian sections at E15.5 (A–H) and E18.5 (I–N). E15.5 sections were stained for GATA4 (green) and GATA6 (red) (A and E), for the granulosa cell marker, FOXL2 (green), and the phosphorylated histone family protein H2A (Gamma-H2AX; red) (B and F), or for the universal germ cell marker mouse vasa homolog (MVH; red) (D, H). C and G are higher magnifications of B and F, respectively, visualized for FOXL2 staining. Bars = 50 μm (A, B, E, F) and 20 μm (C, D, G, H). Ovarian sections at E18.5 were stained for GATA4 (green) and GATA6 (red) (I and L), for FOXL2 (green) and MVH (red) (J and M), or for bromodeoxyuridine to measure cell proliferation (BrdU; green) (K and N). Nuclei are stained by DAPI (blue). Note in J that germ cells are mainly localized to the cortical region in control ovaries (shown by arrows) but not in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (arrowheads in M). Bars = 50 μm (I–N). O, P) Quantitative RT-PCR analysis of changes in gene expression in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at E15.5 (O) and E18.5 (P). Transcripts examined were Irx3, Fst, Foxl2, Mvh, Wnt4, Bmp2, and Gata4. The results are shown as the means ± SEM of fold change relative to wild-type controls from at least three biological replicates. Data were analyzed by Student t-test (two-tailed) with significance considered at *P < 0.05, **P < 0.01, and ***P < 0.001.
Unlike SPRR2 expression that sharply decreases in the ovary after E13.5 [19], the expression of FOXL2 persists through later stages of embryonic ovarian development (E15.5 and E18.5). While abundant FOXL2-positive cells were detected in wild-type ovaries at both stages of development, FOXL2 protein staining was drastically reduced by the simultaneous deletion of Gata4 and Gata6 within the ovary, both at E15.5 (compare Fig. 2, B, C, F, and G) and at E18.5 (compare Fig. 2, J and M). In addition, we performed qPCR and detected an ∼5-fold reduction in the expression of the Foxl2 transcript (P < 0.05) at E15.5. Likewise, the expression of other ovarian-specific genes in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries was strongly reduced as well, that is, Fst (P < 0.05) and Irx3 (P < 0.01) (Fig. 2O). At E18.5, a similar prominent down-regulation in the ovarian gene expression program persisted (Fig. 2P), with Foxl2, Irx3, and Fst significantly down-regulated in the conditional double mutant ovaries (P < 0.001, P < 0.001, and P < 0.01, respectively). Not all the genes associated with ovarian development were down-regulated; for example, the expression of Wnt4 and Bmp2 was significantly up-regulated (P < 0.01 and P < 0.05, respectively; Fig. 2O). To corroborate these results, we performed whole-mount in situ hybridization for the Fst, Bmp2, and Foxl2 genes; these RNA expression studies were in full agreement with the qPCR and immunofluorescence analyses in Figure 2 (Supplemental Fig. S2).
While the gene expression program of the somatic ovarian cells in the Sf1Cre; Gata4flox/flox Gata6flox/flox mutants was clearly affected, the development of germ cells appeared to proceed normally. We observed no notable differences between control and conditional double mutant ovaries in meiotic entrance as assessed by the expression of the meiotic marker, phosphorylated histone 2A (Gamma-H2AX) (Fig. 2, B and F) or overall number of oogonia as assessed by the oocyte-specific protein mouse vasa homolog, MVH (Fig. 2, D, H, J, and M). Similar results were obtained by qPCR analysis where the expression of the Mvh gene was comparable between wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (Fig. 2, O and P). However, at E18.5 the rearrangement of developing germ cells within the Sf1Cre; Gata4flox/flox Gata6flox/flox differs from the control ovary where large numbers of oogonia are positioned at the cortical region (Fig. 2J); in the conditional double mutant, very few MVH-positive cells localize to the developing ovarian cortex (Fig. 2M). Similar to earlier developmental stages, no apparent differences in cell proliferation at E18.5 were noted between wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries as shown by the BrdU assay (compare Fig. 2, K and N).
Lack of Follicular Development and Clusters of Germ Cells in Sf1Cre; Gata4flox/flox Gata6flox/flox Ovaries at PND 4
During follicular assembly, oocyte nests separate by a process called germ-cell nest breakdown and become enclosed in primordial follicles consisting of one oocyte and several flattened granulosa cells (for review, see [28]). As folliculogenesis proceeds, flattened granulosa cells become cuboidal, giving rise to primary follicles. The proliferation of granulosa cells leads to the formation of secondary follicles in which two to four layers of granulosa cells surround the oocyte. Finally, large preantral and antral follicular stages are reached, with several layers of granulosa cells encircling the oocyte. Once secondary follicles are formed, steroidogenic theca cells appear and form a loose layer around the granulosa–oocyte complex (reviewed in [29]).
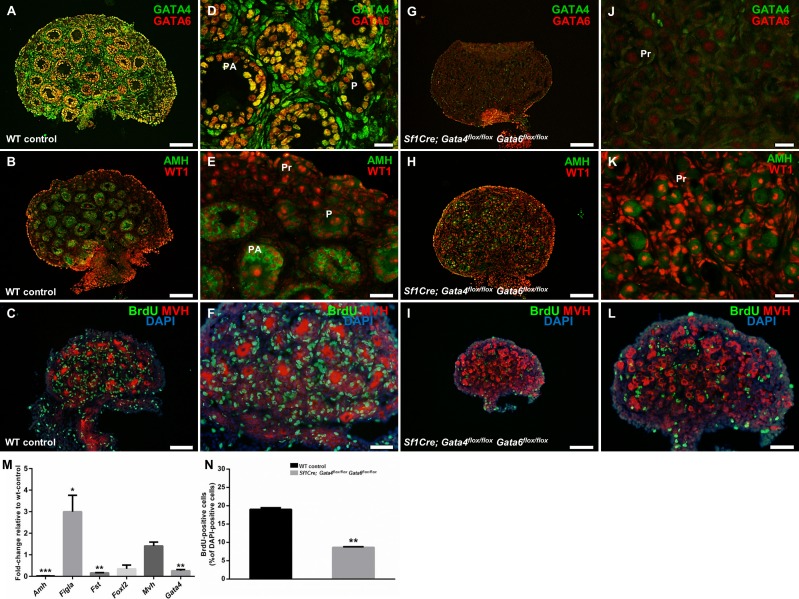
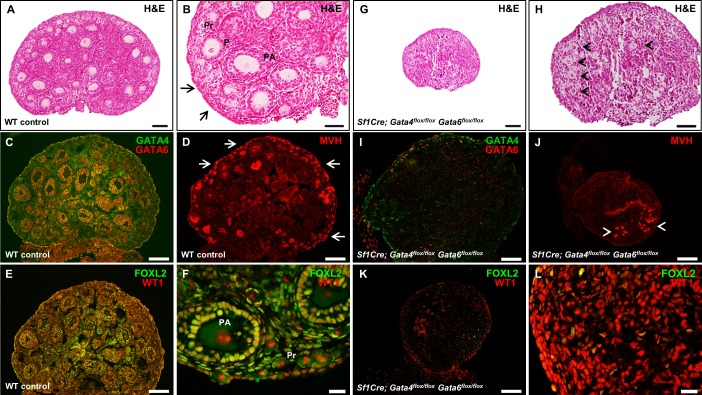
To determine the effect of the simultaneous deletion of Gata4 and Gata6 genes within the ovary on folliculogenesis, sections of wild-type and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries were examined at PND 4. To confirm efficient gene deletion, we inspected GATA4 and GATA6 protein expression in the control and conditional double mutant ovaries. In the wild-type samples, both GATA4 and GATA6 are robustly expressed in somatic cells of the ovary, with coexpression in granulosa cells (Fig. 3, A and D). In contrast, coexpression was not observed in the Sf1Cre; Gata4flox/flox; Gata6flox/flox ovaries. Very few GATA4-positive cells remained within the ovary and are presumably mostly interstitial cells that do not express Sf1Cre. Moderate GATA6 expression in oocytes was present, similar to what was reported previously for these cells [30] (Fig. 3, G and J).
FIG. 3.
The loss of Gata4 and Gata6 expression causes an early block in follicular development and somatic cell proliferation. A–L) Sections of wild-type control (A–F) and Sf1Cre; Gata4flox/flox Gata6flox/flox (G–L) ovaries at Postnatal Day 4 (PND 4). Sections were stained for GATA4 (green) and GATA6 (red) (A and G), for anti-Müllerian hormone (AMH; green) and Wilm's Tumor 1 (WT1; red) (B and H), or for bromodeoxyuridine (BrdU; green) and mouse vasa homologue (MVH; red) (C and I). Nuclei are stained by DAPI (blue). D–F and J–L are higher magnifications of A–C and G–I, respectively. P, primary follicle; PA, preantral follicle; Pr, primordial follicle. Bars = 100 μm (A–C, G–I), 50 μm (E, F, L), and 20 μm (D, J, K). M) Quantitative changes in gene expression of Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries. Genes examined were Amh, Figla, Fst, Foxl2, Mvh, and Gata4; the results are shown as the means ± SEM of fold change relative to wild-type controls (n = 4). N) Changes in somatic cell proliferation in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries. Results are shown as the percentage of BrdU-positive cells relative to the number of DAPI-positive cells from three different females. Data were analyzed by Student t-test (two-tailed) with significance considered at *P < 0.05, **P < 0.01, and ***P < 0.001.
Ovarian AMH is thought to protect the ovarian follicle reserve by inhibiting the recruitment of the primordial follicles and decreasing the responsiveness of the growing follicles to follicle-stimulating hormone (FSH) ([31, 32] and reviewed in [33]). AMH is produced in the granulosa cells of primary, preantral, and small antral follicles and is a useful marker of ovarian follicular progression. AMH staining revealed follicles at different stages of development in wild-type neonatal ovaries (Fig. 3, B and E). In contrast, the lack of follicles in advanced stages of development was evident in the Sf1Cre; Gata4flox/flox; Gata6flox/flox ovaries (Fig. 3, H and K). These morphological changes were corroborated by a significant decrease in the expression of Amh and Fst genes by qPCR (P < 0.01 and P < 0.001, respectively; Fig. 3M). In addition, the expression of Foxl2 in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries was reduced but not significantly (expression of this gene is low at that developmental stage). The factor in the germline-alpha (FIGLA) is a germ cell factor required for the formation of primordial follicles [34]. Its transcript abundance peaks at approximately PND 2, a time in ovarian development at which oocytes are becoming enclosed in primordial follicles [34]. We examined the expression of two oocyte transcripts, Mvh and Figla, but only Figla was significantly increased in the conditional double mutant ovaries (P < 0.05; Fig. 3M). Thus, we conclude that follicles that are formed in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries remain in the primordial state.
PND 4 conditional double mutant ovaries appeared markedly smaller. BrdU-labeling experiments were performed in wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox animals to inspect cell proliferation. Ovarian sections stained with an antibody against BrdU uncovered abundant somatic cell proliferation in the wild-type control (Fig. 3, C and F). Furthermore, a large number of BrdU-positive cells, which are presumably proliferating granulosa cells, enveloped the oocytes (Fig. 3F). In contrast, a significant reduction in somatic cell proliferation (P < 0.01; Fig. 3N) was observed in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (Fig. 3, I and L). Oocyte growth, a notable feature of follicular progression measured by the increase of oocyte diameter (Fig. 3F), was not observed in oocytes from conditional double mutant ovaries (Fig. 3L).
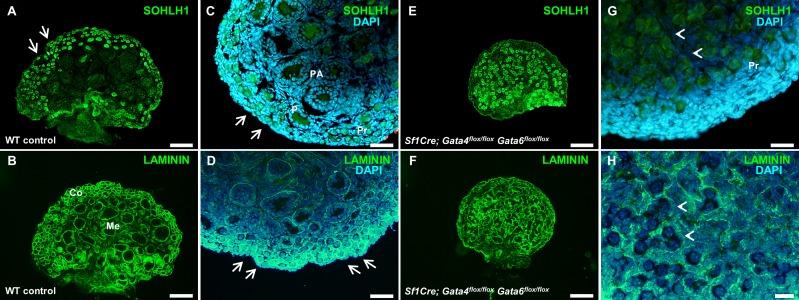
To further examine follicular development in the mutants, sections of wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries were stained with antibodies against spermatogenesis and oogenesis specific basic helix-loop-helix 1 (SOHLH1) and Laminin. SOHLH1 expression is restricted to germ cell clusters and primordial and primary follicles. Sohlh1 gene disruption blocks the primordial-to-primary follicle transition [35]. SOHLH1 and Laminin proteins were detected both in wild-type control and conditional double mutant ovaries (Fig. 4); however, their expression pattern highlighted notable differences in ovarian organization. Oocytes in the medullary region of the wild-type ovaries are enclosed by either cuboidal granulosa cells or by several layers of granulosa cells (Fig. 4, C and D). In contrast, germ cells from Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries are either surrounded by flattened granulosa cells forming primordial follicles or remain grouped in ovigerous cords (Figs. 4, G and H, and 3K), where germ cell nests are loosely surrounded by somatic cells [36]. Moreover, while we noticed abundant primordial follicles in the cortical region of wild-type ovaries (Fig. 4A–D), in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries, primordial follicles as well as clusters of germ cells were localized predominately within the central region (Fig. 4E–H). Here again, the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries do not present a clear delimitation between the medullary and cortical regions, which is clearly established in wild-type ovaries at this stage of development (compare Fig. 4, C and G).
FIG. 4.
Structural organization of control and Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at PND 4. Representative sections of wild-type control (A–D) and Sf1Cre; Gata4flox/flox Gata6flox/flox (E–H) ovaries at Postnatal Day 4 (PND 4) stained either for spermatogenesis- and oogenesis-specific basic helix-loop-helix 1 protein (SOHLH1; green) (A and E) or Laminin (green) (C and G). C, D, G, and H are higher magnifications of A, B, E, and F, respectively, visualized for both marker and nuclear staining (DAPI, blue). Bars = 100 μm (A, B, E, F), 50 μm (C, D, G), and 20 μm (H). Note primordial follicles in the cortical region of wild-type ovaries (arrows in A, C, and D) and clusters of germ cells in the center of Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (arrowheads in G and H). Pr, primordial follicle; P, primary follicle; PA, preantral follicle; Co, cortex; Me, medulla.
Increased Cell Death and Early Oocyte Depletion in Sf1Cre, Gata4flox/flox Gata6flox/flox Ovaries
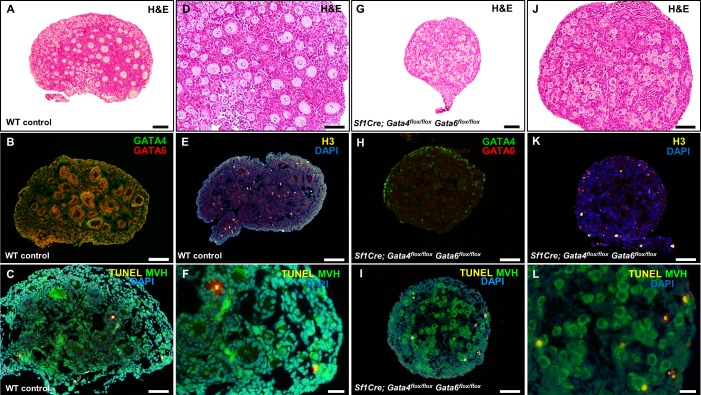
The marked reduction in somatic cell proliferation observed in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at PND 4 (Fig. 3, I, L, and N) had noticeable effects at later stages of development (PND 6 and 9), where conditional double mutant ovaries are remarkably smaller (Figs. 5G and 6G) than wild-type ovaries (Figs. 5A and 6A). Moreover, TUNEL analysis revealed more apoptotic nuclei in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at PND 6 (Fig. 5, I and L) than in wild-type ovaries (Fig. 5, C and F), suggesting a major imbalance between cell proliferation and death in conditional double mutant ovaries.
FIG. 5.
Increased cell death at PND 6 in conditional double mutant ovaries. Sections of wild-type control (A–F) and Sf1Cre; Gata4flox/flox Gata6flox/flox (G–L) ovaries at Postnatal Day 6 (PND 6). Sections were stained with hematoxylin and eosin (H&E) (A and G), for GATA4 (green) and GATA6 (red) (B and H), or for the cell proliferation marker, histone 3 (yellow) (E and K). Detection of apoptotic cells was performed by TUNEL analysis using TMR Red-conjugated dUTP to identify apoptotic nuclei (yellow) and MVH (green) to identify germ cells (C and I). D, J, F, and L are higher magnifications of A, G, C, and I, respectively. Bars = 100 μm (A, B, E, G), 50 μm (C, D, H–K), and 20 μm (F, L). Nuclei are stained by DAPI (blue).
FIG. 6.
Loss of oocytes by PND 9 in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries. Sections of wild-type control (A–F) and Sf1Cre; Gata4flox/flox Gata6flox/flox (G–L) ovaries at Postnatal Day 9 (PND 9). Sections were stained with hematoxylin and eosin (H&E) (A and G), antibodies against GATA4 (green) and GATA6 (red) (C and I), mouse vasa homolog (MVH; red) (D and J), or FOXL2 (green) and Wilm's tumor 1 (WT1; red) (E and K). B, H, F, and L are higher magnifications of A, G, E, and K, respectively. In B and D, arrows show primordial follicles in the ovarian cortex, and in H and J, arrowheads point to the few primordial follicles remaining in the central region of the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries. Bars = 100 μm (A, C–E, G, J, K), 50 μm (B, I, H), and 20 μm (F, L). Pr, primordial follicle; P, primary follicle; PA, preantral follicle.
We noted that the majority of oocytes disappeared from the ovaries of Sf1Cre; Gata4flox/flox Gata6flox/flox animals by PND 9 (Fig. 6, G, H, and J), with the few remaining cells localized in the central region still surrounded by flattened granulosa cells (primordial follicular stage). Conversely, numerous oocytes from wild-type ovaries are enclosed by several layers of granulosa cells, and abundant primordial follicles are present in the ovarian cortex (Fig. 6, A–D and F). In addition, extremely few FOXL2-positive cells are visible in Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries (Fig. 6, K and L) compared to wild-type controls (Fig. 6, E and F). The efficiency of the Sf1Cre excision is corroborated by staining for GATA4 and GATA6 expression in the PND 9 conditional double mutant ovaries, with very few GATA4-positve cells in the epithelium and few in the medullary region of the ovary (compare Fig. 6, C and I).
DISCUSSION
The transcription factors GATA4 and GATA6 have been recently acclaimed as critical regulators of adult ovarian function [14, 37], but their common roles during early ovarian development have not been established. In this study, we provide evidence that a major disruption of the ovarian development necessitates conditional deletion of both GATA factors within the ovary. Previous work from this laboratory demonstrated that early developmental loss of Gata4 gene expression led to limited disturbances during ovarian development. Moreover, the effect of Gata4 gene loss on ovarian gene expression and function was not fully manifested until after birth [8]. Sf1Cre-mediated deletion of Gata6 alone did not result in any notable effect on ovarian morphology or function and Sf1Cre; Gata6flox/flox mice were fertile (data not shown). In contrast, the effect of the simultaneous deletion of Gata4 and Gata6 in the ovary was detected at as early as E13.5 with a reduction in the expression of SPRR2, a pregranulosa cell marker (Fig. 1). Another gene expressed in pregranulosa cells, Irx3, was also down-regulated by the lack of both transcription factors. IRX3 is a member of the Iroquois homeobox family and colocalized with GATA4 in pregranulosa cells [38].
At E13.5, conditional deletion of Gata4 and Gata6 resulted in a down-regulation of other essential ovarian genes such as Fst and Foxl2. Experimental evidence has shown that expression of Foxl2, but not Wnt4, was significantly decreased in mutant mouse ovaries containing a deletion of Irx3 and other genes of the IrxB cluster [39]. In addition, Fst expression in newborn mouse ovaries was partially down-regulated in the absence of either Foxl2 (Foxl2−/−) or Wnt4 (Wnt4−/−), but Wnt4 was up-regulated in ovaries lacking Foxl2 [40]. At E15.5, we observed a similar gene expression pattern in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries where Foxl2 and Fst were down-regulated and Wnt4 and Bmp2 were significantly up-regulated. The down-regulation (but not a complete loss) of Irx3, Fst, and Foxl2 expression in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries suggests the role for both GATA4 and GATA6 as the transcriptional regulators of these granulosa-specific genes. Taken together, these observations, as well as down-regulation of the early pregranulosa cell markers SPRR2 and FOXL2, strongly indicates overlapping function of the two GATA factors in early ovarian development.
Previous work has shown that the loss of β-catenin had an impact on the expression of crucial ovarian genes such as Fst, Wnt4, and Foxl2 (reviewed in [27]). In this study, we used an Axin2LacZ transgenic line to determine the activity of the WNT/β-catenin pathway in the conditional double mutant ovaries. No apparent difference was found in Axin2LacZ expression between wild-type control and Sf1Cre; Gata4flox/flox Gata6flox/flox embryonic ovaries. The most parsimonious explanation for this finding is that WNT/β-catenin activity in the ovary is independent of GATA protein expression at this developmental stage. These data also suggest that the active Wnt/β-catenin pathway alone is insufficient to support ovarian differentiation. Moreover, it is conceivable that the WNT/β-catenin pathway, while critical for ovarian sex determination, is dispensable for subsequent ovarian differentiation. Its activity could be similar to the function of the Fog2 gene in ovarian development or that of Sox9 in the male. While these genes are required for sex determination [41–45], they are dispensable for later development [8, 43, 46].
The loss of GATA4 in the ovaries results in a prominent, but incomplete block of folliculogenesis, with some transitional primary follicles present that develop beyond the primary stage [8]. In contrast, deletion of both Gata4 and Gata6 produced an immature ovarian phenotype with an early and comprehensive block in follicular development where oocytes remained either in germ cell clusters or enclosed by flattened granulosa cells. Primordial follicles and germ cell nests are centrally localized in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries; however, the ovaries do not possess a clear definition of the medullary versus cortical region. A similar phenotype has been described in Fst−/− ovaries in which the boundary between the cortex and medulla is lost [47]. Importantly, loss of Fst expression has been associated with early neonatal germ cell loss [47], and strong reduction in Fst expression (Fig. 2) may explain the loss of germ cells in the conditional double mutant ovaries. Moreover, follistatin has been demonstrated as a factor critical to the process of germ cell nest breakdown [48].
Experimental evidence shows that GATA4 and GATA6 regulate cell proliferation. Conditional deletion of Gata6 and Gata4 produced a decrease in cellular proliferation in crypts of the small intestine [49] as well as a reduction in proliferating cell numbers in the pancreatic epithelium [50, 51]. In addition, Gata4 is thought to regulate cardiomyocyte proliferation through direct activation of the Cyclin D2 and Cdk4 promoters [52], although this does not appear to be a general mechanism (Tevosian et al., unpublished results). Here we assessed cell proliferation by the standard BrdU incorporation assay at different stages of development. We did not observe notable changes in cell proliferation in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries at both embryonic stages studied (E15.5 and 18.5). In contrast, a drastic decrease in cell proliferation was obvious in the postnatal conditional double mutant ovaries, suggesting that both transcription factors are involved in somatic cell proliferation later in development.
We believe that GATA proteins contribute to the regulation of several critical genes in ovarian development that are essential for granulosa cell differentiation and proliferation. The sum effect of the loss of GATA regulation is the demise of normal ovarian development and folliculogenesis. Given the critical role for granulosa cells as supporting cells for oocyte growth and survival, one would predict that deficiencies in granulosa cell differentiation and proliferation observed in the Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries would result in the loss of oocytes, which we detect by PND 9. The mechanism of this loss remains unclear. Although we were able to detect some TUNEL-positive oocytes (i.e., TUNEL- and MVH-doubly positive cells), most TUNEL-positive cells appeared to be either somatic cells or, possibly, degenerating oocytes that already lost their MVH expression. Late embryonic or perinatal oocyte demise is a common theme in rodent genetic models where the interaction between granulosa and germ cells is interrupted (e.g., [20, 47, 53, 54]). Similar to this work, the specific mechanism (or mechanisms) of germ cell loss in these models remains enigmatic. Previous research established that death by the classic apoptotic pathway is insignificant in oocytes of primordial follicles [55, 56]. Moreover, lysosome amplification in oocytes occurs at birth, suggesting that additional mechanisms could be involved in perinatal oocyte loss [56]. Decaying oocytes in primordial and primary follicles from newborn wild-type rat ovaries possessed apoptotic markers (active caspase-3 and TUNEL-positive staining) as well as autophagic markers (increased numbers of autophagosomes) [57]. However, compromised autophagy by the genetic loss of either Becn1 or Atg7 decreased (rather than increased) the number of oocytes in PND 1 mouse ovaries [58]. Thus, it remains unclear if there is a different mechanism for the elimination of atretic oocytes from primordial follicles.
Finally, Sf1Cre; Gata4flox/flox Gata6flox/flox ovaries do not undergo sex reversal. We observed no difference in the intensity of SOX9 staining between controls and conditional double mutant ovaries at PND 4, 15, and 17 (Supplemental Fig. S3 and data not shown). Interestingly, the up-regulation of testis-specific genes (i.e., Sox9, Dmrt1, Hsd17b3) was detected only in early postnatal ovaries deficient for both Wnt4 and Foxl2 [40]. Furthermore, Sf1Cre; Gata4flox/flox Gata6flox/flox females do not survive long after birth, and the majority of them die within the first 2 weeks of age (presumably due to the lack of adrenal glands; Tevosian et al., unpublished results); therefore, live postnatal conditional double mutant females are exceedingly rare. In summary, in the present study we have shown that the transcription factors GATA4 and GATA6 are key regulators of granulosa cell differentiation and proliferation and therefore are required for appropriate follicular assembly and normal ovarian development and function.
Footnotes
Current address: Department of Applied Physiology and Kinesiology, College of Health and Human Performance, University of Florida, Gainesville, Florida.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, a division of the NIH through HD042751 to S.G.T.
REFERENCES
- Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- Zaytouni T, Efimenko EE, Tevosian SG. GATA transcription factors in the developing reproductive system. Adv Genet. 2011;76:93–134. doi: 10.1016/B978-0-12-386481-9.00004-3. [DOI] [PubMed] [Google Scholar]
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggers P, Smith L, Greenfield A. Sexually dimorphic expression of Gata-2 during mouse gonad development. Mech Dev. 2002;111:159–162. doi: 10.1016/s0925-4773(01)00602-5. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Rao MK, Kaur R, Buttigieg MR, Wilkinson MF. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol Cell Biol. 2008;28:2138–2153. doi: 10.1128/MCB.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 are coexpressed in the mouse ovary and can modulate Mullerian-inhibiting substance expression. Biol Reprod. 2003;68:1333–1340. doi: 10.1095/biolreprod.102.008599. [DOI] [PubMed] [Google Scholar]
- Efimenko E, Padua MB, Manuylov NL, Fox SC, Morse DA, Tevosian SG. The transcription factor GATA4 is required for follicular development and normal ovarian function. Dev Biol. 2013;381:144–158. doi: 10.1016/j.ydbio.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice PLoS Genetics 2013. 9 e1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA factors and the nuclear receptors, Steroidogenic Factor 1/Liver Receptor Homolog 1, are key mutual partners in the regulation of the human 3beta-hydroxysteroid dehydrogenase type 2 promoter. Mol Endocrinol. 2005;19:2358–2370. doi: 10.1210/me.2004-0257. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol. 1999;13:1388–1401. doi: 10.1210/mend.13.8.0330. [DOI] [PubMed] [Google Scholar]
- Kyrönlahti A, Vetter M, Euler R, Bielinska M, Jay PY, Anttonen M, Heikinheimo M, Wilson DB. GATA4 deficiency impairs ovarian function in adult mice. Biol Reprod. 2011;84:1033–1044. doi: 10.1095/biolreprod.110.086850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Wu YG, Gossen J, Zhou P, Stocco C. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology. 2012;153:2474–2485. doi: 10.1210/en.2011-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CP, Li J, Duncan SA. Generation of mice harbouring a conditional loss-of-function allele of Gata6 BMC Dev Biol 2006. 6 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Pazin DE, Kahlon RS, Correa SM, Albrecht KH. Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Dev Dyn. 2009;238:812–825. doi: 10.1002/dvdy.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To β or not to β: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Gillio-Meina C, Hui YY, LaVoie HA. GATA-4 and GATA-6 transcription factors: expression, immunohistochemical localization, and possible function in the porcine ovary. Biol Reprod. 2003;68:412–422. doi: 10.1095/biolreprod.102.009092. [DOI] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril. 2013;99:963–969. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, FIGalpha Dean J. a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A. 2006;103:8090–8095. doi: 10.1073/pnas.0601083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Bennett J, Baumgarten SC, Stocco C. GATA4 and GATA6 silencing in ovarian granulosa cells affects levels of mRNAs involved in steroidogenesis, extracellular structure organization, IGF-I activity, and apoptosis. Endocrinology. 2013;154:4845–4858. doi: 10.1210/en.2013-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–762. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Kim B, Kim Y, Cooke PS, Ruther U, Jorgensen JS. The fused toes locus is essential for somatic-germ cell interactions that foster germ cell maturation in developing gonads in mice. Biol Reprod. 2011;84:1024–1032. doi: 10.1095/biolreprod.110.088559. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Chang H, Chaboissier MC, Schedl A, Behringer RR. Sox9 in testis determination. Ann N Y Acad Sci. 2005;1061:9–17. doi: 10.1196/annals.1336.003. [DOI] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Bonomi LM, Schneyer AL. Follistatin regulates germ cell nest breakdown and primordial follicle formation. Endocrinology. 2011;152:697–706. doi: 10.1210/en.2010-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuling E, Aronson BE, Tran LM, Stapleton KA, ter Horst EN, Vissers LA, Verzi MP, Krasinski SD. GATA6 is required for proliferation, migration, secretory cell maturation, and gene expression in the mature mouse colon. Mol Cell Biol. 2012;32:3392–3402. doi: 10.1128/MCB.00070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Delgado I, Soria B, Martin F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122:3504–3515. doi: 10.1172/JCI63240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan S, Borok MJ, Decker KJ, Battle MA, Duncan SA, Hale MA, Macdonald RJ, Sussel L. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122:3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28:5420–5431. doi: 10.1128/MCB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol Reprod. 2009;81:16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137:709–720. doi: 10.1530/REP-08-0203. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Ortiz R, Vazquez-Nin GH. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis. 2008;13:1253–1266. doi: 10.1007/s10495-008-0248-z. [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB., III. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141:759–765. doi: 10.1530/REP-10-0489. [DOI] [PubMed] [Google Scholar]