ABSTRACT
The blastocyst consists of the outer layer of trophectoderm and pluripotent inner cell mass (ICM), the precursor of the placenta and fetus, respectively. During blastocyst expansion, the ICM adopts a compact, ovoidal shape, whose proper morphology is crucial for normal embryogenesis. Rho-associated kinase (ROCK), an effector of small GTPase RHO signaling, mediates the diverse cellular processes of morphogenesis, but its role in ICM morphogenesis is unclear. Here, we demonstrate that ROCK is required for cohesion of ICM cells and formation of segregated tissues called primitive endoderm (PrE) and epiblast (Epi) in the ICM of the mouse blastocyst. Blastocyst treatment with ROCK inhibitors Y-27632 and Fasudil caused widening or spreading of the ICM, and intermingling of PrE and Epi. Widening of ICM was independent of trophectoderm because isolated ICMs as well as colonies of mouse embryonic stem cells (mESC) also spread upon Y-27632 treatment. PrE, Epi, and trophectoderm cell numbers were similar between control and treated blastocysts, suggesting that ROCK inhibition affected ICM morphology but not lineage differentiation. Rock1 and Rock2 knockdown via RNA interference in mESC also induced spreading, supporting the conclusion that morphological defects caused by the pharmacological inhibitors were due to ROCK inactivation. When blastocysts were transferred into surrogates, implantation efficiencies were unaffected by ROCK inhibition, but treated blastocysts yielded greater fetal loss. These results show that proper ICM morphology is dependent on ROCK activity and is crucial for fetal development. Our studies have wider implication for improving efficiencies of human assisted reproductive technologies that diminish pregnancy loss and promote successful births.
Keywords: assisted reproductive technologies (ART), cell lineage, developmental biology, early development, embryo, fetal loss, implantation, preimplantation
INTRODUCTION
In preimplantation development of placental mammals, the early conceptus generates extraembryonic tissues to support fetal development and survival. The first extraembryonic tissue to emerge is the trophectoderm (TE), which is the outer epithelial layer of the blastocyst, and it segregates from the pluripotent inside cells, the inner cell mass (ICM), which gives rise to the fetus [1]. The TE is the progenitor of trophoblasts, which mediate blastocyst implantation in the uterine wall as well as contribute to placentation. The molecular mechanisms by which TE lineage is generated during preimplantation development have been extensively studied. Regulators of apico-basal cell polarity and the Hippo signaling pathway are responsible for TE epithelialization and activation of TE-specific transcription factors, such as CDX2 [2–6]. CDX2, a caudal-type homeodomain transcription factor, suppresses expression of a key regulator of pluripotency, POU-domain transcription factor POU5F1, and thus further promotes segregation of the TE lineage from the ICM [7–12].
The second extraembryonic tissue to emerge is the primitive endoderm (PrE), and it originates from the ICM. During the expansion of the blastocyst cavity (embryonic stages between E3.5 and E4.5), a mixture of cell progenitors of the PrE and the pluripotent lineage epiblast (Epi) appear in the ICM [13–15]. By E4.5, the PrE is clearly segregated from the Epi and forms the superficial layer of the ICM, facing the blastocyst cavity. After implantation of the blastocyst, the PrE gives rise to visceral and parietal endoderm, which contributes to the yolk sac. The PrE derivatives not only perform nutritive functions for the early conceptus, but they also play critical roles in specifying the body axis in the Epi-derived tissues [16, 17]. The molecular mechanisms of PrE formation have also been studied rigorously. The PrE cells are marked by the expressions of zinc finger transcription factors GATA-binding protein 4 and 6 (GATA4, GATA6), both of which are essential in PrE differentiation [18–20]. In contrast, the Epi cells are marked by the expression of NANOG, a homeobox transcription factor that is essential for the maintenance of pluripotency [21, 22]. Studies using knockout mice and pharmacological inhibitors have revealed that cell-cell signaling via the FGF-GRB2-MAPK pathway is essential for PrE differentiation because the interference with this pathway results in ICM that is composed entirely of Epi cells but not PrE cells [13, 23]. However, how the FGF-GRB2-MAPK pathway is activated specifically in the presumptive PrE cells is still unclear.
As the blastocyst cavity expands between E3.5 and E4.5, the ICM adopts a highly compact morphology and is arranged in an oval shape. Formation of the ICM as a cohesive single mass is crucial for normal development, and it needs to segregate into two distinct layers of PrE and Epi, whose interactions are essential for germ layer formation and body axis specification [24, 25]. Splitting or distortion of the ICM is suggested to result in complete or partial twinning of the fetus or failure of fetal development [26–28]. Such disruptions in the ICM morphology have been observed during in vitro culture of human embryos, which may be a factor contributing to higher incidence of monozygotic twinning in human embryos derived by in vitro fertilization (IVF) [29–32]. Currently, however, the mechanism of ICM morphogenesis is not well understood. How ICM is normally maintained as a single discrete mass, and whether its compact morphology is a prerequisite for PrE and Epi tissue segregation are still unclear. Thus, knowledge about the mechanisms of ICM morphogenesis has major clinical implications for assisted reproductive technologies (ART) to improve pregnancy and birth rates in humans.
Rho-associated kinase (also known as, Rho-associated coiled-coil containing protein kinase or ROCK) is a serine/threonine kinase, and there are two isoforms ROCK1 and ROCK2, which are collectively referred to as ROCK. ROCK functions as an effector of small GTPase RHO signaling, which plays diverse roles in various cellular functions. For example, RHO signaling assembles the mechanical forces required for cell adhesion, migration, polarity, division, and apoptosis, by regulating actin filaments and myosin motors in a spatiotemporal manner [33–36]. Previous studies using the mouse embryo suggested the importance of RHO-ROCK signaling at the early stages of preimplantation development. Inhibition of RHO with C3-transferase of Clostridium botulinum impairs cell polarization and compaction at the eight-cell stage (E2.5) [37]. Moreover, interference with ROCK activity by a pharmacological inhibitor Y-27632 from the two-cell stage (E1.5) disrupts blastocyst cavity formation [38]. However, the role of ROCK in later stages of preimplantation development, particularly with respect to ICM aggregation and differentiation and segregation of PrE and Epi, has not been investigated.
Here, to explore the role of ROCK in ICM morphogenesis, we employed pharmacological inhibitors and RNA interference to target both isoforms as tools to interrogate ROCK function in the blastocyst. Our data showed that ROCK activity is necessary for the cohesive aggregation of ICM cells, segregation of PrE and Epi tissues, and fetal development, but is dispensable for cell proliferation and PrE and Epi differentiation. We propose that our data have wider implication for human ART, by providing morphological and molecular parameters with which to assess the efficacy and safety of ART procedures that may affect ICM morphogenesis.
MATERIALS AND METHODS
Animals
B6D2F1 (C57BL/6 × DBA/2; National Cancer Institute) and CD-1 (Charles River Laboratories) mice were used. The protocol for animal handling and use was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Hawaii. The animals were maintained and treated according to the regulations and guidelines of the Animal and Veterinary Service at the University of Hawaii and the Committee for the Update of the Guide for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research of the National Research Council of the National Academies (8th ed., 2011).
Embryo Collection
B6D2F1 female mice were injected with equine chorionic gonadotropin and human chorionic gonadotropin (hCG; EMD Millipore) at 48 h apart and mated with B6D2F1 male mice. At 44 h after hCG injection, two-cell stage embryos were flushed from the oviducts with FHM HEPES-buffered medium (MR-024-D; EMD Millipore) and cultured to the early blastocyst stage (E3.5) in KSOM-AA medium (MR-121-D; EMD Millipore) at 37°C with 5% CO2 in humidified air.
Pharmacological Treatment of Blastocysts with ROCK Inhibitors
Stocks of ROCK inhibitors Y-27632 (10 mM) and Fasudil (20 mM) (EMD Millipore) were dissolved in dimethyl sulfoxide (DMSO) and water, respectively, and were stored at −20°C until ready for use. Y-27632 (10, 15, or 20 μM) and Fasudil (5 μM) were freshly diluted in KSOM-AA and pipetted as 20 μl drops under mineral oil that were equilibrated at 37°C with 5% CO2 in air prior to use for embryo treatment. Control 20 μl drops were prepared by adding DMSO or water to KSOM-AA at a volume equal to that of the inhibitor. E3.5 blastocysts whose cavity volume was at least half the total size of the embryo were selected for use in the experiments. Blastocysts were cultured in ROCK inhibitor and control drops up to E4.5 (24 h) and processed for immunostaining, or E4.5 inhibitor-treated and control blastocysts were further cultured in drug-free KSOM-AA for 24 h and processed for immunostaining.
Immunofluorescent Staining
Samples were fixed in 4% paraformaldehyde solution in phosphate-buffered saline (PBS) for 30 min and permeabilized in PBS containing 0.5% Triton X-100 for 15 min. After blocking with 5% bovine serum albumin in PBS containing 0.1% Tween-20, samples were incubated in the primary antibody overnight at 4°C and incubated in secondary antibody for 2–3 h at 25°C. Primary antibodies used were rabbit anti-CDH1 (1:400; 24E10, #3195; Cell Signaling Technology) [39], mouse anti-CDX2 (1:200; CDX2-88; BioGenex) [10], goat anti-GATA4 (1:400; C-20, #sc-1237; Santa Cruz Biotechnology) [40], rabbit anti-NANOG (1:800; #RCAB0002P-F; Cosmo Bio) [12], goat anti-POU5F1 (1:200; N-19, #sc-8628; Santa Cruz Biotechnology) [3], and mouse anti-β-tubulin (1:5000; TUB 2.1, #T4026; Sigma-Aldrich) [41]. Secondary antibodies (1:1000; Life Technologies) were conjugated with Alexa Fluor 488, namely, donkey anti-rabbit, goat anti-mouse, and rabbit anti-goat, and conjugated with Alexa Fluor 546, namely, donkey anti-goat, goat anti-rabbit, and rabbit anti-mouse. F-actin filaments were visualized by adding phalloidin conjugated with Alexa 633 (Life Technologies) at a final concentration of 33 nM in the secondary antibody solution. Stained samples were mounted in ProLong Gold antifade reagent containing 4′,6′-diamidino-2-phenylindole (DAPI) (Life Technologies).
Microscopy and Image Analysis
Embryos from the same experiment were imaged in the same session, using an Axiovert 200 fluorescence microscope (Carl Zeiss) and FV1000 confocal laser scanning microscope (Olympus). For confocal microscopy, serial optical sections were imaged at 2 μm intervals under a 40× objective lens with oil. Z-axis projections of serial optical sections, measurement of ICM length, and examination of every optical section to count nuclei were performed with the aid of Fluoview Viewer software (Olympus). Three-dimensional reconstructions of the ICM were performed with the aid of Imaris software (Bitplane) to observe cell distribution.
Time-Lapse Video Microscopy
Embryo development was captured in real-time using time-lapse video microscopy, as previously described [42]. A 20 μl drop of KSOM-AA medium was placed on a poly-D-lysine-coated cover glass (BD Biosciences), which was placed in a Petri dish and overlaid with mineral oil. The dish was then placed in Heating Insert P (PeCon), whose temperature and CO2 concentration were regulated by Tempcontrol 37-2 and CO2-Controller (PeCon), respectively. The Heating Insert P was enclosed in Incubator XL-3 (PeCon), which was attached to Axiovert 200 inverted microscope (Carl Zeiss). Blastocysts were placed in the KSOM-AA drop after it equilibrated. Images were captured every 15 min, using the AxioCam MRm digital camera, which was controlled by the AxioVision software (Carl Zeiss). The Incubator XL-3 was covered with a black plastic sheet during time-lapse recording. The diameter of the blastocyst cavity was measured in images with the aid of AxioVision software, using the scalings and length tools.
Cell Culture
P19 mouse embryonal carcinoma cells (American Type Culture Collection) were cultured in alpha-Minimum Essential Medium (Life Technologies) with 2.5% fetal bovine serum and 7.5% calf serum. RW.4 mouse embryonic stem cells (mESC; American Type Culture Collection) were cultured in ESGRO Complete Clonal Grade Medium (EMD Millipore) on gelatin-coated dish without feeder cells. Twenty thousand P19 cells were plated in each well of a four-well plate (Nunc) and transfected with short hairpin RNA (shRNA) plasmids (200 ng) using Lipofectamine 2000 (Life Technologies), according to the manufacturer's instruction. One day after transfection, P19 cells were cultured in the presence of puromycin (10 μg/ml) for 2 days to eliminate untransfected cells, followed by RNA extraction. For colony formation, 50 000 mESC were plated in each well of gelatin-coated four-well plate, and cultured for 2 days in the presence or absence of 10 μM Y-27632. For knockdown of Rock1 and Rock2, mESC were transfected with a mixture of the two plasmids, (500 ng each of TRCN0000022903 and TRCN0000022923; Sigma-Aldrich) 1 day after plating using Lipofectamine LTX (Life Technologies), according to the manufacturer's instruction. Enhanced green fluorescent protein (Egfp) shRNA plasmid (SHC005; Sigma-Aldrich) was used as the control. One day after transfection, culture medium was replaced with fresh medium containing puromycin (2 μg/ml) for an additional 2 days of culture.
Short Hairpin RNA Plasmid Injection
Short hairpin RNA plasmids were injected into one-cell embryos as previously described [3]. The shRNA plasmids were diluted to a total concentration of 10 ng/μl (i.e., 5 ng/μl Rock1 plus 5 ng/μl Rock2 shRNA plasmids, or 10 ng/μl control shRNA plasmid) in the injection buffer, which consisted of 5 mM Tris, pH 7.4, 0.1 mM ethylenediaminetetraacetic acid, and 0.1 mg/ml of Fast Green FCF (Sigma-Aldrich). With the aid of FemtoJet air pump (Eppendorf), approximately 0.1–0.2 fg/egg of shRNA plasmids were injected into a pronucleus of fertilized eggs, which were collected from oviducts of B6D2F1 mice. Injected eggs were transferred into KSOM-AA drops for further culture.
Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was extracted with TRI reagent (Sigma-Aldrich) and used for cDNA synthesis. For P19 cells, cDNA was synthesized from 1 μg of total RNA. For embryos, cDNA was synthesized from the entire total RNA extracted from each sample (15–25 embryos). Quantitative PCR was performed, using iCycler Thermal Cycler with MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). Amplification of 0.5 μl cDNA was done with iQ SYBR Green Supermix (Bio-Rad) in a 20-μl reaction volume as follows: initial denaturation at 94°C for 5 min, followed by up to 45 cycles of 94°C for 15 sec, 60°C for 20 sec, and 72°C for 40 sec. Gapdh was used to normalize gene expression levels. The following primers were used: Rock1, F-CAA GCT TGA AGA GCA ACT GC, R-CTT GTC TGC TTG TGA CTT GG; Rock2, F-AGA ACA CCT TAG CAG TGA GG, R-TTT GGA ACT TTC TGC CTG GG; Rhoa, F-CTT TAT AAG TGA TGG CTG CC, R-TGG TCT TTG CTG AAC ACT CC; and Gapdh, F-GCA TGG CCT TCC GTG TTC CT, R-CCC TGT TGC TGT AGC CGT ATT CAT.
Collapsing the Blastocyst Cavity
Procedures were done as previously described [38, 43]. A stock of cytochalasin D (10 mg/ml in DMSO) (Sigma-Aldrich) was prepared and stored at −20°C until ready for use. Cytochalasin D (0.5 or 2 μg/ml) was freshly diluted in KSOM-AA and pipetted as 20 μl drops under mineral oil to equilibrate at 37°C with 5% CO2 in air prior to use. Control 20 μl drops were prepared by adding DMSO to KSOM-AA at a volume equal to that of the cytochalasin D. E3.5 blastocysts whose cavity volume was at least half the total size of the embryo were used for the experiments. We had observed that the reduction in size of the blastocyst cavity occurred within 5 h after the start of Y-27632 treatment. We therefore incubated the blastocysts in cytochalasin D and control drops within this time frame for 1 h, followed by washing in drug-free KSOM-AA, and culture in drug-free KSOM-AA up to E4.5. The E4.5 blastocysts were processed for immunostaining.
ICM Isolation by Immunosurgery
Procedures were done as previously described [44]. Briefly, E3.5 blastocysts still inside the zona pellucida were incubated in rabbit anti-mouse serum diluted in KSOM-AA followed by guinea pig complement (Invitrogen) diluted in KSOM-AA. Lysed TE cells were removed, along with zona pellucida, by treating embryos with 0.5% pronase (Roche) in FHM. Isolated ICMs were cultured with or without 10 μM Y-27632 in KSOM-AA medium in dishes coated with Matrigel matrix (BD Biosciences) for 1 day. Images of cultured ICMs were photographed using the AxioCam MRm digital camera, and ICM areas were measured in the images using outline tool in the AxioVision software (Carl Zeiss).
Embryo Transfer into Surrogate Mice
Y-27632-treated and control blastocysts at E4.5 were transferred separately into the uterine horns of pseudopregnant CD-1 females that had been mated with vasectomized CD-1 males at three nights previously [44]. Numbers of implantation sites and normal developing fetuses were observed on Day 14.5 of gestation, the time point at which extent of early embryonic loss after implantation can be assessed and when normal fetuses rarely fail to develop to full term [45].
Statistical Analysis
In each set of experiment, embryos were collected from three to five superovulated female mice and pooled together, which were then divided into two groups (i.e., control and experimental). Numbers of replications for each experiment are indicated in the corresponding figure legends, and they are based on independent collections of embryos on separate occasions. Data were presented as mean ± standard error of the mean, unless indicated otherwise. Data for embryo transfers presented in Table 1 were arcsine-transformed and analyzed by Student t-test, which are detailed in Supplemental Tables S1–S3 (all the Supplemental Data is available online at www.biolreprod.org). Differences were considered statistically significant at P < 0.05.
TABLE 1.
Effects of Y-27632 treatment during blastocyst expansion on implantation and fetal development.
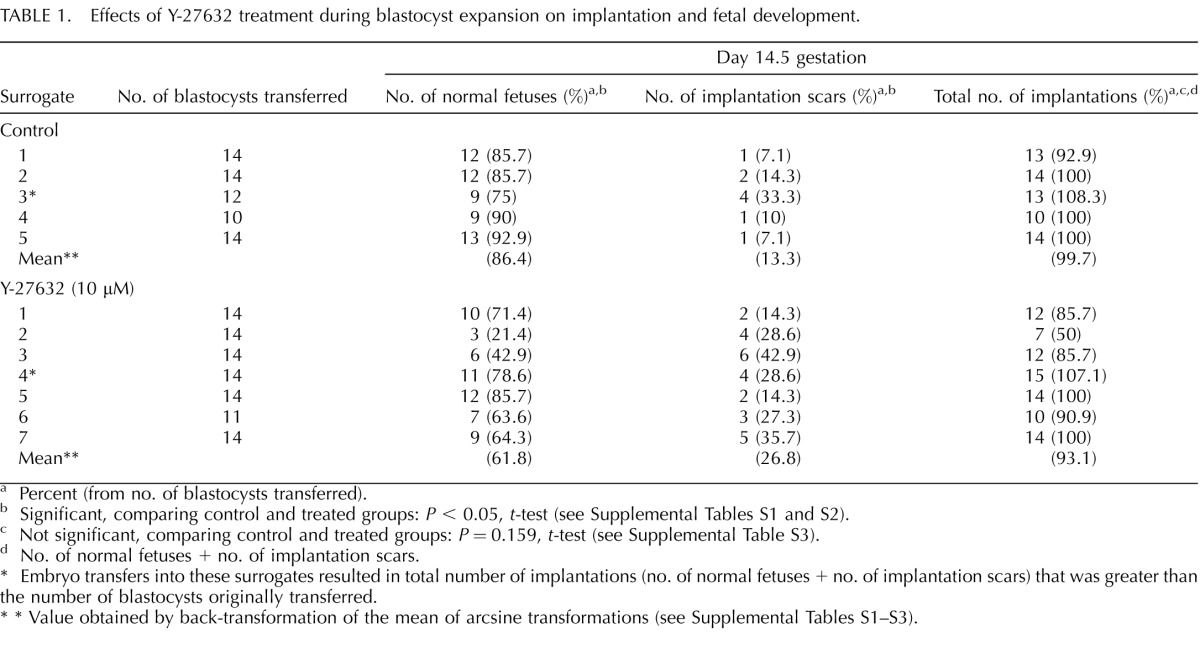
Percent (from no. of blastocysts transferred).
Significant, comparing control and treated groups: P < 0.05, t-test (see Supplemental Tables S1 and S2).
Not significant, comparing control and treated groups: P = 0.159, t-test (see Supplemental Table S3).
No. of normal fetuses + no. of implantation scars.
Embryo transfers into these surrogates resulted in total number of implantations (no. of normal fetuses + no. of implantation scars) that was greater than the number of blastocysts originally transferred.
* Value obtained by back-transformation of the mean of arcsine transformations (see Supplemental Tables S1–S3).
RESULTS
Treatment with Y-27632 Alters the ICM Morphology and Disrupts PrE and Epi Tissue Segregation
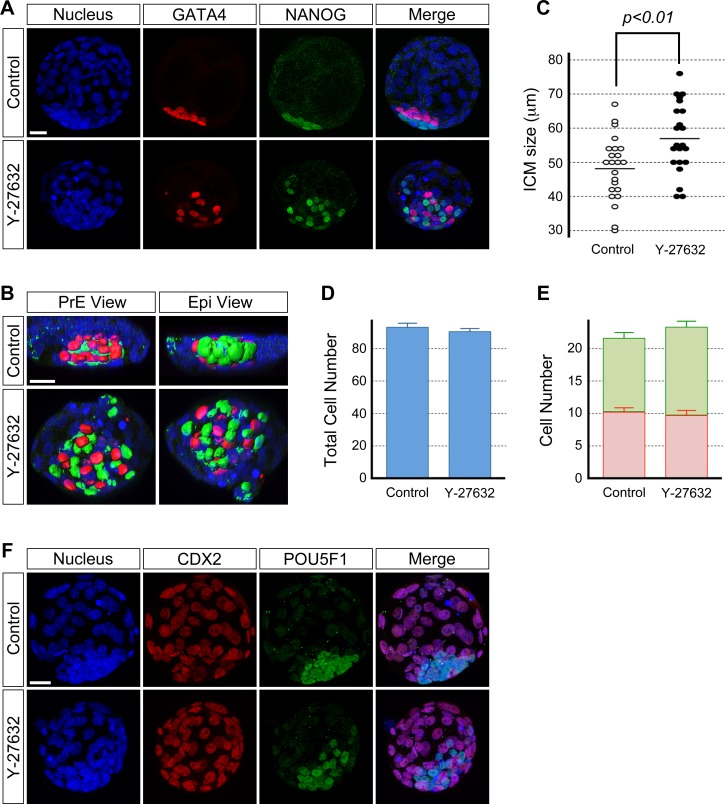
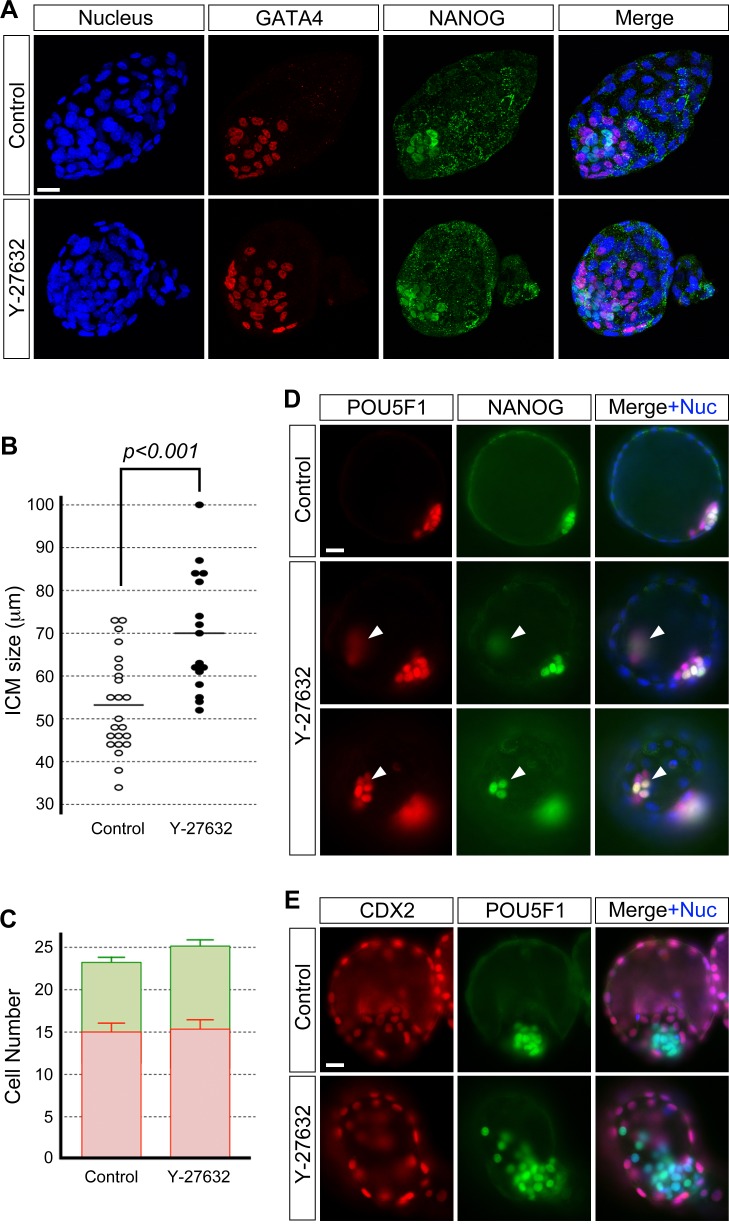
To test the requirement for ROCK in ICM morphogenesis, embryos were treated with the specific inhibitor Y-27632 during blastocyst cavity expansion (E3.5–E4.5, 24 h) and were immunostained for transcription factor proteins that are PrE- and Epi-specific markers, that is, GATA4 and NANOG [14], respectively. Embryos that were treated with 20 μM of Y-27632 led to the permanent collapse of most of the blastocyst cavity (data not shown), whereas blastocysts retained their cavity in 15 μM or less of Y-27632. This suggests that the high concentration of Y-27632 may have resulted in effects other than on the ICM, possibly impairment in the epithelial integrity of the TE. To focus on its impact on ICM morphology, the results presented here were based on experiments using 10 or 15 μM of Y-27632. At E4.5, control (untreated) blastocysts (n = 23) had an ICM whose cells were packed in an aggregate that consisted of an outer layer of PrE cells and a deeper layer of Epi cells (Fig. 1, A and B, and Supplemental Fig. S1, A and B). In contrast, blastocysts treated with 10 μM (n = 22, Fig. 1, A and B) and 15 μM (Supplemental Fig. S1, A and B) of Y-27632 had an ICM with altered morphology such that the ICM cells were less tightly aggregated and were scattered. The PrE and Epi tissue layers were disrupted, and the cells of the two lineages appeared to be intermingled to varying extent. We compared the ICM size between treated and control blastocysts by measuring the ICM at the widest dispersion of its cells. The ICM was significantly wider or more spread out in Y-27632-treated blastocysts (Fig. 1C; Supplemental Fig. S1C). These results show that ROCK inhibition led to the loosening of the ICM aggregate. This suggests that ROCK is required for cell-cell cohesion to enable the tight aggregation of ICM cells during blastocyst cavity expansion. Interestingly, requirement of ROCK activity for tight cell aggregation appears to be specific to ICM but not for cell adhesion in general because treatment of embryos with Y-27632 at earlier stages, namely from two-cell stage onward, does not interfere with cell adhesion or compaction, as shown previously [38].
FIG. 1.
Inhibition of ROCK activity during blastocyst cavity expansion disrupts the ICM morphology but not cell proliferation and differentiation. Blastocysts were cultured in the absence (control) or presence of 10 μM Y-27632 from E3.5 to E4.5 and immunostained for cell lineage-specific transcription factors. A) GATA4-positive PrE (red) and NANOG-positive Epi (green) cells are less tightly packed together and appear scattered in the inhibitor-treated blastocyst than in the control blastocyst. Confocal images are z-series projections. B) Three-dimensional reconstructions of confocal microscopic images of the ICM of embryos in A. Reconstructed three-dimensional images are rotated to show views from the PrE and Epi side of the ICM. C) Comparison of ICM size based on the widest length of ICM cell dispersion. Circles represent the ICM size in individual embryos, and horizontal bars represent the mean value for each group. The ICM is significantly wider (Student t-test) in inhibitor-treated embryos (n = 22) compared to the control embryos (n = 23). D) Comparison of total cell numbers. Graph shows the mean and standard error. There is no statistically significant difference (P = 0.49; Student t-test) between control (n = 24) and inhibitor-treated (n = 25) embryos. E) Comparison of the number of PrE (red bar) and Epi (green bar) cells shows no statistically significant differences (P[PrE] = 0.61, P[Epi] = 0.07; Student t-test) between control (n = 24) and inhibitor-treated (n = 25) embryos. Graph shows the mean and standard error. Data presented in C, D, and E are compilations of two replicates of the experiment that were performed on different occasions. F) The treated blastocyst shows no differences to the control blastocyst with respect to CDX2 (red) protein immunostaining of the TE cells. The ICM is immunostained for pluripotency marker POU5F1 (green) protein. Confocal images are z-series projections. Blue, DAPI. Bars = 20 μm (A, B, F).
To examine whether the abnormal morphology of Y-27632-treated ICM is merely due to delay or arrest in developmental progress, cell numbers were compared between the control and treated blastocysts at E4.5. The total number of cells, as well as the number of PrE and Epi cells in the embryos, were unchanged by Y-27632 treatment (Fig. 1, D and E, and Supplemental Fig. S1, D and E). Thus, cell proliferation and cell-type differentiation were unaffected by ROCK inhibition, suggesting that the failure of tight aggregation and PrE and Epi segregation were not due to delayed or arrested development of the embryo but was due specifically to disturbance in the ICM morphology.
We also examined the impact of Y-27632 on TE differentiation by immunostaining blastocysts for the TE lineage-specific transcription factor, CDX2 [7]. CDX2-positive cells were distinct in inhibitor-treated embryos (n = 9) with no apparent loss in expression, and they were similar to those in the control embryos (n = 8) (Fig. 1F). This suggests that the TE lineage differentiation during blastocyst expansion is independent of ROCK.
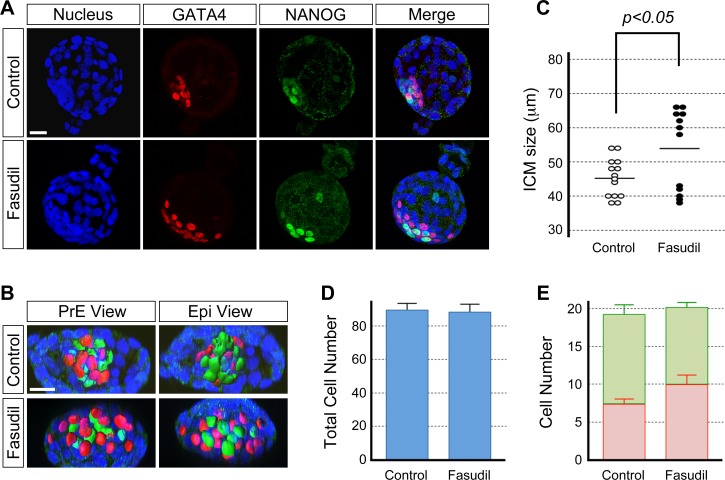
To verify that the phenotype caused by the Y-27632 treatment was due to inhibition of the ROCK activity, the effect of another type of ROCK inhibitor, Fasudil, was analyzed. We found that Fasudil treatment showed essentially the same effects on the ICM morphology as Y-27632 treatment. In Fasudil-treated blastocysts (n = 12), the PrE and Epi cells were more spread out and the ICM was significantly wider than in the control blastocysts (n = 14) (Fig. 2, A–C). Total cell number and PrE and Epi cell numbers were similar between treated and control embryos (Fig. 2, D and E). Thus, Fasudil treatment phenocopied the Y-27632-induced alterations of the ICM morphology. Taken together, the maintenance of the ICM cohesion and the segregation of the PrE and Epi tissue layers require ROCK activity.
FIG. 2.
Fasudil treatment phenocopies the Y-27632-induced disruption of the ICM morphology. Blastocysts were cultured in the absence (control) or presence of 5 μM Fasudil from E3.5 to E4.5 and immunostained for cell lineage-specific transcription factors. A) GATA4-positive PrE (red) and NANOG-positive Epi (green) cells are less tightly packed together and are more spread out in the Fasudil-treated embryo than in the control embryo. Confocal images are z-series projections. B) Three-dimensional reconstructions of confocal microscopic images of the ICM of embryos in A. Reconstructed three-dimensional images are rotated to show views from the PrE and Epi side of the ICM. C) Comparison of ICM size based on the widest length of ICM cell dispersion. Circles represent the ICM size in individual embryos, and horizontal bars represent the mean value for each group. The ICM is significantly wider (Student t-test) in inhibitor-treated embryos (n = 12) than in the control embryos (n = 14). D) Comparison of total cell numbers. Graph shows the mean and standard error. There is no statistically significant difference (P = 0.87; Student t-test) between control (n = 14) and inhibitor-treated (n = 14) embryos. E) Comparison of the number of PrE (red bar) and Epi (green bar) cells shows no statistically significant differences (P[PrE] = 0.16, P[Epi] = 0.29; Student t-test) between control (n = 14) and inhibitor-treated (n = 14) embryos. Graph shows the mean and standard error. Data presented in C, D, and E are compilations of two replicates of the experiment that were performed on different occasions. Blue, DAPI. Bars = 20 μm (A, B).
Transient Reduction in Blastocyst Cavity Size Does Not Interfere with Proper Morphogenesis of the ICM
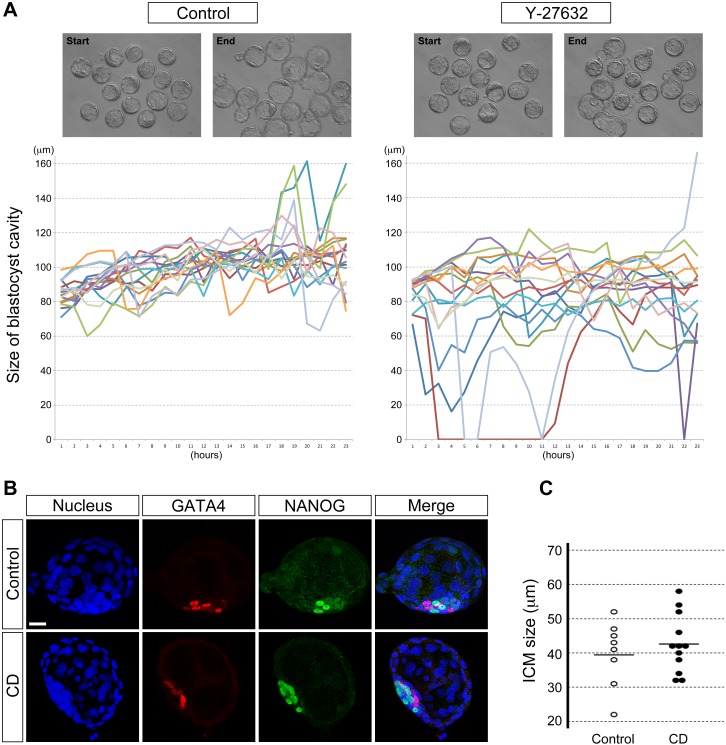
To gain insight into how Y-27632 interferes with normal ICM morphogenesis, we monitored the behavior of blastocysts continuously throughout the culture period (E3.5–E4.5) by time-lapse cinematography (Fig. 3A and Supplemental Movies). We noticed that the size of the blastocyst cavity transiently decreased more prominently in Y-27632-treated embryos compared to control. At the start of the time-lapse recording (E3.5), all the blastocysts had a cavity size greater than 60 μm, which was measured as the longest diameter (see Materials and Methods). During 24 h of culture, none of the control blastocysts (n = 16) collapsed below 60 μm in cavity size, whereas 53% of Y-27632-treated blastocysts (n = 15) did, half of which took place within 5 h after the initiation of treatment (Fig. 3A). Reduction in cavity size, however, was transient, and the cavity reexpanded in most embryos even in the continuous presence of Y-27632.
FIG. 3.
Transient reduction of the blastocyst cavity does not interfere with ICM morphogenesis. A) Time-lapse recordings show the changes in the size of the blastocyst cavity between E3.5 and E4.5. Top panels show still images from time-lapse movies (Supplemental Movies), specifically of the start and end points of recordings. In the graphs, each colored line represents the cavity size of individual blastocysts. Y-27632-treated blastocysts (n = 15) tend to show more drastic reduction in cavity size than control blastocysts (n = 16). B) Blastocyst cavity is transiently reduced by brief cytochalasin D (CD, 0.5 μg/ml) treatment. Nonetheless, the E4.5 blastocyst shows segregated GATA4-positive PrE (red) and NANOG-positive Epi (green) tissue layers, similar to that in the control blastocyst. Confocal images are z-series projections. C) Comparison of ICM size based on the widest length of ICM cell dispersion. Circles represent the ICM size in individual embryos, and horizontal bars represent the mean value for each group. There is no statistically significant difference (P = 0.50; Student t-test) in the ICM size between CD-treated (n = 12) and control (n = 8) embryos. Data presented in C are compilations of two replicates of the experiment that were performed on different occasions. Blue, DAPI. Bar = 20 μm (B).
It had been observed previously that during in vitro culture of human blastocysts, the size of the cavity exhibits repeated reduction and reexpansion, or pulsing behavior. It was suggested that the cavity reduction may contribute to distortion of the ICM morphology and result in dissociation of ICM cells that can produce ectopic ICM [26, 30]. Thus, it is possible that the Y-27632-induced transient reduction in cavity size (Fig. 3A) may have led to the loosening of ICM cells and the interference with PrE and Epi cell sorting (Fig. 1, A and B). To test this, we induced transient cavity reduction using a different method, namely by briefly treating blastocysts with cytochalasin D as previously done [38, 43]. Blastocysts were treated with 0.5 and 2 μg/ml of cytochalasin D for 1 h at E3.5 plus 3 h (corresponding to the approximate timing when cavity reduction mostly occurred in Y-27632-treated blastocysts). The cavity collapsed in all the blastocysts (n = 18) during the cytochalasin D treatment. However, after transfer back to normal culture medium, the cavity reexpanded robustly by E4.5. We found that the PrE and Epi tissue layers showed segregation (n = 12 at 0.5 μg/ml, Fig. 3B; data for 2 μg/ml is found in Supplemental Fig. S2A). The ICM shape was similarly compact in cytochalasin D-treated and control embryos (n = 8, Fig. 3B and Supplemental Fig. S2A). Moreover, the ICM size was not statistically different between the two groups (Fig. 3C and Supplemental Fig. S2B). Thus, reduction in blastocyst cavity size per se does not interfere with proper ICM morphogenesis, including the PrE and Epi tissue segregation, excluding it as the primary cause for the abnormal ICM morphology induced by ROCK inhibition.
ROCK Inhibition Causes Spreading of ICM without TE
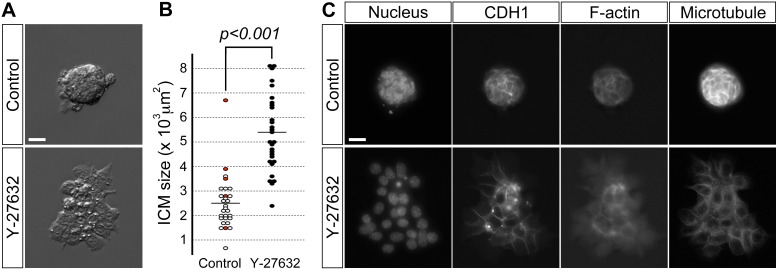
Even though we showed that transient cavity reduction did not cause the ICM defects, there is still a possibility that ROCK inhibition may first affect TE, which in turn causes spreading of ICM. To test whether ROCK inhibition directly induces ICM to spread without TE, ICMs were isolated by immunosurgically removing TE from E3.5 blastocysts and were then cultured on a Matrigel-coated dish for 1 day (i.e., up to the equivalent stage of E4.5 blastocyst) in the presence or absence of Y-27632. Control ICMs (n = 29) were firmly attached to the culture dish while maintaining their spherical shape of aggregated cells (Fig. 4A). In contrast, Y-27632-treated ICMs (n = 29) showed dramatic changes in morphology: they flattened and spread as a monolayer on the culture dish, resulting in significantly increased surface area than in control ICMs (Fig. 4, A and B). Thus, ROCK inhibition in ICM is sufficient to cause its spreading, and this morphological change is not dependent on TE.
FIG. 4.
ROCK inhibition by Y-27632 treatment induces spreading of isolated ICMs. A) Representative examples of morphology of control and treated ICMs. B) Comparison of surface area in control and treated ICMs. Circles represent surface area in individual ICMs, and horizontal bars represent the mean value for each group. Red circles represent ICMs that formed a cavity during the course of the experiment. Treated ICMs (n = 29) have significantly increased surface area (Student t-test) than control ICMs (n = 29). C) Fluorescence microscopic images of control and treated ICMs immunostained for the cell adhesion molecule CDH1 and cytoskeletal components, namely filamentous actin (F-actin) and microtubules. Data presented in B are compilations of two replicates of the experiment that were performed on different occasions. Nuclei were stained with DAPI. Bars = 20 μm (A, C).
We took further advantage of isolated ICMs to investigate how ROCK inhibition led to dramatic changes in ICM morphology, particularly focusing on the key adhesion molecule CDH1 (E-cadherin) and cytoskeletal components, namely filamentous actin (F-actin) and microtubules. Although cells were spread out in ROCK-inhibited isolated ICM, CDH1 was distinctly expressed and remained localized at sites of cell-cell contact (Fig. 4C), suggesting that spreading of ICM cells was not due to loss of CDH1. In contrast, F-actin, which was localized in the cell cortex of control ICMs, was diminished in Y-27632-treated ICMs, particularly in cells near the periphery (Fig. 4C). Microtubules also showed cortical localizations in control ICMs, whereas they were mainly found in the cytoplasm in Y-27632-treated ICMs (Fig. 4C). These results suggest that ROCK inhibition caused substantial remodeling of cytoskeletal components, which might have contributed to the spread morphology of the ICM.
Disruption of the ICM Morphology by ROCK Inhibition Is Irreversible
We then asked whether the normal morphology of the ICM can be restored in Y-27632-treated blastocysts after removal of the inhibitor. After culture with or without Y-27632 for 24 h (E3.5–E4.5), blastocysts were washed and cultured in inhibitor-free medium for another 24 h (E4.5+1day). The results showed that treated/washed embryos (n = 16) retained a significantly wider ICM than control embryos (n = 23) (Fig. 5, A and B). The numbers of PrE and Epi cells were similar between treated/washed and control embryos (Fig. 5C). Interestingly, an additional clump of NANOG/POU5F1-positive cells (Fig. 5D) was observed on occasion, whereas single scattered ICM cells were relatively more common (Fig. 5E). The TE cells were examined by immunostaining for CDX2 protein. There were no apparent differences in CDX2-staining levels between treated/washed embryos (n = 9) and control embryos (n = 8) (Fig. 5E). Thus, the ROCK inhibitor-induced disruption of the ICM morphology is not reversible.
FIG. 5.
Disruption of the ICM morphology by Y-27632 (10 μM) treatment is not reversible. A) GATA4-positive PrE (red) and NANOG-positive Epi (green) cells appear more widely spread apart in the treated/washed blastocyst (E4.5+1day) than in the control blastocyst. Confocal images are z-series projections. B) Comparison of ICM size of blastocysts (E4.5+1day) based on the widest length of ICM cell dispersion. Circles represent the ICM size in individual embryos, and horizontal bars represent the mean value for each group. The ICM is significantly wider (Student t-test) in treated/washed embryos (n = 16) than in control embryos (n = 23). C) Comparison of the number of PrE (red bar) and Epi (green bar) cells shows no statistically significant differences (P[PrE] = 0.83, P[Epi] = 0.22; Student t-test) between control (n = 24) and treated/washed (n = 21) embryos. Graph shows the mean and standard error. Data presented in B and C are compilations of two replicates of the experiment that were performed on different occasions. D) Fluorescence microscopic images of blastocysts (E4.5+1day) immunostained for pluripotency markers. Top row shows POU5F1-positive ICM (red) cells and a subset of NANOG-positive Epi (green) cells in the control blastocyst. Second and third rows show an ectopic clump of NANOG/POU5F1-positive cells (arrowhead) at different planes of focus in the treated/washed blastocyst. E) Fluorescence microscopic images of blastocysts (E4.5+1day). The treated/washed blastocyst shows no differences to the control blastocyst with respect to CDX2 (red) protein immunostaining of the TE nuclei. The ICM is immunostained for POU5F1 (green) protein. Note that ICM cells are scattered in the Y-27632-treated blastocyst. Blue, DAPI (for nuclear staining [+Nuc]). Bars = 20 μm (A, D, E).
Alteration of the ICM Morphology Is Incompatible with Fetal Development
The above studies indicate that ROCK inhibition during blastocyst expansion impairs the ICM morphology, while the lineage formation of PrE, Epi, and TE is unaffected. This situation provides an opportunity to investigate how abnormal ICM morphology would influence postimplantation development, specifically with respect to the efficiency of implantation and fetal development. Thus, Y-27632-treated and control blastocysts at E4.5 were transferred into surrogate mothers, and the contents of the uterine horns were examined at Day 14.5 of gestation. Most control blastocysts generated normal fetuses, whereas treated blastocysts developed significantly fewer fetuses (P < 0.05) (Table 1). Conversely, treated blastocysts resulted in significantly more implantation scars (P < 0.05) that indicated aborted development. Interestingly, however, the total number of implantation sites (i.e., fetus plus scar) was not significantly different (P = 0.159) between control and treated blastocysts, suggesting that the efficiency of implantation was not compromised by ROCK inhibition. This is consistent with the lack of interference by Y-27632 on the TE lineage formation (Figs. 1F and 5E). These results show that ROCK-dependent normal morphogenesis of the ICM plays an essential role in fetal development.
Knockdown of Rock1 and Rock2 Impairs Morphology of Embryonic Stem Cell Colonies
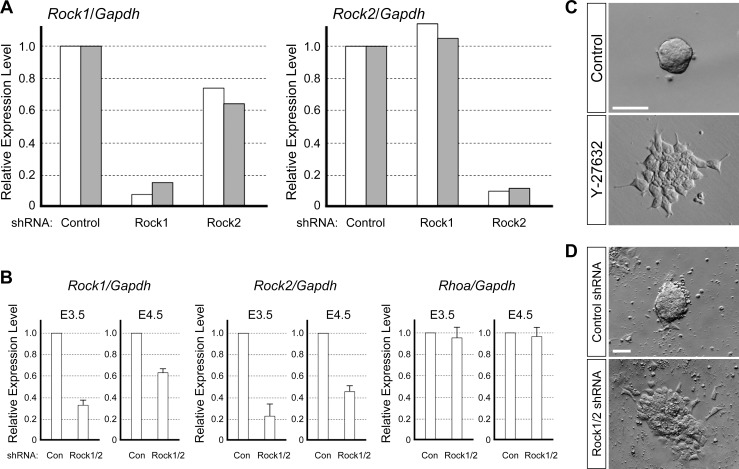
Y-27632 has been used as a specific inhibitor of ROCK in many studies, and the effects of inhibitor treatment are consistent with loss-of-function of ROCK1 and ROCK2 in various experimental systems [35, 36, 46–50]. To verify that the impact of Y-27632 on the ICM morphology is largely due to impairment of Rock1 and Rock2, we attempted to knock down these two genes simultaneously in the blastocyst. The most conventional methodology to knock down gene expression during mouse preimplantation development is to introduce RNA interference agents, such as plasmids encoding gene-specific shRNA, into fertilized eggs. This method has been performed by ourselves as well as by other researchers [3, 51, 52]. Thus, we first identified specific shRNA plasmids that can effectively down-regulate the level of Rock1 or Rock2 transcripts, using P19 mouse embryonal carcinoma cell line. P19 cells were transiently transfected with shRNA plasmids and cultured for 1 day in the presence of puromycin to select those cells that maintained transfected plasmids. Quantitative RT-PCR analysis showed that about 90% of Rock1 transcripts were eliminated by the Rock1-specific shRNA plasmid, and about 90% of Rock2 transcripts were eliminated by the Rock2-specific shRNA plasmid (Fig. 6A), indicating that these two plasmids effectively knock down Rock1 and Rock2.
FIG. 6.
Loss-of-function of ROCK induces spreading of mESC colonies. A) Quantitative RT-PCR analysis of Rock1 and Rock2 transcript levels in P19 cells. P19 cells have been transfected with control, Rock1-specific, or Rock2-specific shRNA plasmid. Expressions of Rock1 and Rock2 are normalized with a house-keeping gene Gapdh, and expression levels are presented relative to the control. White and gray bars represent independent set of experiments performed on different occasions. B) Quantitative RT-PCR analysis of Rock1, Rock2, and Rhoa transcript levels at E3.5 and E4.5 in mouse embryos that have been injected with control or a mixture of Rock1-specific and Rock2-specific shRNA plasmids at E0.5. Expressions are normalized with respect to Gapdh and presented relative to the control at each developmental stage. Graphs show average values and standard deviation of triplicates using a set of cDNAs that have been synthesized from pooled RNA samples of 15–25 embryos. Note that the levels of Rhoa, encoding a component of RHO/ROCK signaling, are not affected by Rock1 and Rock2 shRNA plasmids. C) Representative examples of morphology of control and Y-27632-treated mESC colony. D) Representative examples of morphology of mESC colony that have been transfected with the control shRNA plasmid or a mixture of Rock1 and Rock2 shRNA plasmids. Bars = 50 μm (C, D).
We then microinjected these two shRNA plasmids together into fertilized eggs (E0.5), and examined the transcript levels of Rock1 and Rock2 at E3.5 and E4.5. At E3.5, the levels of Rock1 and Rock2 were reduced by about 65% and 75%, respectively, compared to embryos injected with the control shRNA plasmid (Fig. 6B). At E4.5, reduction in the transcript level was no longer substantial because the knockdown of Rock1 and Rock2 was only by about 35% and 55%, respectively (Fig. 6B). Thus, with this methodology, we were unable to maintain substantially low levels of Rock1 and Rock2 beyond E3.5, possibly due to dilution and/or degradation of the shRNA plasmids over 3–4 days of culture after injection at the one-cell stage. Our present study focuses on the role of ROCK in proper ICM morphogenesis at later stages of preimplantation development, specifically between E3.5 to E4.5. Thus, this methodology may not be suited to compare the effect of the pharmacological inhibitor and that of Rock1/Rock2 knockdown on ICM morphology.
To overcome this limitation, we used mESC, which are derivatives of ICM and retain various molecular and developmental characteristics of ICM, as a model to investigate the impact of ROCK inhibition on ICM morphology. We cultured mESC in the absence or presence of Y-27632 for 2 days. Control mESC formed round, cohesive, dome-shaped colonies (Fig. 6C) that were similar in appearance to isolated ICM cultured in the absence of Y-27632 (Fig. 4A). In contrast, colonies of Y-27632-treated mESC were flat and monolayered (Fig. 6C), which were reminiscent of the morphology of isolated ICM cultured with Y-27632 (Fig. 4A). Thus, ICM and mESC colonies responded to Y-27632 in a similar manner in terms of spread morphology. We then knocked down both Rock1 and Rock2 in mESC and examined their colony morphology. The mESC were transiently transfected with the Rock1 and Rock2 shRNA plasmids, followed by 2 days of culture in the presence of puromycin to select those that retained shRNA plasmids. The mESC transfected with control shRNA plasmid formed round, dome-shaped colonies, whereas those transfected with Rock1 and Rock2 shRNA plasmids formed flat, spread colonies (Fig. 6D). These results support the hypothesis that morphological defects caused by Y-27632 were largely due to inactivation of ROCK.
DISCUSSION
Here, we investigated the requirement for ROCK activity during the later stages of preimplantation development (E3.5–E4.5), particularly focusing on ICM morphogenesis. Our data showed that ROCK activity is required for the tight aggregation of ICM cells, and for PrE and Epi segregation into distinct tissue layers. On the other hand, cell proliferation and differentiation of the PrE, Epi, and TE lineages were ROCK-independent. Our data also showed that in spite of the scattering of ICM cells after inhibition of ROCK activity, such blastocysts were still capable of implantation as efficiently as normal blastocysts. However, blastocysts with scattered ICM cells resulted in significant fetal loss, showing that ICM morphology is a critical determinant of successful fetal development.
The present study showed that inhibition of ROCK impaired the two key aspects of ICM morphogenesis: cohesion of ICM into a tight aggregate and segregation between PrE and Epi. Were these two aspects independently affected by ROCK inhibition, or did impairment of one aspect cause the other? It is unlikely that impairment of ICM cohesion was a result of failed segregation between PrE and Epi because mESC colonies, which are essentially composed of Epi, also exhibited spread morphology in response to ROCK inhibition. Nonetheless, this does not exclude the possibility that segregation between PrE and Epi is also dependent on ROCK activity. Although the molecular mechanisms of PrE/Epi segregation are not fully understood, they are likely to involve differential cell adhesion and directional cell migration [13–15]. It has been shown in various systems that ROCK regulates cell adhesion and cell migration [34]. Thus, it is possible that PrE/Epi segregation is regulated by ROCK independently from ICM cohesion. To test this idea in future studies, an experimental approach is required to rescue the cohesion defect and then to investigate the lineage segregation.
Although no investigations other than the present study have been reported on the roles of RHO-ROCK signaling in ICM morphogenesis, several in vitro studies have suggested that RHO-ROCK signaling regulates cell adhesion and motility in mESC and mouse embryonal carcinoma cells. These cell lines can be indefinitely cultured while maintaining characteristics similar to the ICM and are often used as in vitro models of the ICM to study the mechanisms of cell differentiation [53, 54] as we did in the present study. A previous study showed that inhibition of the RHO-ROCK-myosin signaling axis causes mESC to dissociate from one another and to become motile, resulting in dispersal of the colony [46]. In addition, PrE cells that are differentiated from F9 mouse embryonal carcinoma cell line exhibit increased migration when RHO-ROCK signaling is inhibited [55]. These findings in the cell line models are consistent with the present study, showing that tight aggregation of the ICM is dependent on ROCK activity.
A classic model explicates that cell-type differentiation in the ICM is dependent on cell position with cells facing the blastocyst cavity committing to the PrE fate and deeper cells committing to the Epi fate [24, 25]. With ROCK inhibitor treatment, PrE and Epi were spread out so that some Epi cells appeared to be exposed to the cavity. Nevertheless, the number of Epi and PrE cells were unchanged, which suggested several important aspects of ICM morphogenesis and differentiation: 1) cell position in the ICM is not sufficient to determine cell-type differentiation; 2) cell interactions through FGF-GRB2-MAPK signaling that normally produce PrE [13, 23] can occur independently of ROCK activity; and 3) tight aggregation of the ICM is required for formation of distinct layers of PrE and Epi tissues. More recently, a mosaic/cell-sorting model has been proposed based on real-time tracing of PrE cells in live blastocysts that express green fluorescent protein under the control of the PrE-specific Pdgfra promoter [14]. In this model, the ICM is initially composed of PrE and Epi progenitor cells that are intermingled in the ICM, and later a combination of distinct cell behaviors, namely, directed migration, sorting, and apoptosis, separate the PrE from Epi as a segregated layer. While validity and applicability of this model are still actively investigated, the ROCK-inhibited blastocyst may provide a unique tool to interrogate the mechanisms of PrE and Epi formation with respect to cell behaviors.
The present study also showed that inhibition of ROCK activity in the blastocyst prior to implantation adversely affected fetal development, possibly due to abnormal ICM morphogenesis. Interestingly, this finding is consistent with a recent study of double knockout mice deficient in both Rock1 and Rock2 genes [56]. The double null mutant embryos can develop to the blastocyst stage but show lethality after implantation, as evidenced by the fact that the embryos are resorbed before E9.5, which is the stage when body turning into fetal position has normally completed [57]. Importantly, single knockout mice for either Rock1 or Rock2 do not show any abnormalities until the late fetal stage [58, 59]. Thus, both isoforms need to be eliminated to reveal ROCK functions during early development, which can be achieved by using pharmacological inhibitors, as in the present study. The ROCK activity is absent continuously throughout development in the double knockout embryos, whereas the activity was inhibited only between E3.5 and E4.5 in our study. However, a normal ICM morphology was not restored after removal of ROCK inhibitor, indicating that interference with ROCK during this developmental window results in irreversible detrimental effects that led to fetal wastage. It is of note that the size of ICM varied among blastocysts, whether they were control or ROCK-inhibited. As presented in Figure 5B, some of the ROCK-inhibited blastocysts contained ICM whose size was larger than any of the control blastocysts, whereas the others contained ICM that was within the range of the control ICM (Fig. 5B). We speculate that ROCK-inhibited blastocysts containing abnormally large ICM resulted in fetal loss, whereas those with ICM of normal range were still able to develop into normal fetuses. Further investigations on the inhibitor-treated blastocysts and the development of extraembryonic and embryonic tissues after implantation are necessary to determine abnormalities in connection with the fetal loss. It is noteworthy that in our studies we occasionally observed an additional clump of NANOG/POU5F1-positive cells that have dissociated from the main mass after ROCK inhibitor treatment. We speculate that formation of such ectopic clump of ICM-like cells due to ROCK misregulation in the expanding blastocyst may be a potential mechanism for generating monozygotic twins, which also warrant further investigations.
Our findings on the ICM morphology and fetal wastage bear relevance to human ART, namely with respect to the in vitro culture of human preimplantation embryos. Various clinics practice the method of culture up to the blastocyst stage after which the embryo is transferred into the uterus of the patient. Before transfer, blastocyst quality can be scored based on morphological criteria that include ICM size and shape [60, 61]. There are reports that good quality ICM and blastocysts are compatible with efficient production of pregnancies and births [60, 62]. In our present study, we closely analyzed the ICM morphology using morphological and molecular parameters, as discussed above. These parameters may be used to assess whether newly devised procedures (e.g., culture conditions, micromanipulations) are conducive to development of normal blastocysts. In fact, observations have been reported that during in vitro culture, human blastocysts undergo repeated reduction and reexpansion behaviors of the cavity, leading to ICM cells adhering to ectopic sites [26, 30]. Currently, it is unclear whether human blastocysts with atypical ICM morphology results in abnormal fetal development, although incidence of monozygotic twinning is reported to be higher in embryos conceived by IVF [29–32]. Thus, more vigorous investigations on the quality of blastocysts, particularly with respect to the ICM morphology, should contribute to promoting healthy human preimplantation development in vitro, thereby reducing or preventing pregnancy loss, and consequently, leading to the successful birth of a baby.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to Yusuke Marikawa for technical assistance and critical discussions during the course of our investigations. We also thank Yukiko Yamazaki for thoughtful discussions.
Footnotes
Supported by NIH grant 8P20GM103457 (V.B.A.). A.M.A.L. was supported in part by scholarships from the Hawaii Community Foundation and the Manoa Opportunity Grant.
REFERENCES
- Marikawa Y, Alarcon VB. Creation of trophectoderm, the first epithelium, in mouse preimplantation development Results Probl Cell Differ 2012. 55 165– 184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst Dev Dyn 2006. 235 2301– 2314 [DOI] [PubMed] [Google Scholar]
- Alarcon VB. Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo Biol Reprod 2010. 83 347– 358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RO, Yamanaka Y, Rossant J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin Development 2010. 137 3383– 3391 [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass Dev Cell 2009. 16 398– 410 [DOI] [PubMed] [Google Scholar]
- Home P, Ray S, Dutta D, Bronshteyn I, Larson M, Paul S. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression J Biol Chem 2009. 284 28729– 28737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst Development 2005. 132 2093– 2102 [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4 Cell 1998. 95 379– 391 [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo Development 2007. 134 4219– 4231 [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation Cell 2005. 123 917– 929 [DOI] [PubMed] [Google Scholar]
- Wu G, Gentile L, Fuchikami T, Sutter J, Psathaki K, Esteves TC, Arauzo-Bravo MJ, Ortmeier C, Verberk G, Abe K, Scholer HR. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2 Development 2010. 137 4159– 4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blij S, Frum T, Akyol A, Fearon E, Ralston A. Maternal Cdx2 is dispensable for mouse development Development 2012. 139 3969– 3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway Dev Cell 2006. 10 615– 624 [DOI] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst Development 2008. 135 3081– 3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Adams RJ, Morris SA, Danckaert A, Le Garrec JF, Zernicka-Goetz M. Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst Dev Biol 2009. 331 210– 221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo Curr Biol 1996. 6 1487– 1496 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H. Origin and role of distal visceral endoderm, a group of cells that determines anterior-posterior polarity of the mouse embryo Nat Cell Biol 2011. 13 743– 752 [DOI] [PubMed] [Google Scholar]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors Genes Dev 2002. 16 784– 789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai KQ, Capo-Chichi CD, Rula ME, Yang DH, Xu XX. Dynamic GATA6 expression in primitive endoderm formation and maturation in early mouse embryogenesis Dev Dyn 2008. 237 2820– 2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Smedberg JL, Cai KQ, Capo-Chichi DC, Xu XX. Ectopic expression of GATA6 bypasses requirement for Grb2 in primitive endoderm formation Dev Dyn 2011. 240 566– 576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells Cell 2003. 113 643– 655 [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells Cell 2003. 113 631– 642 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst Development 2010. 137 715– 724 [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse Development 2009. 136 701– 713 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Hamada H. Cell fate decisions and axis determination in the early mouse embryo Development 2012. 139 3– 14 [DOI] [PubMed] [Google Scholar]
- Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos Am J Obstet Gynecol 2008. 199:660 e1– e5 [DOI] [PubMed] [Google Scholar]
- Duncan FE, Stein P, Williams CJ, Schultz RM. The effect of blastomere biopsy on preimplantation mouse embryo development and global gene expression Fertil Steril 2009. 91 (4 Suppl): 1462– 1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr B, Milki AA. Visualization of atypical hatching of a human blastocyst in vitro forming two identical embryos Fertil Steril 2003. 80 1502– 1503 [DOI] [PubMed] [Google Scholar]
- Milki AA, Jun SH, Hinckley MD, Behr B, Giudice LC, Westphal LM. Incidence of monozygotic twinning with blastocyst transfer compared to cleavage-stage transfer Fertil Steril 2003. 79 503– 506 [DOI] [PubMed] [Google Scholar]
- Aston KI, Peterson CM, Carrell DT. Monozygotic twinning associated with assisted reproductive technologies: a review Reproduction 2008. 136 377– 386 [DOI] [PubMed] [Google Scholar]
- Sharara FI, Abdo G. Incidence of monozygotic twins in blastocyst and cleavage stage assisted reproductive technology cycles Fertil Steril 2010. 93 642– 645 [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Fatemi H, Venetis C, Donoso P, Kolibianakis E, Tournaye H, Tarlatzis B, Devroey P. Monozygotic twinning is not increased after single blastocyst transfer compared with single cleavage-stage embryo transfer Fertil Steril 2010. 93 592– 597 [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization Nat Rev Mol Cell Biol 2008. 9 846– 859 [DOI] [PubMed] [Google Scholar]
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity Cytoskeleton 2010. 67 545– 554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells Cell Stem Cell 2010. 7 240– 248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells Cell Stem Cell 2010. 7 225– 239 [DOI] [PubMed] [Google Scholar]
- Clayton L, Hall A, Johnson MH. A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres Dev Biol 1999. 205 322– 331 [DOI] [PubMed] [Google Scholar]
- Kawagishi R, Tahara M, Sawada K, Ikebuchi Y, Morishige K, Sakata M, Tasaka K, Murata Y. Rho-kinase is involved in mouse blastocyst cavity formation Biochem Biophys Res Commun 2004. 319 643– 648 [DOI] [PubMed] [Google Scholar]
- Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene Cancer Cell 2011. 19 470– 483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo Development 2009. 136 3215– 3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vent J, Wyatt TA, Smith DD, Banerjee A, Luduena RF, Sisson JH, Hallworth R. Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating J Cell Sci 2005. 118 4333– 4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon VB, Marikawa Y. Spatial alignment of the mouse blastocyst axis across the first cleavage plane is caused by mechanical constraint rather than developmental bias among blastomeres Mol Reprod Dev 2008. 75 1143– 1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manejwala FM, Cragoe EJ, Jr,, Schultz RM. Blastocoel expansion in the preimplantation mouse embryo: role of extracellular sodium and chloride and possible apical routes of their entry Dev Biol 1989. 133 210– 220 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed New York: Cold Spring Harbor Laboratory Press; 2003. 252– 260, 268,– 271, 501– 502 [Google Scholar]
- Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa Proc Natl Acad Sci USA 2001. 98 13501– 13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb N, Archer TK, Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PO, Wong HK, Oyama F, Goswami A, Okuno M, Kino Y, Miyazaki H, Nukina N. Inhibition of Rho kinases enhances the degradation of mutant huntingtin J Biol Chem 2009. 284 13153– 13164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock NA, Harrison B, Mixon T, Yu XQ, Wilson A, Gerhardt B, Eberhart DE, Abuin A, Rice DS. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK J Ocul Pharmacol Ther 2009. 25 187– 194 [DOI] [PubMed] [Google Scholar]
- Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D'Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis FASEB J 2010. 24 3186– 3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SY, Faux CH, Turbic A, Dixon KJ, Turnley AM. The Rho kinase pathway regulates mouse adult neural precursor cell migration Stem Cells 2011. 29 332– 343 [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Saga Y, Naito K, Inoue H, Seto A. Specific gene silencing in the pre-implantation stage mouse embryo by an siRNA expression vector system Mol Reprod Dev 2004. 68 17– 24 [DOI] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, Ohno S, Marikawa Y, et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos Curr Biol 2013. 23 1181– 1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research Nat Rev Genet 2006. 7 319– 327 [DOI] [PubMed] [Google Scholar]
- Yu J, Thomson JA. Pluripotent stem cell lines Genes Dev 2008. 22 1987– 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, LaMonica K, Hong T, Pagliaruli T, Mulrooney J, Grabel L. Roles for Rho/ROCK and vinculin in parietal endoderm migration Cell Commun Adhes 2005. 12 9– 22 [DOI] [PubMed] [Google Scholar]
- Kamijo H, Matsumura Y, Thumkeo D, Koike S, Masu M, Shimizu Y, Ishizaki T, Narumiya S. Impaired vascular remodeling in the yolk sac of embryos deficient in ROCK-I and ROCK-II Genes Cells 2011. 16 1012– 1021 [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. New York: Academic Press. 1999:26. [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death Mol Cell Biol 2003. 23 5043– 5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles J Cell Biol 2005. 168 941– 953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape Fertil Steril 2001. 76 1157– 1167 [DOI] [PubMed] [Google Scholar]
- Urman B, Yakin K, Ata B, Balaban B. How can we improve current blastocyst grading systems? Curr Opin Obstet Gynecol 2007. 19 273– 278 [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer Fertil Steril 2000. 73 1155– 1158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.