ABSTRACT
Endometriosis is characterized by the presence of endometrial glands and stroma in extrauterine sites. Our objective was to determine whether endometriotic lesions (ELs) from women with endometriosis have altered retinoid levels compared with their eutopic endometrium, and to test the hypothesis that defects in all-trans retinoic acid (ATRA) biosynthesis in EL is related to reduced expression of cellular retinol-binding protein type 1 (RBP1). Retinoids were evaluated by liquid chromatography-tandem mass spectrometry and high-performance liquid chromatography in eutopic endometrial biopsies (EBs) and ELs from 42 patients with pathologically confirmed endometriosis. The ATRA levels were reduced, whereas the retinol and retinyl ester concentrations were elevated in EL compared with EB tissue. Similar results were found in a mouse model of endometriosis that used green fluorescent protein-positive endometrial tissue injected into the peritoneum of syngeneic hosts to mimic retrograde menses. The ATRA biosynthesis in vitro in retinol-treated primary human endometrial stromal cell (ESC) cultures derived from ELs was reduced compared with that of ESCs derived from patient-matched EBs. Correspondingly, RBP1 expression was reduced in tissue and ESCs derived from EL versus EB. Rbp1−/− mice showed reduced endometrial ATRA concentrations compared with wild type, associated with loss of tissue organization and hypercellularity. These findings provide the first quantitative measurements of ATRA in human endometrium and endometriosis, demonstrating reduced ATRA in ectopic tissue and corresponding ESC cultures. Quantitation of retinoids in murine endometriosis and in Rbp1−/− mice supports the contention that impaired ATRA synthesis caused by reduced RBP1 promotes an “endometriosis phenotype” that enables cells to implant and grow at ectopic sites.
Keywords: endometriosis, RBP1, retinoic acid
INTRODUCTION
Endometriosis is an enigmatic disease in which endometrial cells translocate outside of the uterine cavity. This disease affects more than 8 million women in North America alone and gives rise to a variety of symptoms, the most common being chronic pelvic pain and infertility [1, 2]. Although endometriosis is not considered a premalignant condition, it has mixed traits of benign disease and malignancy; its pathogenesis involves loss of control of cell proliferation and is associated with local invasion and distant metastasis. It is well known that normal endometrium is responsive to physiologic fluctuations of steroid hormones with highly orchestrated proliferative, secretory, and inflammatory changes [3, 4]. However, in cases of endometriosis, numerous reports have demonstrated aberrant hormonal regulation of the eutopic (intrauterine) endometrial tissue as well as the ectopic implants [5, 6]. In addition to regulation by steroid hormones, studies have shown that retinoids also play fundamental roles in the normal maintenance of the endometrium [7–9]. To this end, the action of all-trans retinoic acid (ATRA) produced from metabolic conversion of retinol (ROL) has long been recognized as being necessary for normal endometrial cell differentiation and function [10, 11]. This activity is mediated by the expression of nuclear and cytoplasmic retinoid receptors and localized ATRA synthesis in endometrial stromal cells (ESCs) [12, 13]. During the human menstrual cycle, expression of retinoid receptors and synthesis of ATRA are influenced by the changing patterns of steroid exposure. Among the numerous aspects of endometrial behavior regulated by local ATRA production are matrix metalloproteinase (MMP) secretion, gap junctional intracellular communication, and the expression of a variety of cytokines involved in stromal cell growth, adhesion, and differentiation [14, 15]. Some examples of ATRA-regulated genes are IL-6, MCP-1, TNFα, VEGF, connexin43, various integrins, and fas ligand [15–19], genes which are also known to be aberrantly expressed in endometriotic lesions (ELs) [20]. Thus, a number of seemingly discordant features of endometriosis, including decreased cell death, increased growth and migration, inflammation, and enhanced invasive properties of intraperitoneally seeded endometrial cells, could be accounted for by dysregulation of ATRA synthesis. This contention was recently supported in a mouse model of endometriosis where treatment with ATRA suppressed the establishment and growth of peritoneal implants, promoted macrophage differentiation, and inhibited peritoneal fluid accumulation of IL-6 and MCP-1 [21]. Together with previous reports [16, 22, 23], these studies suggest that defective retinoid metabolism and ATRA production in intraperitoneally seeded endometrial cells play a role in the pathophysiology of endometriosis. However, this contention has not been confirmed, because direct quantitation of ATRA in this tissue has heretofore not been reported.
Cellular retinol-binding protein type 1 (RBP1), encoded by RBP1, is an ROL chaperone protein, and RBP1-bound ROL is the preferred substrate for ROL dehydrogenase, the rate-limiting enzyme in ATRA biosynthesis [24]. Reduced RBP1 results in significantly less efficient metabolism of ROL to retinal and subsequent oxidation of retinal to ATRA. Thus, because of the regulatory influence of RBP1 on ATRA production, loss of RBP1 consequentially results in defective ATRA biosynthesis and signaling in a variety of tissues [24, 25]. RBP1 and other genes involved in ROL uptake and metabolism have been shown to be aberrantly expressed in endometriosis [22, 23], as well as in various cancers [25–27] and some developmental diseases of the brain, bone, and skin [24, 28]. These studies, along with the demonstrated ability of ATRA to regulate endometrial cell differentiation and proliferation, suggest that defects in RBP1 gene expression result in abnormal retinoid biosynthesis and may play a role in the etiology and/or progression of endometriosis. To further address this question, we directly measured ATRA levels and RBP1 expression in endometrial tissue and lesions from patients with endometriosis. In addition, we have used animal model systems of endometriosis and RBP1 deficiency to support conclusions reached using these human tissues.
MATERIALS AND METHODS
Human Endometrial Tissue and Cell Cultures
Tissues were obtained from patients (n = 42) undergoing surgery for infertility or pelvic pain, according to protocols approved by the Institutional Review Boards of the Emory University School of Medicine and Northside Hospital (Atlanta, GA). Written informed consent was obtained from all participants prior to surgery. Women who had been on estrogen- or progesterone-containing medications or other forms of pituitary suppression in the previous 3 mo were excluded. At surgery (laparoscopy or laparotomy), a full visual inspection of the pelvic cavity was performed by senior gynecologic surgeons with extensive experience in the recognition and treatment of typical and atypical ELs [29]. Following surgery, women were classified as having endometriosis and entered into the study if their surgeon noted laparoscopic evidence of ELs that were histopathologically confirmed to contain endometrial-type glands and stroma. The stage of endometriosis (I–IV) was classified according to the Revised American Society for Reproduction Medicine classification of endometriosis, 1996 [30]. The phase of the menstrual cycle was determined by histological evaluation of the patient's endometrium by senior pathologists.
Biopsies of eutopic endometrium and endometriosis implants were flash frozen at −80°C for extraction and quantitation of retinoids. In some cases, fresh tissue was used to isolate endometrial biopsy (EB) and EL cells. Separation of epithelial and stromal cells from either eutopic or ectopic endometrial tissue was performed using the procedure originally developed by Ryan et al. [31] and Hornung et al. [32]. Primary ESC cultures were prepared from biopsies of the eutopic endometrium (ESC-B) or ectopic lesions (ESC-L) according to our published procedures [16]. All cultures were grown in complete medium: Dulbecco modified Eagle medium/F12 (Cellgro) containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 1 mM HEPES. For treatment, cultures were washed with PBS and then cultured in serum-free medium in the presence of 2 μM ROL (Sigma Chemical Co.) or solvent control (dimethyl sulfoxide) for 16 h. The final concentration of dimethyl sulfoxide as solvent was always less than 0.1%.
Mouse Model of Endometriosis
The immunocompetent mouse endometriosis model used in these experiments has been described previously [21]. This study was approved by the Emory Institutional Animal Care and Use Committee and performed according to the National Institutes for Health Guidelines for the Care and Use of Laboratory Animals. Briefly, endometrial tissue from 2- to 4-mo-old C57BL/6-Tg (Tg-GFP) donor mice, which express enhanced green fluorescent protein (GFP) under the direction of the human ubiquitin C promoter, was minced and injected into the peritoneal cavity of syngeneic recipient mice as described previously [21]. The mice were purchased from Jackson Laboratories and fed a chow diet ad libitum (Harlan Teklad Global 18% protein-extruded rodent diet no. 2018SX with the equivalent of 30 IU/g vitamin A). The Tg-GFP donor mice were treated s.c. with 100 μg/kg estradiol valerate (dissolved in corn oil) 1 wk before being killed, in order to stimulate proliferation of their endometrial tissue for transplantation. Recipient mice also received 100 μg/kg per week estradiol valerate s.c. in corn oil each week, starting 1 wk before i.p. inoculation of endometrial tissue, to synchronize their estrus cycles. Two to three weeks after uterine fragment inoculation, we killed the recipient mice and examined their peritoneal cavity under 488-nm light (excitation wavelength peak of GFP) by use of interference-filter eyeglasses to observe the GFP+ endometrial implants (emission wavelength peak: 502 nm). All implants, including those on visceral organs (e.g., large bowel, ovaries), were then carefully excised and processed for retinoid determination. As in our previous study, we confirmed the authenticity of the implants by verifying the histopathologic criteria for endometriosis [21].
Rbp1−/− Mice
Rbp1 global knockout mice (Rbp1−/−) on a C57BL/6 background [33] and wild-type (WT; C57BL/6) mice aged 2–4 mo were used according to institutional guidelines. The Rbp1−/− mice were bred in-house from breeders obtained from Pierre Chambon and Norbert Ghyselinck. The fertility of these mice was comparable to that of WT mice in terms of age of first litter, frequency of litters during their breeding course, number of pups per litter, ratio of sexes born per litter, and pup and adult mouse weight. Female WT or Rbp1−/− mice were treated s.c. with 100 μg/kg estradiol valerate (dissolved in corn oil) each week for 2 wk before being killed, in order to stimulate proliferation of their endometrial tissue. After this time period, whole uterus, endometrium, and myometrium tissues were dissected from the uteri and immediately flash frozen and stored at −80°C until retinoid analysis. For histological analysis, uteri from 2-mo-old wild-type and Rbp1−/− mice were preserved in formalin upon collection until tissue was embedded in paraffin, sliced, and stained by the University of Maryland Core Facility for Histology. Hematoxylin-eosin (HE) and Masson trichrome (MTC) stain images were captured using an EVOS XL digital microscope (AMG).
Determination of Retinoid Levels
Biopsy specimens, cultured cell pellets, or culture supernatants were prepared for retinoid analysis under yellow lights. Processing and extraction of samples have been described in detail previously [34–36]. Total protein concentrations were determined using the Bradford method (Bio-Rad Laboratories). Retinoic acid was quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with atmospheric pressure chemical ionization in positive-ion mode on an API-4000 or 5500 Qtrap instrument (AB Sciex). Retinol and total retinyl ester (RE) were quantified by high-performance liquid chromatography with ultraviolet detection (HPLC-UV) on an Alliance 2690 or Aquity H-Class apparatus (Waters).
Evaluation of RBP1 mRNA by Quantitative Real-Time RT-PCR
Total RNA from patient tissue or cultured ESCs was extracted using TRIzol Reagent (Invitrogen) following the manufacturer's protocol. For RBP1 mRNA quantitation, RT was used to synthesize cDNA from RNA template as described previously [37]. For real-time PCR, a total of 20 μl of reaction mix was prepared using iQSYBR Green Supermix (Bio-Rad) and specific primer sets (0.3 μM each). Primer sequences used were as follows: RBP1, sense (5′-AATGTGGCCTTGCGCAAAAT-3′) and antisense (5′-CAGCTCATCACCCTCGATCC-3′); and RPL17, sense (5′-TGAACAAAGCACCTAAGATGCGCC-3′) and antisense (5′-TGGGCAACCTCCTCTTCTGGTTTA-3′. The PCR was set for 40 cycles in an Opticon 2 real-time thermocycler (Bio-Rad) under the following conditions: one denaturation cycle of 95°C for 30 sec followed by amplification cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. The data were analyzed after normalization with RPL17 mRNA levels using the formula 2ΔΔCT, where ct is the cycle threshold.
Statistical Analysis
Statistical analysis was performed using GraphPad Software. Data are presented as mean ± SEM. Differences between groups as indicated were analyzed by t-test (two-tailed), where P < 0.05 was considered statistically significant. Each experiment was replicated a minimum of three times.
RESULTS
Endogenous ATRA Is Reduced in Human Endometriotic Lesions
Direct quantitation was performed on human eutopic EBs and ectopic ELs after tissue extraction using LC-MS/MS and HPLC-UV (Fig. 1). Mean ATRA levels in ELs were 49% lower than those detected in EBs (P < 0.001; Fig. 1A). Retinoic acid isomers without known biological activity—13-cis and 9,13-di-cis RA—were also detected, but they did not differ based on the source. No other RA isomers, including 9-cis RA, were detected above the LC-MS/MS assay limit of detection in biological matrices (∼12 pmol/g protein). Retinol and retinyl esters (REs) were both >2-fold higher in ELs compared with EBs (P < 0.0001; Fig. 1, B and C). Patients were subgrouped according to minimal/mild (I–II) and moderate/severe (III–IV) stages of endometriosis or menstrual cycle phase (proliferative or secretory). Results showed that mean ATRA levels were significantly reduced in EL versus EB (Table 1), whereas ROL and RE were correspondingly higher in EL compared with EB (data not shown) in all subgroups. When comparing the concentrations of these retinoids in the ectopic and eutopic tissues from the same patient (matched samples), ATRA levels were lower in EL versus EB, whereas ROL and RE were higher in EL versus EB in most cases (Supplemental Fig. S1; Supplemental Data are available online at www.biolreprod.org).
FIG. 1.
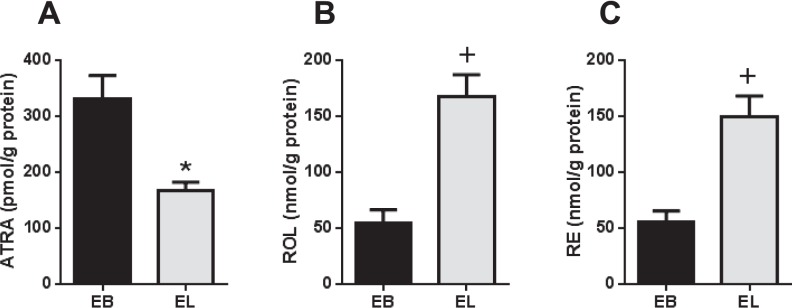
Altered retinoid levels in ectopic endometrial implants compared with eutopic endometrium. Direct quantitation of ATRA (A), ROL (B), and RE (C) levels was performed on extracted human eutopic EBs and ELs from patients with endometriosis (n = 42). Values represent mean ± SEM of retinoid concentrations. *P < 0.005; +P < 0.0001 when compared with EB.
TABLE 1.
Comparison of ATRA levels in eutopic versus ectopic endometrial tissue grouped according to stage of endometriosis or phase of menstrual cycle.
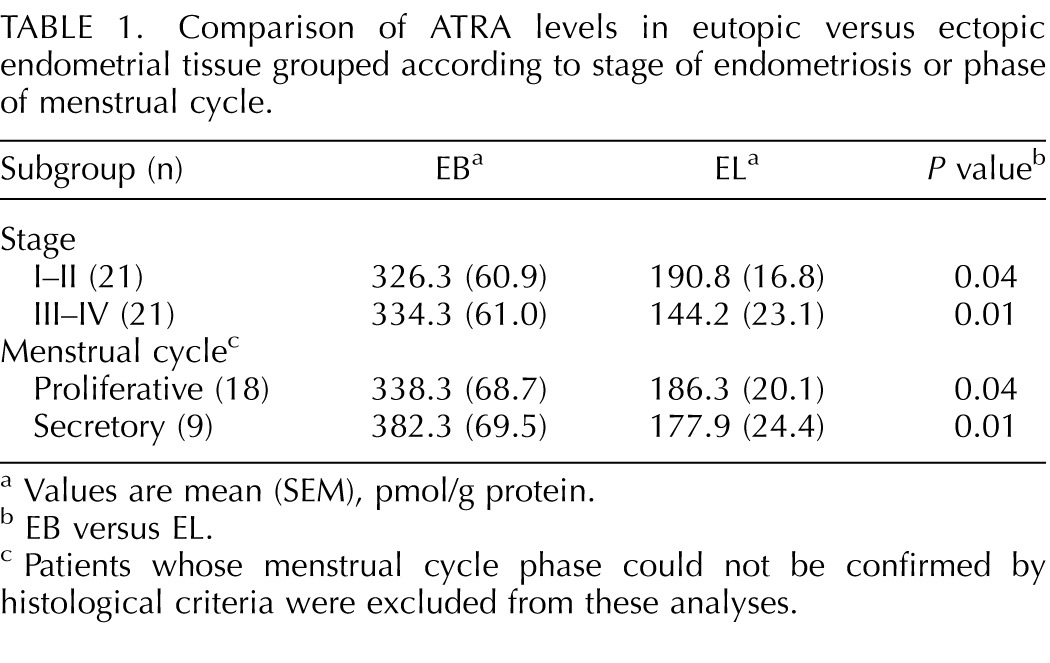
Values are mean (SEM), pmol/g protein.
EB versus EL.
Patients whose menstrual cycle phase could not be confirmed by histological criteria were excluded from these analyses.
Reduced ATRA Production and RBP1 Expression in ELs
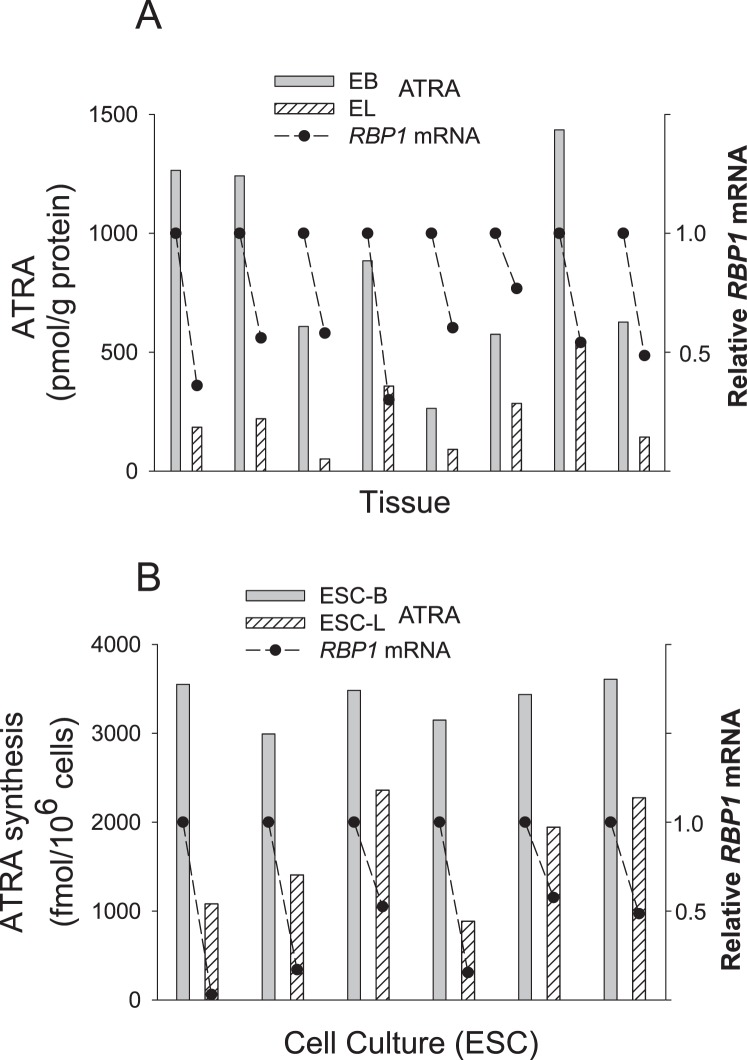
We compared RBP1 mRNA expression in EB versus EL from eight patients with endometriosis, as well as the possible association of this expression with ATRA production in the tissue and in cultured ESCs derived from EBs and ELs. Figure 2A shows reduced levels of ATRA of >50% in EL versus EB that was associated in all cases with a reduction of RBP1 mRNA expression. Correspondingly, ESC-Ls showed a reduction in RBP1 expression and an impaired ability to synthesize ATRA from ROL compared with ESC-Bs (Fig. 2B).
FIG. 2.
All-trans retinoic acid production and RBP1 gene expression are reduced in human endometriotic lesions. A) Relative RBP1 mRNA levels (•) and ATRA concentrations (bars) in matched eutopic EB and EL tissue from eight patients with endometriosis. B) RBP1 mRNA levels (•) and ATRA production (bars) in cultured ESCs derived from ELs (ESC-L) relative to those derived from patient-matched EBs (ESC-B). Total ATRA production in the cultures was assessed by LC-MS/MS analysis of ATRA in cell pellets plus supernatant following addition of 2 μM ROL for 16 h. Results were normalized to 106 cells. In the absence of ROL, ATRA values were <27 fmol per 106 cells in all cases.
Endogenous ATRA Is Lower in Induced Murine ELs
Previous work demonstrated the ability of ATRA to inhibit EL development in the immunocompetent mouse model of endometriosis [21]. Having found that ATRA levels were reduced in EL versus EB in humans, we questioned whether ELs induced in our mouse model also showed a reduction in ATRA compared with eutopic endometrial tissue. Our previous studies showed that 100% of the animals develop prominent peritoneal lesions in this time period, with many lesions containing well-formed vasculature, characteristic of invasive ELs in humans. We then compared retinoid levels in the lesions with those in the eutopic tissue from which the lesions were derived (i.e., the endometrium of the GFP+ donor mice). Figure 3A shows a 37% reduction in mean ATRA levels in the mouse lesions compared with eutopic endometrium. Detection by HPLC-UV indicated that ROL was 40% higher in the lesions, but no significant changes in RE were noted (Fig. 3, B and C). There was no significant difference between ATRA levels in endometrial tissue from the GFP+ donor mice and that found in the eutopic endometrium of WT recipient mice in which the lesions developed (data not shown).
FIG. 3.
Levels of ATRA are lower in induced endometriotic mouse lesions. Quantitation of ATRA (A), ROL (B), and RE (C) was performed on peritoneal implants that developed in recipient mice 14–21 days after challenge with uterine fragments from GFP+ syngeneic donors. Results are compared with retinoid levels in the original GFP+ tissue inoculum. For all data sets, n = 8; values represent mean ± SEM. *P < 0.05.
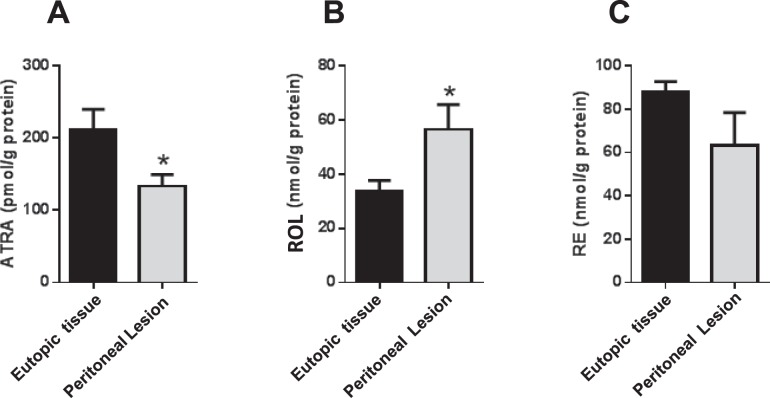
Endogenous ATRA Is Reduced in Rbp1−/− Mouse Endometrium
Loss of RBP1 has been associated with defective retinoid metabolism and ATRA synthesis in a variety of human cancers [25–27]. This fact, along with our finding of a similar association of low RBP1 expression and ATRA levels in clinical endometriotic implants, supports the contention that reduced expression of RBP1 in this tissue plays a causative role leading to reduced ATRA production. To test this hypothesis, we quantified retinoid levels in the dissected endometrial tissue of a previously established Rbp1 global knockout (Rbp1−/−) mouse model [33]. As shown in Figure 4A, endogenous ATRA levels in the endometrium of Rbp1−/− mice were reduced by 37% compared with WT. Quantitation of endogenous ROL and RE in the Rbp1−/− endometrium showed an increase of 42% and no change, respectively (Fig. 4, B and C). Myometrium and whole uterus were also analyzed, with data shown in Supplemental Figure S2. In both myometrium and uterus, ATRA was not changed, whereas ROL and RE were both significantly elevated.
FIG. 4.
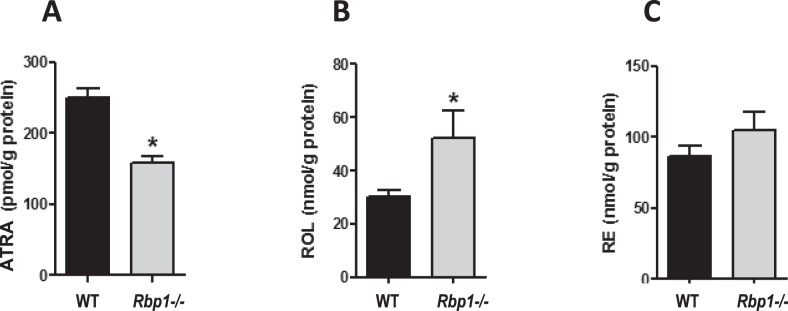
Reduced ATRA in Rbp1−/− endometrium. Quantitation of ATRA (A), ROL (B), and RE (C) was performed on the dissected endometrium of Rbp−/− mice and compared to WT endometrium. For all data sets, n = 15; values represent mean ± SEM. *P < 0.005.
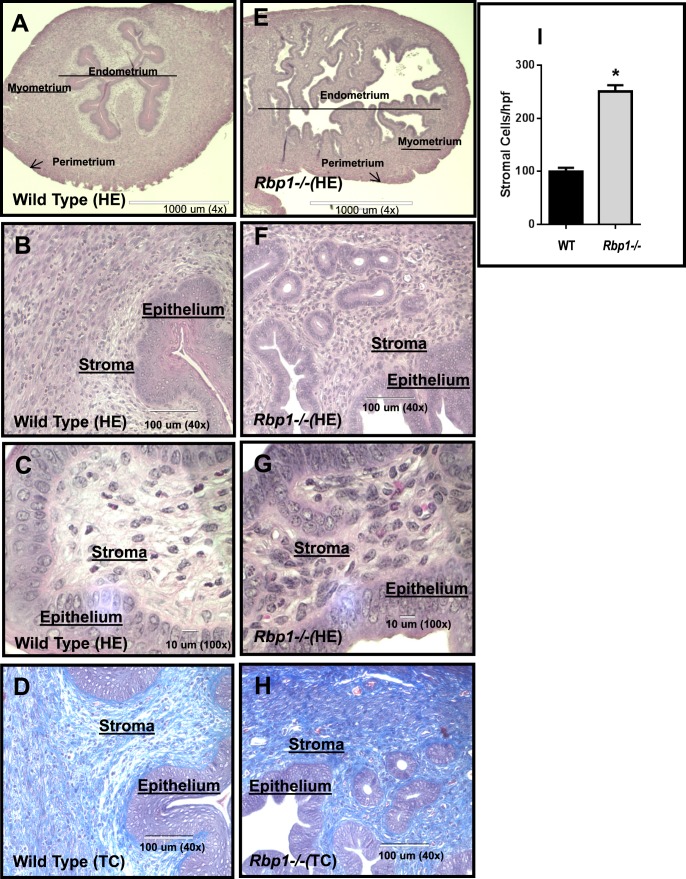
Rbp1−/− Uterus Histology Exhibits Loss of Tissue Organization and Hypercellularity
Because ATRA biosynthesis was found to be deficient in Rbp1−/− mouse endometrium, we analyzed tissue histology to determine whether the morphology of the Rbp1−/− uterus and/or endometrium was altered. For this analysis, HE and MTC stains were applied to evaluate cellular and extracellular matrix morphology of the endometrium, respectively (Fig. 5). In WT uterus, the endometrial/myometrial layers of the uterus were clearly defined and organized (Fig. 5A). However, the uteri of Rbp1−/− were seen to lack the clear separation of the endometrium and myometrium while displaying numerous regions of hypercellularity. Higher magnification also indicated a loss of order in the epithelial layer of the Rbp1−/− endometrium as well as hypercellularity of the stroma. Stromal cell counts provided statistical evidence to confirm hypercellularity in Rbp1−/− endometrium (Fig. 5I). In addition, MTC staining indicated excess collagen accumulation in the stroma of Rbp1−/− uteri (Fig. 5, D and H).
FIG. 5.
Morphological disorganization and hypercellularity of Rbp1−/− endometrium. C57BL/6 WT (A–D) and Rbp1−/− uterus (E–H) were stained with HE (A–C, E–G) or MTC (D and H). Low-power magnification (×4) shows a distinct endometrial-myometrial interface (lighter-darker areas, respectively) in the uterus of WT mice (A). In contrast, Rbp1−/− uterus lacks a clear delineation of these regions (E). Higher-power magnifications B and F (×40) and C and G (×100) show hypercellularity of both epithelial and stromal layers of the endometrium (F and G) compared with the WT (B and C). The blue intensity in the MTC-stained images (×40) indicates excess stromal collagen accumulation in the Rbp1−/− uterus (H) compared with the WT (D). I) Mean (±SEM) stromal cell counts of the endometrium per high-power field (hpf) at ×100 with n = 10 total fields from three mice in each group. *P < 0.0001.
DISCUSSION
Originally proposed by Sampson [38] in the 1920s, retrograde menstruation is the most accepted theory to explain the migration of endometrial tissue outside the uterine cavity. This occurs in most women throughout their reproductive years [39], but the reason that only ∼10% of women develop endometriosis is not known. Investigators from a number of laboratories have demonstrated the presence of retinoid-binding proteins and metabolic enzymes in endometrial tissue and have established that synthesis of ATRA is essential for normal cellular differentiation and function [7–9, 40]. To this end, studies have shown that the ability of endometrial stromal cells to synthesize ATRA from ROL correlates well with their degree of decidualization, and that most of the requirement for ATRA in this process is met by local synthesis [13]. This biosynthesis of ATRA is mediated by two sequential oxidations, first producing retinal from ROL by ROL dehydrogenases using RBP1-ROL complexes as the preferred substrate. Retinal is then oxidized to ATRA by retinal dehydrogenases of the aldehyde dehydrogenase family [24]. Although numerous reports have elaborated on altered hormone responses (progesterone and estrogen) within ectopic endometrial implants compared with eutopic tissue [3–6], few studies have investigated potential retinoid metabolic defects in these lesions. Pavone and colleagues [22, 23] showed altered expression of genes involved in retinoid signaling, including RBP1, in lesion cells from endometriosis patients compared with normal eutopic endometrium from patients without endometriosis. Although their study did not measure ATRA levels directly, the results were consistent with decreased retinoid uptake, metabolism, and action. In contrast, the present work uses matched samples from patients with endometriosis (i.e., lesions vs. eutopic tissue from the same person) and has directly quantified ATRA levels and metabolic conversion of ROL to ATRA in ESCs, the primary source of ATRA biosynthesis in endometrial tissue [22]. Our results demonstrate reduced ATRA levels in EL versus EB from patients with endometriosis on a population basis (Fig. 1) and in a matched sample comparison from the majority of patients (Supplemental Fig. S1). With regard to the latter, it is noted that there was a high degree of variability among the 42 specimens with respect to relative ATRA levels in EB versus EL. This heterogeneity is comparable to that seen in certain cancers, such as breast cancer, in which reduced RBP1 levels result in varying degrees of ATRA deficiency (Pierzchalski and Kane, unpublished results). In addition, a source of variability in our determination of ATRA levels in EB is unavoidable sampling artifacts inherent in eutopic EB collection (i.e., varying amounts of blood and mucus collected with the biopsy samples), as reported previously [41, 42]. In contrast to ATRA, relative tissue concentrations of ROL and RE were reversed, such that EL showed higher concentrations of these retinoids than EB. This finding of elevated ROL in EL is consistent with a “roadblock” in metabolic conversion of ROL to retinal and is supported by our data demonstrating reduced RBP1 expression in EL and in ESCs derived from this tissue (Fig. 2). These results also support the contention that dysfunctional retinoid biosynthesis is responsible for the suppressed ATRA levels in EL, rather than reduced ROL transport or uptake into ectopic sites. Impaired ATRA synthesis was directly confirmed by treating primary ESC cultures with ROL; results showed a significant reduction of ATRA production in lesion-derived cultures compared with those derived from patient-matched eutopic tissue. Reduced ATRA biosynthesis in ESC-L cultures was associated with a significant decrease in mRNA levels of RBP1. Importantly, reduced ATRA levels were also found in peritoneal lesions that developed in a mouse model of endometriosis in which recipient mice were inoculated i.p. with syngeneic endometrial tissue to mimic a massive retrograde menses (Fig. 3). As in the human samples, the mouse endometriotic implants showed an increase in ROL concentration consistent with a decrease in ATRA biosynthesis from ROL. These human and animal data suggest two possible scenarios to account for aberrant retinoid metabolism in ectopically growing endometrial cells: 1) among the cells that reach the peritoneal cavity via retrograde menstruation, those with intrinsically defective ATRA synthesis preferentially populate the ectopic sites because of downstream effects of reduced ATRA levels (e.g., increased MMP and proinflammatory cytokine synthesis); or 2) the peritoneal milieu provides environmental cues that induce defects in ATRA synthesis in some shed endometrial cells, as opposed to impaired retinoid metabolism being an intrinsic property of the cells. Evidence from studies showing alterations in cytokine and MMP profiles of eutopic endometrium from some women with endometriosis support the former possibility [42, 43]. However, support for the latter possibility comes from the observation that oxidative stress, known to be elevated in peritoneal fluid from patients with endometriosis [44], can inhibit biosynthesis of ATRA [25] and induce transcriptional repression of RBP1 [45]. It has also been reported that cellular retinoic acid-binding protein 2 (CRABP2), which delivers ATRA to retinoic acid receptor-alpha (RARA) [46], is reduced in endometriosis, potentially as a consequence of defective progesterone responses [22, 23]. Whereas it is unknown whether CRABP2 loss precedes RBP1 reduction, it is likely significant to the persistence of reduced RBP1 expression, because loss of CRABP2 function can result in heritable chromatin repression of multiple loci downstream of RARA, including RBP1 [45].
Our animal studies demonstrated that eutopic endometrial cells from normal healthy donors can implant and grow at ectopic sites following i.p. injection into ostensibly “normal” recipients [21]. Extrapolated to humans, this finding suggests that the endometrium in all women inherently contains cells capable of forming ectopic lesions. As such, the predisposition to develop endometriosis may depend on the frequency of such cells within the eutopic population and/or the mass of cells that are shed into the peritoneal cavity during menses. Support for the latter hypothesis is provided by reports indicating that women with “heavy” menstrual cycles and those with obstructed menstrual outflow resulting in increased menstrual reflux are at increased risk for developing endometriosis [47].
Consistent with the associative relationship in human tissue and cells (Fig. 2), a direct causal relationship between reduced RBP1 expression and depleted ATRA levels in endometrial tissue was established by analysis of Rbp1−/− mice. Results demonstrated a similar reduction of ATRA and an increase of ROL in the endometrium of the Rbp1−/− mice compared with WT animals. The myometrium and whole uteri were also evaluated in this model, showing significantly elevated ROL and RE but no differences in ATRA between Rbp1−/− and WT (Supplemental Fig. S2). These analyses show that a primary role for RBP1 in the biosynthesis of ATRA has tissue specificity. Based on a comparison of absolute retinoid levels in the endometrium (Fig. 4) versus the myometrium (Supplemental Fig. S2), the former appears to be the primary source of ATRA production in the uterus in the WT animals. To address the question of whether reduced RBP1 and ATRA production plays a causative role in inducing phenotypic and functional changes in endometrial tissue consistent with endometriotic implants, histochemical comparisons of the uteri of Rbp1−/− versus wild-type mice were performed. Hematoxylin-eosin-stained tissues showed an overall lack of tissue organization in the Rbp1−/− mice, as well as numerous regions of hypercellularity. There was a loss of definitive borders between endometrium and myometrium, and stromal hypercellularity was observed in Rbp1−/− endometrium. In addition, in comparison with WT, the Rbp1−/− endometrial stroma showed gross accumulation of collagenous extracellular matrix. Fibrosis and collagen deposition around clinical endometriosis implants is well established, and upregulation of Fas ligand by extracellular matrix has been hypothesized to promote lesion survival [48]. Despite these alterations in uterine morphology, the fertility of the Rbp1−/− mice was seen to be comparable to that of WT. Previous studies have demonstrated that although ATRA is decreased in Rbp1−/− embryos and fetuses, they do not exhibit the abnormalities characteristic of a vitamin A-deficiency syndrome [49]. These authors concluded that although RBP1 is involved in ATRA homeostasis, it is not critically required for fetal development, at least under conditions of dietary vitamin A sufficiency. Because the fertility of Rbp1−/− mice does not appear to be impaired, we conclude that the altered uterine features that we observed in these mice (Fig. 5) do not interfere with functions related to embryo implantation and early placentation, as well as later development of the fetus. Thus, although reduced levels of RBP1 and ATRA biosynthesis appear to play a role in the ability of endometrial cells to implant and grow at ectopic sites, these deficiencies per se may not be directly involved in the subfertility of patients with endometriosis. This conclusion is consistent with the long-standing hypothesis that endometriosis-associated infertility may be due to the influence of certain immunological factors (e.g., increased peritoneal cytokine levels) triggered by endometriotic lesion development [50].
In summary, these studies provide the first direct quantitative measurements of ATRA levels in human endometrium and have demonstrated reduced ATRA concentrations in ectopic versus eutopic tissue and in corresponding ESC cultures. Our findings showed a direct association between ATRA biosynthesis and RBP1 expression. Quantitation of retinoids in a mouse model of endometriosis and in Rbp1−/− endometrium supports the contention that impaired ATRA synthesis caused by reduced RBP1 promotes an “endometriosis phenotype” that is able to implant and grow at ectopic sites. Future studies using GFP+ Rbp1−/− mice as donors of endometrial cells in our transplant animal model will directly address questions related to the loss of RBP1 and the attachment, invasion, and growth of endometriotic lesions. Although it is unlikely that ATRA itself can be used as a drug to treat endometriosis, the present findings may lead to a new appreciation of the cause(s) of endometriosis, and the search for new diagnostic and treatment modalities that target the retinoid metabolic pathway.
ACKNOWLEDGMENT
The authors are grateful to Karalaine Barrett, RN, for her tireless efforts in specimen collection. We thank Pierre Chambon and Norbert Ghyselinck (Institut de Genetique et de Biologie Moleculaire et Cellulaire, Institut National de la Santé et de la Recherche Médicale, Illkirch, France) for the Rbp1−/− mice.
Footnotes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health grants R01HD55379 and U01HD66439 to N.S., National Institute of Allergy and Infectious Diseases contract HHSN272202000046C to M.A.K., the University of Maryland School of Pharmacy Mass Spectrometry Center grant SOP1841-IQB2014 to M.A.K., and National Institutes of Health grants DK090522 and AA017927 to J.L.N.
These authors are co-senior authors.
REFERENCES
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Littman ED, Lathi RB, Berker B, Westphal LM, Giudice LC, Milki AA. The dilemma of endometriosis: is consensus possible with an enigma? Fertil Steril. 2005;84:1587–1588. doi: 10.1016/j.fertnstert.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. doi: 10.1016/0002-9378(92)91706-g. [DOI] [PubMed] [Google Scholar]
- Dizerega GS, Barber DL, Hodgen GD. Endometriosis: role of ovarian steroids in initiation, maintenance, and suppression. Fertil Steril. 1980;33:649–653. doi: 10.1016/s0015-0282(16)44780-1. [DOI] [PubMed] [Google Scholar]
- Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19:406–418. doi: 10.1093/humupd/dmt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Cao S, Wang X, Feng Y, Billig H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6:104–113. [PMC free article] [PubMed] [Google Scholar]
- Kumarendran MK, Loughney AD, Prentice A, Thomas EJ, Redfern CP. Nuclear retinoid receptor expression in normal human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1996;2:123–129. doi: 10.1093/molehr/2.2.123. [DOI] [PubMed] [Google Scholar]
- Loughney AD, Kumarendran MK, Thomas EJ, Redfern CP. Variation in the expression of cellular retinoid binding proteins in human endometrium throughout the menstrual cycle. Hum Reprod. 1995;10:1297–1304. doi: 10.1093/oxfordjournals.humrep.a136137. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Keller NR, Feltus FA, Melner MH. Paracrine regulation of matrix metalloproteinase expression in the normal human endometrium Gynecol Obstet Invest 1999. 48 (suppl 1): 2 13 [DOI] [PubMed] [Google Scholar]
- Bo WJ, Smith MS. The effect of retinol and retinoic acid on the morphology of the rat uterus. Anat Rec. 1966;156:5–9. doi: 10.1002/ar.1091560103. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Howell JM, Pitt GA. Vitamin A and reproduction in rats. Proc R Soc Lond B Biol Sci. 1964;159:510–535. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- Li XH, Kakkad B, Ong DE. Estrogen directly induces expression of retinoic acid biosynthetic enzymes, compartmentalized between the epithelium and underlying stromal cells in rat uterus. Endocrinology. 2004;145:4756–4762. doi: 10.1210/en.2004-0514. [DOI] [PubMed] [Google Scholar]
- Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE. Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology. 2000;141:802–808. doi: 10.1210/endo.141.2.7323. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- Wu J, Taylor RN, Sidell N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J Cell Physiol. 2013;228:903–910. doi: 10.1002/jcp.24241. [DOI] [PubMed] [Google Scholar]
- Sawatsri S, Desai N, Rock JA, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril. 2000;73:1012–1019. doi: 10.1016/s0015-0282(00)00483-0. [DOI] [PubMed] [Google Scholar]
- Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, Napoli JL, Taylor RN. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clin Immunol. 2006;119:272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Sago K, Teitelbaum SL, Venstrom K, Reichardt LF, Ross FP. The integrin alphavbeta5 is expressed on avian osteoclast precursors and regulated by retinoic acid. J Bone Miner Res. 1999;14:32–38. doi: 10.1359/jbmr.1999.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- Wieser F, Wu J, Shen Z, Taylor RN, Sidell N. Retinoic acid suppresses growth of lesions, inhibits peritoneal cytokine secretion, and promotes macrophage differentiation in an immunocompetent mouse model of endometriosis. Fertil Steril. 2012;97:1430–1437. doi: 10.1016/j.fertnstert.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, Bulun SE. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26:2157–2164. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95:E300–E309. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821:152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierzchalski K, Yu J, Norman V, Kane MA. CrbpI regulates mammary retinoic acid homeostasis and the mammary microenvironment. FASEB J. 2013;27:1904–1916. doi: 10.1096/fj.12-219410. [DOI] [PubMed] [Google Scholar]
- Mendoza-Rodriguez M, Arreola H, Valdivia A, Peralta R, Serna H, Villegas V, Romero P, Alvarado-Hernandez B, Paniagua L, Marrero-Rodriguez D, Meraz MA, Salcedo M. Cellular retinol binding protein 1 could be a tumor suppressor gene in cervical cancer. Int J Clin Exp Pathol. 2013;6:1817–1825. [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic D, Williams SJ, Hamilton TC. Loss of cellular retinol-binding protein 1 gene expression in microdissected human ovarian cancer. Clin Cancer Res. 2003;9:1013–1020. [PubMed] [Google Scholar]
- Xu L, Song C, Ni M, Meng F, Xie H, Li G. Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRalpha-induced beta-catenin degradation. Int J Biochem Cell Biol. 2012;44:612–619. doi: 10.1016/j.biocel.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Silfen S, Nezhat F, Martin D. Surgery for endometriosis. Curr Opin Obstet Gynecol. 1991;3:385–393. [PubMed] [Google Scholar]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997. 67 817 821 [DOI] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–369. doi: 10.1042/BJ20041867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods Mol Biol. 2010;652:1–54. doi: 10.1007/978-1-60327-325-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Greene ME, Pitts J, Wada RK, Sidell N. Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARgamma) in human neuroblastoma cells. Clin Cancer Res. 2001;7:98–104. [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Zheng WL, Ong DE. Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol Reprod. 1998;58:963–970. doi: 10.1095/biolreprod58.4.963. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Nie G, Rainzcuk A, Rombauts LJ, Salamonsen LA. Uterine lavage or aspirate: which view of the intrauterine environment? Reprod Sci. 2012;19:1125–1132. doi: 10.1177/1933719112443879. [DOI] [PubMed] [Google Scholar]
- Yu J, Boicea A, Barrett KL, James CO, Bagchi IC, Bagchi MK, Nezhat C, Sidell N, Taylor RN. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod. 2014;20:260–270. doi: 10.1093/molehr/gat087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31:109–124. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol Endocrinol. 2009;25:75–81. doi: 10.1080/09513590802485012. [DOI] [PubMed] [Google Scholar]
- Corlazzoli F, Rossetti S, Bistulfi G, Ren M, Sacchi N. Derangement of a factor upstream of RARalpha triggers the repression of a pleiotropic epigenetic network. PLoS One. 2009;4:e4305. doi: 10.1371/journal.pone.0004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhu A, Gillilan R, Noy N. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J Mol Biol. 2001;305:939–949. doi: 10.1006/jmbi.2000.4340. [DOI] [PubMed] [Google Scholar]
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Selam B, Kayisli UA, Garcia-Velasco JA, Arici A. Extracellular matrix-dependent regulation of Fas ligand expression in human endometrial stromal cells. Biol Reprod. 2002;66:1–5. doi: 10.1095/biolreprod66.1.1. [DOI] [PubMed] [Google Scholar]
- Matt N, Schmidt CK, Dupe V, Dennefeld C, Nau H, Chambon P, Mark M, Ghyselinck NB. Contribution of cellular retinol-binding protein type 1 to retinol metabolism during mouse development. Dev Dyn. 2005;233:167–176. doi: 10.1002/dvdy.20313. [DOI] [PubMed] [Google Scholar]
- Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300–315. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]