ABSTRACT
Mitochondria are home to many cellular processes, including oxidative phosphorylation and fatty acid metabolism, and in steroid-synthesizing cells, they are involved in cholesterol import and metabolism, which is the initiating step in steroidogenesis. The formation of macromolecular protein complexes aids in the regulation and efficiency of these mitochondrial functions, though because of their dynamic nature, they are hard to identify. To overcome this problem, we used Blue-Native PAGE with whole-gel mass spectrometry on isolated mitochondria from control and hormone-treated MA-10 mouse tumor Leydig cells. The presence of multiple mitochondrial protein complexes was shown. Although these were qualitatively similar under control and human chorionic gonadotropin (hCG)-stimulated conditions, quantitative differences in the components of the complexes emerged after hCG treatment. A prominent decrease was observed with proteins involved in fatty acid import into the mitochondria, implying that mitochondrial beta-oxidation is not essential for steroidogenesis. To confirm this observation, we inhibited fatty acid import utilizing the CPT1a inhibitor etomoxir, resulting in increased steroid production. Conversely, stimulation of mitochondrial beta-oxidation with metformin resulted in a dose-dependent reduction in steroidogenesis. These changes were accompanied by changes in mitochondrial respiration and in the lactic acid formed during glycolysis. Taken together, these results suggest that upon hormonal stimulation, mitochondria efficiently import cholesterol for steroid production at the expense of other lipids necessary for energy production, specifically fatty acids required for beta-oxidation.
Keywords: beta-oxidation, etomoxir, metformin, proteomics, Respiratory Bioflux Analysis, steroid biosynthesis, translocator protein
INTRODUCTION
Protein-protein interactions play many important roles in the cell, from the transmission of intracellular signals to the transfer of intermediates needed for biological functions [1, 2]. The intersection between intracellular signaling and movement of metabolites occurs in the mitochondria during steroid biosynthesis, which is the focus of our research [3–5]. Steroidogenesis in steroidogenic Leydig cells initiates at the plasma membrane by the binding of the luteinizing hormone (LH) to the G-protein-coupled LH receptor. The activation of the LH receptor results in an increase in intracellular cAMP formation, protein phosphorylation, and free cholesterol present in the cell [6]. These three alterations in these cellular activities converge on the mitochondria to increase the rate of intramitochondrial import of cholesterol, the precursor of all steroids. Cholesterol is translocated into the inner mitochondrial membrane (IMM), where it is metabolized by the cytochrome P450 side chain cleavage (CYP11A1) enzyme into pregnenolone [6].
Previous work in our laboratory, utilizing Blue-Native PAGE (BN-PAGE), demonstrated that the import of cholesterol into the mitochondria occurs through an 800-kDa protein complex containing outer mitochondrial membrane (OMM) proteins, including the translocator protein (TSPO; 18 kDa) and the voltage-dependent anion channel (VDAC), along with IMM proteins, including the AAA+ ATPase ATAD3A and the CYP11A1 proteins [7]. The import and metabolism of cholesterol for steroid production has previously been shown to be dependent upon a functioning electron transport chain (ETC) and on intramitochondrial ATP production [8, 9]. Because of this observation, we examined whether mitochondrial protein complexes previously identified via BN-PAGE [5, 10], the mitochondrial ETC and fatty acid import protein complexes, are altered upon initiation of steroid production. Moreover, we investigated whether the alteration of these protein complexes can play a role in the regulation of cholesterol import and steroid production.
The ETC is composed of four different IMM protein complexes, complexes I, II, III, and IV, which function in the transfer of electrons from the reduced nicotinamide adenine dinucleotides (predominantly NADH) to the terminal electron acceptor, oxygen, forming water and using the free energy of electron transport to generate an electrical and proton gradient that is utilized by the cell to generate ATP through the F1F0 ATPase (complex V) [11]. These different protein complexes are demonstrated to interact with each other to form a protein supercomplex composed of ETC complexes I and III or of complexes I, III, and IV together [12]. The formation of this complex is proposed to encourage a more efficient transfer of the electrons between complexes, resulting in the decrease of free radical formation with increasing ATP production [13, 14]. These enzymatically active protein complexes have been identified and purified together via BN-PAGE where it has been demonstrated that ETC complexes can regulate their own stability in forming supercomplexes, responding to the energetic needs of the cell [10],
Mitochondrial beta (β)-oxidation metabolizes long-chain fatty acids in the mitochondrial matrix, where it produces acetyl-CoA utilized in the citric acid cycle and donates reducing electrons to NADH and FADH2 utilized by the ETC [15]. The import of long-chain fatty acids used for β-oxidation into the mitochondria is the first committed and regulated step in fatty acid oxidation [15]. This process occurs through a large molecular weight OMM protein complex consisting of long-chain acyl-CoA synthase (ACSL), carnitine palmitoyltransferase 1a (CPT1a), and VDAC [5]. ACSL activates the long-chain fatty acids, forming acyl-CoA esters, which are transferred to CPT1a. CPT1a then converts the activated fatty acids into acylcarnitines and assists in the transfer of the acylcarnitines into the mitochondria for utilization in fatty acid oxidation [5]. VDAC is demonstrated to function in the transport and stabilization of the complex.
To determine if these protein complexes are altered upon the initiation of steroid biosynthesis, we used BN-PAGE to separate the protein complexes present in the mitochondria before and after hormone treatment. We then analyzed the gels via mass spectrometry, identifying protein complex changes in the mitochondria treated with and without human chorionic gonadotropin (hCG). The data obtained indicated that there was an increase in ETC protein complex associations, but a decrease in proteins required for fatty acid import for β-oxidation. These results suggested that β-oxidation, as compared to mitochondrial respiration, is not necessary for steroidogenesis. The pharmacological inhibition of fatty acid import resulted in an increase in steroid production, whereas prolonged stimulation of β-oxidation resulted in reduced steroid production, suggesting that upon hormonal stimulation, long-chain β-oxidation of fatty acids appears to be detrimental to steroid-producing mitochondria.
MATERIALS AND METHODS
Materials
Antibodies against ACSL4 were obtained from Santa Cruz Biotechnology Inc.; antibodies against Cox IV, VDAC1, PECI, ACDVL, and CPT1a were from Abcam; antibodies against PMP-70 were from Sigma-Aldrich Co.; and antibodies against TSPO was generated as previously described [16]. Etomoxir (E1905), metformin (PHR1084), and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (CCCP; C2920) were purchased from Sigma-Aldrich Co.
Cell Culture
MA-10 Leydig cells were a gift from Mario Ascoli (University of Iowa, Ames) and were maintained in Dulbecco modified Eagle medium (DMEM)/Ham F12 (50:50) supplemented with 5% fetal bovine serum and 2.5% horse serum maintained at 37°C and 3.7% CO2.
Mitochondrial Isolation and BN-PAGE
Confluent MA-10 cells were harvested in Buffer A (10 mM Hepes-KOH, pH 7.5, 0.2 M mannitol, 0.07 M sucrose, 1 mM EDTA, 1× Complete Protease Inhibitor Cocktail Tablet; Roche Applied Science) using a cell lifter, and centrifuged at 500 × g for 10 min. The cell pellet was resuspended in five volumes of Buffer A, incubated at 4°C for 10 min, and then centrifuged at 500 × g for 10 min. The cell pellet was resuspended in five volumes of Buffer B (40 mM Hepes-KOH, pH 7.5; 500 mM sucrose; 160 mM potassium acetate; and 10 mM magnesium acetate, 1× Complete Protease Inhibitor Cocktail Tablet) and sonicated for 10 sec. Once complete, cells were centrifuged at 500 × g for 10 min. The supernatant was pooled and centrifuged at 10 000 × g for 10 min at 4°C to form a mitochondrial pellet. This step was repeated until the desired mitochondrial purity was achieved. The mitochondrial sample was then solubilized with a 1% digitonin buffer at 1 mg/ml and centrifuged at 10 000 × g for 10 min. A loading dye (5% w/v Coomassie Brilliant Blue G-250, 500 mM ε-amino-n-caproic acid, and 160 mM BisTris, pH 7.0) was added to the sample supernatants, loaded onto a 4%–16% native gel (Life Technologies, Thermo Fisher Scientific), and run at 130 V. Once complete, the gel was transferred using a semidry transfer apparatus for 1 h at 25 V. Following that, the membrane was then used for immunodetection.
In-Gel Digestion and Mass Spectrometry
For mass spectrometry analysis of MA-10 isolated mitochondria protein complexes, mitochondrial preparations were separated in triplicate by BN-PAGE. Each BN-PAGE gel was divided into 18 horizontal slices starting at 20 kDa, cut into 3-mm slices, increasing in molecular mass until reaching 1200 kDa, and subjected to in-gel digestion and mass spectrometry, as previously described [7]. Samples identified via mass spectrometry were run through the MitoMiner mitochondrial protein database to identify mitochondrial pathways [17] Normalization of MS data was corrected by calculating the slope of the averaged control versus treated hits, linearity was corrected by multiplying the control hits by 1.32, and the final quantification of total hits was plotted using Excel.
Immunoblot (Western) Analysis
Proteins separated by BN-PAGE or SDS-PAGE were electrophoretically transferred to a nitrocellulose membrane, as previously described [7]. SDS-PAGE membranes were incubated with primary antibodies against TSPO (1:2500), VDAC (1:2000), PMP70 (1:1000), and PECI (1:1000), and SDS-PAGE and BN-PAGE membranes were probed with ACSL4 (1:500), ACDVL (1:1000), CPT1a (1:1000), and cytochrome c oxidase subunit IV (CoxIV; 1:1000). Immunoreactive proteins were visualized using an enhanced chemiluminescence kit (Amersham Biosciences) and horseradish peroxidase-goat anti-rabbit and horseradish peroxidase-rabbit anti-donkey secondary antisera used at 1:5000 and 1:3000 dilutions, respectively.
MA-10 Cell Drug Treatments
We plated 2.5 × 104 MA-10 cells into 96-well plates. Serial dilution of etomoxir or metformin was generated from 100, 50, 10, 1, and 0.1 μM in MA-10 cell media. Each dilution of stated drug was then added in triplicate to MA-10 cells in the 96-well plates to assess their effect on basal steroid production or the stated drug concentration was added together with either 50 ng/ml hCG or 40 μM 22R-hydroxycholesterol (22R-HC) to the plate in triplicate, for a total of nine wells for each stated drug concentration. For the 24-h drug incubations, 2.5 × 104 MA-10 cells were plated in 96-well plates and cell media were changed after 4 h to include the indicated drug dilutions. After 22 h of incubation with the indicated drug concentration, the media was changed to drug only to assess its effect on basal steroid measurement, or the drug with either 50 ng/ml hCG or 40 μM 22R-HC for the final 2-h incubation of the 24 h incubation. At the end of the incubation media were harvested for radioimmunoassay (RIA) analysis or the cells were treated for the biological assays described below.
Radioimmunoassay
Cells were plated into 96-well plates at a density of 2.5 × 104 cells per well and treated with stated concentrations of etomoxir and metformin. After 22 h, selected wells were treated with the stated drug and simultaneously stimulated with 50 ng/ml hCG or 40 μM 22R-HC. Basal levels of steroid production were treated with the stated concentration of the drug and measured after 2 or 24 h of incubation. RIA analysis was performed as previously mentioned [18], with antiprogesterone serum purchased from MP Biomedicals.
Respiratory Bioflux Analysis
MA-10 cells were plated at 2 × 104 cells/well in XF96 plates and incubated with DMEM/F12 regular cell culture medium in a 3.5% CO2 atmosphere at 37°C. Selected wells were treated with stated concentrations of a drug, etomoxir or metformin, for 20 h. The medium was then replaced with unbuffered DMEM XF assay medium (pH adjusted to 7.4 using 1 N sodium hydroxide) supplemented with 2 mM glutamine and 1 g/L glucose with or without stated concentrations of etomoxir or metformin. After media changes, the cells were placed in a 37°C CO2-free incubator for 2 h. The basal oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were then determined using the XF96 plate reader (Seahorse Bioflux Analyzer; Seahorse Bioscience) using a 2-min mix followed by 5-min measurement. After baseline readings were obtained, the stated drug with or without hCG prepared in the reagent ports was injected into the wells, and the subsequent measurement of OCR and ECAR was obtained. Then, a 1 mM final concentration of CCCP or etomoxir was added to the reagent ports to monitor the alteration in mitochondrial function.
MTT assay
MA-10 cells were seeded at 2.5 × 104 cells/well into a 96-well plate and treated with the stated concentration of etomoxir or metformin for 22 h. After 22 h, selected wells were treated only with the stated concentration of the drug or simultaneously stimulated with 50 ng/ml of hCG and the drug. At the end of the treatment, cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as described in the CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega Corporation) based on the conversion of water-soluble MTT to insoluble formazan. Formazan blue formation was quantified at 590 and 690 nm using the Victor multilabel plate reader, and the results were expressed as (OD590 − OD690). For all experiments, samples were analyzed in replicates of three, for three separate experiments.
ApoTox-Glo Assay
MA-10 cells were plated and treated with specific drug concentrations. At the end of the basal or hCG stimulation, we incubated the cells with reagents from the ApoTox-Glo kit (Promega Corporation), specifically peptide substrate glycyl-phenylalanyl-aminofluorocoumarin (GF-AFC) and bis-alanylalanyl-phenylalanyl-rhodamine 110 (bis-AAF-R110). GF-AFC is a cell-permeant substrate that is cleaved by live-cell protease, generating a fluorescent signal that is representative of the number of living cells, and bis-AAF-R110 is a non-cell-permeant substrate that can only be cleaved from proteases that have been released from cells whose membranes have become permeable. A total of 20 μl of the reagent mixture containing GF-AFC and bis-AAF-R110 was added to each well, mixed briefly via orbital shaking for 30 sec, and incubated at 37°C for 30 min. Fluorescence was measured at 405ex/535em for viability and 485ex/535em for cytotoxicity, as measured by a Victor multilabel plate reader. We then added 100 μl of Caspase-Glo 3/7 reagents to each well, mixed by orbital shaking for 30 sec, and incubated for 30 min at room temperature. Luminescence was measured for 1 sec with the Victor multilabel plate reader to determine caspase activation for a measure of apoptosis.
Statistical Analysis
Statistical analysis was performed using the Student t-test and one-way ANOVA, followed by the Dunnett multiple comparison test, using the Prism 4.02 software package from GraphPad Software, Inc.
RESULTS
Identification of Protein Complexes in the Mitochondria Using BN-PAGE
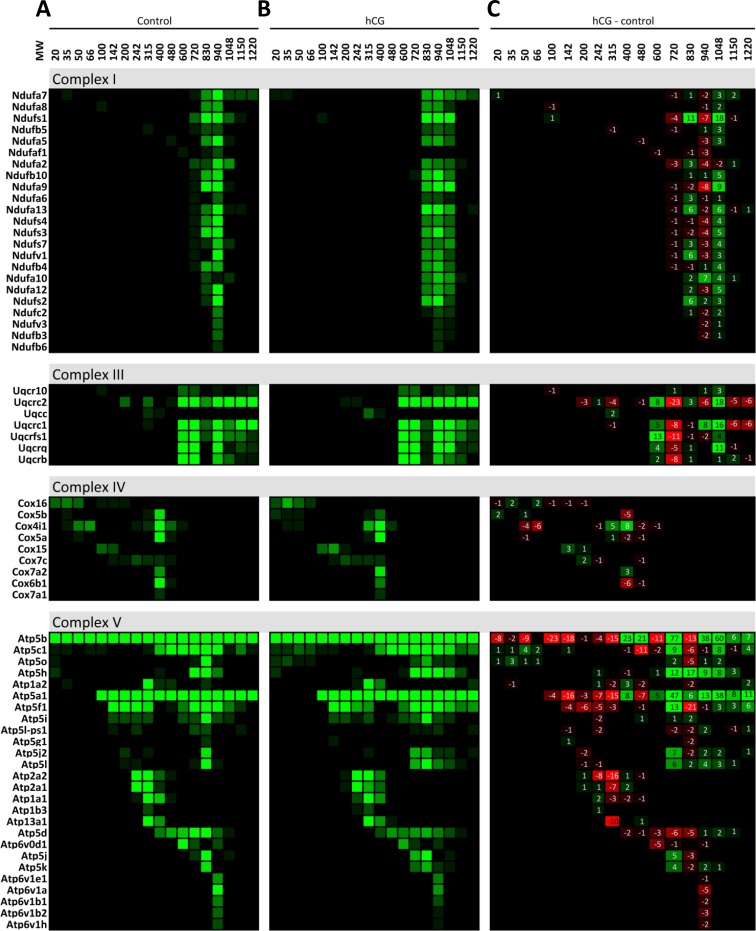
We initiated our characterization of the protein complexes that are present in the mitochondria of MA-10 tumor Leydig cells under basal conditions and after 2 h of hCG stimulation. The mitochondria were isolated from the basal and stimulated cells, and the mitochondrial proteins were separated by BN-PAGE followed by mass spectrometry. We used MitoMiner, an integrated database for the analysis of mitochondrial proteins [17], to identify the proteins involved in specific mitochondrial pathways identified via the Kyoto Encyclopedia of Genes and Genomes with the number of hits in each gel slice charted (Supplemental Fig. S1, available online at www.biolreprod.org). The initial analysis of the data generated from BN-PAGE was used to analyze the appearance of protein complexes I, III, and IV of the ETC, which were previously demonstrated to be associated under native conditions as a supercomplex [10, 13]. We were able to identify protein complexes I, III, and IV migrating separately in protein complex form in the mitochondria from both control (Fig. 1A) and hCG-treated cells (Fig. 1B). Subtracting the number of control mass spectrometry hits at each band from the corresponding number of hCG hits to identify the differences between the conditions, we were able to identify the presence of a higher-molecular-weight protein complex that forms following hCG stimulation, consisting of ETC complexes I and III (Fig. 1C). We also identified complex V and ATP synthase (which was present around 600 kDa), which increased in a higher-molecular-weight complex after hCG stimulation. These results confirm that the methodology used is able to both identify protein complexes present in the mitochondria and expose changes in the protein complex formation after hormonal stimulation.
FIG. 1.
Proteins identified to be present in the mitochondria ETC BN-PAGE protein complexes. Isolated mitochondria from control and hCG-treated MA-10 cells were solubilized and run on a BN-PAGE gel. The gel was divided into the indicated molecular weights (MWs) and analyzed via mass spectrometry. Identified proteins were grouped via MitoMiner to be present in the ETC complexes in both control (A) and hCG-treated (B) isolated mitochondria. The differences in the proteins identified from hCG-treated minus the control (C) samples demonstrate a larger-molecular-weight molecular complex containing complexes I, III, and V at MW 1048. Proteins identified to be present in complex I: Ndufa7, Ndufa8, Ndufs1, Ndufb5, Ndufa5, Ndufaf1, Ndufa2, Ndufb1, Ndufa9, Ndufa6, Ndufa13, Ndufs4, Ndufs3, Ndufs7, Ndufv1, Ndufb4, Ndufa10, Ndufa12, Ndufs2, Ndufc2, and Ndufv3. Complex III: Uqcr10, Uqcrc2, Uqcc, Uqcrc1, Uqcrfs1, Uqcrq, and Uqcrb. Complex IV: Cox16, Cox5b, Cox4i1, Cox5a, Cox15, Cox7c, Cox7a2, Cox6b1, and Cox7a1. Complex V: Atp5b, Atp5c1, Atp5o, Atp5h, Atp1a2, Atp5a1, Atp5f1, Atp5i, Atp5l-ps1, Atp5g1, Atp5j2, Atp5l, Atp2a2, Atp2a1, Atp1a1, Atp1b3, Atp13a1, Atp5d, Atp6v0d1, Atp5j, Atp5k, Atp6v1e1, Atp6v1a, Atp6v1b1, Atp6v1b2, and Atp6v1h.
Reduction in β-Oxidation Proteins in Steroid-Producing Mitochondria
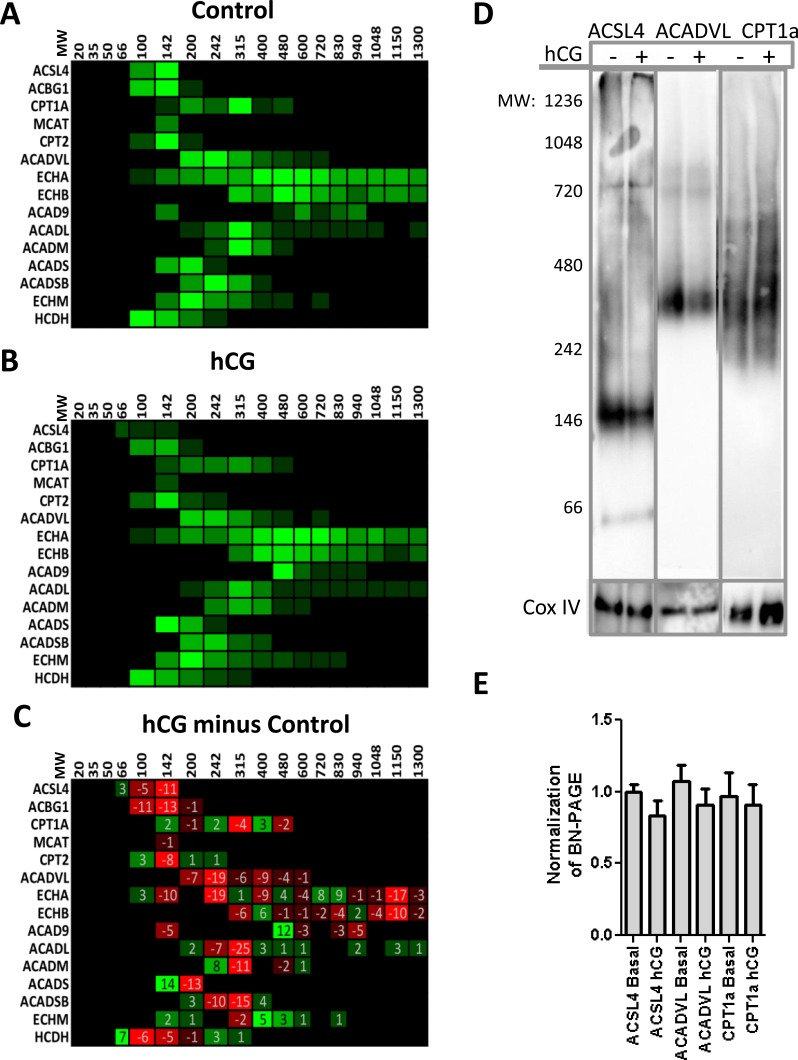
Quantification of the protein complexes involved in β-oxidation by mass spectrometry failed to identify a clear protein complex necessary for metabolism in either control (Fig. 2A) or hCG-treated (Fig. 2B) samples, with many of the proteins distributed throughout the gel. Upon analysis of the hCG minus the control data (Fig. 2C), we observed a decrease in the amount of proteins necessary for the initiating step in β-oxidation, specifically long-chain ACSL and acyl-CoA dehydrogenase (Fig. 2C). We then performed BN-PAGE immunoblot analysis of both the control and hCG-treated isolated mitochondria targeted against the proteins identified to be altered upon hormonal stimulation, so as to confirm the mass spectrometry results. The immunoblot analysis demonstrated that long-chain ACSL, ACSL4, and acyl-CoA dehydrogenase, ACADVL, exhibited a reduced mitochondrial association upon hormonal stimulation (Fig. 2, D and E). We observed no changes in the integral membrane protein, CPT1a, as seen in the mass spectrometry analysis. Cox IV was used as our loading control, as we observed no change in its expression levels by mass spectrometry analysis. As importing fatty acids into the mitochondria is essential in initiating β-oxidation, these results suggest that the rate of β-oxidation may decrease during hormone stimulation, negatively correlating with the concomitant import of mitochondrial cholesterol and subsequent steroid production.
FIG. 2.
Mitochondrial proteins identified via mass spectrometry to play a role in fatty acid metabolism decrease upon hormonal stimulation. Mass spectrometry results were analyzed via MitoMiner to identify proteins that play a role in β-oxidation in either control (A) or hCG-treated MA-10 (B) isolated mitochondria. C) The difference in the identified β-oxidation proteins from hCG-treated samples minus control identified no novel protein complexes, though a decrease in the proteins ACSL4 and ACADVL, which function in long-chain fatty acid import into the mitochondria, was observed. D) Immunoblot analysis of BN-PAGE membrane confirms the presence of ACSL4, ACADVL, and CPT1a at the indicated molecular weights as identified via mass spectrometry analysis. E) Quantification of BN-PAGE immunoblot analysis; no significant changes were observed. The graph shown represents the mean ± SEM of the three experiments; samples were normalized to control from each experiment. The following are proteins that were identified to play a role in β-oxidation: ACSL4, ACBG1, CPT1a, MCAT, CPT2, ACADVL, ECHA, ECHB, ACAD9, ACADL, ACADM, ACADS, ACADSB, ECHM, HCDH, and ACAA2.
Inhibition of β-Oxidation Does Not Affect Steroid Production
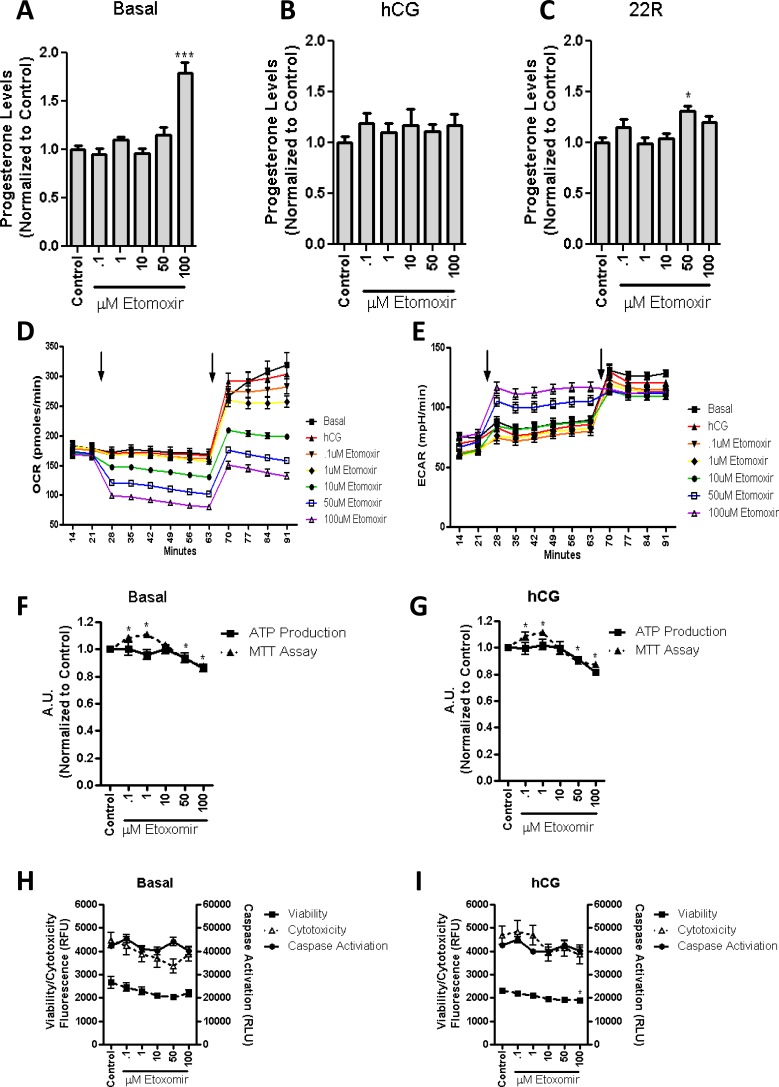
To investigate whether β-oxidation is required for steroid production, the import of fatty acids into the mitochondria was inhibited using the CPT1a inhibitor etomoxir. Etomoxir works by binding to the malonyl-CoA binding site of CPT1a, which acts by preventing the import of fatty acids [19]. As etomoxir is proposed to rapidly inhibit fatty acid import into the mitochondria, we incubated etomoxir at the indicated concentration and measured the basal levels of steroid production after 2 h. The treatment resulted in an increase in steroidogenesis at the highest dose of etomoxir (Fig. 3A). Concomitant treatment of MA-10 cells with etomoxir and hCG did not alter steroid production (Fig. 3B), corroborating the hypothesis that β-oxidation is not necessary for steroid production to occur. To ensure that the various treatments used did not alter mitochondrial function, we incubated the cells with 22R-HC, a membrane-permeable cholesterol analog whose metabolism ensures that CYP11A1 is functional. In the presence of 22R-HC and etomoxir, we noted no change in progesterone formation by the MA-10 cells (Fig. 3C).
FIG. 3.
β-oxidation of long-chain fatty acids is not necessary for steroid biosynthesis in MA-10 cells. MA-10 cells were treated for 2 h with stated concentrations of the CPT1a inhibitor etomoxir, with or without hCG present. Steroid production was measured under basal conditions (A; control = 27.9 ng progesterone/mg protein), 50 ng/ml of hCG (B; control = 481 ng progesterone/mg protein), or 22R-HC (C; control = 2402 ng progesterone/mg protein). No significant decrease of steroid production was observed upon the incubation of etomoxir in either basal or hCG-stimulated MA-10 cells. Respiratory extracellular flux analysis demonstrated that the OCR (D) was decreased and the ECAR (E) was increased upon the addition of etomoxir with or without hCG (first arrow), confirming the inhibition of fatty acid import into the mitochondria. Upon addition of CCCP (second arrow), we observed the rise in OCR, demonstrating the total oxygen consumption from the mitochondria, and a rise in ECAR, representing the increase in glycolysis. The points shown represent the mean ± SEM of three experiments, with six replicates per experiment. Incubation of the stated etomoxir concentrations resulted in the decrease in basal (F) and hCG-stimulated MTT (G) readings, but they did not result in significant alterations in ATP production. Overall, cellular health was observed in basal (H) or hCG-stimulated MA-10 (I) cells in the presence of etomoxir, demonstrating no changes in either cytotoxicity or caspase activation at the stated concentration of etomoxir as compared to control MA-10 cells. In addition, a slight decrease in viability was observed at the highest concentration of etomoxir upon hCG stimulation. The points shown represent the mean ± SEM of the three experiments, with three replicates per experiment. Samples are normalized to control steroid production from each experiment.*P < 0.05, ***P < 0.001.
Confirmation of β-Oxidation-Oxidation Alteration by Etomoxir
To confirm that etomoxir was inhibiting fatty acid import and, therefore, β-oxidation, we used the Respiratory Bioflux analyzer that monitors the real-time OCR and the ECAR of the cell. OCR is a measure of mitochondrial respiration, and ECAR provides a readout of the production of lactic acid formed during glycolysis [20]. Confirmation of the inhibition of β-oxidation has been demonstrated to result in a decrease in oxygen consumption, leading to reduced OCR. The decrease in mitochondrial oxygen consumption results in a decrease in ATP; to maintain ATP levels, the rate of glycolysis is increased, as can be observed through an increase in ECAR [20]. Upon incubation of etomoxir with the MA-10 cells, we were able to observe a dose-dependent decrease in OCR (Fig. 3D) and a corresponding increase in ECAR (Fig. 3E). To determine the total mitochondrial utilization of oxygen, we then incubated the cells with the mitochondrial protonophore CCCP, which dissipates the protonic mitochondrial membrane potential and promotes futile respiration. Increasing amounts of etomoxir reduced the maximal OCR, which was seen upon the addition of CCCP, while not altering ECAR. These results suggest that the cells are generating more of their ATP from glycolysis and not from the mitochondria (Fig. 3, D and E, second arrow).
Because of the altered rate of oxygen consumption, we wanted to ensure that the health of the cell was not altered. MTT, a compound that is metabolized to an insoluble purple formazan by oxidoreductase enzymes present in the ETC [21], was used to measure mitochondrial health. Cells were incubated with increasing concentrations of etomoxir for a 2-h incubation period with or without hCG, after which the MTT assay was performed. The results obtained showed a dose-dependent increase of absorbance at lower concentrations of etomoxir, indicating a higher rate of MTT metabolism and, therefore, increased mitochondrial activity followed by a decrease in MTT metabolism at higher doses of etomoxir (Fig. 3, F and G). To determine whether these changes represented an alteration in ATP production, we measured the amount of ATP present in the cells after a 2-h incubation period with etomoxir. No significant changes in basal (Fig. 3F) or hCG-stimulated cells (Fig. 3G) were observed. To ensure that there is no change in cell viability, and that the reduction in the MTT readings are due to altered mitochondrial function, we measured the viability, cytotoxicity, and caspase activation of the treated cells. Upon treatment with etomoxir, we noticed no significant change in either basal (Fig. 3H) or hCG-treated cells (Fig. 3I) in terms of cell viability, cytotoxicity, or caspase activation. Taken together, these data suggest that β-oxidation is not required for steroid production, and that the inhibition of β-oxidation is not detrimental to overall MA-10 cell health.
Long-Term Inhibition of β-Oxidation Increases Steroid Production
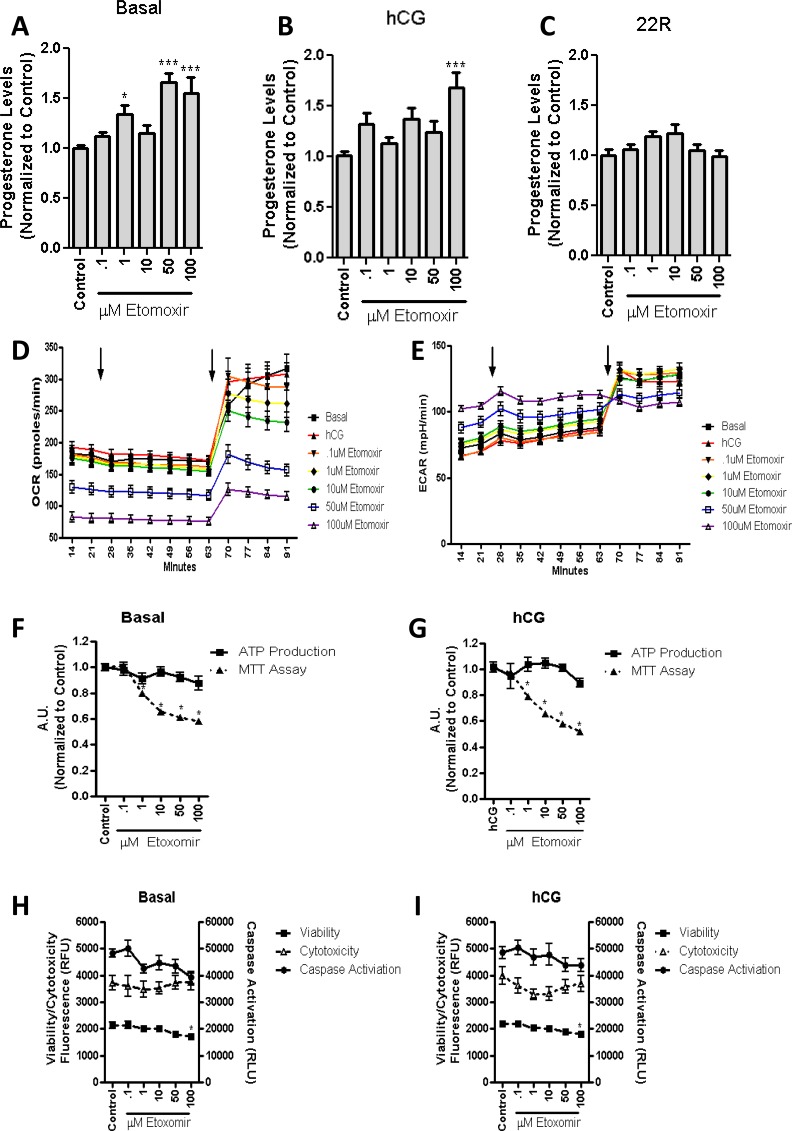
Given that a 2-h inhibition of β-oxidation did not alter hormone-stimulated steroid production, we subsequently pretreated MA-10 cells with increasing concentrations of etomoxir for 24 h and measured both basal and hormone-stimulated levels of steroid production. In both conditions, etomoxir induced a dose-dependent increase in steroid production (Fig. 4, A and B), whereas cotreatment with 22R-HC showed no alteration in steroid production (Fig. 4C). These data indicate the increased mitochondrial import of cholesterol for steroid production under both control and hCG-stimulated conditions. Respiratory Bioflux analysis after 24-h treatment with etomoxir indicated a similar decrease in the OCR (Fig. 4D), along with an increase in ECAR (Fig. 4E). After the addition of CCCP, we observed a decrease in the maximal oxygen consumption, with no alterations observed in ECAR at the highest doses of etomoxir (Fig. 4, D and E, second arrow), confirming the results from the previous experiment, indicating that the mitochondria are not energetically utilizing oxygen to its full capacity.
FIG. 4.
Long-term inhibition of β-oxidation in MA-10 cells increases hormone-stimulated steroid production. MA-10 cells were treated for 24 h with stated concentrations of etomoxir. Steroid production was measured in basal conditions (A; control = 32 ng progesterone/mg protein), 50 ng/ml of hCG (B; control = 662.9 ng progesterone/mg protein), or 22R-HC (C; control = 2496 ng progesterone/mg protein) for 2 h. We then demonstrated a statistically significant increase in both basal and hormone-stimulated steroid production. Respiratory extracellular flux analysis confirmed that the OCR (D) was decreased and the ECAR (E) was increased as compared to control (media/hCG; first arrow). Upon the addition of CCCP (second arrow), we observed the rise in both OCR and ECAR. The points shown represent the mean ± SEM of the three experiments, with six replicates per experiment. Incubation of stated etomoxir concentrations for 24 h results in significant decreases in basal (F) and hCG-stimulated MTT (G) readings, but this does not result in significant alterations in ATP production. Overall cellular health was observed in basal (H) and hCG-stimulated MA-10 (I) cells, demonstrating no change in either cytotoxicity or caspase activation at the stated concentration of etomoxir, with a slight decrease in viability at the highest concentration of etomoxir. The points shown represent the mean ± SEM of the three experiments, with three replicates per experiment. Samples are normalized to control steroid production from each experiment. *P < 0.05, ***P < 0.001.
We then measured ATP production rates and found no significant alterations in either basal (Fig. 4F) or hCG-stimulated cells (Fig. 4G), but a significant decrease was observed in MTT metabolism (Fig. 4, F and G). These findings further support a reduced role of mitochondria in the energy production in these cells. However, the overall health of the cells was not altered, as we observed no alterations in cell viability, cytotoxicity, or caspase activation in the basal (Fig. 4H) or hCG-stimulated MA-10 cells (Fig. 4I).
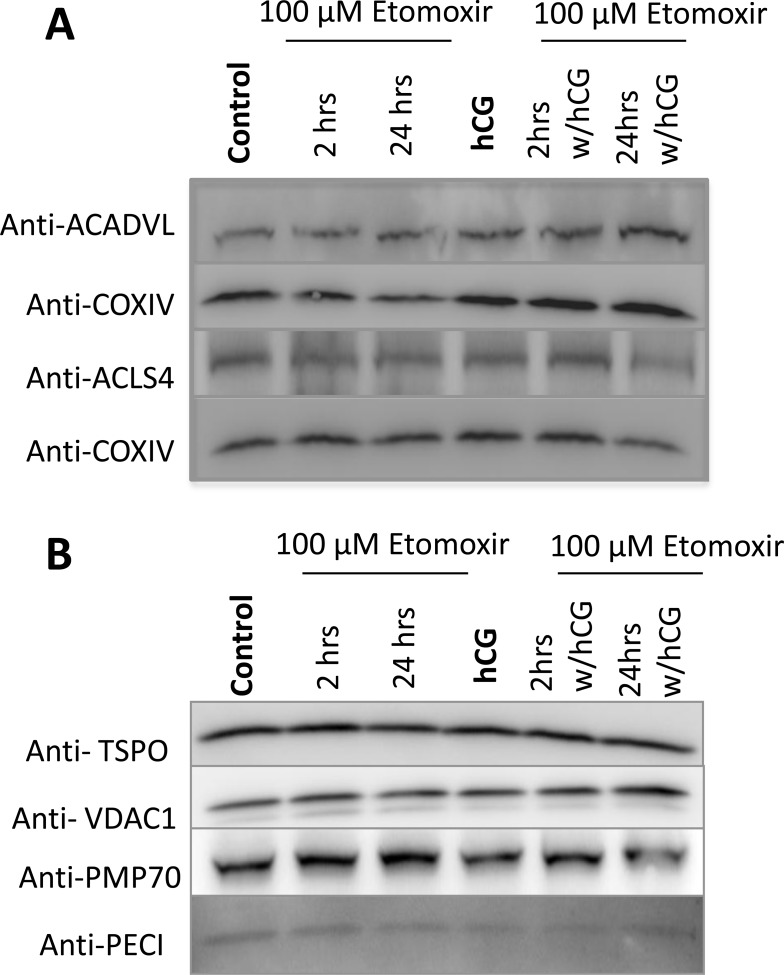
Etomoxir treatment did not affect the expression of either ACSL4 or ACADVL, normalized to mitochondrial ETC protein CoxIV, as shown by immunoblot analysis (Fig. 5A); change in the expression of either protein would alter β-oxidation. Given that etomoxir also functions as a peroxisome proliferator-activated receptor (PPAR)-α antagonist, we then wanted to assess whether the treatment was affecting peroxisomal proliferation. This could be potentially confounding, as peroxisomal proliferation can also contribute to short-chain fatty acids that are permissible to the mitochondria, bypassing CPT1a [22]. We identified no changes in the peroxisomal membrane protein, PTP70, along with PECI (a peroxisomal matrix protein) (Fig. 5B). In addition, no changes were observed in the mitochondrial proteins VDAC and TSPO. These findings indicate that etomoxir is functionally active at its main target site, the CPT1a fatty acid import complex.
FIG. 5.
No alterations in mitochondrial or peroxisomal proteins are observed upon incubation of etomoxir. A) Immunoblot analysis demonstrated no changes in β-oxidation proteins ACADVL or ACSL4, normalized to mitochondrial ETC protein COX IV, under hCG or etomoxir treatment for the stated time points. B) No changes in either mitochondrial or peroxisomal levels were observed determined by assessing the levels of the OMM proteins, TSPO and VDAC1, and peroxisomal proteins, PMP70 and PECI, at the stated time points of etomoxir incubation.
Stimulation of β-Oxidation Results in a Decrease in Steroid Production
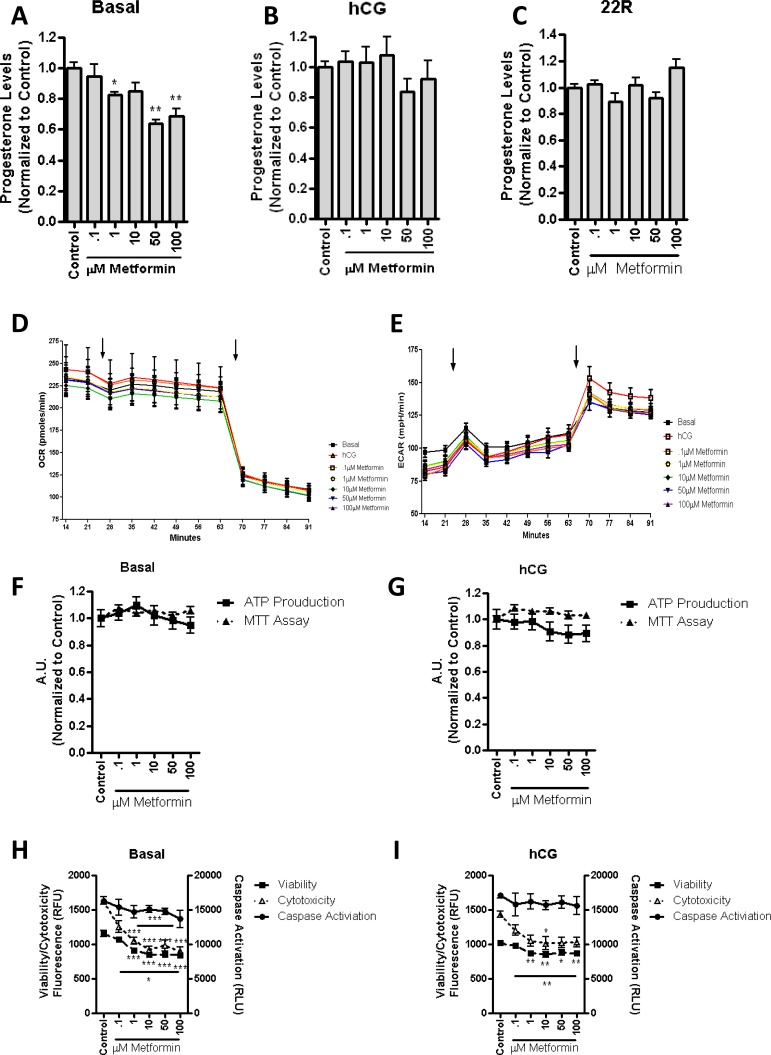
To confirm that mitochondrial β-oxidation is not necessary for MA-10 cell steroid production, and to verify that it could possibly be detrimental, cells were treated with metformin. Metformin has been proposed to stimulate β-oxidation by activating the 5′ AMP-activated protein kinase pathway [23], which results in the phosphorylation of acetyl-CoA carboxylase, decreasing the production of the CPT1a inhibitor malonyl-CoA, and resulting in the increase of imported mitochondrial fatty acid [24]. Upon the addition of increasing concentrations of metformin to the MA-10 cells for 2 h, we observed a decrease in basal (Fig. 6A) but not in hCG-treated (Fig. 6B) or 22R-HC-supported (Fig. 6C) steroid production. Considering that these results are the opposite of what we observed with etomoxir, we propose that the short-term regulation of mitochondrial energy production affects basal steroid production, but not hormone-induced steroidogenesis.
FIG. 6.
Stimulation of β-oxidation for 2 h results in a decrease in basal steroid production. MA-10 cells were treated for 2 h with stated concentrations of metformin. Steroid production was measured in basal conditions (A; control = 22.7 ng progesterone/mg protein), 50 ng/ml hCG (B; control = 317.8 ng progesterone/mg protein), or 22R-HC (C; control = 2659 ng progesterone/mg protein), demonstrating a significant decrease in basal steroid production. Respiratory extracellular flux analysis identified that the OCR (D) and the ECAR (E) were unchanged upon the addition of metformin (first arrow). Upon the addition of etomoxir (second arrow), we observed a decrease in OCR and an increase in ECAR, as previously seen. The points shown represent the mean ± SEM of the three experiments, with six replicates per experiment. Incubation of the stated metformin concentrations for 2 h resulted in no alterations in basal (F) or hCG-stimulated MTT (G) readings or measurement of ATP production. Overall cellular health was observed, demonstrating no change in caspase activation, but a decrease in both cell viability and cytotoxicity in basal (H) and hCG-stimulated MA-10 (I) cells at stated concentrations of metformin. The points shown represent the mean ± SEM of the three experiments, with three replicates per experiment. Samples are normalized to control steroid production from each experiment. *P < 0.05, **P < 0.01, ***P < 0.001.
Respiratory Bioflux analysis did not show any significant alterations in oxygen consumption or pH in the extracellular media (Fig. 6, D and E) in response to metformin treatment. Upon the addition of etomoxir, a slight increase in ECAR was observed (Fig. 6E, second arrow), suggesting that there is a trend towards increased mitochondrial respiration that is altered upon the inhibition of fatty acid import. No significant changes in response to metformin treatment were observed in terms of mitochondrial function and ATP production in control (Fig. 6F) or hCG-treated cells (Fig. 6G). Cellular viability analysis indicated that there was a decrease in response to the addition of metformin in terms of both cytotoxicity and cell viability with no effect on caspase activation in control (Fig. 6H) and hCG-treated cells (Fig. 6I). The cell viability assay is representative of the total living cells, whereas the cytotoxicity measurement represents the cells with permissible plasma membranes. As the viability signal decreases, the cytotoxicity signal should increase; therefore, these results suggest that metformin is not toxic, but it possibly alters the cellular proliferation rate or cell membrane properties.
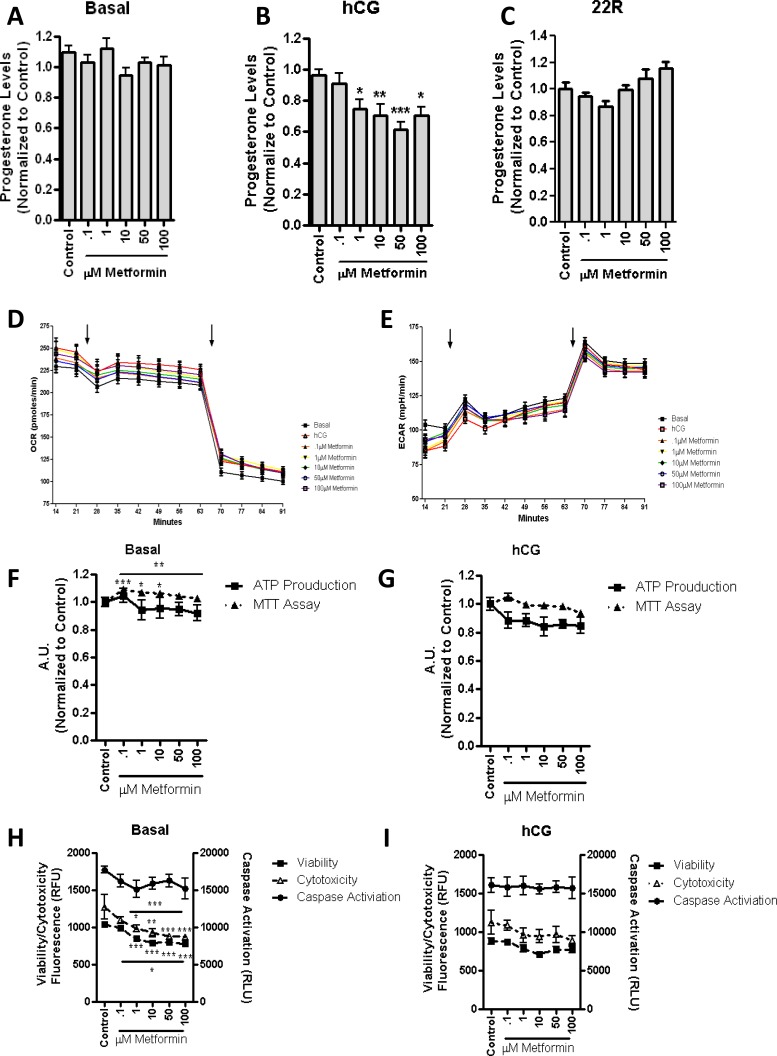
Long-Term Incubation of Metformin Decreases Hormone-Mediated Steroid Production
Twenty-four-hour incubation with increasing concentrations of metformin resulted in no changes in basal (Fig. 7A) and 22R-HC-supported (Fig. 7C) steroid production (Fig. 7A). However, the treatment resulted in a significant decrease in hCG-stimulated steroid production (Fig. 7B). No significant changes were observed in the OCR or ECAR rates measured by the Respiratory Bioflux analysis following 24-h treatment with metformin (Fig. 7, D and E). Although mitochondrial function was increased in control cells in response to metformin treatment (Fig. 7F), in hCG-stimulated cells (Fig. 7G), both mitochondrial function and ATP levels were unaltered. Reductions in cell viability and cytotoxicity were observed in basal conditions (Fig. 7H), as previously seen with the 2-h incubation with the drug. The overall health of cells treated with metformin was not altered upon hCG stimulation, as demonstrated by the fact that no changes were observed in caspase activation, cell viability, and cytotoxicity (Fig. 7I). However, a decrease in steroid production was observed, suggesting that the increase in mitochondrial β-oxidation might be the primary cause of these phenomena.
FIG. 7.
Long-term incubation of metformin results in a decrease in basal and hCG-stimulated steroid production. MA-10 cells were treated for 24 h with stated concentrations of metformin. Steroid production was measured in basal conditions (A; control = 14.7 ng progesterone/mg protein), 50 ng/ml hCG (B; control = 319 ng progesterone/mg protein), or 22R-HC (C; control = 2603 ng progesterone/mg protein). Respiratory extracellular flux analysis identified that the OCR (D) and the ECAR (E) were unchanged upon the addition of metformin (first arrow). Upon the addition of etomoxir (second arrow), we observed a decrease in OCR and an increase in ECAR, as previously seen. The points shown represent the mean ± SEM of the three experiments, with six replicates per experiment. Incubation of the stated metformin concentrations for 24 h resulted in MTT activity in basal conditions (F), but no significant changes in ATP production or hCG stimulation (G) were noted. Overall cellular health was observed upon the basal incubation of metformin (H), which resulted in a decrease in cell viability and cytotoxicity. I) Upon hCG stimulation, no demonstrated changes were observed in caspase activation, cell viability, or cytotoxicity at the stated concentrations of metformin. The points shown represent the mean ± SEM of the three experiments, with three replicates per experiment. Samples are normalized to control steroid production from each experiment. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
We report herein that BN-PAGE paired with mass spectrometry allowed for the identification of changes in known protein complexes, which occur in MA-10 Leydig cell mitochondria in response to hormone (hCG) treatment. Although in this report we have focused our efforts on the documented protein complexes of the ETC and fatty acid import for β-oxidation, this process can be used to study other mitochondrial processes [5, 10, 15, 25].
Upon the analysis of ETC complexes, we were able to establish the formation of a supramolecular complex of ETC components between complexes I and III following hormonal stimulation. We identified no changes in complex IV location both before and after hormonal stimulation. It has been demonstrated that the lack of an interaction between complex IV and the complex I and III supercomplex results in the altered stability of complex I [26]. In previous studies, we demonstrated that the inhibition of MA-10 cell complex I via rotenone did not alter the membrane potential or ATP production at doses that affected primary mouse Leydig cells [9]. This observation suggests that the lack of complex IV interacting with other complexes could be one of the reasons for the altered complex I function. As complexes I and III still interact to start the initial formation of a respirasome, these complexes could result in increased efficiency of electron transport, resulting in decreased generation of free radicals [10]. As the generation of free radicals has been observed to be increased upon the initiation of steroid production [27], the initiation of the formation of a supercomplex identified here could suggest that the increased electron leakage from the mitochondria might not be due primarily to the ETC, but to other intracellular sources, specifically the hydroxylation of cholesterol required for steroid biosynthesis [28].
Given that the IMM is normally cholesterol poor, the increase of cholesterol levels imported into the IMM in response to hormone treatment could function in the thickening and stiffening of the membranes. This increase in the rigidity of the IMM is proposed to decrease the enzymatic activity or mobility of the components of the ETC [29]. As we observed, there were no alterations in the oxygen consumption in basal versus hCG-stimulated cells (Fig. 3D), indicating that it is likely that the ETC supercomplex formation could compensate for the changes in membrane composition, ensuring more efficient transfer of electrons between complexes.
We then continued investigating the effect of hormonal stimulation on β-oxidation in MA-10 cells, attempting to identify the protein complex required for the import of fatty acids (as described by Lee et al. [5]), which includes ACSL4, CPT1a, and VDAC. We identified these proteins in the BN-PAGE datasets, but we were unable to show whether they were interacting as a unitary protein complex. This could be due either to the different solubilization techniques used herein, as compared to those used by Lee et al. [5] (e.g., digitonin compared to Triton X-100), or to the use of a Leydig cell line, as compared to mouse liver primary cells. Nevertheless, upon hormonal stimulation, we did observe a decrease in ACSL4, which is the protein required for the activating step of fatty acid import, with no significant changes found in CPT1 and CPT2. Because mitochondrial β-oxidation cannot occur without the import of fatty acids via ACSL4, these results suggest that β-oxidation is not critical for steroidogenesis.
To confirm this observation, we used the β-oxidation inhibitor etomoxir, which binds to CPT1a and prevents the import of fatty acids into the mitochondria. Short-term (2-h) treatment of MA-10 cells with etomoxir increased basal steroid production, but it did not alter hormone-stimulated steroid production, confirming the observation that β-oxidation is not necessary for steroid production. However, long-term (24-h) incubation of MA-10 cells with etomoxir resulted in increased basal and hCG-stimulated steroid production. Incubation of etomoxir at both time points also demonstrated a decrease in oxygen consumption and an increase in lactic acid production. As we also observed no alteration of ATP production, we concluded that a shift to glycolysis is occurring upon the inhibition of β-oxidation in the mitochondria. This shift to glycolysis appears to be beneficial in steroid production, possibly because of the fact that the extramitochondrial energy supply would allow the mitochondria to focus on steroid production and not on the cell's energetic demands. Conversely, we also demonstrated that increasing the rate of β-oxidation in the cells via treatment with metformin decreases steroid production. However, as metformin treatment does not alter the rate of glycolysis or mitochondrial ATP production, as seen by the Respiratory Bioflux analysis, it suggests that the act of importing fatty acids itself is inhibitory to steroid production.
Fatty acids have previously been shown to be inhibitory towards primary Leydig cell steroid production [30], but it is unknown whether the fatty acid import into the mitochondria or the β-oxidation is detrimental to steroid production. We have previously shown that the primary isoform of VDAC in the mitochondria, VDAC1, is associated with the protein complex that is necessary for cholesterol import in MA-10 cells, and Lee et al. demonstrated that VDAC1 is associated with the fatty acid import complex [5, 7]. In fatty acid import, VDAC1 is proposed to function in the regulation of the import of activated fatty acids into the inner membrane space. Upon analysis of our mass spectrometry results, it was observed that CPT1a was not affected by hormone treatment, but VDAC1 shifted into a higher-molecular-weight complex where TSPO is known to associate with and import cholesterol into the IMM [7]. This observation suggests that VDAC1 might provide the regulatory mechanism through which the import of lipids into the mitochondria could be selected. Indeed, it is likely that VDAC1 could select the lipid that would most benefit the cell by sensing or controlling the energy requirements of the mitochondria.
Our results also suggest that upon hormonal stimulation, the mitochondria function in the import of cholesterol, decreasing the overall import of fatty acids into the mitochondria. This raises the question of the role of acyl-CoA-binding protein 1 (ACBD1), also known as diazepam-binding inhibitor, which has been demonstrated to be necessary for steroidogenesis to occur in the Leydig cells [31]. ACBD1 binds and facilitates the transport of long-chain acyl-CoA esters in the cytosol, along with the regulation of cytosolic pool formation [32]. Therefore, it could be proposed that ACBD1 binds fatty acids, pooling them at the OMM after activation by ACSL, preventing them from being imported into the mitochondria. However, ACBD1 is not a mitochondrial protein, and as such it was not found in the mass spectrometry results; thus, at present, it is currently unknown what role ACBD1 would play in this process.
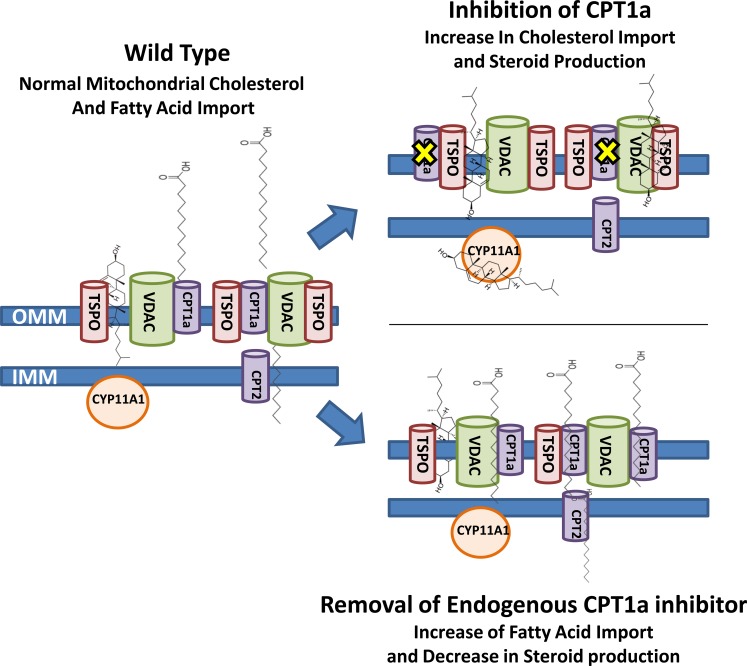
The data presented here demonstrate the power of the methodology used to identify the changes in protein complexes that could regulate the transport of metabolic intermediates and electron transport in the production of ATP in the mitochondria under various treatments in MA-10 mouse Leydig cells. Our initial results generated by mass spectrometry identified a decrease in the proteins associated with β-oxidation, a result that was later confirmed through the inhibition of fatty acid import via etomoxir. As the rate-limiting step in steroid production has been demonstrated to be the import of cholesterol into the mitochondria for metabolism by CYP11A1 [6], our results suggest that the ability of the mitochondria to efficiently import cholesterol for steroidogenesis at the expense of other lipids can be regulated by the energetics of the mitochondria (Fig. 8). Furthermore, through the use of BN-PAGE, we identified multiple mitochondrial protein complexes that function in the regulation of steroidogenesis, demonstrating that the overall structural framework of the mitochondria is important in the regulation of steroid production and many other mitochondrial processes.
FIG. 8.
Schematic representation of the role of fatty acid import into the mitochondria in the regulation of mitochondrial steroid production. The import of fatty acids into the mitochondria under wild-type conditions has been proposed to occur through VDAC and CPT1a, whereas cholesterol has been proposed to be imported via VDAC and TSPO. Upon inhibition of CPT1a via etomoxir, a decrease in fatty acid import is observed with an increase in imported cholesterol, which can be observed via an increased steroid production. Increasing fatty acid import through the use of metformin results in a decrease in the amount of cholesterol imported, which is determined via a decrease in steroid production.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (MOP102647 & MOP125983) and a Canada Research Chair in Biochemical Pharmacology to V.P. M.B.R. was supported in part by a postdoctoral fellowship from the Research Institute of the McGill University Health Centre. A.M. was supported in part by a postdoctoral fellowship from Les Fonds de la recherche du Québec-santé. The Research Institute of MUHC was supported by a Center grant from Les Fonds de la recherche du Québec-santé.
REFERENCES
- Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they're apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issop L, Rone MB, Papadopoulos V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol Cell Endocrinol. 2013;371:34–46. doi: 10.1016/j.mce.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Vartak R, Porras CA, Bai Y. Respiratory supercomplexes: structure, function and assembly. Protein Cell. 2013;4:582–590. doi: 10.1007/s13238-013-3032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kerner J, Hoppel CL. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem. 2011;286:25655–25662. doi: 10.1074/jbc.M111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, Ye X, Blonder J, Veenstra T, Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006;147:3924–3935. doi: 10.1210/en.2005-1204. [DOI] [PubMed] [Google Scholar]
- Midzak AS, Chen H, Aon MA, Papadopoulos V, Zirkin BR. ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells. Biol Reprod. 2011;84:976–985. doi: 10.1095/biolreprod.110.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina NV, Kouril R, Peters K, Braun HP, Boekema EJ. Structure and function of mitochondrial supercomplexes. Biochim Biophys Acta. 2010;1797:664–670. doi: 10.1016/j.bbabio.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quant Biol. 2011;76:1–16. doi: 10.1101/sqb.2011.76.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Schagger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim Biophys Acta. 2009;1787:672–680. doi: 10.1016/j.bbabio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schagger H. Respiratory chain supercomplexes. IUBMB Life. 2001;52:119–128. doi: 10.1080/15216540152845911. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Genova ML. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int J Biochem Cell Biol. 2009;41:1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci U S A. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Blackshaw JA, Robinson AJ. MitoMiner: a data warehouse for mitochondrial proteomics data. Nucleic Acids Res. 2012;40:D1160–D1167. doi: 10.1093/nar/gkr1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Lilly K, Chung C, Kerner J, VanRenterghem R, Bieber LL. Effect of etomoxiryl-CoA on different carnitine acyltransferases. Biochem Pharmacol. 1992;43:353–361. doi: 10.1016/0006-2952(92)90298-w. [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Sumantran VN. Cellular chemosensitivity assays: an overview. Methods Mol Biol. 2011;731:219–236. doi: 10.1007/978-1-61779-080-5_19. [DOI] [PubMed] [Google Scholar]
- Violante S, Ijlst L, Te BH, Koster J, Tavares de Almeida I, Wanders RJ, Ventura FV, Houten SM. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim Biophys Acta. 2013;1831:1467–1474. doi: 10.1016/j.bbalip.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O'Neill HM, Ford RJ, Palanivel R, O'Brien M, Hardie DG, Macaulay SL, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Winge DR. Sealing the mitochondrial respirasome. Mol Cell Biol. 2012;32:2647–2652. doi: 10.1128/MCB.00573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88:100. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Keefee EB, Scharschmidt BF, Blankenship NM, Ockner RK. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979;64:1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle AW, Benson SJ, Liu XH, Boam WD, Stringham JD. Nonesterified fatty acids modulate steroidogenesis in mouse Leydig cells. Am J Physiol. 1989;257:E937–E942. doi: 10.1152/ajpendo.1989.257.6.E937. [DOI] [PubMed] [Google Scholar]
- Knudsen J, Hojrup P, Hansen HO, Hansen HF, Roepstorff P. Acyl-CoA-binding protein in the rat. Purification, binding characteristics, tissue concentrations and amino acid sequence. Biochem J. 1989;262:513–519. doi: 10.1042/bj2620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal J, Ertbjerg P, Knudsen J. Characterization of ligand binding to acyl-CoA-binding protein Biochem J 1993. 290 (pt 2): 321 326 [DOI] [PMC free article] [PubMed] [Google Scholar]