ABSTRACT
Oocytes isolated from cows of reproductive age with reduced antral follicle counts (AFC) have a diminished capacity of embryonic development, which may be related to alterations in the mechanism that directs the proper segregation of chromosomes. Because we demonstrated that progesterone receptor membrane component 1 (PGRMC1) is involved in chromosome congression and metaphase II (MII) plate formation, the present study was designed to determine 1) if the decrease in oocyte developmental competence observed in dairy cows with a reduced AFC is due to a higher incidence of aneuploidy and 2) whether alterations in PGRMC1 contributes to the incidence of aneuploidy. Oocytes from ovaries with reduced AFC and age-matched controls were matured in vitro and the occurrence of aneuploidy determined as well as the mRNA level and localization of PGRMC1. Although oocytes from ovaries with reduced AFC were capable of undergoing meiosis in vitro, these oocytes showed a 3-fold increase in aneuploidy compared to oocytes isolated from control ovaries (P < 0.05). Although Pgrmc1 mRNA levels were not altered, PGRMC1 and aurora kinase B (AURKB) failed to localize to precise focal points on MII chromosomes of oocytes from ovaries with reduced AFC. Furthermore, when oocytes of control ovaries were cultured with an inhibitor of AURKB activity, their MII plate was disrupted and PGRMC1 was not properly localized to the chromosomes. These results suggest that alterations in PGRMC1 and/or AURKB localization account in part for the increased aneuploidy and low development competence of oocytes from ovaries with reduced AFC.
Keywords: aneuploidy, antral follicle count, AURKB, infertility, meiosis, oocyte, ovary, PGRMC1, premature ovarian failure
INTRODUCTION
Premature ovarian senescence as estimated by a reduced number of antral follicle count (AFC) is a common cause of infertility in both women and cattle [1, 2]. Low AFC has been described in women in the perimenopausal period [3, 4] as well as in young infertile and subfertile women affected by premature ovarian failure [1, 5], suggesting that the factors that reduce follicular reserve also affect the quality of the remaining oocytes [6].
Recently, we demonstrated that about 5% of culled dairy cows 4–8 yr old had a reduced AFC compared to age-matched controls [7]. Moreover, oocytes isolated from low AFC ovaries had limited developmental capability compared to age-matched controls [7–9]. The mechanism responsible for the reduced developmental competence of these oocytes is still unclear and it may be related to alterations in the mechanism that directs the proper segregation of chromosomes.
During oocyte meiosis, the cell division machinery must function sequentially during a reductive (meiosis I) and equational (meiosis II [MII]) cell division to ultimately produce a haploid oocyte that can be fertilized and give rise to the next generation. Errors in this process can result in aneuploidy, which can have adverse reproductive outcomes such as infertility, miscarriages, and birth defects [10–13].
Aurora kinase B (AURKB) is a component of the chromosomal passenger complex, which together with the spindle assembly checkpoint ensures faithful chromosome segregation [14, 15]. During mitosis, AURKB plays a critical role in chromosome-microtubule interactions [16]. This kinase localizes to the kinetochores from prophase to metaphase and relocates to the central spindle and the midbody during cytokinesis [17]. Alterations in the localization of AURKB are implicated in defects of centrosome function and spindle assembly and in chromosomal instability [18, 19].
Interestingly, progesterone receptor membrane component 1 (PGRMC1) and the phosphorylated (active) form of AURKB colocalize at the centromere of chromosomes in MII oocytes [20]. Moreover, an injection of a PGRMC1 antibody impairs the ability of oocytes to successfully mature and causes abnormalities in chromosomal segregation such as chromosomal misalignment and disorganization [20], suggesting that PGRMC1 plays a key role in chromosome congression. Therefore, the present study was designed to determine 1) if the decrease in oocyte developmental competence observed in dairy cows with a reduced AFC is due to a higher incidence of aneuploidy and 2) whether alterations in PGRMC1 and AURKB localization contribute to the incidence of aneuploidy.
MATERIALS AND METHODS
Oocyte Collection and Culture
All the chemicals used in this study were purchased from Sigma Chemical Company except for those specifically mentioned. Bovine ovaries were recovered at an abattoir (INALCA SpA) from pubertal females (4–8 yr old) and subjected to routine veterinary inspection in accordance to the specific health requirements stated in Council Directive 89/556/ECC and subsequent modifications. As previously reported [21], ovaries were transported at 26°C, and all the subsequent procedures were performed at 35°C–38°C. From each slaughtered animal, ovaries were isolated and classified into two previously described categories [7–9]: ovaries with reduced AFC (<10 midsized antral follicles of 2–6 mm) and age-matched controls (>10 follicles of 2–6 mm).
Cumulus-oocyte complexes (COCs) were retrieved from midsized antral follicles (2–6 mm) and examined under a stereomicroscope. Only COCs that were medium brown in color with five or more complete layers of cumulus cells with oocytes with finely granulated homogenous ooplasm were used [21].
Groups of 15–30 COCs were in vitro matured in TCM-199 supplemented with 0.68 mM L-glutamine, 25 mM NaHCO3, 0.4% fatty acid-free bovine serum albumin, 0.2 mM sodium pyruvate and 0.1 international units/ml of recombinant human follicle-stimulating hormone (r-hFSH, Gonal-F, Merck Serono SpA) in humidified air under 5% CO2 at 38.5°C as previously described [22].
Aneuploidy Assessment
Karyotype analysis was performed as previously described [23] with some modifications, and all the chromosome preparation procedures were performed at 26°C unless otherwise stated [24]. After in vitro maturation (IVM), oocytes were mechanically separated from cumulus cells by 5-min vortexing. Only oocytes with a polar body were selected for karyotype analysis and incubated for 20 min. Each oocyte was placed in a hypotonic solution (0.075 M KCl) for 20 min to induce oocyte swelling. Subsequently, the oocytes were gently transferred to a well with the first fixative (methanol:acetic acid:distilled water, 5:1:4, v/v/v) for 5 min. Then they were dropped from 5 cm to a wet slide heated at 37°C and at a slope of 45°. When the drop was spread out, the oocytes were covered with the second fixative (methanol:acetic acid, 3:1, v/v), and then the slides were immersed in a coplin jar containing the second fixative for 10 min. After that they were transferred to a second jar containing the third fixative (methanol:acetic acid:distilled water, 3:3:1, v/v/v) for 1 min, and then the slides were slowly removed and air-dried overnight. Chromosomes staining was performed using a 4% Giemsa solution for 15 min. Then the slides were extensively washed with tap and distilled water and air-dried at 37°C for 30 min and mounted. Slides were examined by light microscopy (40× magnification), and the chromosome number for each metaphase plate was counted using Leica CW4000 Karyo software (Leica Microsystems). Two investigators examined each chromosome preparation.
Real-Time PCR for PGRMC1
The levels of Pgrmc1 mRNA expression were assessed in oocytes from ovaries with low AFC and from ovaries of age-matched control group at the time of collection from the follicle (GV stage) and after 24 h of IVM (MII stage) using a real-time multiplex PCR assay. COCs were collected and cultured as described above, and cumulus cells were mechanically removed by the use of the vortex. Denuded oocytes were stored in RNAlater solution (Ambion, Life Technologies) until assayed. Total RNA was extracted from groups of 20 oocytes using the RNeasy Plus Mini Kit (Qiagen Inc.) according to the manufacturer's instruction. Total RNA was then retrotranscribed with oligo dT using the M-MLV Reverse Transcriptase System (Invitrogen, Life Technologies). A total amount of cDNA equivalent to six oocytes was used in each amplification reaction. Reactions were run in triplicates on four different biological replicates for each experimental group. Amplification was performed with specific primers and TaqMan probes for bovine Pgrmc1 (NCBI RefSeq NM_001075133.1; forward primer: 5′-CTGGAAGAGATGCATCCAGA-3′; reverse primer: 5′-GAGATCCCAGTCACTCAGGGT-3′; probe: 5′ d FAM-TCCGACCTCACTCCTGCCCA-BHQ-1 3′) and β-actin (NCBI RefSeq: NM_173979.3; forward primer: 5′-CACTCTTCCAGCCTTCCTTC-3′; reverse primer: 5′-GGATGTCCACGTCACACTTC-3′; probe: 5′ CAL Fluor Gold 540-TGCCACAGGACTCCATGCCC-BHQ-1 3′) using an CFX96 PCR Thermal Cycler (Bio-Rad). Beta actin was used as the internal standard, and the relative level of Pgrmc1 mRNA was determined using the ΔCT method.
Immunofluorescence Staining
Indirect immunofluorescence was carried out to evaluate the cellular localization of PGRMC1 and AURKB in MII-stage paraformaldehyde-fixed oocytes as previously described [20, 25]. The samples were incubated overnight at 4°C either with a rabbit polyclonal anti-PGRMC1 (dilution 1:50, Prestige Antibodies; Sigma) or a mouse monoclonal anti-AIM-1 antibody (dilution 1:50, aurora B and Ipl1-like midbody associated protein; BD Transduction Laboratories). Secondary antibodies used were: TRITC-labeled donkey anti-rabbit antibody (dilution 1:100; Vector Laboratories, Inc.) or Alexa Fluor 488-labeled donkey anti-mouse antibody (dilution 1:500; Invitrogen, Life Technologies) for 30 min at room temperature. The samples were mounted on slides in the antifade medium Vecta Shield (Vector Laboratories) supplemented with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI). In each experiment, negative controls were performed by omitting the primary antibodies; they did not reveal any staining.
Samples stained for PGRMC1 were analyzed on a C1si confocal laser-scanning microscope (Nikon Corp.) with a ×60 objective. Z-stacks were compiled with 0.25 μm intervals encompassing the entire MII plate. PGRMC1 localization was classified as regular when PGRMC1 localized at the centromeric region of each chromosome or irregular when presented with one or more of the following aspects: more than one point on a chromosome, shape different from the punctuated, not in the centromeric region, and/or lack of PGRMC1. Samples stained for AURKB were analyzed on an epifluorescence microscope (Eclipse E600; Nikon) equipped with a ×60 objective, a digital camera, and deconvolution software (NIS elements Imaging Software; Nikon). AURKB localization was classified as regular or irregular according to the criteria adopted for PGRMC1 localization.
ZM447439 Treatment
ZM447439 (Tocris) was dissolved in dimethyl sulfoxide (DMSO) at 10 mM and stored in aliquots at −20°C. Appropriate concentrations were prepared in culture medium so that the final concentration of DMSO never exceeded 1 μl/ml. This DMSO concentration has been previously reported to have no effect on bovine oocyte maturation and fertilization [26, 27].
In a first set of experiments, GV-stage oocytes were cultured in the presence of the aurora kinase activity inhibitor ZM447439 in concentrations ranging from 0 to 10 μM. After 24 h, COCs were freed of cumulus cells, fixed, and stained with 1 μg/ml DAPI. Samples were analyzed on a epifluorescence microscope (Eclipse E600; Nikon) for polar body extrusion, maturation rate, and chromosome alignment. In a second set of experiments GV-stage oocytes were cultured in presence of 5 μM ZM447439, then fixed and stained for PGRMC1 immunolocalization as described above.
All the MII-stage oocytes analyzed, either following three-dimensional reconstruction of the metaphasic plate by confocal microscopy or with conventional fluorescence microscopy, that failed to align all their chromosome along the equator of the metaphasic plate were classified as misaligned as previously described [28].
Statistical Analysis
All the experiments were repeated three to five times. Observations from all the experiments were pooled. Statistical significance was determined by the Fisher exact test. Values of P < 0.05 were considered significant.
RESULTS
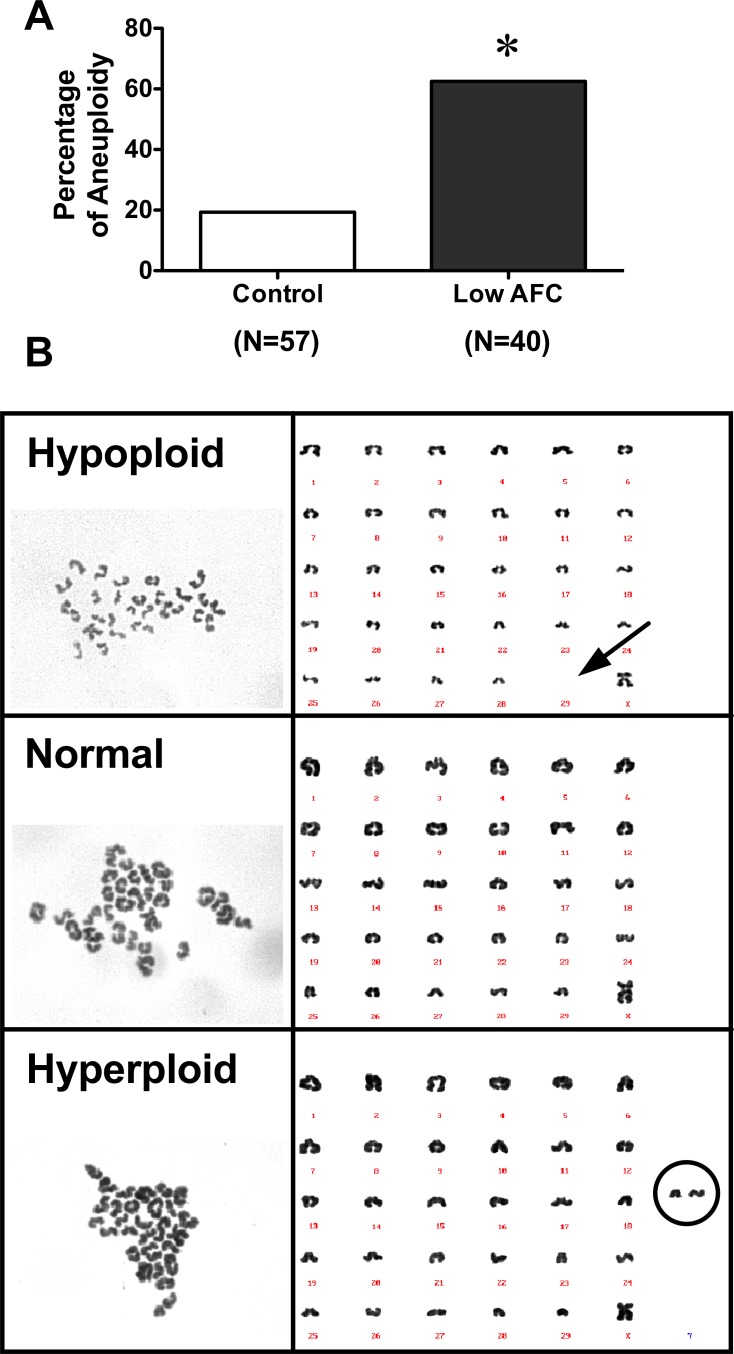
Maturation rates were similar between oocytes isolated from reduced AFC (89%, 67/75) and age-matched control ovaries (94%, 119/127). Importantly, karyotype analysis (Fig. 1) indicated that although oocytes from ovaries with reduced AFC were capable of undergoing meiosis in vitro, these oocytes showed a 3-fold increase in aneuploidy compared to oocytes isolated from age-matched control ovaries (Fig. 1A). The analysis of karyotypes detected both hyperploid and hypoploid (Fig. 1B) oocytes within each group, but there was not a difference in the percentage of hypoploid or hyperploid oocytes between the reduced AFC and age-matched control groups. Moreover, no specific chromosome-dependence aneuploidy was detected.
FIG. 1.
A) Percentage of aneuploidy in oocytes isolated from control and low AFC ovaries. Data were analyzed by the Fisher exact test (*P < 0.05). B) Representative images of hypoploid, normal, and hyperploid karyograms.
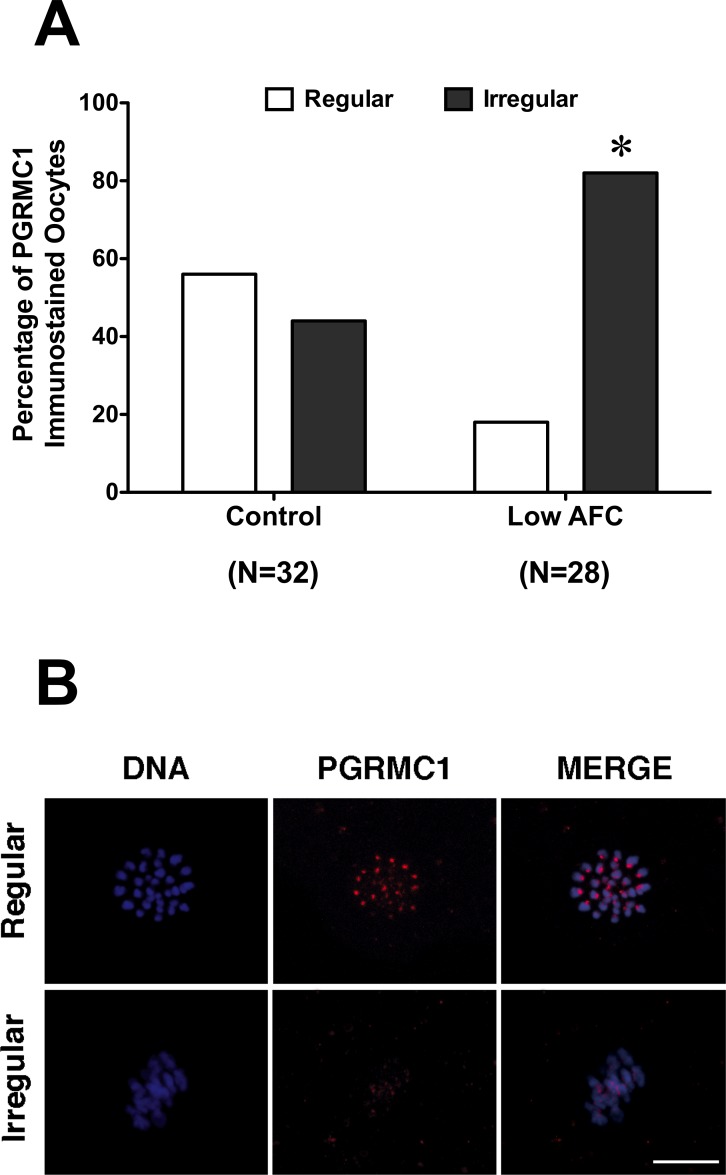
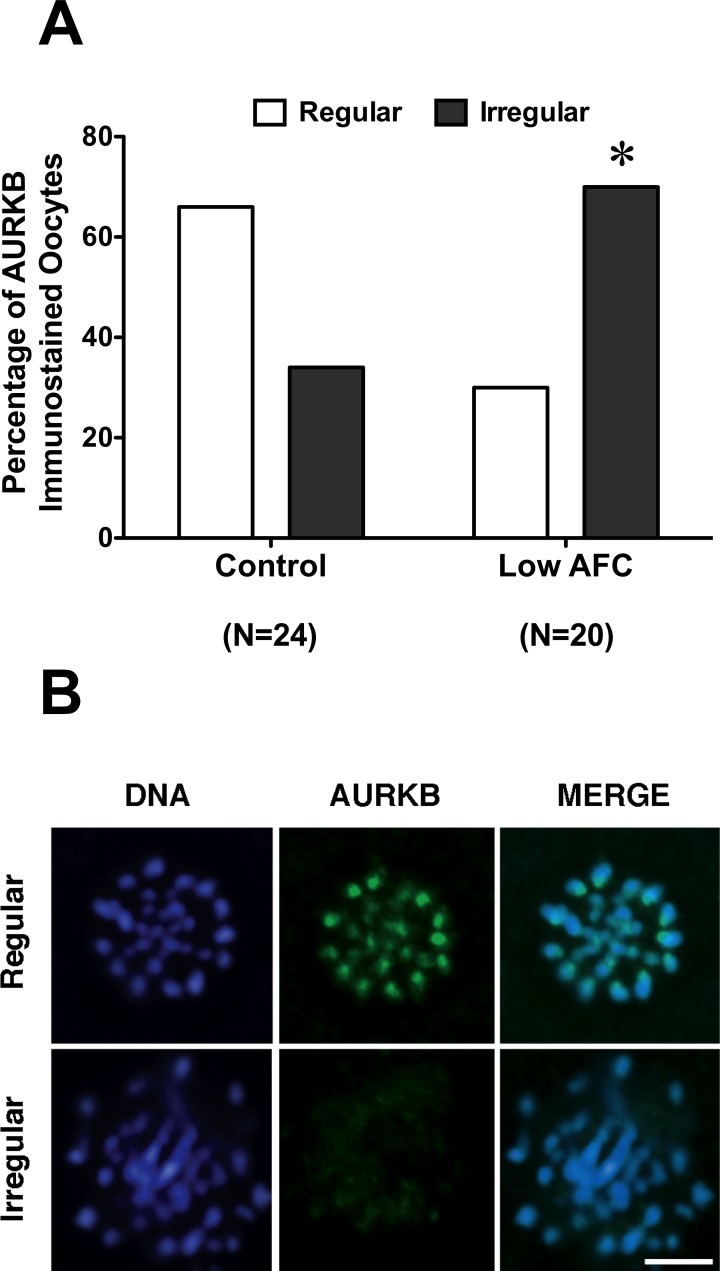
Because PGRMC1 plays a role in the formation of the MII plate, Pgrmc1 mRNA levels and the colocalization of PGRMC1 with AURKB was monitored at the GV and at the MII stage. Pgrmc1 mRNA levels were not altered in either GV- or MII-staged oocytes of ovaries with reduced ovarian reserve when compared to the age-matched control group (Supplemental Fig. S1, available online at www.biolreprod.org). Moreover, PGRMC1 and AURKB colocalized at the centromeric region of MII-stage bovine oocyte, as confirmed in a control experiment that corroborated our previous observations (Fig. 2). However, PGRMC1 failed to localize to precise focal points on MII chromosomes in a significantly higher percentage of oocytes isolated from reduced AFC ovaries when compared to oocytes of age-matched control ovaries (Fig. 3, A and B). Similarly, the percentage of oocytes in which AURKB failed to properly localize on the MII chromosomes was significantly higher in oocytes from ovaries with reduced AFC compared to oocytes isolated from ovaries of age-matched controls (Fig. 4, A and B).
FIG. 2.
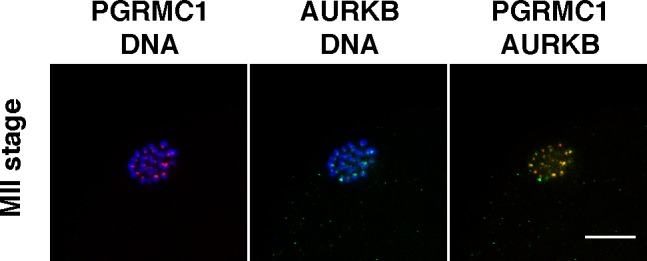
PGRMC1 and AURKB colocalization on the centromere of chromosomes in metaphase II control oocytes. Red, PGRMC1; green, AURKB; blue, DNA. Bar = 10 μm.
FIG. 3.
PGRMC1 localization in oocytes isolated from low AFC and age-matched control ovaries. A) Percentage of oocytes isolated from control and low antral follicle count ovaries showing regular and irregular PGRMC1 localization. Data were analyzed by the Fisher exact test (*P < 0.05). B) Representative images of regular and irregular localization of PGRMC1 in MII-stage oocytes. Blue, DNA; red, PGRMC1. Bar = 10 μm.
FIG. 4.
AURKB localization in oocytes isolated from low AFC and age-matched control ovaries. A) Percentage of oocytes isolated from control and low antral follicle count ovaries showing regular and irregular AURKB localization. Data were analyzed by the Fisher exact test (*P < 0.05). B) Representative images of regular and irregular localization of AURKB in MII-stage oocytes. Blue, DNA; green, AURKB. Bar = 5 μm.
To further investigate the relationship between PGRMC1 and the AURKB function during oocyte maturation, oocytes isolated from control ovaries were matured in the presence of increasing concentrations of ZM447439 [28], an AURKB inhibitor [29]. At all the concentrations tested (1–10 μM), the percentages of oocytes that reached MII with polar body extrusion after 24 h of treatment did not differ from control oocytes (Table 1). However at the concentrations of 5 and 10 μM, a significantly higher percentage of oocytes exhibited misaligned chromosomes when compared to controls (Table 1). In another set of experiments, when oocytes of age-matched control ovaries were cultured with 5 μM of ZM447439, the chromosomal organization of the MII plate was disrupted (Fig. 5) and PGRMC1 was not properly localized to the chromosomes (Table 2) even though the ability of oocytes to mature was not affected (Table 2).
TABLE 1.
Effect of ZM447439 on meiotic progression, polar body (PB) extrusion, and chromosome alignment.*
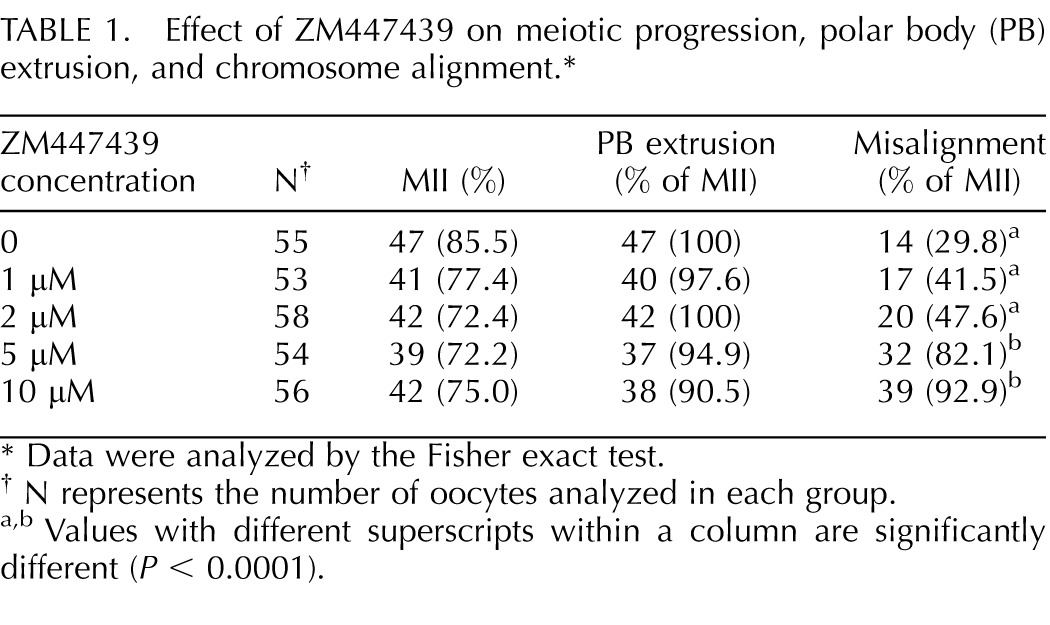
FIG. 5.
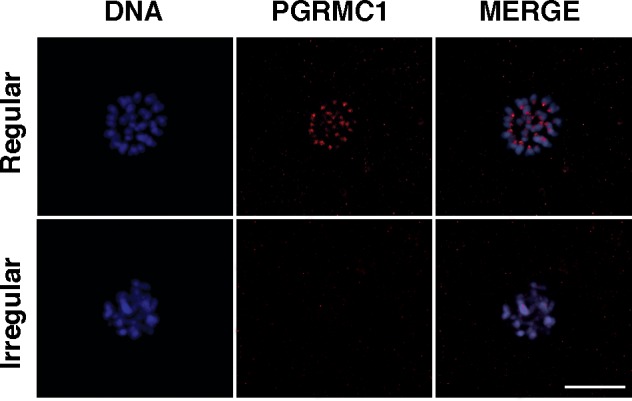
PGRMC1 localization in oocytes of control ovaries treated with the AURKB inhibitor ZM447439. Representative images of regular and irregular localization of PGRMC1 in MII-stage oocytes. Blue, DNA; red, PGRMC1. Bar = 10 μm.
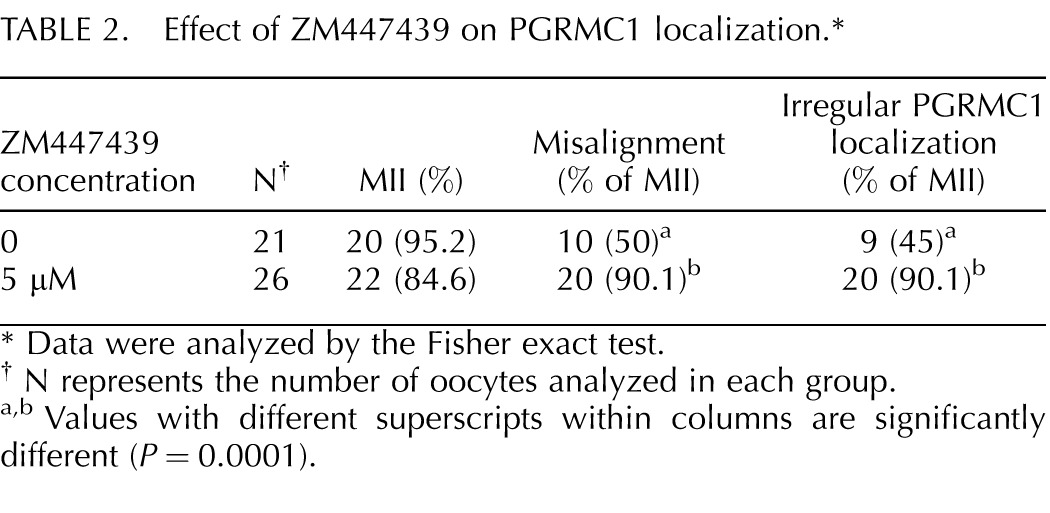
TABLE 2.
Effect of ZM447439 on PGRMC1 localization.*
DISCUSSION
During oocyte meiotic division, defects in spindle formation and/or function can generate chromosome instability and aneuploidy, a condition that is the major cause of defective early embryonic development, miscarriages, and birth defects [30]. While errors in chromosomal segregation can occur at any time during embryonic development, aneuploidy occurs frequently during oocyte meiotic division with about 20% of human oocytes having chromosomal segregation errors [31]. While aneuploidy is an important cause of reproductive wastage, the factors that promote chromosomal segregation errors have not been clearly defined. In mice, the frequency of chromosomal segregation errors (i.e., aneuploidy) increases with the gradual depletion of ovarian follicle reserve, which is associated with increasing chronological age [32]. The present work also reveals a relationship between ovarian reserve and chromosomal segregation errors by demonstrating that oocytes collected from cows with reduced ovarian reserve (i.e., reduced AFC) have a 3-fold increase in aneuploidy compared with oocytes collected from cows with a normal AFC. In contrast to the well-known age-related increase in chromosomal segregation errors, the present study examined animals of the same approximate age. Therefore, there may be some defect(s) within oocytes isolated from ovaries with premature decreases in ovarian reserve that are distinctly different from age-related alterations.
As evidence of functional molecular alterations in oocytes harvested from ovaries with reduced ovarian reserve, the present study reveals that PGRMC1 fails to properly associate with the MII chromosomes of oocytes isolated from cows with a reduced ovarian reserve. This inability to target PGRMC1 to the MII chromosomes occurs even though Pgrmc1 is expressed at levels similar to that of oocytes with normal ovarian reserve. Moreover, failure of PGRMC1 to associate with the MII chromosomes likely accounts, at least in part, for their higher incidence of aneuploidy because disrupting PGRMC1's action with an antibody to PGRMC1 interferes with the formation of the MII plate [20].
Although PGRMC1 influences the formation of the MII plate, its mechanism of action is not known. PGRMC1 colocalizes with the active (Thr-232 phosphorylated) form of AURKB. AURKB is gradually activated during oocyte maturation and phosphorylates essential proteins that are involved in chromosome segregation such as the meiotic cohesin protein Rec8 [33], which functions to hold sister chromatids together [34].
Although AURKB colocalizes with PGRMC1, a functional relationship between these two proteins has not been established. The present study demonstrates that the AURKB inhibitor, ZM447439, causes chromosome misalignment and alters PGRMC1's localization at centromeres in oocytes obtained from ovaries with high or normal AFC. We argue that AURKB activity is required to properly localize PGRMC1 to the MII chromosomes. However, some caution must be exercised in drawing this conclusion because ZM447439 could inhibit AURKA or AURKC as well as AURKB. Importantly, at all the concentrations tested, ZM447439 did not inhibit maturation and polar body extrusion, which are known to be dependent on AURKA [28, 35]. This argues that ZM447439 does not inhibit AURKA even at the maximum concentration used in this study (10 μM). Interestingly, the expression of the AURKC isoform is negligible in immature and mature bovine oocytes [36]. It is likely then that even though the inhibition of AURKC cannot be completely excluded, the biological effect of ZM447439 in bovine oocyte should be principally ascribed to the inhibition of AURKB, and it is therefore logical to conclude that AURKB is required for PGRMC1 to properly localize to the MII chromosomes.
Because our data indicate that Pgrmc1 mRNA levels were not reduced in oocytes of ovaries with reduced AFC, the hypothesis that PGRMC1 regulates oocyte maturation through the association with AURKB raises the question as to whether posttranslational modification of PGRMC1 could be regulated by AURKB. Several proteomic-based studies indicate that PGRMC1 can be phosphorylated at several serine residues [37, 38]. In fact, phosphorylated PGRMC1 is associated with the mitotic spindle [39, 40]. Because AURKB is a serine/threonine kinase [41], it is possible that AURKB either directly or indirectly phosphorylates PGRMC1 with phosphorylation directing PGRMC1 to the MII chromosomes. This hypothesis is currently being assessed.
In addition to phosphorylation, recent studies reveal that PGRMC1 is sumoylated (i.e., posttranscriptional addition of small ubiquitin-related modifier [SUMO] proteins) in ovarian cells [42]; sumoylation involves covalent attachment of SUMO proteins to lysine residues in substrate proteins. Substrate modification by sumoylation can alter protein-protein interactions, change protein intracellular localization, or direct changes in the activities of the protein to which SUMO is attached [43]. Importantly, sumoylation may play an essential role in regulating mouse oocyte meiosis because SUMO1, or more likely proteins that are covalently coupled to SUMO1, localize to the spindle poles in prometaphase I, metaphase I and II, and around the separating chromosomes in anaphase I and telophase I, while SUMO2/3 is mainly concentrated near the centromeres [44]. These observations are consistent with the concept that part of mechanism that localizes PGRMC1 to MII chromosomes involves both phosphorylation and sumoylation.
In conclusion, our data support the hypothesis that PGRMC1 and AURKB interaction during oocyte meiosis plays an essential role in the process of chromosome segregation because the inhibition of AURKB causes changes in the localization of PGRMC1 and alterations in the MII chromosomal plate. Moreover, alteration in the localization of PGRMC1 and AURKB could account in part for the increased aneuploidy and low development competence of oocytes of ovaries isolated from cows with reduced ovarian reserve.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to Dr. Pietro Parma of the Department of Agricultural and Environmental Sciences-Production, Landscape, Agro-energy, University of Milan, for expert contribution on the karyotype analysis; Prof. Cristiano Rumio of the Department of Medical Biotechnology and Translational Medicine, University of Milan, for support in LSCM; and Xiufang Liu, Department of Cell Biology, University of Connecticut Health Center, for excellent assistance with the real-time PCR analysis.
Footnotes
Supported by grants from OECD Co-operative Research Programme: Biological Resource Management for Sustainable Agricultural Systems (Contract: JA00064361 to A.M.L.), by a CIG-Marie Curie International Reintegration Grant within the 7th European Community Framework Programme (Contract: 303640, “Pro-Ovum,” to V.L. and A.M.L.), and by NIH grant R01 HD052740 (to J.J.P.). F.F. received a “L'Oreal Women for Science” 2012 fellowship; I.T. was supported by “Dote Ricercatori” FSE, Regione Lombardia, Italy.
REFERENCES
- Monget P, Bobe J, Gougeon A, Fabre S, Monniaux D, Dalbies-Tran R. The ovarian reserve in mammals: a functional and evolutionary perspective. Mol Cell Endocrinol. 2012;356:2–12. doi: 10.1016/j.mce.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JL, Mossa F, Lonergan P, Evans AC. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev. 2011;23:1–14. doi: 10.1071/RD10226. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Johnstone E, Addauan-Andersen C, Cedars MI. A lower antral follicle count is associated with infertility. Fertil Steril. 2011;95:1950–1954. doi: 10.1016/j.fertnstert.2011.01.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modina SC, Tessaro I, Lodde V, Franciosi F, Corbani D, Luciano AM. Reductions in the number of mid-sized antral follicles are associated with markers of premature ovarian senescence in dairy cows Reprod Fertil Dev 2013. (in press). Published online 18 January 2013; http://dx.doi.org/10.1071/RD12295. [DOI] [PubMed] [Google Scholar]
- Modina S, Borromeo V, Luciano AM, Lodde V, Franciosi F, Secchi C. Relationship between growth hormone concentrations in bovine oocytes and follicular fluid and oocyte developmental competence. Eur J Histochem. 2007;51:173–180. [PubMed] [Google Scholar]
- Tessaro I, Luciano AM, Franciosi F, Lodde V, Corbani D, Modina SC. The endothelial nitric oxide synthase/nitric oxide system is involved in the defective quality of bovine oocytes from low mid-antral follicle count ovaries. J Anim Sci. 2011;89:2389–2396. doi: 10.2527/jas.2010-3714. [DOI] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Yanowitz J. Meiosis: making a break for it. Curr Opin Cell Biol. 2010;22:744–751. doi: 10.1016/j.ceb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragouli E, Wells D, Delhanty JD. Chromosome abnormalities in the human oocyte. Cytogenet Genome Res. 2011;133:107–118. doi: 10.1159/000323801. [DOI] [PubMed] [Google Scholar]
- Jones KT, Lane SI. Chromosomal, metabolic, environmental, and hormonal origins of aneuploidy in mammalian oocytes. Exp Cell Res. 2012;318:1394–1399. doi: 10.1016/j.yexcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Sun SC, Kim NH. Spindle assembly checkpoint and its regulators in meiosis. Hum Reprod Update. 2012;18:60–72. doi: 10.1093/humupd/dmr044. [DOI] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- Ota T, Suto S, Katayama H, Han ZB, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M. Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Cancer Res. 2002;62:5168–5177. [PubMed] [Google Scholar]
- Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Lodde V, Franciosi F, Ceciliani F, Peluso JJ. Progesterone receptor membrane component 1 expression and putative function in bovine oocyte maturation, fertilization, and early embryonic development. Reproduction. 2010;140:663–672. doi: 10.1530/REP-10-0218. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Franciosi F, Modina SC, Lodde V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s) Biol Reprod. 2011;85:1252–1259. doi: 10.1095/biolreprod.111.092858. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a Co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′, 5′-monophosphate, and glutathione. Mol Reprod Dev. 2005;71:389–397. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Ergun B, Huang TH, Rosenwaks Z, Palermo GD. A reliable technique of nuclear transplantation for immature mammalian oocytes. Hum Reprod. 1999;14:1312–1317. doi: 10.1093/humrep/14.5.1312. [DOI] [PubMed] [Google Scholar]
- Lundsteen C, Lind AM. A test of a climate room for preparation of chromosome slides. Clin Genet. 1985;28:260–262. doi: 10.1111/j.1399-0004.1985.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Modina S, Beretta M, Lodde V, Lauria A, Luciano AM. Cytoplasmic changes and developmental competence of bovine oocytes cryopreserved without cumulus cells. Eur J Histochem. 2004;48:337–346. [PubMed] [Google Scholar]
- Lazzari G, Tessaro I, Crotti G, Galli C, Hoffmann S, Bremer S, Pellizzer C. Development of an in vitro test battery for assessing chemical effects on bovine germ cells under the ReProTect umbrella. Toxicol Appl Pharmacol. 2008;233:360–370. doi: 10.1016/j.taap.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Franciosi F, Lodde V, Corbani D, Lazzari G, Crotti G, Galli C, Pellizzer C, Bremer S, Weimer M, Modina SC. Transferability and inter-laboratory variability assessment of the in vitro bovine oocyte maturation (IVM) test within ReProTect. Reprod Toxicol. 2010;30:81–88. doi: 10.1016/j.reprotox.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Shuda K, Schindler K, Ma J, Schultz RM, Donovan PJ. Aurora kinase B modulates chromosome alignment in mouse oocytes. Mol Reprod Dev. 2009;76:1094–1105. doi: 10.1002/mrd.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going Hum Mol Genet 2007. 16 (Spec No. 2): R203 R208 [DOI] [PubMed] [Google Scholar]
- Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:206–212. doi: 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol. 2002;157:219–229. doi: 10.1083/jcb.200110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Jessberger R. Keeping sister chromatids together: cohesins in meiosis. Reproduction. 2005;130:783–790. doi: 10.1530/rep.1.00864. [DOI] [PubMed] [Google Scholar]
- Vogt E, Kipp A, Eichenlaub-Ritter U. Aurora kinase B, epigenetic state of centromeric heterochromatin and chiasma resolution in oocytes. Reprod Biomed Online. 2009;19:352–368. doi: 10.1016/s1472-6483(10)60169-1. [DOI] [PubMed] [Google Scholar]
- Uzbekova S, Arlot-Bonnemains Y, Dupont J, Dalbies-Tran R, Papillier P, Pennetier S, Thelie A, Perreau C, Mermillod P, Prigent C, Uzbekov R. Spatio-temporal expression patterns of aurora kinases a, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol Reprod. 2008;78:218–233. doi: 10.1095/biolreprod.107.061036. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Adam G, Seeger H, Mueck AO, Solomayer E, Wallwiener D, Cahill MA, Fehm T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12:230–239. doi: 10.1080/13697130802635637. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Clare SE, Wozny W, Schwall GP, Poznanovic S, Stegmann W, Vogel U, Sotlar K, Wallwiener D, Kurek R, Fehm T, Cahill MA. Breast cancer proteomics reveals correlation between estrogen receptor status and differential phosphorylation of PGRMC1. Breast Cancer Res. 2008;10:R85. doi: 10.1186/bcr2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci U S A. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodde V, Peluso JJ. A novel role for progesterone and progesterone receptor membrane component 1 in regulating spindle microtubule stability during rat and human ovarian cell mitosis. Biol Reprod. 2011;84:715–722. doi: 10.1095/biolreprod.110.088385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Lodde V, Liu X. Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells. Endocrinology. 2012;153:3929–3939. doi: 10.1210/en.2011-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Wang ZB, Ou XH, Tong JS, Li S, Wei L, Ouyang YC, Hou Y, Schatten H, Sun QY. The SUMO pathway functions in mouse oocyte maturation. Cell Cycle. 2010;9:2640–2646. doi: 10.4161/cc.9.13.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.