Abstract
Background
Anorexia is a common symptom among cancer patients and contributes to malnutrition and strongly impinges on quality of life. Cancer-induced anorexia is thought to be caused by an inability of food intake-regulating systems in the hypothalamus to respond adequately to negative energy balance during tumour growth. Here, we show that this impaired response of food-intake control is likely to be mediated by altered serotonin signalling and by failure in post-transcriptional neuropeptide Y (NPY) regulation.
Methods
Two tumour cachectic mouse models with different food intake behaviours were used: a C26-colon adenocarcinoma model with increased food intake and a Lewis lung carcinoma model with decreased food intake. This contrast in food intake behaviour between tumour-bearing (TB) mice in response to growth of the two different tumours was used to distinguish between processes involved in cachexia and mechanisms that might be important in food intake regulation. The hypothalamus was used for transcriptomics (affymetrix chips).
Results
In both models, hypothalamic expression of orexigenic NPY was significantly higher compared with controls, suggesting that this change does not directly reflect food intake status but might be linked to negative energy balance in cachexia. Expression of genes involved in serotonin signalling showed to be different between C26-TB mice and Lewis lung carcinoma-TB mice and was inversely associated with food intake. In vitro, using hypothalamic cell lines, serotonin repressed neuronal hypothalamic NPY secretion while not affecting messenger NPY expression, suggesting that serotonin signalling can interfere with NPY synthesis, transport, or secretion.
Conclusions
Altered serotonin signalling is associated with changes in food intake behaviour in cachectic TB mice. Serotonins' inhibitory effect on food intake under cancer cachectic conditions is probably via affecting the NPY system. Therefore, serotonin regulation might be a therapeutic target to prevent the development of cancer-induced eating disorders.
Keywords: Anorexia, Serotonin, Neuropeptide Y, Cancer, Cachexia
Introduction
Anorexia and consequently a reduced caloric intake1 affects a majority of all cancer patients.2 Cancer patients with good appetite and adequate caloric intake have higher survival rates than patients who suffer from anorexia and hence a lower energy intake.3,4 Cancer-induced anorexia is considered to be predominantly caused by failure of orexigenic and anorexigenic food intake-regulating systems in the hypothalamus to respond adequately to changes in energy balance.5 This merits further elucidation of the specific mechanisms involved in this hypothalamic resistance to peripheral neuroendocrine triggers. Several food intake-regulating systems have been mentioned to play a role in the onset of hypothalamic resistance, including the orexigenic triggers neuropeptide Y (NPY) and ghrelin, and anorexigenic melanocortin and CART systems. Cancer anorexia often develops during the progression of cachexia,6 and the combined clinical picture is often called anorexia–cachexia syndrome.7 Cachexia is a complex metabolic syndrome associated with underlying illness and is characterized by progressive loss of muscle (muscle wasting) with or without loss of fat mass. This results in weight loss, a reduced quality of life and a shortened survival time.6,8,9 Although anorexia is often linked to cachexia and likely to be initiated by similar pathologies (tumour growth), it is unclear as to what extent anorexia and the metabolic alterations of cachexia affect each other, or to what extent these are distinct entities with their own pathology. Here, we use two tumour-bearing (TB) mouse models displaying similar development of cachexia. At the same time, they show opposite effects on food intake in response to tumour growth. This provides opportunities to disentangle processes involved in anorexia from those causing cachexia. By applying transcriptomics, we show that tumour-induced cachexia is associated with several distinct changes in food-intake, regulating mediators in the hypothalamus that appear to be independent of food intake status. Furthermore, we found alterations in hypothalamic serotonin signalling, and these changes were inversely associated with food intake. In addition, we used hypothalamic cell lines to explore the mechanisms involved in the interaction between serotonin and the NPYergic system.
Materials and methods
Lewis lung tumour model (LLM)
C57Bl/6 male mice (Harlan, Barcelona, Spain), weighing approximately 20 g, were placed on a standard ad libitum diet (Panlab, Barcelona, Spain) and had free access to water. Lewis Lung carcinoma (LLC) cells were obtained from exponential tumours. Under general anaesthesia, 0.5 × 106 cells were injected intramuscularly in the hind leg. Four groups were included: C, control mice sacrificed at Day 14 (n = 6); TB-10, TB mice sacrificed at Day 10 (n = 6); TB-14, TB mice sacrificed at Day 14 (n = 8), and TB-17, TB mice sacrificed at Day 17 (n = 6). Body weight and food intake were measured daily. At the day of sacrifice, mice were anesthetized with a ketamine/xylazine mixture (i.p., Imalgene® and Rompun®, respectively), and blood was collected by cardiac puncture.
C26 colon adenocarcinoma tumour model
Part of the data (Figure 1A) from this experimental arm with C26 TB mice have been published previously10 but are briefly described again here for easy comparison with Lewis lung TB mice.
Figure 1.
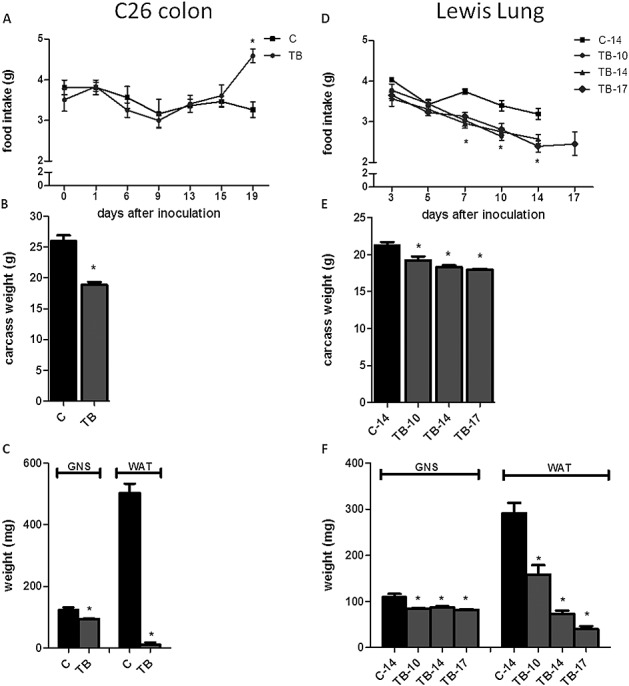
Effect of tumour inoculation on food intake, carcass weight and muscle, and fat weight in two cachectic models. (A) Time course of change in food intake in mice bearing the C26 tumour. (B) Carcass weight of C26 tumour-bearing mice at Day 19. (C) Weight of m. gastrocnemius and epididymal fat pads in C26 tumour-bearing mice at Day 19. (D) Time course of change in food intake of tumour-bearing mice bearing the Lewis lung carcinoma. (E) Carcass weight of Lewis lung carcinoma tumour-bearing mice at Days 10, 14, and 17. (F) Weight of m. gastrocnemius and epididymal fat pads in Lewis lung carcinoma tumour-bearing mice at Days 10, 14, and 17. *Significantly different from C (P < 0.05). Data are expressed as mean ± SEM. C, sham-injected control; TB, C26-injected tumour bearing; C-14, sham-injected control sacrificed at Day 14; TB-10, Lewis lung carcinoma-injected tumour bearing sacrificed at Day 10; TB-14, Lewis lung carcinoma-injected tumour bearing sacrificed at Day 14; and TB-17, Lewis lung-injected tumour bearing sacrificed at Day 17. Carcass weight was calculated by body weight at day of section minus tumour weight.
Male CDF1 (BALB/cx DBA/2) mice aged 6–7 weeks (Harlan Nederland, Horst, The Netherlands) were placed on a standard ad libitum diet (AIN93M, Research Diet Services, The Netherlands) and had free access to water.
Murine C26 adenocarcinoma cells were cultured and suspended as described previously.11 Under general anaesthesia (isoflurane/N2O/O2), 1 × 106 tumour cells in 0.2 mL Hank's balanced salt solution (HBSS) were inoculated subcutaneously into the right inguinal flank. Controls were sham-injected with 0.2 mL HBSS.
Two groups were included: C, control mice (n = 6) and TB (n = 9).
Body weight and food intake were measured three times a week. At Day 19, blood was collected by cardiac puncture under general anaesthesia.
In both tumour models, animals were individually housed 1 week before start of the experiment in a climate-controlled room (12:12 dark-light cycle, 21°C ± 1°C).
After sacrifice, brain, hypothalamus, tumour, organs, and lower leg skeletal muscles (non-tumour leg) were weighted and frozen in liquid nitrogen and were stored at −80°C. Carcass weight was calculated by body weight at day of section minus tumour weight.
All experimental procedures were made in accordance with the European community guidelines for the use of laboratory animals and complied with the principles of good laboratory animal care.
Cell culture and reagents
Murine-derived hypothalamic neuronal cell lines hypoE-46 and hypo-A2/12 (CELLutions Biosystems Inc., Canada) were grown and maintained in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% heat-inactivated foetal calf serum, 100 unit/mL penicillin and 100 µg/mL streptomycin at 37°C under 5.0% CO2. Cells were grown in monolayers to 90% confluency. Then, medium was replaced by serum-free DMEM containing penicillin and streptomycin. After 4 h, cells were exposed to serotonin (100 µg/mL, 1 µg/mL, 10 ng/mL, or 100 pg/mL) for 24 h or 60 mM KCl for 15 min. After exposure, supernatant was collected and used for NPY measurements using an enzyme immunoassay (NPY EIA, Phoenix Pharmaceuticals, CA, USA). Cells were homogenized in 40 mM Tris, 1 mM ethylenediaminetetraacetic acid, 5 mM ethylene glycol tetraacetic acid, and 0.50% Triton X-100. Homogenates were used to measure protein content (Pierce Bicinchoninic acid Rockford, IL, USA). Serotonin cytotoxicity was determined by measuring lactate deshydrogenase leakage and effects on cell viability using a tetrazolium salt XTT assay (Roche Diagnostics, Mannheim, Germany).
Serotonin levels
Hypothalamic samples were used for microarray experiments, while remaining brain parts were used to determine serotonin levels. Brains were homogenized in 1 mL containing in 40 mM Tris, 1 mM ethylenediaminetetraacetic acid, 5 mM ethylene glycol tetraacetic acid, and 0.50% Triton X-100. Serotonin levels were measured using enzyme-immunoassay kits (BAE-5900, LDN, Nordhorn, Germany) and were corrected for the amount of protein (nanodrop spectrophotometer, Thermo Scientific).
Statistics
Data were analysed by statistical analysis of variance followed by a post hoc Bonferroni test or by a Student's t-test. Differences were considered significant at a two-tailed P < 0.05. Statistical analyses were performed using GraphPad Prism 5. For statistical analysis of microarray data, see microarray section.
Microarray studies
Total RNA from the hypothalamus was isolated by using RNeasy lipid tissue kit (Qiagen, Venlo, The Netherlands). RNA concentrations were measured by absorbance at 260 nm (nanodrop). RNA quality was checked using the RNA 6000 n assay on the Agilent 2100 bioanalyser (Agilent Techologies, Amsterdam, The Netherlands) according to the manufacturer's protocol. For each mouse, total RNA (100 ng) was labelled using the Ambion WT expression kit (Life Technologies, Bleiswijk, The Netherlands). Microarray experiments were performed by using affymetrix mouse gene ST 1.1.
From the C26 model experiment, four control samples and five samples from TB mice were included in this experiment; however, one control sample gave multiple spots on the array and was therefore excluded from analysis. The Lewis lung experiment included four controls, five TB-10, seven TB-14, and six TB-17 samples.
Array data were analysed using an in-house, online system.12Briefly, probesets were redefined according to Dai et al.13 using remapped computable document format version 15.1 based on the Entrez gene database. In total, these arrays target 21 225 unique genes. Robust multi-array analysis was used to obtain expression values.14,15 We only took genes into account that had an intensity >20 on at least two arrays, had an interquartile range throughout the samples of >0.1, and had at least seven probes per gene. Genes were considered differentially expressed at P < 0.05 after intensity-based moderated t-statistics.16 Further functional interpretation of the data was performed through the use of Ingenuity Pathway Analysis (IPA) (Ingenuity® Systems, www.ingenuity.com). Canonical pathway analysis identified the pathways from the IPA library of canonical pathways that were most significant to the data set. Genes from the data set that met the cut-off of 1.3-fold change and P-value cut off of 0.05 and were associated with a canonical pathway in the ingenuity knowledge base were considered for the analysis. To list up genes involved in food intake regulation, genes included in gene ontology (GO) 0002023, GO 0007631, GO 0008343, GO 0060259, and GO 0042755 (food intake, feeding behaviour, and eating behaviour) were analysed (gene ontology, tool for the unification of biology. The Gene Ontology Consortium (2000) Nature Genet. 25: 25–29). Also, genes pro-opiomelanocortin (ID: 18976), growth hormone (ID: 14599), Sst (ID: 20604), and pro-melanin-concentrating hormone (PMCh) (ID: 110312) were additionally looked at,since they also have been described to play a role in food intake behaviour.17
Array data have been submitted to the gene expression omnibus, accession numbers GSE44082 (C26 tumour) and GSE57190 (Lewis Lung tumour and hypothalamic cell lines). Data set containing the array data on hypothalamic tissues of C26 TB mice has been published previously.10
Results
Body weight and food intake
In both tumour models, TB mice showed a lower carcass weight, muscle weight, and white adipose fat mass (Figure 1) compared with their healthy controls, reflecting that cachexia was present in mice bearing either the C26 or the Lewis Lung tumour.
Food intake changes in TB animals were found to differ between the two cachectic models. Mice bearing the C26 tumour was found to increase their food intake synchronously to loss of body weight,10 while mice bearing the LLC showed a lowered food intake from Day 7 after tumour inoculation compared with controls (Figure 1 A and D). These opposing changes in food intake in TB mice in response to tumour growth of the two different tumours are used to disentangle shared mechanisms of cachexia from those important for food intake regulation.
Microarray analysis of the hypothalamus: comparison of C26 and LLM
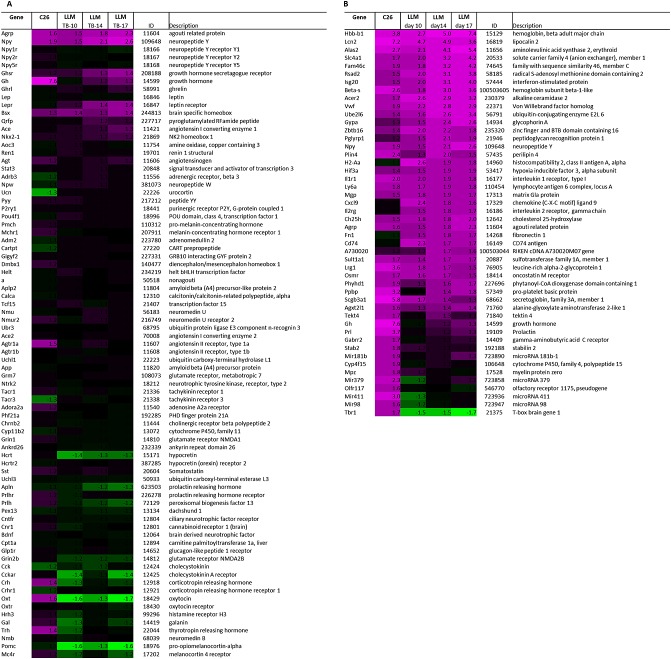
Expression of genes involved in food intake was analysed to determine if these could explain the opposing changes in food intake between C26 TB and LLC TB mice. A list of genes involved in food intake was generated by using GO categories involved in food intake regulation (M&M). Fold changes of expression of orexigenic and anorexigenic genes were compared between the two tumour models (Figure 2A). Interestingly, changes in gene expression of important mediators of food intake regulation, NPY, AgRP, and pro-opiomelanocortin showed to be similar in C26 TB mice and LLC TB mice compared with their controls even though food intake behaviour was different between the two models. Furthermore, gene expression of oxytocin showed to be altered in TB mice compared with controls and differed between the two models.
Figure 2.
Heat map representation of gene expressions in the hypothalamus in C26 and Lewis lung carcinoma tumour-bearing mice. (A) Fold changes of orexigenic and anorexigenic genes relative to their control group were calculated and compared between C26 and Lewis lung carcinoma tumour-bearing mice. (B) Top 30 up-regulated genes in C26 tumour-bearing mice compared with their controls and top 30 up-regulated genes in Lewis lung carcinoma tumour-bearing mice compared with their controls. Twelve genes were overlapping between the C26 and Lewis lung carcinoma tumour model. Each row represents a gene, and each column represents a group of animals. Red colour indicates genes that were higher expressed as controls, and green colour indicates genes that were lower expressed as the controls. Black indicates genes whose expression was similar to compared with controls. ID: Entrez ID. C, sham-injected control; TB, C26-injected tumour-bearing; C-14, sham-injected control sacrificed at Day 14; TB-10, Lewis lung carcinoma-injected tumour bearing sacrificed at Day 10; TB-14, Lewis lung carcinoma-injected tumour bearing sacrificed at Day 14; and TB-17, Lewis lung carcinoma-injected tumour bearing sacrificed at Day 17.
In addition, analysis of highly up-regulated genes (top 30 up-regulated genes in TB mice compared with controls) in both tumour models was performed. There was a strong overlap of genes that was highly up-regulated in both C26 TB and LLC TB mice compared with their controls, including NPY and AgRP. Furthermore, expression of inflammatory markers lipocalin 2, leucin-rich α2-glycoprotein 1, secretoglobin 3a1, and oncostatin M receptor showed to be highly up-regulated in TB mice in both models. Taken together, this suggests that major alterations in hypothalamic mechanisms are similar for both tumour models.
Microarray analysis of the hypothalamus: serotonin signalling
We hypothesized serotonin to play a crucial role in the opposing food intake behaviour between the C26 tumour and LLC tumour model. Therefore, expression levels of genes involved in serotonin signalling were compared between the two tumour models. C26 TB mice expressing compensatory eating behaviour in response to weight loss showed altered serotonin signalling and lower brain serotonin levels compared with their controls. In LLC TB mice, genes involved in serotonin signalling showed a different expression pattern compared with C26 TB mice. Expression of tryptophan hydroxylase (tph) was strongly down-regulated in C26 TB mice compared with controls, while expression of this gene was not different from controls in LLC TB mice. Expression of ddc and vmat, genes involved in serotonin synthesis and serotonin release, respectively, showed to be down-regulated in C26 TB mice, whereas LLC TB mice showed higher expression of these genes (Figure 3B). Serotonin brain levels in LLC TB mice showed to be slightly elevated at Day 10, just after the onset of anorexia, while levels were lower in C26 TB mice compared with controls (Figure 3C).
Figure 3.
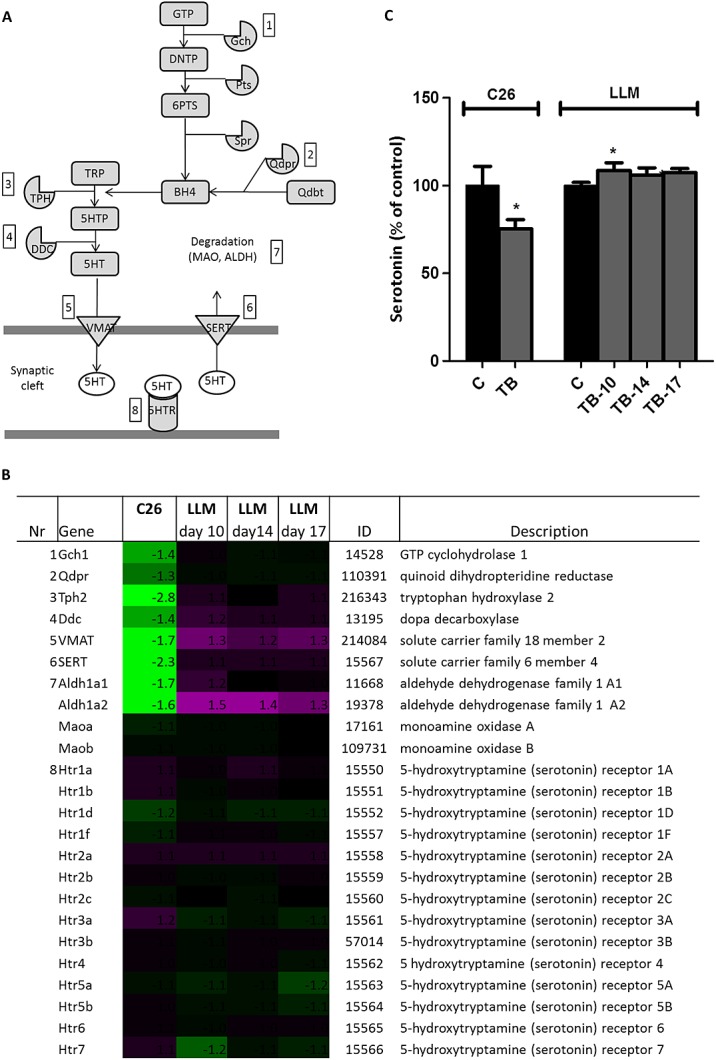
Serotonin signalling in C26 and Lewis lung carcinoma tumour-bearing mice. (A) Schematic view of genes involved in serotonin signalling. (B) Heat map of fold changes of expressions of genes involved in serotonin signalling. (C) Serotonin level in brain relative to control mice in C26 and Lewis lung carcinoma tumour-bearing mice. Values are expressed as mean ± SEM. C, sham-injected control; tumour bearing, injected with 1 × 106 tumour cells; DNTP, 7,8-dihydroneopterin triphosphate; 6PTS, 6-pyruvoyl-tetrahydropterin; q-dbt, q-dihydrobiopterin; TRP, tryptophan; 5HT, 5-hydroxytryptamine (serotonin); MAO, monoamine oxidase; and ALDH, aldehyde dehydrogenase.
Hypothalamic cell lines: effect of serotonin on messenger neuropeptide Y and neuropeptide Y secretion
Murine-derived hypothalamic cell lines hypoE-46 and hypoA-2/12 were 24 h exposed to serotonin (100 pg/mL–100 µg/mL). Serotonin showed to decrease NPY secretion in the hypoE-46 cells, while it did not have any effect on messenger NPY (Figure 4). In hypoA-2/12 cells, serotonin did not have an effect on either NPY secretion or NPY gene expression. Basal expression level of serotonin receptors between the two cell lines showed to be significantly different for receptors 5HT1b, 5HT1d, 5HT2a, and 5HT2b (Table 1). The effect of serotonin on gene expression of 5HT receptors was not different between the two cell lines, except for 5HT2b, which was down-regulated in hypoE-46 cells and up-regulated in hypoA-2/12 cells after serotonin exposure.
Figure 4.
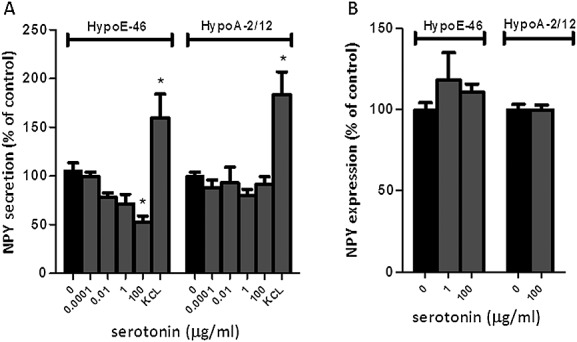
Effect of serotonin on messenger neuropeptide Y and neuropeptide Y secretion in hypothalamic cells. Murine-derived hypothalamic cell lines were 24 h exposed to various concentrations serotonin. Potassium chloride was used to depolarize cells (positive control). (A) Effect of serotonin on NPY secretion in hypoE-46 and hypoA-2/12 cells. (B) Effect of serotonin on neuropeptide Y gene expression in hypoE-46 and hypoA-2/12 cells. *Significantly different from C (P < 0.05). Data are expressed as mean ± SEM.
Table 1.
Effect of serotonin on gene expression in hypothalamic cells
| Gene | HypoE-46 | HypoA-2/12 | ID | Description | ||
|---|---|---|---|---|---|---|
| Control | 100 µg 5HT | Control | 100 µg 5HT | |||
| Htr1a | 12.0 | 12.5 | 11.8 | 12.7 | 15 550 | 5-Hydroxytryptamine receptor 1A |
| Htr1b | 19.0 | 17.6 | 41.8b | 31.9 | 15 551 | 5-Hydroxytryptamine receptor 1B |
| Htr1d | 20.2 | 13.0a | 15.8b | 14.5 | 15 552 | 5-Hydroxytryptamine receptor 1D |
| Htr1f | 17.3 | 15.7 | 16.7 | 15.9 | 15 557 | 5-Hydroxytryptamine receptor 1F |
| Htr2a | 32.1 | 24.4a | 104.0b | 213.7c | 15 558 | 5-Hydroxytryptamine receptor 2A |
| Htr2b | 18.6 | 16.2 | 12.9b | 16.4 | 15 559 | 5-Hydroxytryptamine receptor 2B |
| Htr2c | 9.3 | 9.5 | 9.0 | 10.3 | 15 560 | 5-Hydroxytryptamine receptor 2C |
| Htr3a | 10.2 | 9.5 | 9.6 | 10.5 | 15 561 | 5-Hydroxytryptamine receptor 3A |
| Htr3b | 12.1 | 13.1 | 12.1 | 11.9 | 57 014 | 5-Hydroxytryptamine receptor 3B |
| Htr4 | 20.9 | 19.9 | 19.0 | 19.2 | 15 562 | 5-Hydroxytryptamine receptor 4 |
| Htr5a | 13.9 | 14.8 | 13.5 | 14.0 | 15 563 | 5-Hydroxytryptamine receptor 5A |
| Htr5b | 21.6 | 18.3 | 18.5 | 17.7 | 15 564 | 5-Hydroxytryptamine receptor 5B |
| Htr6 | 21.6 | 19.4 | 21.9 | 19.8 | 15 565 | 5-Hydroxytryptamine receptor 6 |
| Htr7 | 17.9 | 17.8 | 13.9 | 14.7 | 15 566 | 5-Hydroxytryptamine receptor 7 |
Murine-derived hypothalamic cell lines hypoE-46 were 24 h exposed to various concentrations serotonin. Gene expression of serotonin receptors (5HTRs) were determined by using microarrays.
Significantly different from control in hypoE-46 cells.
Significantly different from hypoE-46 cells basal expression.
Significantly different from control in hypoA-2/12 cells (P > 0.01).
Discussion
Cancer anorexia has been explained by an inability of the hypothalamus to respond adequately to triggers from the periphery during a situation of negative energy balance.18 Here, we show that this impaired response of food-intake control is associated with altered serotonin signalling and probably stunted NPY post-transcriptional regulation in the hypothalamus. In addition, this serotonin regulation and brain serotonin levels were inversely associated with food intake. In vitro, serotonin was found to modulate NPY release by hypothalamic neurons. Taken together, this suggests that serotonin is likely to play a crucial role in the failure of the NPYergic system during cancer anorexia.
By comparing two cancer models sharing common characteristics of tumour-induced muscle wasting and fat loss while displaying opposite effects on food intake behaviour, we are able to disentangle anorexia from cachectic processes. Peripheral markers for cachexia reflecting weight loss, muscle loss, and fat loss were comparable between the two tumour models. In addition, the most prominently (top 30-based) induced hypothalamic genes compared with healthy controls showed to be similar between the two models. This suggests that there is a common subset of genes that is strongly induced during tumour growth and progression of cachexia, despite the differences in type of tumour, inoculation site, strain of mice, and independent from effects on food intake behaviour. These changes might represent markers for cachexia or tumour-induced inflammation as gene expression of strongly up-regulated inflammatory genes was overlapping between the two models.
Interestingly, also a majority of genes involved in food intake regulation showed to be similar between the models, even though ultimate effects on food intake were different for the two models. Food intake and drive to eat are the outcomes of numerous metabolic, physiological, and behavioural cues including satiety, hunger and reward, and learning. Therefore, changes on individual genes are difficult to interpret, as the impact on food intake is not similar between these single genes. Furthermore, it might be that, considering the complexity of food intake regulation, by using GO classification on food intake behaviour, some genes are ignored that might be important as well. Finally, a limitation of the current set-up is that we study expression in the hypothalamus only. Although the hypothalamus is a crucial site of food intake regulation, not all processes involved in food intake are regulated in the hypothalamus.19 Gene expression of NPY, one of the most potent food stimulating neuropeptides in the hypothalamus,20 showed to increase in both C26 TB and LLC TB mice and was among the strongest induced genes in both models. This suggests that expression of NPY in cachectic TB mice is independent of food intake status. NPY is considered to act as a sensor and regulator of energy balance by altering food intake in energy deficits, which is supported by findings that NPY is stimulated in conditions of negative energy balance like food restriction,21 food deprivation,22,23 and exercise.24,25 Several studies have shown that in cachectic TB rodents, NPY messenger RNA (mRNA) in the hypothalamus is elevated.26–29 However, this elevation of mRNA NPY does not reflect food intake behaviour27,29 and does not correspond to NPY levels in the hypothalamus.27 In contrast to these elevated messenger NPY levels, anorectic TB rodents have lower paraventricular nucleus (PVN) hypothalamic NPY levels30,31 and lower NPY immunostaining of fibres innervating various hypothalamic nuclei.32 On the other hand, mice bearing an A375 tumour that do not suffer from cachexia and weight loss did not show this elevation of NPY mRNA,26 suggesting that cachexia is an important trigger in stimulation of NPY mRNA. This might explain that in both the C26 model and LLC model, messenger NPY was highly up-regulated in TB mice compared with controls, despite their difference in food intake behaviour. Consequently, we hypothesize that failure of the NPYergic system taking place at a post-transcriptional level, for example, by impaired translation, synthesis, transport, or secretion, plays a role in the development of tumour-induced anorexia.
In this study, we also found serotonin signalling to be differently regulated between two types of tumour-induced cachexia models, in addition to their opposed effects on food intake behaviour. In C26 TB mice, serotonin signalling was strongly down-regulated and inversely associated with an increased food intake. Anorectic Lewis lung TB mice displayed less pronounced changes in transcript levels of gene involved in serotonin signalling compared with C26 TB mice. Moreover, transcriptional regulation was different from mice-bearing C26 tumour. Furthermore, brain serotonin levels were significantly elevated in TB mice at Day 10 compared with controls, shortly after the manifestation of anorexia. This effect was less clear at Days 14 and 17, suggesting that effects on serotonin are more prominent during the initial stages of anorexia development. It has been postulated that cancer anorexia develops by waves of brain activation,33 each of them not necessarily mediated by the same mediators. It could be that serotonin might play a role in the initiation of anorexia. Hypothalamic serotonin is an important mediator in the regulation of satiety and hunger.34 In cancer-induced anorexia, serotonin is considered a key player in the induction of anorexia.35,36 In rodents, tumour-driven elevation of hypothalamic serotonin has been associated with reduced food intake.31,37,38 Furthermore, this elevation of serotonin was not present in the pair-fed control group, meaning that reduced food intake itself does not result in an elevation of hypothalamic serotonin. In humans, a practical and non-invasive method to measure site-specific changes on serotonergic activity in the hypothalamus is not yet available, but increased levels of tryptophan, the precursor of serotonin, have been measured in cerebral spinal fluid of cancer anorectic patients.39
The exact mechanism on how serotonin is able to regulate food intake is not yet understood. A cross interaction of serotonin with NPYergic system has been discussed.31 Serotonin signalling is negatively associated with NPY activity.40–42 Active lowering of serotonin levels results in increased NPY levels,43 while induction of 5-HT signalling showed to reduce NPY levels in rat hypothalamus.44 More importantly, serotonin is able to repress food intake, despite weight loss-induced elevation of messenger NPY,45 suggesting that serotonin might act as an interfering factor between transcription and NPY secretion. Therefore, we hypothesized that serotonin might interfere with NPY signalling at a post-transcriptional level. In this way, it could play a role in post-transcriptional failure of the NPY system during certain forms of cancer.
To determine serotonin's ability to repress NPY secretion, we used two murine-derived hypothalamic cell lines. We showed that serotonin is able to reduce NPY secretion in hypoE-46 cells, a cell line suggested to originate from the PVN,46 without affecting NPY gene expression, supporting the hypothesis that serotonin acts post transcriptionally.
Serotonin did not have an effect on NPY secretion in hypoA-2/12 cells, a cell line suggested to originate from the arcuate nucleus (ARC) or supraoptic nucleus.47 In lean and obese Zucker rats, increased levels of serotonin, induced by chronic administration of fluoxetine, resulted in reduced food intake and reduced levels of NPY in the PVN, but not in the ARC and other areas of the hypothalamus.40 This suggests that NPY release by PVN neurons is more sensitive to serotonin and might explain the difference in response to serotonin between the two cell lines.
The two cell lines did not show differences in their general response to serotonin as reflected by expression of genes involved in serotonin signalling. An exception to this was the effect of serotonin on expression of the 5HT2a receptor, which was down-regulated in response to serotonin in the hypoE-46 and up-regulated in the hypoA-2/12 cell line. Moreover, basal expression of 5HT1b and 5HT2a, and 5HT2b and 5HT2c differed between the two cell lines. In the ARC nucleus, serotonin has been reported to inhibit NPY release by hyperpolarization of AgRP/NPY neurons via 5HT1B receptors.48 The role of 5HT2 receptors might be a target for further research on their role in NPY regulation. However, as NPY secretion is via granule exocytosis,49 also, a receptor-independent process like serotonylation of guanosine triphosphatases (GTPases) might be implicated. Serotonylation is a process, where intracellular serotonin is able to activate small GTPases via transamination. Dependent on the type of GTPases that are activated, several processes dependent on granule exocytosis can be affected, which has been reported for insulin secretion by pancreatic β cells50 or platelet aggregation.51
In this study, we provide evidence that cancer anorexia might be due to post-transcriptional failure of the NPYergic system in the hypothalamus to respond to increased energy requirement in cancer cachexia. We show that serotonin is able to affect NPY secretion in vitro. In addition, serotonin signalling is found to be altered in TB mice, suggesting that serotonin might play a crucial role in failure of hypothalamic NPY signalling and subsequently the development or sustainment of cancer anorexia.
Funding
The research leading to these results has received funding from the European Union's Seventh Framework Programme for research, technological development, and demonstration under grant agreement 266408 (Full4Health). The authors confirm that they comply with the principles of ethical publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley J.E., Coats A.J., and Anker S.D.).
Conflict of interest
J. Dwarkasing, M. Boekschoten, J. Argilès, S. Busquets, F. Penna, M. Toledo, A. Laviano, and R. Witkamp have nothing to declare. M. van Dijk is an employee of Nutricia Research, a medical nutrition company. K. van Norren is a guest employee of Nutricia Research, a medical nutrition company.
References
- Sarhill N, Mahmoud F, Walsh D, Nelson KA, Komurcu S, Davis M, LeGrand S, Abdullah O, Rybicki L. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11:652–659. doi: 10.1007/s00520-003-0486-0. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Olivan M, Busquets S, Lopez-Soriano FJ. Optimal management of cancer anorexia-cachexia syndrome. Cancer Manag Res. 2010;2:27–38. doi: 10.2147/cmar.s7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaka T, Okada S, Ishii H, Ikeda M, Kosakamoto H, Yoshimori M. Prognosis of advanced pancreatic cancer patients with reference to calorie intake. Nutr Cancer. 1998;32:55–58. doi: 10.1080/01635589809514717. [DOI] [PubMed] [Google Scholar]
- Tamburini M, Brunelli C, Rosso S, Ventafridda V. Prognostic value of quality of life scores in terminal cancer patients. J Pain Symptom Manage. 1996;11:32–41. doi: 10.1016/0885-3924(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Laviano A, Inui A, Meguid MM, Molfino A, Conte C, Rossi Fanelli F. NPY and brain monoamines in the pathogenesis of cancer anorexia. Nutrition. 2008;24:802–805. doi: 10.1016/j.nut.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- Churm D, Andrew IM, Holden K, Hildreth AJ, Hawkins C. A questionnaire study of the approach to the anorexia-cachexia syndrome in patients with cancer by staff in a district general hospital. Support Care Cancer. 2009;17:503–507. doi: 10.1007/s00520-008-0486-1. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Moore-Carrasco R, Fuster G, Busquets S, Lopez-Soriano FJ. Cancer cachexia: the molecular mechanisms. Int J Biochem Cell Biol. 2003;35:405–409. doi: 10.1016/s1357-2725(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Dwarkasing JT, van Dijk M, Dijk FJ, Boekschoten MV, Faber J, Argiles JM, Laviano A, Muller M, Witkamp RF, van Norren K. Hypothalamic food intake regulation in a cancer-cachectic mouse model. J Cachexia Sarcopenia Muscle. 2013;5:159–169. doi: 10.1007/s13539-013-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren K, Kegler D, Argiles JM, Luiking Y, Gorselink M, Laviano A, Arts K, Faber J, Jansen H, van der Beek EM, van Helvoort A. Dietary supplementation with a specific combination of high protein, leucine, and fish oil improves muscle function and daily activity in tumour-bearing cachectic mice. Br J Cancer. 2009;100:713–722. doi: 10.1038/sj.bjc.6604905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Kools H, de Groot PJ, Gavai AK, Basnet RK, Cheng F, Wu J, Wang X, Lommen A, Hooiveld GJ, Bonnema G, Visser RG, Muller MR, Leunissen JA. MADMAX—management and analysis database for multiple ∼omics experiments. J Integr Bioinform. 2011;8:160. doi: 10.2390/biecoll-jib-2011-160. [DOI] [PubMed] [Google Scholar]
- Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F. Evolving gene/transcript definitions significantly alter the interpretation of genechip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of affymetrix genechip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Sartor MA, Tomlinson CR, Wesselkamper SC, Sivaganesan S, Leikauf GD, Medvedovic M. Intensity-based hierarchical Bayes method improves testing for differentially expressed genes in microarray experiments. BMC Bioinformatics. 2006;7:538. doi: 10.1186/1471-2105-7-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome—when all you can eat is yourself. Nat Clin Pract Oncol. 2005;2:158–165. doi: 10.1038/ncponc0112. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16:S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Hagimoto M, Matsuura T, Ohkubo J, Ohno M, Maruyama T, Ishikura T, Hashimoto H, Kakuma T, Yoshimatsu H, Terawaki K, Uezono Y, Toyohira Y, Yanagihara N, Ueta Y. Effects of food deprivation on the hypothalamic feeding-regulating peptides gene expressions in serotonin depleted rats. J Physiol Sci. 2014;64:97–104. doi: 10.1007/s12576-013-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DE, Shellard L, Koeslag DG, Boer DE, McCarthy HD, McKibbin PE, Russell JC, Williams G. Intense exercise and food restriction cause similar hypothalamic neuropeptide Y increases in rats. Am J Physiol. 1993;264:E279–E284. doi: 10.1152/ajpendo.1993.264.2.E279. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Scott KA, Moran TH, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1800–R1805. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- Nara-ashizawa N, Tsukada T, Maruyama K, Akiyama Y, Kajimura N, Nagasaki K, Iwanaga T, Yamaguchi K. Hypothalamic appetite-regulating neuropeptide mRNA levels in cachectic nude mice bearing human tumor cells. Metabolism. 2001;50:1213–1219. doi: 10.1053/meta.2001.26706. [DOI] [PubMed] [Google Scholar]
- Chance WT, Xiao C, Dayal R, Sheriff S. Alteration of NPY and Y1 receptor in dorsomedial and ventromedial areas of hypothalamus in anorectic tumor-bearing rats. Peptides. 2007;28:295–301. doi: 10.1016/j.peptides.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Gayle D. Brain cytokine mRNAs in anorectic rats bearing prostate adenocarcinoma tumor cells. Am J Physiol. 1998;275:R566–R573. doi: 10.1152/ajpregu.1998.275.2.R566. [DOI] [PubMed] [Google Scholar]
- Nara-ashizawa N, Tsukada T, Maruyama K, Akiyama Y, Kajimura N, Yamaguchi K. Response of hypothalamic NPY mRNAs to a negative energy balance is less sensitive in cachectic mice bearing human tumor cells. Nutr Cancer. 2001;41(1-2):111–118. doi: 10.1080/01635581.2001.9680621. [DOI] [PubMed] [Google Scholar]
- McCarthy HD, McKibbin PE, Perkins AV, Linton EA, Williams G. Alterations in hypothalamic NPY and CRF in anorexic tumor-bearing rats. Am J Physiol. 1993;264:E638–E643. doi: 10.1152/ajpendo.1993.264.4.E638. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Ramos EJ, Laviano A, Varma M, Sato T, Chen C, Qi Y, Das UN. Tumor anorexia: effects on neuropeptide Y and monoamines in paraventricular nucleus. Peptides. 2004;25:261–266. doi: 10.1016/j.peptides.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Makarenko IG, Meguid MM, Gatto L, Chen C, Ugrumov MV. Decreased NPY innervation of the hypothalamic nuclei in rats with cancer anorexia. Brain Res. 2003;961:100–108. doi: 10.1016/s0006-8993(02)03850-7. [DOI] [PubMed] [Google Scholar]
- Laviano A, Meguid MM, Cascino A, Molfino A, Rossi Fanelli F. Tryptophan in wasting diseases: at the crossing between immune function and behaviour. Curr Opin Clin Nutr Metab Care. 2009;12:392–397. doi: 10.1097/MCO.0b013e32832b73af. [DOI] [PubMed] [Google Scholar]
- Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Laviano A, Inui A, Marks DL, Meguid MM, Pichard C, Rossi Fanelli F, Seelaender M. Neural control of the anorexia-cachexia syndrome. Am J Physiol Endocrinol Metab. 2008;295:E1000–E1008. doi: 10.1152/ajpendo.90252.2008. [DOI] [PubMed] [Google Scholar]
- Rossi Fanelli F, Laviano A. Cancer anorexia: a model for the understanding and treatment of secondary anorexia. Int J Cardiol. 2002;85:67–72. doi: 10.1016/s0167-5273(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Ramos EJ, Suzuki S, Meguid MM, Laviano A, Sato T, Chen C, Das U. Changes in hypothalamic neuropeptide Y and monoaminergic system in tumor-bearing rats: pre- and post-tumor resection and at death. Surgery. 2004;136:270–276. doi: 10.1016/j.surg.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Blaha V, Yang ZJ, Meguid MM, Chai JK, Oler A, Zadak Z. Ventromedial nucleus of hypothalamus is related to the development of cancer-induced anorexia: in vivo microdialysis study. Acta Medica (Hradec Kralove) 1998;41:3–11. [PubMed] [Google Scholar]
- Cangiano C, Cascino A, Ceci F, Laviano A, Mulieri M, Muscaritoli M, Rossi-Fanelli F. Plasma and CSF tryptophan in cancer anorexia. J Neural Transm Gen Sect. 1990;81:225–233. doi: 10.1007/BF01245044. [DOI] [PubMed] [Google Scholar]
- Dryden S, Frankish HM, Wang Q, Pickavance L, Williams G. The serotonergic agent fluoxetine reduces neuropeptide Y levels and neuropeptide Y secretion in the hypothalamus of lean and obese rats. Neuroscience. 1996;72:557–566. doi: 10.1016/0306-4522(95)00566-8. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. 5-Hydroxytryptaminergic receptor agonists: effects on neuropeptide Y potentiation of feeding and respiratory quotient. Brain Res. 1998;803:212–217. doi: 10.1016/s0006-8993(98)00643-x. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Kim SJ, Joh TH, Son JH. Neuropeptide Y mRNA and serotonin innervation in the arcuate nucleus of anorexia mutant mice. Brain Res. 1998;790:67–73. doi: 10.1016/s0006-8993(98)00049-3. [DOI] [PubMed] [Google Scholar]
- Dryden S, Frankish HM, Wang Q, Williams G. Increased feeding and neuropeptide Y (NPY) but not NPY mRNA levels in the hypothalamus of the rat following central administration of the serotonin synthesis inhibitor p-chlorophenylalanine. Brain Res. 1996;724:232–237. doi: 10.1016/0006-8993(96)00329-0. [DOI] [PubMed] [Google Scholar]
- Shinozaki T, Kimura M, Hosoyamada M, Shibasaki T. Fluvoxamine inhibits weight gain and food intake in food restricted hyperphagic Wistar rats. Biol Pharm Bull. 2008;31:2250–2254. doi: 10.1248/bpb.31.2250. [DOI] [PubMed] [Google Scholar]
- Moon YW, Choi SH, Yoo SB, Lee JH, Jahng JW. 5-Hydroxy-L-tryptophan suppressed food intake in rats despite an increase in the arcuate NPY expression. Exp Neurobiol. 2010;19:132–139. doi: 10.5607/en.2010.19.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon SS, Belsham DD. Leptin differentially regulates NPY secretion in hypothalamic cell lines through distinct intracellular signal transduction pathways. Regul Pept. 2011;167:192–200. doi: 10.1016/j.regpep.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy P, Wang Q, Whim MD. Cell type-dependent trafficking of neuropeptide Y-containing dense core granules in CNS neurons. J Neurosci. 2011;31:14783–14788. doi: 10.1523/JNEUROSCI.2933-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]