Abstract
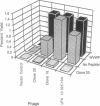
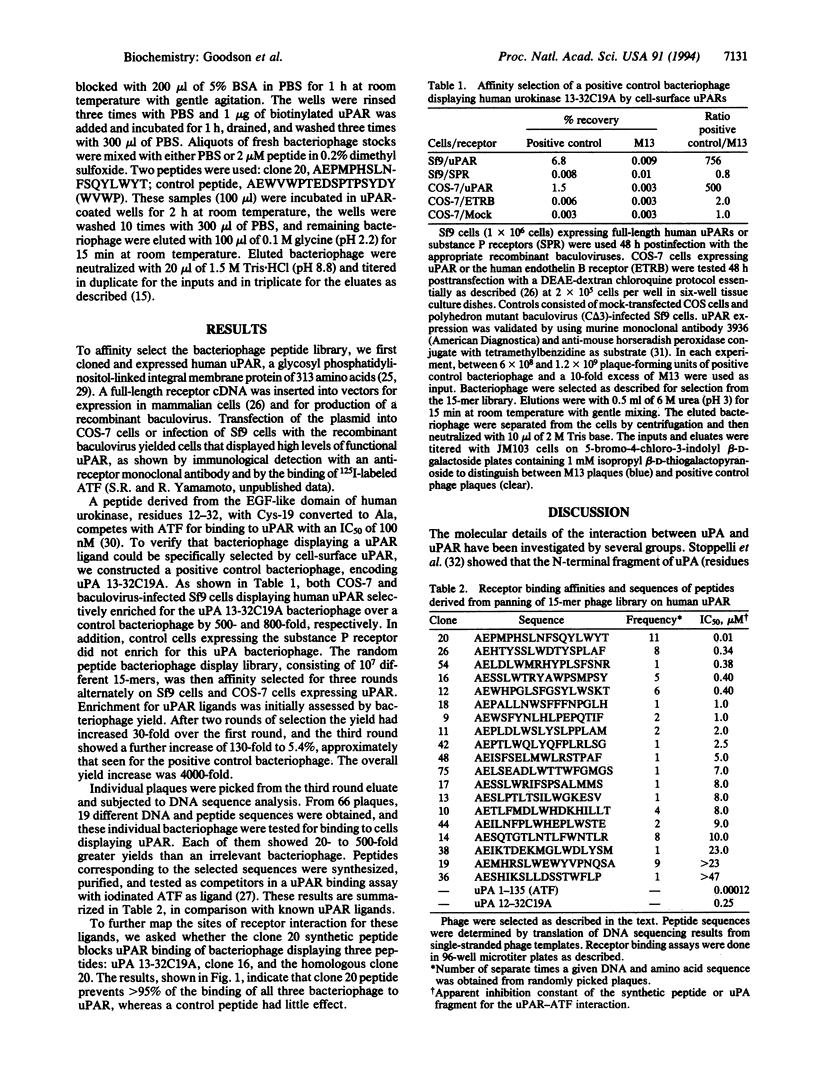
Affinity selection of a 15-mer random peptide library displayed on bacteriophage M13 has been used to identify potent ligands for the human urokinase receptor, a key mediator of tumor cell invasion. A family of receptor binding bacteriophage ligands was obtained by sequentially and alternately selecting the peptide library on COS-7 monkey kidney cells and baculovirus-infected Sf9 insect cells overexpressing the human urokinase receptor. Nineteen peptides encoded by the random DNA regions of the selected bacteriophage were synthesized and tested in a urokinase receptor binding assay, where they competed with the labeled N-terminal fragment of urokinase with IC50 values ranging from 10 nM to 10 microM. All of the isolated peptides were linear and showed two relatively short conserved subsequences: LWXXAr (Ar = Y, W, F, or H) and XFXXYLW, neither of which is found in urokinase or its receptor. Competition experiments demonstrated that the most potent peptide, clone 20, prevented binding of bacteriophage displaying the urokinase receptor binding sequence (urokinase residues 13-32). In addition, this peptide blocked other apparently unrelated receptor binding bacteriophage, suggesting overlapping receptor interaction sites for all of these sequences. These results provide a demonstration of bacteriophage display identifying peptide ligands for a receptor expressed on cells and yield leads for the development of urokinase receptor antagonists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Arkin A. P., Youvan D. C. Optimizing nucleotide mixtures to encode specific subsets of amino acids for semi-random mutagenesis. Biotechnology (N Y) 1992 Mar;10(3):297–300. doi: 10.1038/nbt0392-297. [DOI] [PubMed] [Google Scholar]
- Barrett R. W., Cwirla S. E., Ackerman M. S., Olson A. M., Peters E. A., Dower W. J. Selective enrichment and characterization of high affinity ligands from collections of random peptides on filamentous phage. Anal Biochem. 1992 Aug 1;204(2):357–364. doi: 10.1016/0003-2697(92)90252-3. [DOI] [PubMed] [Google Scholar]
- Behrendt N., Ploug M., Patthy L., Houen G., Blasi F., Danø K. The ligand-binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem. 1991 Apr 25;266(12):7842–7847. [PubMed] [Google Scholar]
- Blasi F., Behrendt N., Cubellis M. V., Ellis V., Lund L. R., Masucci M. T., Møller L. B., Olson D. P., Pedersen N., Ploug M. The urokinase receptor and regulation of cell surface plasminogen activation. Cell Differ Dev. 1990 Dec 2;32(3):247–253. doi: 10.1016/0922-3371(90)90037-w. [DOI] [PubMed] [Google Scholar]
- Crowley C. W., Cohen R. L., Lucas B. K., Liu G., Shuman M. A., Levinson A. D. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Ellis V., Behrendt N., Danø K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991 Jul 5;266(19):12752–12758. [PubMed] [Google Scholar]
- Ellis V., Pyke C., Eriksen J., Solberg H., Danø K. The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Ann N Y Acad Sci. 1992 Dec 4;667:13–31. doi: 10.1111/j.1749-6632.1992.tb51591.x. [DOI] [PubMed] [Google Scholar]
- Emonard H. P., Remacle A. G., Noël A. C., Grimaud J. A., Stetler-Stevenson W. G., Foidart J. M. Tumor cell surface-associated binding site for the M(r) 72,000 type IV collagenase. Cancer Res. 1992 Oct 15;52(20):5845–5848. [PubMed] [Google Scholar]
- Estreicher A., Wohlwend A., Belin D., Schleuning W. D., Vassalli J. D. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem. 1989 Jan 15;264(2):1180–1189. [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Harvey T. S., Wilkinson A. J., Tappin M. J., Cooke R. M., Campbell I. D. The solution structure of human transforming growth factor alpha. Eur J Biochem. 1991 Jun 15;198(3):555–562. doi: 10.1111/j.1432-1033.1991.tb16050.x. [DOI] [PubMed] [Google Scholar]
- Hollas W., Blasi F., Boyd D. Role of the urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res. 1991 Jul 15;51(14):3690–3695. [PubMed] [Google Scholar]
- Hommel U., Harvey T. S., Driscoll P. C., Campbell I. D. Human epidermal growth factor. High resolution solution structure and comparison with human transforming growth factor alpha. J Mol Biol. 1992 Sep 5;227(1):271–282. doi: 10.1016/0022-2836(92)90697-i. [DOI] [PubMed] [Google Scholar]
- Kaufman S. E., Brown S., Stauber G. B. Characterization of ligand binding to immobilized biotinylated extracellular domains of three growth factor receptors. Anal Biochem. 1993 Jun;211(2):261–266. doi: 10.1006/abio.1993.1267. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Gay D. A., Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993 Sep 25;268(27):20205–20210. [PubMed] [Google Scholar]
- LaBean T. H., Kauffman S. A. Design of synthetic gene libraries encoding random sequence proteins with desired ensemble characteristics. Protein Sci. 1993 Aug;2(8):1249–1254. doi: 10.1002/pro.5560020807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman H. B., Bass S. H., Simpson N., Wells J. A. Selecting high-affinity binding proteins by monovalent phage display. Biochemistry. 1991 Nov 12;30(45):10832–10838. doi: 10.1021/bi00109a004. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Ouwehand W. H., Bye J. M., Finnern R., Gorick B. D., Voak D., Thorpe S. J., Hughes-Jones N. C., Winter G. Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology (N Y) 1993 Oct;11(10):1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Min H. Y., Semnani R., Mizukami I. F., Watt K., Todd R. F., 3rd, Liu D. Y. cDNA for Mo3, a monocyte activation antigen, encodes the human receptor for urokinase plasminogen activator. J Immunol. 1992 Jun 1;148(11):3636–3642. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., Hoess R. H., Jackson S. A., Ramachandran N. S., Mousa S. A., DeGrado W. F. Identification of novel peptide antagonists for GPIIb/IIIa from a conformationally constrained phage peptide library. Proteins. 1992 Dec;14(4):509–515. doi: 10.1002/prot.340140411. [DOI] [PubMed] [Google Scholar]
- Ossowski L. Invasion of connective tissue by human carcinoma cell lines: requirement for urokinase, urokinase receptor, and interstitial collagenase. Cancer Res. 1992 Dec 15;52(24):6754–6760. [PubMed] [Google Scholar]
- Ploug M., Rønne E., Behrendt N., Jensen A. L., Blasi F., Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991 Jan 25;266(3):1926–1933. [PubMed] [Google Scholar]
- Pyke C., Graem N., Ralfkiaer E., Rønne E., Høyer-Hansen G., Brünner N., Danø K. Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res. 1993 Apr 15;53(8):1911–1915. [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J. J. The N-terminal domain of human urokinase receptor contains two distinct regions critical for ligand recognition. Blood. 1993 Nov 1;82(9):2719–2729. [PubMed] [Google Scholar]
- Roberts B. L., Markland W., Siranosian K., Saxena M. J., Guterman S. K., Ladner R. C. Protease inhibitor display M13 phage: selection of high-affinity neutrophil elastase inhibitors. Gene. 1992 Nov 2;121(1):9–15. doi: 10.1016/0378-1119(92)90156-j. [DOI] [PubMed] [Google Scholar]
- Roldan A. L., Cubellis M. V., Masucci M. T., Behrendt N., Lund L. R., Danø K., Appella E., Blasi F. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 1990 Feb;9(2):467–474. doi: 10.1002/j.1460-2075.1990.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Scott J. K., Loganathan D., Easley R. B., Gong X., Goldstein I. J. A family of concanavalin A-binding peptides from a hexapeptide epitope library. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5398–5402. doi: 10.1073/pnas.89.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Sheldon E. L., Kellogg D. E., Watson R., Levenson C. H., Erlich H. A. Use of nonisotopic M13 probes for genetic analysis: application to HLA class II loci. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9085–9089. doi: 10.1073/pnas.83.23.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Wohlwend A., Belin D. Urokinase-catalyzed plasminogen activation at the monocyte/macrophage cell surface: a localized and regulated proteolytic system. Curr Top Microbiol Immunol. 1992;181:65–86. doi: 10.1007/978-3-642-77377-8_3. [DOI] [PubMed] [Google Scholar]
- Zini J. M., Murray S. C., Graham C. H., Lala P. K., Karikó K., Barnathan E. S., Mazar A., Henkin J., Cines D. B., McCrae K. R. Characterization of urokinase receptor expression by human placental trophoblasts. Blood. 1992 Jun 1;79(11):2917–2929. [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]