ABSTRACT
Embryo implantation in the uterus depends on decidualization of the endometrial stromal cells (ESCs), and glucose utilization via the pentose phosphate pathway is critical in this process. We hypothesized that the amino sugar glucosamine may block the pentose phosphate pathway via inhibition of the rate-limiting enzyme glucose-6-phosphate dehydrogenase in ESCs and therefore impair decidualization and embryo implantation, thus preventing pregnancy. Both human primary and immortalized ESCs were decidualized in vitro in the presence of 0, 2.5, or 5 mM glucosamine for 9 days. Viability assays demonstrated that glucosamine was well tolerated by human ESCs. Exposure of human ESCs to glucosamine resulted in significant decreases in the activity and expression of glucose-6-phosphate dehydrogenase and in the mRNA expression of the decidual markers prolactin, somatostatin, interleukin-15, and left-right determination factor 2. In mouse ESCs, expression of the decidual marker Prp decreased upon addition of glucosamine. In comparison with control mice, glucosamine-treated mice showed weak artificial deciduoma formation along the stimulated uterine horn. In a complementary in vivo experiment, a 60-day-release glucosamine (15, 150, or 1500 μg) or placebo pellet was implanted in a single uterine horn of mice. Mice with a glucosamine pellet delivered fewer live pups per litter than those with a control pellet, and pup number returned to normal after the end of the pellet-active period. In conclusion, glucosamine is a nonhormonal inhibitor of decidualization of both human and mouse ESCs and of pregnancy in mice. Our data indicate the potential for development of glucosamine as a novel, reversible, nonhormonal contraceptive.
Keywords: decidualization, glucosamine, human endometrial stromal cells, pentose phosphate pathway
INTRODUCTION
Unintended pregnancy is a significant global health care problem; in the United States alone, more than three million women between the ages of 15 and 44 experience an unintended or unplanned pregnancy each year [1]. The consensus is that limited contraceptive choices play a critical role in the persistence of these high rates. In a survey conducted in 2004, 40% of women reported being dissatisfied with their current contraceptive method, and many of the perceived side effects were thought to be due to hormones contained in the contraceptives [2]. The most commonly used contraceptive method in the United States is the oral contraceptive pill, which requires daily compliance and has annual failure rates with typical use estimated at 9% for the general population, 13% for teenagers, and 30% or higher for some high-risk subgroups [3]. In contrast, long-acting reversible contraceptive methods, including intrauterine devices (IUDs) and subdermal implants, are not user-dependent and have very low failure rates (less than 1%), which rival those of sterilization [3]. Currently, the only long-acting nonhormonal contraceptive method available is the copper IUD. Additional nonhormonal long-acting contraceptive methods are needed not only to improve compliance, but also for patients unable to tolerate hormonal methods because of other health issues such as clotting disorders or cancer [4, 5].
One mechanism of action by which a contraceptive might work is to prevent the endometrium from becoming receptive to implantation of an embryo. The endometrium is a steroid hormone-dependent tissue that responds to cyclical changes in estrogen and progesterone (P4) during the menstrual cycle [6]. The proliferative stage of the menstrual cycle is associated with follicular growth and increased estrogen secretion leading to endometrial proliferation. This proliferation ceases after ovulation when P4 begins to rise. The P4-dominant luteal phase is characterized by decidualization, a process of endometrial stromal cell (ESC) differentiation typified by changes in the cytoskeleton and glucose metabolism, as well as the up-regulation of prolactin, insulin-like growth factors, and insulin-like growth factor-binding proteins [7, 8]. As a result of these cellular changes, the human endometrium becomes receptive to embryonic implantation 7–10 days after ovulation, a period commonly referred to as the window of implantation. Because differentiation of the ESCs into decidual cells is required for successful embryo implantation and establishment of pregnancy [9], decidualization is a candidate target for the development of new contraceptives.
Previously, our laboratory has shown that decidualization of primary human ESCs is accompanied by significant increases in expression of the ubiquitous facilitative glucose transporter GLUT1, glucose uptake, and glucose utilization via the pentose phosphate pathway [9, 10]. Inhibition of this pathway with dehydroepiandrosterone (DHEA), a known noncompetitive inhibitor of the rate-limiting enzyme of the pentose phosphate pathway, glucose 6-phosphate dehydrogenase (G6PDH), blocked decidualization both in vitro and in vivo [9]. Although DHEA is an endogenous hormone synthesized by the adrenal glands, it may not be ideal for use as a contraceptive. First, it is not known whether DHEA is safe for long-term use; as a sex hormone precursor, DHEA may cause higher than normal levels of androgens and estrogens in the body, and theoretically might increase the risk of hormone-sensitive cancers. Second, high DHEA serum levels are observed in women with polycystic ovary syndrome (PCOS) [11], and rat studies have confirmed that administration of DHEA leads to anovulation and a PCOS-like phenotype [12]. Because of these adverse effects of DHEA, we aimed to determine whether a nonhormonal inhibitor of the pentose phosphate pathway, that is, glucosamine (GlcN), also has an inhibitory effect on decidualization and can prevent pregnancy.
Glucosamine is an amino monosaccharide that is an essential component of glycosaminoglycans, the large carbohydrate chains incorporated into connective tissue, skin, tendons, ligaments, and cartilage [13]. It is readily synthesized in the body from glucose and is widely used as an oral supplement to relieve arthritic complaints [14]. Exogenous GlcN is actively transported from extracellular space into cells by GLUT1, 2, and 4, and is phosphorylated into GlcN-6-phosphate by tissue hexokinases [15, 16]. Once inside the cell, GlcN-6-phosphate acts as a competitive inhibitor of G6PDH [17, 18]. In this study, we demonstrate that GlcN has a significant inhibitory effect on the pentose phosphate pathway in, and hence decidualization of, human ESCs. The process of decidualization is also impaired in mice exposed to GlcN. Furthermore, we show that intrauterine delivery of GlcN reversibly reduces the birth rate in mice. This work suggests the possibility for a new, nonhormonal contraceptive mechanism.
MATERIALS AND METHODS
Isolation of Human Primary ESCs and In Vitro Decidualization
Endometrial tissue was obtained from human uteri removed during hysterectomies conducted for benign indications. Informed consent was obtained from each patient before surgery, and protocols were approved by the Human Research Protection Office of Washington University. Tissue was placed in normal saline and transported to the laboratory. Human ESCs were isolated as previously described, with some modifications [9, 19]. Briefly, endometrial tissue was minced and digested in Dulbecco Modified Eagle Medium (DMEM):F12 (Invitrogen) containing 2 mg/ml collagenase (Invitrogen) and 15 units/ml deoxyribonuclease (Roche) for 30 min at 37°C with agitation. The dispersed endometrial cells were separated by filtration through a 40-μm wire sieve (BD), plated, and cultured in media supplemented with 10% heat-inactivated charcoal dextran-treated fetal bovine serum (HyClone) and 50 μg/ml penicillin/streptomycin (Cambrex Bio Science). The same culture conditions were used for human immortalized ESCs (telomerase-immortalized, ESC-Ts). For hormonal stimulation, approximately 3 × 105 cells were plated per well in six-well cell culture plates (Costar). After reaching 70%–80% confluency, cells were treated with 1 μM medroxyprogesterone-17-acetate (Sigma) and 0.5 mM N6,2′-O-dibutyryladenosine cAMP sodium salt (Sigma) for 3–9 days to induce decidualization [9]. Control samples received no hormone supplementation.
Animal Care and Use
All mouse studies were approved by the Animal Studies Committee at Washington University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Female ICR (The Harlan Laboratories) mice 8–10 wk of age were used for the isolation of primary ESCs. Female ICR mice 12 wk of age were used for in vivo deciduoma induction studies. Female ICR mice 9 wk of age were used for the pellet implantation surgeries.
Isolation of Murine Primary ESCs and In Vitro Decidualization
Murine ESCs were isolated and cultured as previously described [9]. The isolated ESCs were plated in six-well culture plates at a density of 5 × 105 cells per well in DMEM:F12 without phenol red, supplemented with 2% heat-inactivated charcoal dextran-treated fetal bovine serum and 50 μg/ml penicillin/streptomycin. Cells were treated with 10 nM β-estradiol (E2) (Sigma) and 1 μM P4 (Sigma) for 72 h to induce decidualization. Control samples were treated with 0.2% ethanol.
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium Bromide Assay
Human ESC-Ts were seeded in a 96-well culture plate at a density of 5 × 103 cells/well. After 3–9 days (specific to the experiment) of culture in the presence or absence of GlcN, the media was replaced with 110 μl of media containing 0.5 mg/ml MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide)-labeling reagent. After 4 h at 37°C, 100 μl of 10% SDS/0.01 M HCl solubilization solution was added, cells were incubated for 4 h at 37°C, and absorbance at 570 nm was measured. Viability of GlcN-treated cells is reported relative to the viability of cells decidualized in the absence of GlcN. For decidualizing cells, 5 × 104 human ESC-Ts were seeded per well in a 24-well culture plate. After 3–9 days of in vitro decidualization with or without GlcN, MTT assay was performed by the aforementioned methods with 500 μl of MTT and 500 μl of 10% SDS/0.01 M HCl solution.
RNA Extraction and Quantitative Real-Time PCR
Cells were lysed with Trizol Reagent (Invitrogen), and total RNA was extracted according to the vendor's specifications. One microgram of total RNA was reverse transcribed by using the Quantitect Reverse Transcription kit (QIAGEN). Decidualization was monitored by determination of PRL, SMST, IL-15, and LEFTY2 mRNA levels for human cell studies and Prp mRNA levels for murine cell studies by Quantitative RT-PCR using the 7500 Fast Real-Time PCR System (Applied Biosystems). Each reaction was performed in triplicate and consisted of 25 ng of cDNA, 1× Fast SYBR Green master mix (Applied Biosystems), and 300 nM gene-specific primers. The fold change in gene expression was calculated using the comparative cycle threshold method with the housekeeping gene, ribosomal protein 36B4, as the internal control in human cell studies and β-actin as the internal control in murine cell studies. The sequences of primers used for quantitative PCR are shown in Supplemental Table S1 (all the Supplemental Data are available online at www.biolreprod.org).
Metabolite Microanalytic Assays
Cells were seeded in six-well culture plates and decidualized in the presence or absence of GlcN as described above. After 9 days of treatment, cells were harvested using cell dissociation solution (Sigma). Microanalytic assays designed to measure G6PDH activity were performed as previously described [20]. Three independent replicates were performed.
Western Blot Analysis
Western blot analysis was performed as previously described [10]. The rabbit anti-G6PDH antibody (Bethyl Laboratories) and mouse anti-ACTIN (Millipore) were used at 1:1000 and 1:3000 dilutions, respectively, in 5% milk and Tris-buffered saline with Tween 20 (10 mM Tris, 100 mM NaCl, 0.05% Tween 20, pH 7.5). Secondary antibody was horseradish peroxidase-linked goat anti-rabbit or goat anti-mouse antibody (1:10 000; Santa Cruz Biotechnology, Inc.). Secondary antibody was visualized using enhanced chemiluminescence reagent (Amersham). Signal intensities were quantified by the program ImageJ (National Institutes of Health), and the values were normalized to ACTIN for loading variation.
Artificially Induced Deciduoma
The protocol for the induction of deciduoma has been described previously [9]. Briefly, female ICR mice 12 wk of age were ovariectomized and allowed 2 wk for recovery. They were then injected with three daily subcutaneous injections of 100 ng E2 in 100 μl of sunflower seed oil (Sigma), followed by 2 days of no injection and then 3 days of subcutaneous injections of 10 ng of E2 together with 1 mg of P4. At 2 h after the last of the above sensitization injections, the right uterine horn received an intraluminal injection of 100 μl of sunflower seed oil. Mice continued to receive 1 mg of P4 for 4 days. For the GlcN treatment, mice received two daily intraperitoneal injections of 25 μg of GlcN in 100 μl PBS starting on the day the E2/P4 injection were begun, followed by intraluminal injection of GlcN in 100 μl sunflower seed oil to the right uterine horn and 4 days of intraperitoneal injections of GlcN in 100 μl PBS. The whole uterus was dissected out, and the uterine horns were weighed separately on the day after the last injection.
Pellet Implantation and Hysterectomy
Females (9-wk-old) were anesthetized with ketamine and xylene 1 day after their pups were delivered. To mimic a single uterine model, a hysterectomy of one uterine horn was performed according to standard surgical procedures. A sustained-release GlcN-containing pellet or a placebo pellet was implanted into the top of the remaining uterine horn. Pellets measuring 1.5 mm in diameter that release 15, 150, or 1500 μg of GlcN over 60 days were obtained from Innovative Research of America. Although it is difficult to compare dosages administered by oral versus intrauterine routes, standard oral dosing for GlcN to treat osteoarthritis is approximately 20 mg/kg/day. By comparison, the maximal dosage used in the present study was 1 mg/kg/day, delivered intrauterine. Females recovered in an independent cage for 10 days and were then mated with ICR males. The number of pups per litter was recorded until two litters after the 60-day pellet release period. Previous study has shown that GlcN did not affect mouse mating [21]. Because the gestation period for ICR mice ranges from 19 to 21 days, pup number was counted as zero if no pup delivered within the 60-day pellet-active and postpellet periods.
Statistical Analyses
Data are expressed as means ± SEM of at least three separate experiments. Differences between control and experimental values were compared by Student t test. ANOVA with a Bonferroni multiple comparison test was used when comparisons were made between multiple experimental groups (PRISM program, GraphPad). Significance was defined as a P value of <0.05.
RESULTS
Glucosamine Is Not Toxic to Human ESCs
Although GlcN is a well-tolerated monosaccharide that is naturally made from glucose and glutamine in the body [13], a few studies have reported that large doses of GlcN induce cell death in chondrocytes and several malignant cell lines [22–25]. To examine the effect of GlcN on viability of human ESCs, human ESC-Ts [26] were cultured for 3, 6, or 9 days in the presence of 0, 2.5, or 5 mM GlcN, and the MTT assay [27] was used to determine cell viability and proliferation. As shown in Figure 1A, GlcN attenuated ESC-Ts proliferation activity during culture. On the other hand, the numbers of live cells slightly increased during GlcN incubation, implying that GlcN did not induce dramatic cell death or complete cell cycle arrest (Fig. 1A). We next examined the effect of GlcN on viability of decidualizing ESC-Ts. Cells were decidualized for 3, 6, or 9 days with or without GlcN. Consistent with previous findings that decidualizing ESC-Ts demonstrate almost no proliferative activity [9], we observed no evidence of proliferation during the 9-day decidualization period. Relative to mock-treated cells, decidualizing cells exposed to 2.5 or 5 mM GlcN had reduced viability by approximately 15%–20% by Day 3, but the cells recovered by Day 9, suggesting that GlcN could be tolerated by decidualizing ESC-Ts. We also used flow cytometry to determine whether apoptosis was increased in decidualizing ESC-Ts exposed to GlcN. No significant apoptosis was observed in GlcN-treated cells (see Supplemental Materials and Methods and Fig. S1). Similar results were observed from GlcN-treated human immortalized uterine epithelial cells, in which GlcN was not toxic but attenuated cell proliferation during 9 days of culture (Supplemental Materials and Methods and Fig. S2).
FIG. 1.
Glucosamine does not induce significant apoptosis in human ESC-Ts. The MTT assay was used to determine cell viability and proliferation rates in nondecidualized (A) and decidualizing (B) ESC-Ts. All values are represented relative to the viability of cells without GlcN exposure at Day 3 and are the mean ± SEM of triplicate cultures.
Glucosamine Attenuates G6PDH Activity and Expression in Human ESCs
We next sought to examine the effect of GlcN on activity of G6PDH, the rate-limiting enzyme in the first step of the pentose phosphate pathway [28], in decidualizing ESCs as has been shown in vitro and in mouse oocytes [17, 29]. Human ESC-Ts were cultured in decidualization media in the absence or presence of GlcN. G6PDH activity was determined after 9 days of exposure. As shown in Figure 2A, G6PDH enzyme activities in decidualized ESC-Ts were markedly higher than those in nondecidualized cells. In contrast, G6PDH activity in the cells exposed to 5 mM GlcN was reduced to 74% of the level in cells cultured in decidualization media alone (Fig. 2A). We also examined G6PDH expression in human ESC-Ts. Intriguingly, in addition to the role it plays as a competitive inhibitor of G6PDH, GlcN reduced G6PDH mRNA level to 68% and G6PDH protein level to about 80% of control levels in cells (Fig. 2, B and C). These data indicate that GlcN attenuates G6PDH both activity and expression; therefore, the flux via the pentose phosphate metabolic pathway in human ESC-Ts is attenuated.
FIG. 2.
Activity and expression of G6PDH are reduced in human ESC-Ts exposed to GlcN. Human ESC-Ts were collected after in vitro decidualization for 9 days in the presence of 0 or 5 mM GlcN. A) The levels of G6PDH activity were measured by microanalytic assay and normalized to cell counts. B) The levels of G6PDH expression were measured by quantitative real-time PCR. Data is expressed as mean ± SEM of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the decidualized sample. C) Western blot exhibits G6PDH protein expression and the loading control, ACTIN. Lower panel is the quantification of duplicate Western blots.
Glucosamine Inhibits Decidualization of Human ESCs In Vitro
Given the importance of the pentose phosphate pathway to the process of decidualization [9], we wanted to determine whether exposure to GlcN results in a functional change in human ESC-Ts by impairing differentiation. Decidualization was assessed by monitoring the levels of prolactin (PRL), somatostatin (SMST), interleukin-15 (IL-15), and left-right determination factor 2 (LEFTY2) mRNAs, all of which are significantly induced during decidualization [7, 30–32]. PRL has been used widely as phenotypic marker of decidualized cells [33]. SMST, IL-15, and LEFTY2 have the potential to amplify and propagate the decidual process in an autocrine and paracrine fashion [34, 35]. As shown in Figure 3, mRNA levels for these decidual markers were markedly induced during in vitro decidualization. However, these genes were induced to much lower levels in cells exposed to GlcN during the decidualization process. This reduced expression (up to 65%) was evident by Day 3 for SMST and IL-15, Day 6 for PRL, and Day 9 for LEFTY2 (Fig. 3, A–D).
FIG. 3.
Glucosamine inhibits decidualization of human ESC-Ts in vitro. Decidualization of human ESC-Ts was induced in vitro, and differentiation was assessed by monitoring the mRNA expression levels of decidual markers. Glucosamine treatment decreased the expression levels of PRL (A), SMST (B), IL-15 (C), and LEFTY2 (D) starting as early as Day 3 and continuing through Day 9. E) Human primary ESCs were isolated from hysterectomy samples and decidualized in the absence or presence of GlcN. Expression levels of decidual markers were assessed on Day 9. Values are a mean of at least three independent experiments ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the decidualized samples.
To determine whether these results were specific to telomerase-immortalized cells, we investigated the effect of GlcN on decidualization of primary ESCs isolated from human uteri after hysterectomies. Primary ESCs were cultured in decidualization media, with or without GlcN, for 9 days (see Materials and Methods for the details). Similar to our observations with ESC-Ts, the mRNA expression levels of decidual markers decreased in a GlcN dose-dependent manner in primary ESCs (Fig. 3E). Taken together, these results suggest that GlcN is able to inhibit the process of in vitro decidualization of both an immortalized cell line and primary human cells.
Glucosamine Inhibits Decidualization of Murine ESCs Both In Vitro and In Vivo
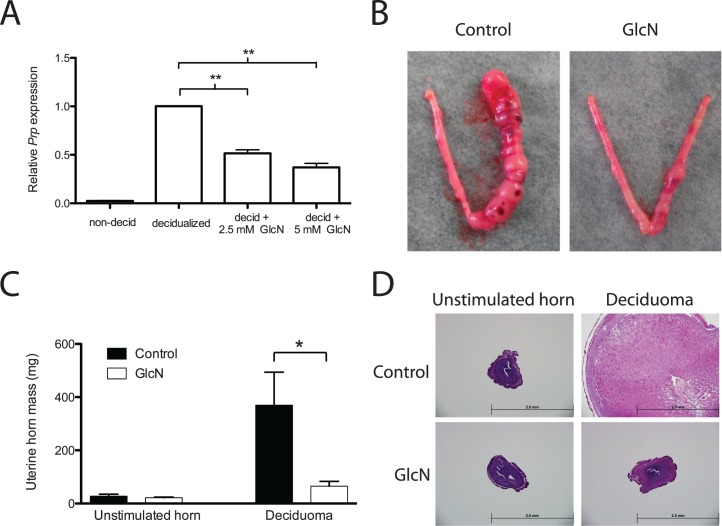
To further confirm the inhibitory effect of GlcN on decidualization, ESCs were isolated from a gravid mouse uterus at Day 4 postcoitus and cultured in decidualization media for 3 days (see Materials and Methods for details). Expression of Prp mRNA, a murine decidual marker, was significantly lower in cells decidualized in the presence of GlcN than in cells decidualized in the absence of GlcN (Fig. 4A). We next employed a previously described mouse model of induced decidualization [9] to examine whether GlcN has similar effects in vivo. Ovariectomized ICR mice received a regimen of E2 and P4, and then the right uterine horn of each animal was mechanically stimulated. The unstimulated left uterine horn served as an internal control. The decidual response, as determined by robust deciduoma formation, was observed consistently in the stimulated uterine horn in control mice (Fig. 4B). By contrast, weak or no deciduoma formation was observed in mice that had been maintained on GlcN (see Materials and Methods for the details) throughout the hormone and deciduoma induction period. We quantified the decidual response by weighing the uterine horns. Those from GlcN-treated mice weighed 5.5-fold less than those from the control mice (Fig. 4C). In addition to the gross anatomies of the uteri, histological examination revealed that stimulated uterine horns from control mice exhibited a marked increase in the number of decidualized cells and in the size of the stromal compartment. In contrast, decidualization was not observed in the stimulated horns from GlcN-treated mice, and the size of the stromal compartment was similar to that of the unstimulated uterine horn (Fig. 4D).
FIG. 4.
Decidualization is inhibited both in vitro and in vivo by GlcN in mice. A) Primary cultures of ESCs isolated from mice were decidualized with E2 and P4 in the absence or presence of GlcN for 3 days in vitro. Decidualization was assessed by monitoring the expression of Prp mRNA. Values are the mean of three independent experiments ± SEM. B) Representative uteri from mice in each treatment group—control and GlcN—are shown. The horn that received a physical stimulus to induce deciduoma formation is on the right, and the unstimulated internal control horn is on the left. C) The mass of deciduoma and control uterine horns was quantified for control and GlcN-treated mice. Values are the mean of three mice in each group ± SEM. *P < 0.05; **P < 0.01. D) Representative cross-section of the uterine horns stained with hematoxylin and eosin. Bar = 2 mm.
Intrauterine GlcN Decreases Birth Rate in Mice
Because differentiation of ESCs into decidual cells is one of the key processes in uterine preparation for embryo implantation, and proper implantation is essential for the continuation of pregnancy, failure of decidualization results in prevention of pregnancy [6, 36]. Thus, we anticipated that exposure of uteri to GlcN would prevent successful pregnancy. To test this hypothesis, we implanted time-release GlcN pellets into one uterine horn of female mice; the other uterine horn was removed to mimic the single reproductive tract found in humans and to avoid the possibility that pups may implant in the empty horn. In preliminary studies not shown, we had determined that this occurs at a fairly equal rate in the contralateral horn to the one containing a pellet regardless of type of pellet. The mice with one horn containing one pellet were mated, and the litter sizes were recorded both during the 60-day pellet-active period and in the postpellet period. After hysterectomy and implantation of placebo pellets, litters were approximately half the size that they were before surgery (5.6 ± 0.66 and 12.7 ± 0.71 pups per litter, respectively), as expected. The litters delivered during the 60-day pellet-active period and the postpellet period were of similar sizes (Fig. 5). Taken together, these results suggest that hysterectomy and placebo pellet implantation do not interfere with fertility. In contrast, mice that received GlcN pellets delivered significantly fewer live pups per litter over a 60-day pellet active period than those that received placebo pellets (15 μg GlcN, 2.75 ± 0.73 pups; 150 μg GlcN, 2.13 ± 0.85 pups; 1500 μg GlcN, 0.25 ± 0.25 pups; placebo, 5.61 ± 0.66 pups) (Fig. 5). The gross morphological appearance of the pups from placebo- and GlcN-treated mice were normal after birth. Meanwhile, the GlcN levels in the blood were measured by using a D-GlcN assay kit (Megazyme), which has a lower limit of detection in the μg/ml range. The serum GlcN levels were similar to the background among placebo and the three different GlcN dosages groups (data not shown), suggesting that the reduction of litter size seen is not due to systemic whole-body GlcN circulation, but rather due to a local intrauterine GlcN effect. After the 60-day pellet release period, there was no statistically significant difference in the litter sizes delivered by GlcN and placebo pellet-implanted mice, except at the highest dosage of GlcN, suggesting reversal of the effects once the pellets were spent. We conclude that, at moderate levels of local GlcN exposure, the GlcN effect did not persist beyond the 60-day release period, and there were no long-term fertility sequelae.
FIG. 5.
Glucosamine decreases litter size in ICR mice. Placebo or GlcN pellets were implanted into one uterine horn of each 9-wk-old ICR female mouse; the other uterine horn was removed. Pups per litter of mice with placebo (n = 9), 15 μg GlcN (n = 4), 150 μg GlcN (n = 4), and 1500 μg GlcN (n = 4) pellet implantations were recorded. Values are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
DISCUSSION
Here we provide evidence that intrauterine administration of GlcN reversibly impairs fertility in mice. Additionally, we show that GlcN, an inhibitor of the pentose phosphate pathway, blocks decidualization of both human and murine endometrial cells. Given that GlcN is a natural metabolite that is safely and widely used in the treatment of osteoarthritis and joint disease [14, 37], these results suggest that GlcN has the potential to be formulated as a nonhormonal contraceptive utilizing a novel metabolic mechanism.
Although our data clearly exclude the possibilities that GlcN inhibited decidualization due to toxicity for human ESCs (Fig. 1) and murine primary ESCs (Supplemental Fig. S3), there are four possible mechanisms to explain the physiologic effects of GlcN on decidualization of ESCs. First, the intermediate metabolite GlcN-6-phosphate could be directly blocking the pentose phosphate pathway via competitive inhibition of G6PDH. This mechanism has previously been described in postimplantation embryos [38] and is consistent with our earlier finding that DHEA, a noncompetitive inhibitor of G6PDH, also inhibits decidualization. In support of this mechanism, we observed decreases of both G6PDH catalytic activity (Fig. 2A) and expression levels (Fig. 2, B and C) in ESC-Ts exposed to GlcN. Prior evidence that the pentose phosphate pathway may play a role in decidualization and embryo implantation exists in both humans and mice. For example, human females heterozygous for G6PDH deficiency are known to have an increased risk of spontaneous abortions [39], and G6PDH-null mice have a 7-fold higher rate of fetal resorptions than their wild-type counterparts [39, 40]. These results suggest that activity of G6PDH affects female fertility, perhaps via a uterine event. Data also exist to suggest that GlcN itself inhibits G6PDH [29, 38], but the hypothesis that GlcN administered in vivo has uterine-specific reproductive consequences had not been tested.
A second possible explanation for the inhibition of decidualization by GlcN is that excess GlcN-6-phosphate could be converted to N-acetyl-GlcN-6-phosphate and be used for protein glycosylation and other posttranslational modifications of proteins involved in decidualization [41]. Third, extensive stromal cell proliferation is one of the hallmark events of decidualization in rodents. We observed that GlcN attenuated cell proliferation of human ESCs (Fig. 1A), human uterine epithelial cells (Supplemental Fig. S2) and murine ESCs (Supplemental Fig. S3) during in vitro culture in this study. Previous study has also shown that GlcN inhibited retinal pigment epithelium proliferation in a dose-dependent manner without affecting cell viability [42]. It is possible that the negative effect of GlcN on cell proliferation led to impaired decidualization in mice because the size of the stromal compartment was similar to that of the unstimulated uterine horn in the GlcN-treated mice (Fig. 4B). Fourth, GlcN could cause reduction in the surface levels of transforming growth factor (TGF)-β receptor and thus the ability of ESCs to bind TGF-β as has been demonstrated in human retinal pigment epithelial cells [43]. TGF-β is a multifunctional cytokine that regulates growth, differentiation, and apoptosis of various cell types and can induce decidualization of ESCs in vitro [44]. Which mechanism, or combination of mechanisms, is responsible for the effects of GlcN on decidualization remains to be determined.
Several studies have shown that deficient decidualization results in early embryonic lethality and infertility. For example, death effector domain-containing protein (Dedd) deficiency female mice are infertile owing to unsuccessful decidualization with reduced polyploidy [45]. Deletion of the IL-11 receptor alpha chain or sphingosine kinases shpk1 and sphk2 also cause defective decidualization, leading to early embryonic lethality [46, 47]. Thus, proper decidualization is essential for successful pregnancy. Because our ESC data clearly demonstrated that GlcN inhibits decidualization both in vitro and in vivo, we propose that the reduction in litter sizes we observed upon intrauterine administration of GlcN in mice was mainly due to inhibition of decidualization and implantation. Although this idea is consistent with our previous finding that knockdown of G6PDH or inhibition of the pentose phosphate pathway with DHEA or 6-aminonicotinamide blocks decidualization in vivo [9], we cannot rule out the possibility that GlcN affected other aspects of fertility because there is a long period between the time of fertilization and fetus delivery. For example, exposure of cumulus-oocyte complexes to 2.5 mM GlcN during in vitro maturation (IVM) inhibits oocyte developmental competence, potentially through up-regulation of hexosamine activation and inhibition of pentose phosphate [29]. Murine and bovine blastocyst developments are impaired by GlcN exposure during embryo culture or IVM [48, 49]. Increase of the rate of resorbed implantations has also been reported by injecting intraperitoneally 20 or 400 mg/kg GlcN into 8-wk-old female mice 3–6 days before and 1 day after mating [21]. Furthermore, administration of a high dose (∼1600–2000 mg/kg) of GlcN on Day 7.5 of pregnancy in mice increased the incidence of neural tube defects through inhibition of pax-3, a gene required for neural tube closure, expression [38]. As shown in Figure 5, the litter sizes of the mice implanted with pellets releasing 1500 μg of GlcN were not fully recovered during the postpellet period, suggesting that a high dose of GlcN triggers another mechanism by which fertility is reduced. Such uncertainty about the mechanism of action of contraceptives is not unusual, however, as there is still some ambiguity about the contributions of pre- and postfertilization mechanisms to the efficacy of copper and hormonal IUDs [50]. In addition, the finding that some pups delivered instead of none suggests that decidualization might be most impaired in the region immediately adjacent to the GlcN pellet implantation site and that the further away that implantation occurs, the better the outcome. Thus, the efficacy of a GlcN-containing IUD needs to be tested in a single uterine horn model, preferably a nonhuman primate.
Given the widespread use of GlcN to treat joint pain, it is important to assess whether oral consumption of this common supplement affects fertility. The LD50 of oral GlcN is about 8 g/kg (body weight) for rats, mice, and rabbits [13], far above the 20 mg/kg regularly taken by patients. One study of 54 women has reported no adverse fetal effects following the use of GlcN during pregnancy [51]. Studies in rats have also shown no adverse side effects of oral GlcN doses of 2.7 g/kg for 12 mo [52]. Moreover, prior mouse studies by our group have shown that mice fed a therapeutic GlcN-supplemented diet (0.01%) had normal litter sizes (Data not shown). Thus, it is unlikely that oral administration of GlcN will reduce fertility.
Our intrauterine pellet implantation experiments were designed to mimic how GlcN might be used in human IUDs; their success at reducing litter sizes indicates that this route takes advantage of local, rather than systemic, effects of GlcN. Importantly, this local inhibition is reversible because the litter sizes from the GlcN pellet-implanted mice returned to control levels following the pellet's active period. Our findings support the potential use of GlcN as a metabolically acting nonhormonal contraceptive. With no systemic effects at moderate dosage and a reversible, localized mechanism of action, future research into the development of a GlcN-containing, reversible contraceptive IUD is warranted.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Graciela Krikun for providing the human immortalized endometrial stromal cell line, Dr. Liang Ma for providing the human immortalized uterine epithelial cell line, and Dr. Deborah J. Frank for critical reading of the manuscript.
Footnotes
Supported by the National Institutes of Child Health and Human Development Grant R01 HD065435 to K.H.M. and by T32 Grant HD 49305-6 A1 to J.-H.T. Presented in part at the 45th Annual Meeting of the Society for the Study of Reproduction, 12–15 August 2012, State College, Pennsylvania.
REFERENCES
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- Frost JJ. The state of hormonal contraception today: overview of unintended pregnancy. Am J Obstet Gynecol. 2011;205:S1–S3. doi: 10.1016/j.ajog.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- Frye CA. An overview of oral contraceptives: mechanism of action and clinical use. Neurology. 2006;66:S29–S36. doi: 10.1212/wnl.66.66_suppl_3.s29. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Grinius L, Kessler C, Schroeder J, Handwerger S. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol. 2006;189:179–187. doi: 10.1677/joe.1.06451. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frolova AI, O'Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol. 2011;25:1444–1455. doi: 10.1210/me.2011-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2009;150:1512–1520. doi: 10.1210/en.2008-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8:331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- Roy S, Mahesh VB, Greenblatt RB. Effect of dehydroepiandrosterone and delta4-androstenedione on the reproductive organs of female rats: production of cystic changes in the ovary. Nature. 1962;196:42–43. doi: 10.1038/196042a0. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43:187–201. doi: 10.1016/j.fct.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Houpt JB, McMillan R, Wein C, Paget-Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol. 1999;26:2423–2430. [PubMed] [Google Scholar]
- Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203. doi: 10.1016/s0014-5793(02)03058-2. [DOI] [PubMed] [Google Scholar]
- Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, Caputo M, McClain D, Del Prato S, Giaccari A, Muggeo M, Bonora E, et al. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes. 2000;49:926–935. doi: 10.2337/diabetes.49.6.926. [DOI] [PubMed] [Google Scholar]
- Glaser L, Brown DH. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955;216:67–79. [PubMed] [Google Scholar]
- Kanji MI, Toews ML, Carper WR. A kinetic study of glucose-6-phosphate dehydrogenase. J Biol Chem. 1976;251:2258–2262. [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Al-Rejjal R, Zheng W, Luleci G, Arici A. Regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) expression by estradiol and progesterone in human endometrium. J Clin Endocrinol Metab. 2003;88:5017–5026. doi: 10.1210/jc.2003-030414. [DOI] [PubMed] [Google Scholar]
- Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab. 2002;283:E226–E232. doi: 10.1152/ajpendo.00046.2002. [DOI] [PubMed] [Google Scholar]
- Schelbach CJ, Robker RL, Bennett BD, Gauld AD, Thompson JG, Kind KL. Altered pregnancy outcomes in mice following treatment with the hyperglycaemia mimetic, glucosamine, during the periconception period. Reprod Fertil Dev. 2013;25:405–416. doi: 10.1071/RD11313. [DOI] [PubMed] [Google Scholar]
- Varghese S, Theprungsirikul P, Sahani S, Hwang N, Yarema KJ, Elisseeff JH. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthritis Cartilage. 2007;15:59–68. doi: 10.1016/j.joca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Chesnokov V, Sun C, Itakura K. Glucosamine suppresses proliferation of human prostate carcinoma DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int. 2009;9:25. doi: 10.1186/1475-2867-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Bekesi JG. Cytotoxic effects of D-glucosamine on the ultrastructures of normal and neoplastic tissues in vivo. Cancer Res. 1972;32:756–765. [PubMed] [Google Scholar]
- Friedman SJ, Skehan P. Membrane-active drugs potentiate the killing of tumor cells by D-glucosamine. Proc Natl Acad Sci U S A. 1980;77:1172–1176. doi: 10.1073/pnas.77.2.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Lockwood C. The immortalization of human endometrial cells. Methods Mol Med. 2006;121:79–83. doi: 10.1385/1-59259-983-4:077. [DOI] [PubMed] [Google Scholar]
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- Schelbach CJ, Kind KL, Lane M, Thompson JG. Mechanisms contributing to the reduced developmental competence of glucosamine-exposed mouse oocytes. Reprod Fertil Dev. 2010;22:771–779. doi: 10.1071/RD09193. [DOI] [PubMed] [Google Scholar]
- Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod. 2007;76:777–783. doi: 10.1095/biolreprod.106.053058. [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics. 2001;7:135–148. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- Godbole G, Modi D. Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol. 2010;85:130–139. doi: 10.1016/j.jri.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983;145:672–678. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics. 2004;4:299–312. doi: 10.2165/00129785-200404050-00003. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskisson EC. Glucosamine and chondroitin for osteoarthritis. J Int Med Res. 2008;36:1161–1179. doi: 10.1177/147323000803600602. [DOI] [PubMed] [Google Scholar]
- Horal M, Zhang Z, Stanton R, Virkamaki A, Loeken MR. Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol. 2004;70:519–527. doi: 10.1002/bdra.20056. [DOI] [PubMed] [Google Scholar]
- Toncheva D, Tzoneva M. Prenatal selection and fetal development disturbances occurring in carriers of G6PD deficiency. Hum Genet. 1985;69:88. doi: 10.1007/BF00295536. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Zielenski J, Tsui LC, Wells PG. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 2000;14:111–127. doi: 10.1096/fasebj.14.1.111. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Chien MW, Chen JT. Glucosamine inhibits epidermal growth factor-induced proliferation and cell-cycle progression in retinal pigment epithelial cells. Mol Vis. 2010;16:2559–2571. [PMC free article] [PubMed] [Google Scholar]
- Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Lu DW, Chen JT. Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol. 2011;89:e505–e514. doi: 10.1111/j.1755-3768.2011.02147.x. [DOI] [PubMed] [Google Scholar]
- Chang HJ, Lee JH, Hwang KJ, Kim MR, Chang KH, Park DW, Min CK. Transforming growth factor (TGF)-beta1-induced human endometrial stromal cell decidualization through extracellular signal-regulated kinase and Smad activation in vitro: peroxisome proliferator-activated receptor gamma acts as a negative regulator of TGF-beta1. Fertil Steril. 2008;90:1357–1365. doi: 10.1016/j.fertnstert.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Mori M, Kitazume M, Ose R, Kurokawa J, Koga K, Osuga Y, Arai S, Miyazaki T. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J Clin Invest. 2011;121:318–327. doi: 10.1172/JCI44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleon M, Tan HY, Kafer GR, Kaye PL. Toxic effects of hyperglycemia are mediated by the hexosamine signaling pathway and o-linked glycosylation in early mouse embryos. Biol Reprod. 2010;82:751–758. doi: 10.1095/biolreprod.109.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Mitchell M, Cetica P, Dalvit G, Pantaleon M, Lane M, Gilchrist RB, Thompson JG. Glucosamine supplementation during in vitro maturation inhibits subsequent embryo development: possible role of the hexosamine pathway as a regulator of developmental competence. Biol Reprod. 2006;74:881–888. doi: 10.1095/biolreprod.105.048553. [DOI] [PubMed] [Google Scholar]
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol. 2002;187:1699–1708. doi: 10.1067/mob.2002.128091. [DOI] [PubMed] [Google Scholar]
- Sivojelezova A, Koren G, Einarson A. Glucosamine use in pregnancy: an evaluation of pregnancy outcome. J Womens Health (Larchmt) 2007;16:345–348. doi: 10.1089/jwh.2006.0149. [DOI] [PubMed] [Google Scholar]
- Setnikar I, Pacini MA, Revel L. Antiarthritic effects of glucosamine sulfate studied in animal models. Arzneimittelforschung. 1991;41:542–545. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.