Abstract
Cellular damage and deregulated apoptotic cell death lead to functional impairment, and a main consequence of these events is aging. Cellular damage is initiated by different stress/risk factors such as oxidative stress, inflammation, and heavy metals. These stress/risk factors affect the cellular homeostasis by altering methylation status of several aging and Alzheimer’s disease associated genes; these effects can be manifested immediately after exposure to stress and at later stages of life. However, when cellular damage exceeds certain threshold levels apoptosis is initiated. This review discusses the stress factors involved in cellular damage and the role and potential of TSPO-mediated cell death in aging as well as in Alzheimer’s disease, which is also characterized by extensive cell death. Mitochondrial-mediated apoptotic death through the release of cytochrome c is regulated by TSPO, and increased expression of this protein is observed in both elderly people and in patients with Alzheimer’s disease. TSPO forms and mediates opening of the mitochondrial membrane pore, mPTP and oxidizes cardiolipin, and these events lead to the leakage of apoptotic death mediators, such as cytochrome c, resulting in cell death. However, TSPO has many proposed functions and can also increase steroid synthesis, which leads to inhibition of inflammation and inhibition of the release of apoptotic factors, thereby decreasing cell damage and promoting cell survival. Thus, TSPO mediates apoptosis and decreases the cell damage, which in turn dictates the process of aging as well as the functionality of organs such as the brain. TSPO modulation with ligands in the Alzheimer’s disease mouse model showed improvement in behavioral symptoms, and studies in Drosophila species showed increased cell survival and prolonged lifespan in flies after TSPO inhibition. These data suggest that since effects/signs of stress can manifest at any time, prevention through change in lifestyle and TSPO modulation could be potential strategies for altering both the aging process and the progression of Alzheimer’s disease.
Keywords: Aging, Alzheimer’s disease, apoptosis, mitochondria, neurodegenerative diseases, TSPO.
INTRODUCTION
Aging is a multifactorial genetic phenomenon manifested by slow functional breakdown of tissues and organs due to deleterious changes in the cellular environment throughout life. Changes from exposure to toxins or other stress/risk factors cause cellular damage and after a certain threshold it leads to apoptosis. Thus in healthy human beings, apoptosis works as a sentinel mechanism to destroy damaged cells resulting from inflammation, heavy metals, and reactive oxygen species (ROS), thereby maintaining tissue homeostasis. However, excessive cellular damage and deregulated apoptosis has been linked to age-related pathologies and specifically neurodegenerative diseases [1-8]. TSPO expression is increased in aging people and in patients with Alzheimer’s disease (AD) and has been implicated as a modulator of inflammation and apoptosis. This review will start by explaining some of the factors associated with cellular damage and their link to apoptosis and neurodegenerative diseases, and also illustrates the role of Translocator Protein (TSPO).
CELLULAR DAMAGE AND APOPTOSIS-AGING/AD
Cellular damage and apoptosis can be caused by several stress factors such as oxidative stress, inflammation, dietary factors and heavy metals, so having resistance to these can prevent damage and cell death, and can improve organ functionality resulting in an increased life span [6, 7, 9]. Oxidative stress is caused when reactive oxygen species (ROS) such as superoxide (O2-), hydrogen peroxide (H2O2) and hydroxyl (OH-) anions are generated excessively in the cell and are not removed by antioxidant enzymes. Mitochondria are major sources of ROS as they consume most of the available (O2) to produce adenosine triphosphate (ATP). Superoxide dismutases (SODs) convert O2- into H2O2, and glutathione peroxidase (Gpx1) and peroxiredoxin (Prx) convert H2O2 into water (H2O) [10]. These ROS react with other cellular components such as membranes, enzymes, proteins, and deoxyribonucleic acid (DNA) leading to disruption of cellular homeostasis, and cell death. Enhanced cell death leads to organ failure, accelerates the aging process and ultimately affects the life span [7, 11]. Aged skeletal muscle volume loss can be attributed to apoptosis as it shows more apoptotic markers such as apoptotic protease activating factor-1 (Apaf-1), caspase 9 and 3 [12]. Extended life span is observed in several animal species that have less ROS compared to animals with more ROS. For example, rats, a species that has naturally high levels of ROS have shorter life spans than pigeons that have less ROS. Furthermore, the deletion of oxidative factors such as p66shc, which is involved in the conversion of O2 to H2O2 in mouse embryonic fibroblasts (MEFs) and mouse models, increased resistance to oxidative stress, and prolonged cell survival and life span. The ROS induce mutations in DNA, and the correlations between mutations and aging, life span, and neurodegenerative diseases have been observed in several cell culture and animal studies. Studies on skeletal muscle of mice showed that 9-month-old mice that have mitochondrial DNA mutations exhibited increased apoptosis compared to normal mice [7]. Additionally, AD patients showed increased levels of 8-oxodeoxyguanine (8-oxodG), indicating possible oxidative damage, and its induced aberrant methylation might be a causative factor for disease progression [13, 14]. Oxidative stress can be reduced by restricting diet, which enhances stress resistance to ROS. This is corroborated in studies with Caenorhabditis elegans, Drosophila melanogaster, and mice, which all showed a correlation between restricted calorie intake and increased lifespan. Additionally, reduction of insulin/insulin-like growth factor-1 (IGF-1) signaling through knockdown of various genes such as daf-2 and age-1 increased cell resistance and life span in worms, flies and other animals [7].
Cell death is the major contributor for neurodegenerative diseases; however, this phenomenon appears in the late stages of disease. Cellular damage and cellular death can be results of acute exposure or earlier exposure to several kinds of toxins and other stress/risk factors. Some of the risk factors associated with AD are aging, oxidative stress, heavy metals (lead-Pb), vitamin B deficiency, dietary cholesterol, lack of exercise, head trauma, genetics (APOEε4 genotype), and inflammation [8, 13, 15, 16]. According to the LEARn model, early exposure to the above-mentioned factors can cause increase in AD-associated genes such as amyloid precursor protein (APP) and beta secretase/beta-site APP cleaving enzyme (BACE) through increased transcription by transcription factor specificity protein 1 (SP1). Amylod beta (Aβ) is produced from APP via the amyloidogenic pathway where it is cleaved by beta and gamma secretases. Increased transcription elevates the levels of Aβ, but it’s not enough to manifest pathological symptoms at that time, and these changes are temporary as the levels returns to normal. However, the changes are maintained epigenetically and make the individuals more susceptible to AD development as they age and are exposed to other triggering factors such as oxidative stress and heavy metals. Studies on mice and Cyanomolgus monkeys with Pb exposure support the above phenomenon. The mice/monkeys that are exposed to Pb at early and later stages showed elevated Aβ compared to animals that are exposed only in later stages. As explained earlier, oxidative stress-induced DNA damage is one of the triggering factors for developing AD. For example, oxidative stress-induced 8-oxodG has been proposed to play a role in AD as it leads to the inhibition of methylation leading to elevated levels of AD genes APP, BACE and Aβ [9, 13, 15]. Elevated levels of 8-oxodG are observed in aging people and AD patients [13, 14], so inhibition of oxidative stress might be beneficial. Such inhibition is accomplished through antioxidants such as melatonin as its administration decreased oxidative stress and Aβ in different disease mouse models [15]. Aβ aggregate levels can also be elevated due to the lack of an efficient chaperone system (heat shock protein 70 - HSP70) that removes aggregated/misfolded proteins [17]. Another key factor is inflammation, which is also considered a risk factor for AD; inflammation can be developed as early as during gestation through exposure to toxins, and during different developmental stages, illness, or injury [18]. Elevated levels of cytokines and microglial activations are also observed in the AD patients, and the aging population also has elevated levels of pro-inflammatory factors, including pro-inflammatory cytokines, such as interleukin6 (IL6), and prolonged inflammation causes damage to neurons [18-20]. Vitamin B12/folate deficiency has been documented as one of the stress/risk factors for AD as well as it increases BACE levels, gamma-secretases and Aβ levels through demethylation which occurs when S-adenosylhomocysteine (SAM) levels are reduced. SAM is produced from methionine, which is produced from homocysteine (HCY) in the presence of vitamin B12 and folate [15]. These observations indicate that modification of lifestyle by increasing the antioxidant intake, exercise and avoiding exposure to heavy metals modify the aging and disease progression [9, 13, 15, 18, 19].
THE MECHANISM OF APOPTOSIS
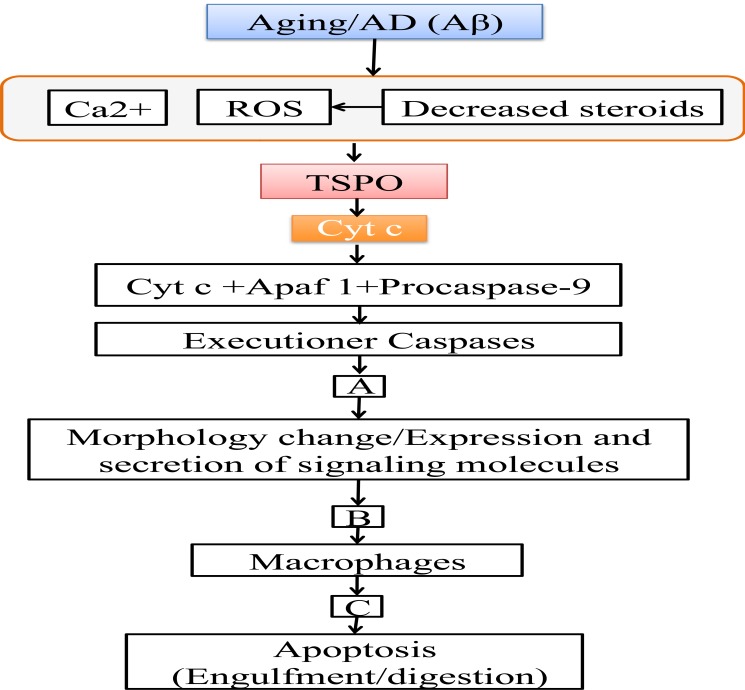
Apoptosis is executed through two regulated pathways: extrinsic and intrinsic. The extrinsic pathway is mediated through transmembrane death receptors, tumor necrosis factor receptor 1 (TNFR1), death receptors 4 and 5 (DR4 and DR5), and Fas, which are activated by proapoptotic ligands, such as TNF, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL). These receptors activate caspases 3, 6, 7, and 8, which then initiate cell death [21]. The intrinsic pathway is carried out in mitochondria and involves the release of cytochrome c (Cyt c), leading to cell death [2]. Mitochondrial-mediated cell death can be increased due to aging [22-24], neurodegenerative diseases [22, 24, 25], DNA damage [26], or the presence of reactive oxygen species (ROS) [27-30]. Mitochondrial-mediated cell death is regulated through TSPO that releases Cyt c from mitochondria through the membrane permeability transition pore (mPTP) formation and cardiolipin (CL) oxidation [30, 31]. Cyt c forms an apoptosome upon binding with Apaf-1 and procaspase 9. The formation of the apoptosome complex is essential for cell death [1, 32]. Additionally, the cleaved caspase 9 molecules from this complex activate executioner caspases 3 and 7, which, in turn, deactivate cell survival mechanisms through inhibition and activation of different enzymes, ultimately leading to apoptosis (Fig. 1) [1-3, 32-37].
Fig.(1).
Schematic representation of steps involved in apoptosis. Aging and AD disturbs the Ca2+ homeostasis, increases ROS and decreases neurosteroids and the release of Cyt c through TSPO, which then activates caspases. (A) Caspases inhibits several substrates such as poly (ADP-ribose) polymerase (PARP), protein kinase Cδ, retinoblastoma, and degrades lamin leading to inhibition of DNA repair, inhibition of cell cycle progression and nuclear condensation. Activates several other substrates such as gelsolin, MEK kinase 1 (MEKK1) leading to F-actin depolymerization, DNA fragmentation, cytoskeleton organization and plasma membrane blebbing. (B) These actions leading to morphological changes and expression/excretion of different signaling molecules such as fractaline, lysophosphatidylcholine (LPC), sphingosine- 1-phosphate (SIP), adenosine triphosphate (ATP)/uridine triphosphate (UTP). These molecules recruit macrophages by binding to receptors on the macrophages CX3CR1, G-protein-coupled receptors G2A, SIP-R1/5, P2Y2. (C) Recruited phagocytes recognize molecules expressed on apoptotic cells such as thrombospondin, complement C1q, intracellular adhesion molecule 3 (ICAM3), phospholipid phosphatidylserine (PtdSer). Then phagocytes bind them through their receptors cluster of differentiation 36 (CD36), low density lipoprotein receptor related protein 1 (LRP 1), cluster of differentiation 14 (CD14), Tyro-3-Axl-Mer family of receptors (TAM receptors). Then the cells are then engulfed and digested them by the phagocytes. Alzheimer’s disease (AD), amyloid beta (Aβ), apoptotic protease activating factor 1 (Apaf 1), calcium2+ (Ca2+), cytochrome c (Cyt c), membrane permeability transition pore (mPTP), reactive oxygen species (ROS), translocator protein (TSPO).
TSPO REGULATES APOPTOSIS
TSPO, an 18 kDa translocator protein, is involved in calcium2+ (Ca2+) signaling cascades, steroidogenesis, and cell survival and death, thereby helping maintain homeostasis in the cell [30, 31, 38, 39]. TSPO is present in many different tissues and organs, including steroidogenic tissues, adrenal tissues, ovaries, testes, liver, kidney and brain [38, 40, 41]. TSPO is activated by high Ca2+ and ROS generation that occurs at an increased rate in the aging population and in people with AD. Activated TSPO mediates the formation of mPTPs, ROS generation, and CL oxidation, causing the release of Cyt c and, ultimately, cell death [23, 24, 29-31, 39, 42, 43]. TSPO is also involved in the synthesis of neurosteroids. TSPO ligands have been reported to increase neurosteroid synthesis and inhibit neurodegeneration, and have also shown improved behavioral symptoms in mice, including decreased anxiety and increased cognition. Decreased levels of some neurosteroids are observed in both AD patients and aging people [31, 39, 44-47].
TSPO-mPTP
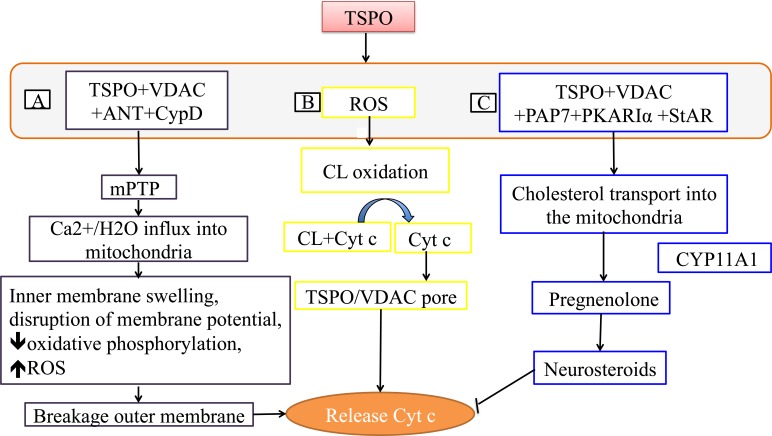
TSPO regulates the mPTP formation as well as its opening, and it is proposed that TSPO forms mPTP through interaction with the outer membrane voltage-dependent anion channels (VDACs), which transport anions, and the inner membrane channel ATP-ADP translocase (ANT)/ cyclophilin D (CypD). The ANT channel transports ADP-ATP, and CypD matrix proteins bind to ANT. mPTP permits the passage of not only calcium but also any other solute below the pore size, which under normal conditions is strictly mediated by specialized channels. This uncontrolled influx of solutes leads to breakage of the outer membrane and the release Cyt c due to inner membrane swelling (Fig. 2) [30, 31, 38, 39, 42, 48, 49].
Fig.(2).
Mechanisms involved in release of cytochrome c. A) Release of Cyt c through mPTP: TSPO forms a pore with VDAC, ANT and CypD and releases Cyt c. B) Release of Cyt c through CL oxidation: TSPO oxidizes cardiolipin through ROS, that causes dissociation of Cyt c, which is then released through the TSPO and VDAC pore. C) TSPO Neurosteroidogenesis: TSPO increases neurosteroidgenesis by transporting cholesterol into the mitochondria. Increased neurosteroids inhibits release of Cyt c. ATP-ADP translocase (ANT), calcium2+ (Ca2+), cardiolipin (CL), cyclophilin D (CypD), cytochrome c (Cyt c), cytochrome P450 enzyme CYP11A1 (CYP11A1), membrane permeability transition pore (mPTP), peripheral-type benzodiazepine receptor (PBR)-associated protein 7 (PAP7), protein kinase A (PKA), PKA-RIalpha (PKARIα), reactive oxygen species (ROS), steroidogenic acute regulatory protein (StAR), translocator protein (TSPO), voltage-dependent anion channels (VDAC), water (H2+O).
TSPO-CARDIOLIPIN OXIDATION
TSPO oxidizes cardiolipin through ROS generation; oxidized lipid no longer binds to Cyt c, resulting in the release of Cyt c into the cytosol through pores formed by TSPO and VDAC (Fig. 2). Additionally, cardiolipin oxidation enhances the mPTP opening [23, 24, 29, 30, 43].
TSPO-NEUROSTEROIDOGENESIS
One function of TSPO is to transport cholesterol into mitochondria from the cytosol through the transduceosome. Cholesterol gets converted into pregnenolone and then into steroids once it exits the mitochondria. Peripheral-type benzodiazepine receptor-associated protein 7 (PAP7) signal transporter from the golgi apparatus initiates the steroidogenesis by binding to TSPO/VDAC, and by activating protein kinase A (PKA) to form homodimer PKARIα in the presence of excessive cAMP (cyclic adenosine monophosphate). PKARIα phosphorylates steroidogenic acute regulatory protein (StAR), which then delivers free cholesterol to the TSPO complex. StAR also mobilizes the TSPO-bound cholesterol for transport into the mitochondria [39, 50-53]. Neurosteroids can exhibit neuroprotective effects and inhibit cell death by several mechanisms such as inhibition of Ca2+ elevation and Cyt c release (Fig. 2) [31, 38, 39, 44-46]. Additionally, neurosteroids can inhibit the neuroinflammation through inhibiting the release of proinflammatory factors such as cytokines [45].
TSPO MEDIATED APOPTOSIS INFLUENCE ON AGING AND NEURODEGENERATIVE DISEASES
Normal brain tissue contains TSPO in the olfactory bulb, the choroid plexus, and the glial cells at low levels. Aging and neurodegenerative brains, such as brains from patients with AD and Parkinson’s disease showed an increased expression of TSPO [31, 38, 54-56], indicating it as a potential culprit for increased apoptosis. AD patients have dementia caused by neuronal dysfunction/loss due to beta amyloid plaques and neurofibrillary tangles and inflammation.
TSPO-MEDIATED APOPTOSIS INFLUENCE ON AGING
Since TSPO mediates apoptosis, its inhibition leads to prolonged cell survival and may improve some of the functions related to cell loss in the aging process. The role of TSPO in cell death and aging was examined by Lin et al., in Drosophila. Drosophila 3rd-instar larval tissues were exposed to gamma-rays at 30 Gray to induce apoptosis and after 3 hours, wing disc cells from TSPO-inactivated (TSPO-/-) and TSPO-knockdown flies showed less apoptosis compared to TSPO wild-type flies. Similarly, cells isolated from 3rd-instar TSPO-/- larval brains exposed to H2O2, which induces oxidative stress, demonstrated significant suppression of apoptosis when compared to TSPO wild-type cells from male and female flies, indicating that TSPO plays a key role in the induction of apoptosis. Furthermore, the authors studied the effect of TSPO deletion or TSPO knockdown on the life span of the fly. Interestingly, increased life span was observed only in male TSPO-/- or TSPO knockdown or TSPO depletion Drosophila flies. When these flies were exposed to stress, such as H2O2, male TSPO-/- flies had longer lifespans than wild-type flies, while no differences were found between the groups in female flies. Additionally, it was found that TSPO ligands, PK11195 (at moderate concentrations) and Ro5-4864, inhibited TSPO and increased the lifespan in male flies [57].
TSPO IN NEURODEGENERATIVE DISEASES
In positron emission tomography (PET) studies, adult human brains showed increased uptake of the 11C-[R]-PK11195 ligand, which specifically binds to TSPO, when compared to the brains of children [54]. PK11195 can be used as an imaging agent by labeling it to map the inflamed areas of brain. It also exhibits agonistic and antagonistic actions based on its concentration, the cell type, and the environment [58-60]. Brains of patients with neurodegenerative diseases such as AD also showed increased uptake of another ligand, [11C]vinpocetine, in diseased regions, thus, TSPO may also be used as a target to inhibit AD [55].
A reduction of TSPO in the brains of Drosophila flies expressing beta amyloid 42 (Aβ42), a biomarker for AD, caused a restoration from shortened life span to a normal lifespan in the flies due to decreased caspase 3 and 7 activity and decreased apoptosis [57]. In studies by Barron et al., improvements in AD symptoms were observed in the 3xTgAD mouse model of AD when treated with R05-4864, which binds with high affinity to TSPO [44]. R05-4864 can have both apoptotic and antiapoptotic effects depending on its concentration and the context (type of cells) [39, 60]. In this case, treatment-induced Aβ42 clearance and decreased inflammatory markers involved in microglial and astrocyte activity were observed, including ionized calcium-binding adaptor molecule-1 (Iba1) or glial fibrillary acidic protein (GFAP), indicating decreased inflammation. Furthermore, the levels of steroidal hormones such as testosterone and progesterone were increased with the treatment. It can be speculated that these steroidal hormones and inhibition of inflammation are mediating the beneficial anxiolytic and cognitive effects [31, 44]. These cumulative actions mediated by TSPO can positively effect neuronal survival. However, the 3xTgAD mouse model does not show significant cell loss, so further studies in different cell culture and AD mouse models need to be conducted to determine the effect on survival [61]. Additionally, neurosteroids can inhibit inflammation and cell death, which has been shown in several studies and described in reference [45]. Translational relevance of these studies can be highlighted by increased behavioral alertness, including improved attention and memory as well as decreased anxiety, measured through elevated plus maze and Y maze tests [44]. Several studies have documented elevated expression of TSPO in activated microglia, and TSPO ligand PK11195, can inhibit inflammation by decreasing microglial activation and the release of proinflammatory factors such as cytokines, as well as inhibiting apoptosis [62, 63]. Studies by Bin et al., revealed that inflamed microglia with TSPO combined with inflamed astrocytes without TSPO is a marker of neuronal loss and irreversible damage. Whereas microglia with low TSPO combined with inflamed astrocytes with high TSPO is a marker of reversible neuron injury [64]. However, further studies are needed to delineate the consequences of expression levels on neuronal survival and death. These results indicate that either modulating or inhibiting TSPO can provide an anti-inflammatory/antiapoptotic effect and improve some functional aspects of brain. However, as explained before, aging and neurodegenerative diseases are the combination of genetics, diet, and environmental factors, so combinations with other interventions can be useful to modulate the process and is discussed in the summary.
SUMMARY
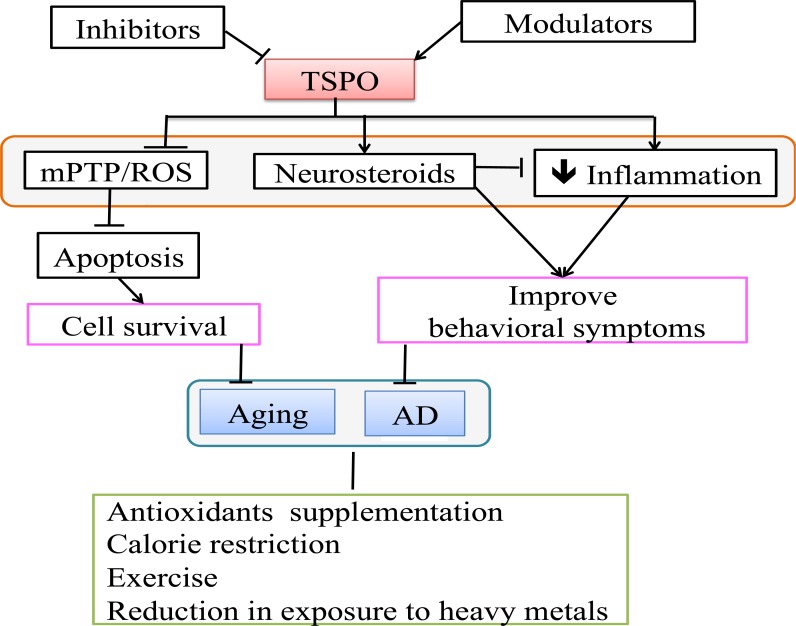
Aging is a systematic deterioration of the human body due to cell death and cell damage caused by exposure to several stress/risk factors throughout life, and it drastically affects the quality of life. Additionally, in neurodegenerative diseases, brain deterioration patterns are similar to aging pathology, so limiting the risk factors through early intervention and inhibition/modulation of apoptosis can potentially prevent/delay aging and/or neurodegenerative diseases. Some of the risks for developing disease can be mitigated by modifying life style, such as restricting calorie intake, preventing exposure to heavy metals, and supplementing with antioxidant agents might inhibit oxidative stress and correct methylation, thereby delaying the aging process and altering/preventing the course of neurodegenerative disease. Modulation/Inhibition of cell death can be a useful tool to slow down the aging process and the progression of neurodegenerative diseases, and TSPO appears to be a promising target (Fig. 3). Several small molecules that affect neurological disorders have been described in references [38, 60]. TSPO is present in the mitochondrial membrane and functions by forming mPTPs and oxidizing cardiolipin, thereby regulating Cyt c release. In addition TSPO is involved in the neurosteroid synthesis, which can inhibit inflammation and decrease Ca2+ elevation, Cyt c release, and cell death. In aging and neurodegenerative diseases, permeability and cardiolipin oxidation is augmented, possibly due to overexpression of TSPO, leading to increased TSPO-mediated apoptosis. These observations indicate that TSPO is inducing apoptosis of damaged cells. However, this may not be beneficial in this case as it leads to excessive loss of neuronal cells and thereby to the functional decline and decreased life span of the affected individuals. Experiments by Lin et al., have provided encouraging results showing an increased cell survival rate and increased life span in Drosophila flies, and Barron et al., have observed decreased inflammation, as well as improved behavioral symptoms, in AD mouse models by inhibition and modulation of TSPO. It could be speculated that inhibition of inflammation/apoptosis may help cells regain homeostasis and perform some of the cell loss related functions, so it is important to evaluate the mechanisms of the TSPO ligands with appropriate controls, and also study the effects on the entire organism.
Fig.(3).
TSPO as a potential drug target: TSPO inhibition can delay the aging progression through inhibition of apoptosis, and its modulation can delay AD disease by decreasing inflammation and increasing neurosteroids. Antioxidant supplementation, calorie restriction, exercise and reduction in exposure to heavy metals delay aging/AD. Alzheimer's disease (AD), membrane permeability transition pore (mPTP), reactive oxygen species (ROS), translocator protein (TSPO).
TSPO ligands are used for wide variety of applications such as neuroimaging agents, psychiatric disorders, brain tumors, neuroinflammation, brain damage, and peripheral nervous system lesions [38]. PK11195 and Ro5-4864 are considered to be prototype compounds and several classes of compounds have been developed such as benzodiazepine derivatives, aryloxyanilide derivatives and isoquinoline carboxamide derivatives [65]. Considerations that are applied to develop imaging agents that target TSPO should also be applied to developing TSPO ligands [66]. The molecules that target TSPO should enter the brain and must show optimal retention, affinity and selectivity towards TSPO with safe pharmakokinetics profile with minimal side effects. Based on the ligand affinity, the action of the compounds and their effect on TSPO would change, so careful delineation of particular ligand binding affinity and site, as well as any conformational changes of the protein need to be elucidated to decrease the toxicity and increase the specificity of the compounds before applying them in the clinical setting. Some ligands such as Alpidem showed liver toxicity, so future compounds must be developed to minimize these side effects. TSPO expression is observed in normal neuronal tissues, so the effects of acute administration and long-term administration can be studied [38]. Inhibition and modulation of TSPO can lead to unexpected effects in the organism as it involved in multiple signaling pathways, so careful studies in different control systems should be performed to learn the effect on the entire system. Even though studies are present on both healthy and diseased brain, more studies on TSPO expression and differences in mechanism of action with inclusion of other factors in healthy and diseased brains are still needed in order to increase the potential of developing a successful drug. Since TSPO ligands can be used to affect different pathways in different stages of disease, it may be possible to use them in combination with other agents for neurodegenerative diseases, which have multiple etiologies. As explained above, aging and AD are complex processes with involvement of multiple causative factors, so the combination of different strategies needs to be applied. Based on evidence from published studies, we can conclude that early prevention with lifestyle changes and TSPO can be used as a potential target to inhibit or modulate cellular damage/apoptosis, thereby slowing the aging process, as well as the progression of neurodegenerative diseases.
PATIENT’S CONSENT
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 8-oxodG =
8-oxodeoxyguanine
- Aβ =
Amyloid beta
- AD =
Alzheimer’s disease
- ANT =
ATP-ADP translocase
- Apaf-1=
Apoptotic protease activating factor-1
- APP =
Amyloid precursor protein
- ATP =
Adenosine triphosphate
- BACE =
Beta-site APP cleaving enzyme
- Ca2+ =
Calcium2+
- cAMP =
Cyclic adenosine monophosphate
- CL =
Cardiolipin
- CypD =
Cyclophilin D
- Cyt c =
Cytochrome c
- DR4 and DR5 =
Death receptors 4 and 5
- FasL =
Fas ligand
- GFAP =
Glial fibrillary acidic protein
- Gpx1 =
Glutathione peroxidase
- H2O =
Water
- H2O2 =
Hydrogen peroxide
- HCY =
Homocysteine
- HSP70 =
Heat shock protein70
- Iba1 =
Calcium-binding adaptor molecule-1
- IGF-1 =
Insulin/insulin-like growth factor-1
- IL6 =
Interleukin6
- IL6 =
Interleukin6
- MEFs =
Mouse embryonic fibroblasts
- mPTP =
Membrane permeability transition pore
- O2 =
Oxygen
- O2- =
Superoxide
- OH- =
Hydroxyl
- PAP7 =
Peripheral-type benzodiazepine receptor-associated protein 7
- Pb =
Lead
- PET =
Positron emission tomography
- PKA =
Protein kinase A
- Prx =
Peroxiredoxin
- ROS =
Reactive oxygen species
- SAM =
S-adenosylhomocysteine
- SODs =
Superoxide dismutases
- SP1 =
Specificity protein 1
- StAR =
Steroidogenic acute regulatory protein
- TNFR1 =
Tumor necrosis factor receptor 1
- TRAIL =
Tumor necrosis factor-related apoptosis-inducing ligand
- TSPO =
Translocator protein
- VDACs =
TVoltage-dependent anion channels
References
- 1.Cooper DM. The balance between life and death: defining a role for apoptosis in aging. J Clin Exp Pathol. 2012. pp. S4–001.
- 2.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 4.Bin Lu H-D, Lu H-G. The relationship between apoptosis and aging. Adv. Biosci. Biotechnol. 2012;3:705–711. doi: 10.4236/abb.2012.326091. [DOI] [Google Scholar]
- 5.Joaquin A.M, Gollapudi S. Functional decline in aging and disease: a role for apoptosis. J. Am. Geriatr. Soc. 2001;49(9):1234–1240. doi: 10.1046/j.1532-5415.2001.04990.x. [DOI] [PubMed] [Google Scholar]
- 6.Vinay K, Abul K.A, Jon C.A. 9th. Saunders: An imprint of Elsevier; 2012. Cell injury, cell death, and adaptations In: Robbins Basic Pathology; pp. 1–30. [Google Scholar]
- 7.Robb E.L, Page M.M, Stuart J.A. Mitochondria, cellular stress resistance, somatic cell depletion and lifespan. Curr. Aging Sci. 2009;2(1):12–27. doi: 10.2174/1874609810902010012. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010. [DOI] [PMC free article] [PubMed]
- 9.Lahiri D.K, Maloney B, Basha M.R, Ge Y.W, Zawia N.H. How and when environmental agents and dietary factors affect the course of Alzheimer s disease: the LEARn model (latent early-life associated regulation) may explain the triggering of AD. Curr. Alzheimer Res. 2007;4(2):219–228. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- 10.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 11.Dai D.F, Chiao Y.A, Marcinek D.J, Szeto H.H, Rabinovitch P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirks-Naylor A.J, Lennon-Edwards S. Cellular and molecular mechanisms of apoptosis in age-related muscle atrophy. Curr. Aging Sci. 2011;4(3):269–278. [PubMed] [Google Scholar]
- 13.Lahiri D.K, Maloney B, Zawia N.H. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry. 2009;14(11):992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterfield D.A, Reed T, Newman S.F, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer s disease and mild cognitive impairment. Free Radic. Biol. Med. 2007;43(5):658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahiri D.K, Maloney B. The LEARn (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer s disease, and proposes remedial steps. Exp. Gerontol. 2010;45(4):291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahiri D.K. Prions: a piece of the puzzle? Science. 2012;337(6099):1172. doi: 10.1126/science.337.6099.1172-a. [DOI] [PubMed] [Google Scholar]
- 17.Shiber A, Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules. 2014;4(3):704–724. doi: 10.3390/biom4030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granholm A.C, Boger H, Emborg M.E. Mood, memory and movement: an age-related neurodegenerative complex? Curr. Aging Sci. 2008;1(2):133–139. doi: 10.2174/1874609810801020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods J.A, Wilund K.R, Martin S.A, Kistler B.M. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. Scientific World Journal. 2012. [DOI] [PMC free article] [PubMed]
- 21.Ricci M.S, El-Deiry W. The extrinsic pathway of apoptosis. In: Gewirtz D, Holt S, Grant S, editors. Apoptosis, senescence, and cancer. New Jersey: Humana Press; 2007. pp. 31–54. [DOI] [Google Scholar]
- 22.Shen J, Tower J. Programmed cell death and apoptosis in aging and life span regulation. Discov. Med. 2009;8(43):223–226. [PubMed] [Google Scholar]
- 23.Paradies G, Petrosillo G, Paradies V, Ruggiero F.M. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic. Biol. Med. 2010;48(10):1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Paradies G, Paradies V, Ruggiero F.M, Petrosillo G. Changes in the mitochondrial permeability transition pore in aging and age-associated diseases. Mech. Ageing Dev. 2013;134(1-2):1–9. doi: 10.1016/j.mad.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1(2):120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 26.Roos W.P, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006;12(9):440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Circu M.L, Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchi S, Giorgi C, Suski JM, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012. 2012. [DOI] [PMC free article] [PubMed]
- 29.Petrosillo G, Ruggiero F.M, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17(15):2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 30.Gatliff J, Campanella M. The 18 kDa translocator protein (TSPO): a new perspective in mitochondrial biology. Curr. Mol. Med. 2012;12(4):356–368. doi: 10.2174/1566524011207040356. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp. Neurol. 2009;219(1):53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boatright K.M, Salvesen G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003;15(6):725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Widmann C, Gibson S, Johnson G.L. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J. Biol. Chem. 1998;273(12):7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry N.A. Caspases: key mediators of apoptosis. Chem. Biol. 1998;5(5):R97–R103. doi: 10.1016/S1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 35.Fischer U, J nicke R.U, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10(1):76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson D.W, Thornberry N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22(8):299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 37.Hochreiter-Hufford A, Ravichandran K.S. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupprecht R, Papadopoulos V, Rammes G, Baghai T.C, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 39.Ukessays.com. United kingdom: Available from www.ukessays.com/essays/biology/translocator-protein-formerlyknown-as-peripheral-benzodiazepine-receptor-biology-essay.php.
- 40.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 2010;327(1-2):1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rampon C, Bouzaffour M, Ostuni M.A, Dufourcq P, Girard C, Freyssinet J.M, Lacapere J.J, Schweizer-Groyer G, Vriz S. Translocator protein (18 kDa) is involved in primitive erythropoiesis in zebrafish. FASEB J. 2009;23(12):4181–4192. doi: 10.1096/fj.09-129262. [DOI] [PubMed] [Google Scholar]
- 42.Du H, Yan S.S. Mitochondrial permeability transition pore in Alzheimer s disease: cyclophilin D and amyloid beta. Biochim. Biophys. Acta. 2010;1802(1):198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J. Bioenerg. Biomembr. 2008;40(3):199–205. doi: 10.1007/s10863-008-9142-1. [DOI] [PubMed] [Google Scholar]
- 44.Barron A.M, Garcia-Segura L.M, Caruso D, Jayaraman A, Lee J.W, Melcangi R.C, Pike C.J. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer s disease. J. Neurosci. 2013;33(20):8891–8897. doi: 10.1523/JNEUROSCI.1350-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borowicz K.K, Piskorska B, Banach M, Czuczwar S.J. Neuroprotective actions of neurosteroids. Front. Endocrinol. (Lausanne) 2011;2:50. doi: 10.3389/fendo.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato-Negishi M, Kawahara M. Neurosteroids block the increase in intracellular calcium level induced by Alzheimer s β-amyloid protein in long-term cultured rat hippocampal neurons. Neuropsychiatr. Dis. Treat. 2008;4(1):209–218. doi: 10.2147/ndt.s2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strous R. Neurosteroids in the Aging Brain. In: Ritsner M, Weizman A, editors. Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders. Netherlands: Springer; 2008. pp. 241–248. [DOI] [Google Scholar]
- 48.Martin L.J. The mitochondrial permeability transition pore: a molecular target for amyotrophic lateral sclerosis therapy. Biochim. Biophys. Acta. 2010;1802(1):186–197. doi: 10.1016/j.bbadis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michael L, Allan D.M, editors. Marks' basic medical biochemistry: a clinical approach. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 383–401. [Google Scholar]
- 50.Miller W.L. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim. Biophys. Acta. 2007;1771(6):663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Li H, Papadopoulos V. PAP7, a PBR/PKA-RIalpha-associated protein: a new element in the relay of the hormonal induction of steroidogenesis. J. Steroid Biochem. Mol. Biol. 2003;85(2-5):275–283. doi: 10.1016/S0960-0760(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Rone M, Papadopoulos V. PAP7 is a steroidogenesis signal transporter from golgi apparatus to mitochondria in mouse MA-10 leydig cells. FASEB J. 2007;21:328–1. [Google Scholar]
- 53.Rone M.B, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim. Biophys. Acta. 2009;1791(7):646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A, Muzik O, Shandal V, Chugani D, Chakraborty P, Chugani H.T. Evaluation of age-related changes in translocator protein (TSPO) in human brain using (11)C-[R]-PK11195 PET. J. Neuroinflammation. 2012;9:232. doi: 10.1186/1742-2094-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guly s B, Vas A, T th M, Takano A, Varrone A, Csel nyi Z, Schain M, Mattsson P, Halldin C. Age and disease related changes in the translocator protein (TSPO) system in the human brain: positron emission tomography measurements with [11C]vinpocetine. Neuroimage. 2011;56(3):1111–1121. doi: 10.1016/j.neuroimage.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Chen M.K, Guilarte T.R. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther. 2008;118(1):1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin R, Angelin A, Da Settimo F, Martini C, Taliani S, Zhu S, Wallace D.C. Genetic analysis of dTSPO, an outer mitochondrial membrane protein, reveals its functions in apoptosis, longevity, and Ab42-induced neurodegeneration. Aging Cell. 2014;13(3):507–518. doi: 10.1111/acel.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojas S, Mart n A, Arranz M.J, Pareto D, Purroy J, Verdaguer E, Llop J, G mez V, Gispert J.D, Mill n O, Chamorro A, Planas A.M. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27(12):1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- 59.Park S.Y, Cho N, Chang I, Chung J.H, Min Y.K, Lee M.K, Kim K.W, Kim S.J, Lee M.S. Effect of PK11195, a peripheral benzodiazepine receptor agonist, on insulinoma cell death and insulin secretion. Apoptosis. 2005;10(3):537–544. doi: 10.1007/s10495-005-1884-1. [DOI] [PubMed] [Google Scholar]
- 60.Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007;13(23):2385–2405. doi: 10.2174/138161207781368710. [DOI] [PubMed] [Google Scholar]
- 61.Medeiros R, Chabrier M.A, LaFerla F.M. Elucidating the triggers, progression, and effects of Alzheimer's disease. J. Alzheimers Dis. 2013;33(Suppl.1):S195–S210. doi: 10.3233/JAD-2012-129009. [DOI] [PubMed] [Google Scholar]
- 62.Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber B.H, Rupprecht R, Langmann T. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflammation. 2014;11:3. doi: 10.1186/1742-2094-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryu J.K, Choi H.B, McLarnon J.G. Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol. Dis. 2005;20(2):550–561. doi: 10.1016/j.nbd.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Ji B, Maeda J, Sawada M, Ono M, Okauchi T, Inaji M, Zhang M.R, Suzuki K, Ando K, Staufenbiel M, Trojanowski J.Q, Lee V.M, Higuchi M, Suhara T. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer s and other CNS pathologies. J. Neurosci. 2008;28(47):12255–12267. doi: 10.1523/JNEUROSCI.2312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taliani S, Pugliesi I, Da Settimo F. Structural requirements to obtain highly potent and selective 18 kDa Translocator Protein (TSPO) Ligands. Curr. Top. Med. Chem. 2011;11(7):860–886. doi: 10.2174/156802611795165142. [DOI] [PubMed] [Google Scholar]
- 66.Ching A.S, Kuhnast B, Damont A, Roeda D, Tavitian B, Doll F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging. 2012;3(1):111–119. doi: 10.1007/s13244-011-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]