ABSTRACT
Insulin, elevated during obesity, regulates xenobiotic biotransformation enzymes, potentially through phosphatidylinositol 3-kinase (PI3K) signaling, in extraovarian tissues. PI3K regulates oocyte viability, follicular activation, and ovarian chemical biotransformation. 7,12-Dimethylbenz[a]anthracene (DMBA), a carcinogen and ovotoxicant, destroys all stages of follicles, leading to premature ovarian failure. Obesity has been reported to promote DMBA-induced tumors, but it remains unknown whether obesity affects ovarian xenobiotic metabolism. Therefore, we investigated ovarian expression of xenobiotic metabolism genes—microsomal epoxide hydrolase (Ephx1), glutathione S-transferase (GST) class Pi (Gstp1) and class mu 1 (Gstm1), and PI3K-signaling members (protein kinase B [AKT] alpha [Akt1], beta [Akt2], and the forkhead transcription factor subfamily 3 [Foxo3])—in lean and obese female mice after DMBA exposure (1 mg/kg; intraperitoneal injection for 14 days). Relative to lean, obese mice had decreased (P < 0.05) healthy primordial and primary follicle numbers but increased (P < 0.05) secondary and preovulatory follicles numbers. Obesity increased (P < 0.05) Akt1, Akt2, Gstm1, and Ephx1 mRNA and pAKTSer473/Thr308, GSTM1, GSTP1, and EPHX1 protein levels. DMBA decreased (P < 0.05) ovarian weight in lean and obese mice, however, obese DMBA-treated females had a greater reduction (P < 0.05) in ovarian weight. In both lean and obese mice, DMBA decreased (P < 0.05) all stages of healthy follicle numbers, increased Gstp1 and Ephx1 mRNA as well as GSTM1, GSTP1, and EPHX1 protein levels, and decreased Akt1 and Akt2 mRNA as well as pAKTSer473 or pAKTThr308, FOXO3, and pFOXO3Ser253 protein expression. There was an additive effect between obesity and DMBA exposure for increased Gstm1 and Ephx1 mRNA as well as GSTM1 and EPHX1 protein expression.
Keywords: 7,12-dimethylbenz[a]anthracene; environmental contaminants and toxicants; obesity; ovary; ovotoxicity
INTRODUCTION
Obesity is positively correlated with a number of health hazards, some of which, including diabetes [1–5], cardiovascular disease [6–9], and cancer [10–14], are the leading causes of preventable death. The prevalence of obesity and obesity-related health complications, such as type 2 diabetes, which were previously considered adult diseases, are now also rising dramatically in children [8, 15, 16], leading to premature death and infertility. In recent years, a strong association between increased body mass index with enhanced incidence in reproductive health impairments has been reported [12, 17–20]. Obese women have an increased likelihood to display signs of polycystic ovarian syndrome, reduced fecundity, and poor quality oocytes [19, 21]. There has also been a strong link between obesity and an increased risk of birth defects, premature and stillbirth [22–28], and gestational diabetes [29, 30]. Although, there is strong association between obesity and compromised reproductive health, the molecular mechanisms involved remain ill-defined. Additionally, despite the alarming prevalence of obesity and obesity-associated maladies, little is known of how this epidemic may influence ovarian xenobiotic metabolism.
Ovaries are important for supplying the germ cell necessary for perpetuation of species and production of hormones essential for female growth and development [31–33]. At birth, females are born with a limited number of primordial follicles, which once depleted, cannot be replenished [33–35]. It is accepted that the process of folliculogenesis is an irreversible process; once follicles are recruited from the resting pool to the growing pool, they will undergo atresia if not selected for further growth to ovulation [36–38]. Unlike the cyclic maturation of follicles to ovulation, initial primordial follicular activation has been identified to be regulated, independent of the pituitary gonadotropins, largely by local ovarian factors including the phosphatidylinositol 3-kinase (PI3K) pathway [38–41]. A balance between dormancy, activation, and atresia of primordial follicles is critical for the female reproductive lifespan [37, 38, 42]. Any environmental factor(s) that could accelerate the rate of primordial follicle activation and the process of atresia would greatly threaten the reproductive potential of the female.
Obesity can alter insulin sensitivity in a number of target tissues, including muscle, liver, adipose tissue, and the ovary [2, 43–46]. Insulin hormone binds to its receptor resulting in autophosphorylation and recruitment of the insulin receptor substrate proteins (Irs) [47–49], which in turn regulate numerous downstream insulin-mediated signaling pathways including PI3K signaling [2, 43]. PI3K-signaling events are largely mediated through protein kinase B (AKT), a subfamily consisting of three mammalian isoforms Akt1, Akt2, and Akt3 [50]. Upon PI3K activation, AKT is recruited to the membrane where AKT is phosphorylated (pAKT). Phosphorylated AKT dissociates from the membrane and shuttles to the cell nucleus where it has the ability to phosphorylate and inactivate several targets, including forkhead transcription factor subfamily 3 (FOXO3).
A number of environmental chemicals can target the ovary and destroy the primordial follicles as well as other follicle types, leading to premature ovarian failure, infertility, and other health impairments [31, 32, 51–53], making ovarian xenobiotic metabolism critical for protection of the female germ cell. The polycyclic aromatic hydrocarbon, 7,12-dimethylbenz[a]anthracene (DMBA), is an environmental carcinogen [54–58] as well as ovotoxicant [51, 52, 59, 60]. Human exposure to DMBA is mainly through smoke or fumes from burning of organic substances such as coal, car exhaust, and cigarette smoke [59, 61]. Relative to nonsmokers, women who are cigarette smokers undergo early onset of menopause and suffer infertility [62–64]. Studies from animal models have demonstrated that DMBA exposure can destroy follicles of all types, resulting in accelerated premature ovarian insufficiency and other reproductive complications [32, 51, 59, 65, 66]. In the liver, the parent compound DMBA is bioactivated to its more toxic metabolite, DMBA-3,4-diol-1,2-epoxide by cytochrome P450 isoforms 1B1 (CYP1B1) and 1A1 (CYP1A1) [59, 61, 67, 68] in conjunction with microsomal epoxide hydrolase (EPHX1) [69, 70]. Several studies have also demonstrated the ovary's capacity to metabolize DMBA to its more ovotoxic metabolite, DMBA-3,4-diol-1,2-epoxide via the action of EPHX1 [51, 59, 61, 68, 70–72].
Insulin can regulate the expression of xenobiotic biotransformation genes products such as the CYP's, glutathione S-transferases (GST), and EPHX1 through PI3K/AKT signaling [73, 74]. Inhibition of PI3K has also been reported to alter the expression of EPHX1, GSTP1, and GSTM1 genes in Postnatal Day 4 cultured rat ovaries [75–77]. GSTP1 and GSTM1 are members of the GST superfamily of proteins involved in phase II metabolism of xenobiotic compounds. These enzymes function in the detoxification of electrophilic compounds, including carcinogens, therapeutic drugs, environmental toxins, and products of oxidative stress, by conjugation with glutathione [78]. Though the effects of obesity on DMBA-induced ovotoxicity have not been previously explored, regulation of EPHX1 by insulin in nonovarian tissues has been reported [73, 74]. Because obesity can alter insulin action to its target tissues, including the ovary, we hypothesized that obesity-induced increased insulin could increase PI3K signaling and alter xenobiotic gene expression leading to accelerated DMBA-induced ovotoxicity.
MATERIALS AND METHODS
Reagents
7,12-Dimethylbenz[a]anthracene (>98% DMBA), 2-β-mercaptoethanol, 30% acrylamide/0.8% bis-acrylamide, ammonium persulfate, glycerol, N,N,N′,N′-tetramethylethylenediamine (TEMED), Tris base, Tris HCl, sodium chloride, Tween-20, bovine serum albumin, ascorbic acid (vitamin C), phosphatase inhibitor, protease inhibitor, and transferrin were purchased from Sigma-Aldrich Inc. (St. Louis, MO). RNAlater was obtained from Ambion Inc. (Austin, TX). Hanks balanced salt solution (without CaCl2, MgCl2, or MgSO4) and superscript III one-step RT-PCR System were obtained from Invitrogen Co. (Carlsbad, CA). RNeasy Mini kit, QIAshredder kit, RNeasy MinElute kit, and QuantitectTM SYBR Green PCR kit were purchased from Qiagen Inc. (Valencia, CA). Custom-designed primers were obtained from the DNA facility of the Office of Biotechnology at Iowa State University. Ponceau S was purchased from Fisher Scientific (Waltham, MA). SignalFire ECL reagent and anti-pAKTSer473 antibody were from Cell Signaling Technology (Danvers, MA). Anti-FOXO3, anti-pFOXO3Ser253, anti-alpha tubulin (TUBA), anti-GSTP1, and anti-GSTM1 antibodies were purchased from Millipore (Temecula, CA). Anti-pAKTThr308 antibody was purchased from Abcam (Cambridge, MA). Goat anti-rabbit and donkey anti-goat secondary antibodies were purchased from Pierce Biotechnology (Rockford, IL). Restore PLUS Western Blot Stripping Buffer was purchased from Thermo Scientific (Rockford, IL).
Animals
Four-wk-old female wild-type normal nonagouti (a/a; designated lean; n = 15) and agouti lethal yellow (KK.Cg-Ay/J; designated obese; n = 15) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed at the animal facility at Iowa State University. All the experimental protocols and procedures were approved by the Iowa State University Animal Care Committee (IACUC). Animals were maintained under controlled lighting (12L:12D) and temperature (21°C–22°C) conditions. Food and water were provided ad libitum. At 6 wk of age, nonagouti and agouti lethal yellow mice (n = 5/genotype) were killed. At 18 wk of age, glucose tolerance testing confirmed that obese mice were less glucose tolerant than their lean littermates and had a higher systemic basal glucose level. Both lean and obese mice (n = 10/genotype) were intraperitoneally (i.p.) dosed with sesame oil or DMBA (> 98%; 1mg/kg/day) for 14 days. This dose was chosen based on destruction of approximately 50% of primary and secondary follicles, with a higher loss of primordial follicles [52].
Tissue Collection
Mice were killed at 6 wk of age or 3 days after the end of dosing (approximately 20 wk of age) during the proestrus stage of cyclicity, and the body weight was recorded. Ovaries were collected, trimmed of excess fat, and weighed. One ovary was fixed in 4% paraformaldehyde for histological analysis while the other ovary was stored in RNAlater at −80°C for RNA and protein expression studies.
Histology and Follicle Counting
The histology work was performed at the Iowa State University Veterinary Medicine Histopathology laboratory. Briefly, one ovary from each animal was fixed in 4% paraformaldehyde overnight, transferred to 70% ethanol, dehydrated, embedded in paraffin blocks, serially sectioned (5 μM) and every sixth section (4–6 sections/slide) was mounted (15–20 slides per animal), and stained with hematoxylin and eosin. Digital images were acquired with a Leica DMI300B Fluorescent Microscope. Numbers of healthy follicles (oocyte-containing follicles showing a distinct oocyte nucleus) were classified and counted in every 12th section according to the procedures as previously described [79, 80]. Briefly, primordial follicles contained an oocyte surrounded with a single layer of squamous-shaped granulosa cells, primary follicles contained an oocyte surrounded by a single layer of cuboidal-shaped granulosa cells, secondary follicles contained an oocyte surrounded by multiple layers of granulosa cells, and antral follicles contained an oocyte surrounded by at least two layers of granulosa cells and a fluid-filled antral space.
RNA Isolation
Total RNA was isolated using Qiagen RNeasy Mini Kit (n = 3 ovaries per treatment) according to the manufacturer's protocol. Briefly, ovaries were lysed and homogenized using a handheld homogenizer followed by applying the homogenate to a QIAshredder column with subsequent centrifugation at 16 100 relative centrifugal force for 2 min at room temperature. The resulting supernatant was applied to an RNeasy Mini column, allowing RNA to bind to the filter cartridge. Following washing, RNA was eluted from the filter and concentrated using an RNeasy MinElute Kit according to the manufacturer's protocol. The final total concentrated RNA was eluted using 14 μl of RNase-free water, and the RNA concentration was determined using an ND-1000 Spectrophotometer (λ = 260/280 nm; NanoDrop Technologies, Inc., Wilmington, DE).
First-Strand cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction
Total RNA (0.5 μg) was reverse transcribed into cDNA utilizing the Invitrogen Superscript III Reverse Transcriptase as detailed in the manufacturer's procedures. Two microliters of diluted cDNA (1:25) was amplified on an Eppendorf Mastercycler using a Quantitect SYBR Green PCR kit and primers specific for mouse Gapdh, Ephx1, Gstp1, Gstm1, and Akt1 sequences, as indicated in Nteeba et al. [81], and Akt2 (forward primer: 5′-TGG ACC ACA GTC ATC GAG AG-3′; reverse primer: 5′-CTT GTA ATC CAT GGC GTC CT-3′). The PCR cycling program consisted of a 15 min hold at 95°C and 45 cycles of: denaturing at 95°C for 15 sec, annealing at 58°C for 15 sec, and extension at 72°C for 20 sec, at which point data was acquired. Product melt conditions were determined using a temperature gradient from 72°C to 99°C with a 1°C increase at each step. Three replicates of each sample (n = 3 wells per treatment) were included. Statistical analysis was performed on the normalized ΔCT for each sample. There was no difference in ovarian Gapdh mRNA level between sesame control and DMBA-treated ovaries in lean or obese mice. Therefore, for each sample, relative mRNA expression of each of the above genes was normalized using Gapdh as a housekeeping gene and relative fold change calculated using the 2−ΔΔCT method. The results are presented as mean fold change ± standard error relative to the sesame control group.
Protein Isolation and Western Blot Analysis
Total ovarian protein was isolated, and immunoblots were performed according to the procedure of Nteeba et al. [81]. Briefly, ovaries (n = 3 per treatment) were homogenized in 300 μl of ice-cold tissue lysis buffer, and protein quantified using a standard bicinchoninic acid protocol on a 96-well assay plate. Total protein (15 μg) was separated on a 10% SDS-PAGE and subsequently transferred to nitrocellulose membranes. Following blocking, membranes were incubated with specific primary antibodies (rabbit anti-GSTP1 [1:250], rabbit anti-GSTM1 [1:200], goat anti-EPHX1 [1:500], rabbit anti-pAKTSer473 [1:500], rabbit anti-pAKTThr308 [1:500], rabbit anti-FOXO3 [1:500], rabbit anti-pFOXO3Ser253 [1:1000]) in 5% bovine serum albumin in TTBS (5 M NaCl, 20 mM Tris-HCl, 0.15% Tween-20; pH 8.0) for 15–20 h at 4°C. Horseradish peroxidase-conjugated secondary antibodies (1:2000–1:5000) were added for 1h at room temperature, and then membrane-bound horseradish peroxidase were washed three times for 5 min in TTBS. Autoradiograms were visualized on x-ray films in a dark room following 7 min incubation of membranes with 1× SignalFire ECL reagent. Equal protein loading was confirmed by Ponceau S staining of nitrocellulose membranes prior to antibody incubation. Additionally, blots were stripped and probed with an anti-TUBA or anti-GAPDH antibody. Densitometry of the appropriate sized bands was measured using Carestream molecular imaging software version 5.0 (Carestream Health Inc., Rochester, NY), which eliminates background noise. Values were normalized to the appropriate loading control (TUBA, GAPDH, or Ponceau S staining).
Statistical Analysis
Statistical analyses were performed using either two-way ANOVA followed by Bonferroni multiple comparisons to assess interaction between strain and drug or one-way ANOVA followed by Tukey multiple pairwise comparison function of GraphPad Prism 5.5 software with a statistical significance level set at P < 0.05. Bars represent means ± SEM. Different letters indicate significant difference from respective pairs; n = 3 per treatment.
RESULTS
Obesity Decreases the Number of Healthy Primordial Follicles in Murine Ovaries
We determined the effect of DMBA exposure on ovarian weight and the number of healthy follicles in lean and obese mice (Fig. 1). There was no effect of DMBA on body weight (Fig. 1A); however, compared to sesame oil, DMBA decreased ovarian weight in both lean (P < 0.05) and obese (P < 0.001) treated groups (Fig. 1B). There was no difference between ovarian weight of lean and obese sesame-treated groups; however, DMBA treatment further reduced (P < 0.05) ovarian weight in the obese compared to lean mice (Fig. 1B). In both lean and obese females, DMBA treatment significantly decreased (P < 0.0001) the number of healthy follicles compared to sesame-treated females (Fig. 1C–F). Interestingly, ovaries from obese mice had both decreased (P < 0.0001) number of healthy primordial (Fig. 1C) and primary (Fig. 1D) follicles but increased (P < 0.001) secondary (Fig. 1E) and preovulatory (Fig. 1F) follicles compared to ovaries from lean sesame-treated mice. No impact of obesity or DMBA on corpora lutea numbers were observed.
FIG. 1.
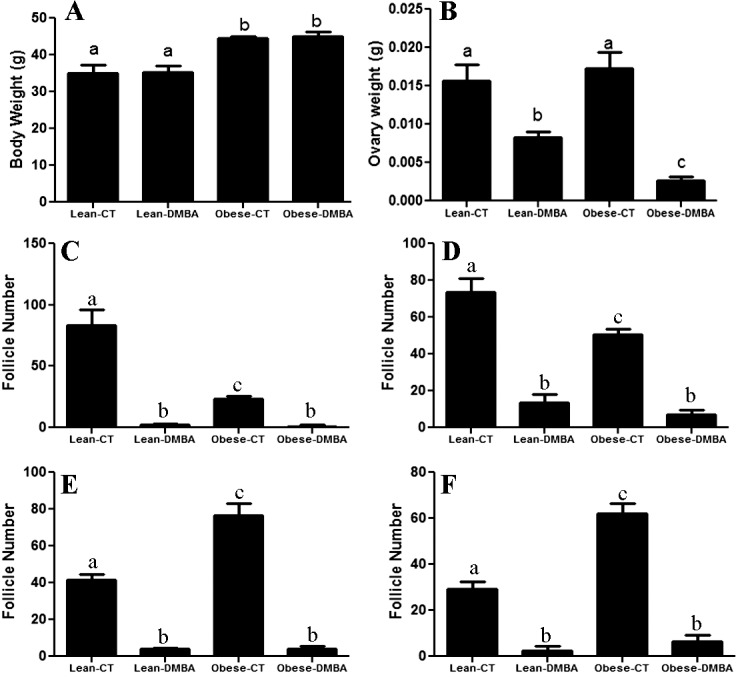
Effect of DMBA on ovarian weight and follicle numbers in lean and obese mice. At 18 wk of age, lean and obese mice were intraperitoneally (i.p) dosed with sesame oil or DMBA (95%; 1mg/kg) for 14 days. Mice were killed 3 days after the end of dosing; body weight was measured (A), and ovaries were collected, trimmed of excess fat, and weighed (B). One ovary was fixed in 4% paraformaldehyde, and complete serial sections were prepared and subjected to histological examination for the number of healthy follicles in both lean and obese treated groups (C–F); (C) healthy primordial follicle number; (D) healthy primary follicle number; (E) healthy secondary follicle number; and (F) healthy preovulatory follicle number. Bars represent mean follicle number per ovary ± SEM. Different letters indicate significant difference between respective pairs (P < 0.05; n = 3).
DMBA and Obesity Have an Additive Effect on Ovarian Ephx1 and Gstm1 Gene Expression
Relative to lean counterparts, ovaries from obese mice had increased ovarian Gstm1 (Fig. 2A; P < 0.01) and Ephx1 (Fig. 2C; P < 0.05) mRNA levels. However, obesity did not affect Gstp1 (Fig. 2B) mRNA levels in murine ovaries. In lean ovaries, DMBA did not alter Gstm1 mRNA expression. Gstp1 and Ephx1 mRNA levels were increased (P < 0.05) in ovaries of both lean and obese treated groups compared to those of their respective control groups (Fig. 2A–C). Compared to lean DMBA-treated females, ovaries from obese DMBA-treated females displayed increased (P < 0.01) Gstm1 and a strong tendency for increased (P = 0.06) Ephx1 but no difference in Gstp1 mRNA levels (Fig. 2A–C). Lack of any impact of genotype on Gstm1, Gstp1, or Ephx1 mRNA level was confirmed in ovaries from 6-wk-old mice (preobese) (Fig. 2D).
FIG. 2.
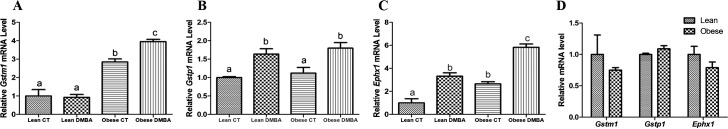
Effects of DMBA on ovarian metabolism gene expression in lean and obese mice. Eighteen-wk-old lean and obese female mice were treated with sesame oil or DMBA (95%; 1mg/kg; i.p) for 14 days. Three days after dosing, ovaries were collected, trimmed of excess fat, and total ovarian RNA was isolated. Using quantitative RT-PCR, relative mRNA levels of (A) Gstm1, (B) Gstp1, and (C) Ephx1 were evaluated after normalization to the housekeeping gene, Gapdh. D) Ovarian Gstm1, Gstp1, and Ephx1 mRNA levels in 6-wk-old lean and obese mice. Values represent relative fold-change means ± SEM. Different letters indicate significant difference from respective pairs (P < 0.05; n = 3).
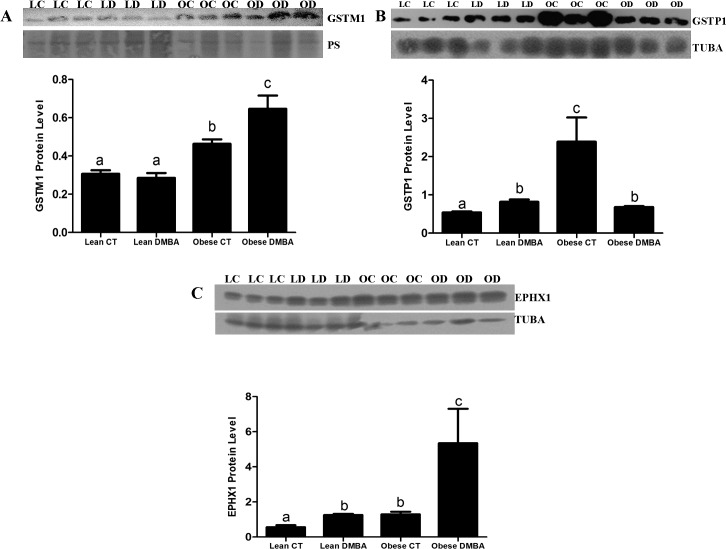
DMBA Increases Ovarian GSTM1, GSTP1, and EPHX1 Protein Levels in Obese but Not in Lean Mice
Ovaries from obese mice had increased (P < 0.05) basal ovarian GSTM1 protein levels relative to their lean counterparts (Fig. 3A). DMBA exposure did not affect GSTM1 protein level in lean ovaries, however, there was increased (P < 0.05) GSTM1 protein levels in ovaries of obese treated groups compared to their respective control groups (Fig. 3A). Relative to lean counterparts, ovaries from obese mice had increased basal ovarian GSTP1 (P < 0.001) protein levels (Fig. 3B). Interestingly, DMBA increased (P < 0.05) GSTP1 protein level in ovaries of lean mice, but decreased (P < 0.05) ovarian GSTP1 protein level in obese treated groups compared to those of their respective control groups (Fig. 3B). Similar to mRNA results, ovaries from obese mice had increased (P < 0.05) basal ovarian EPHX1 protein levels relative to their lean counterparts (Fig. 3C). DMBA increased (P < 0.05) EPHX1 protein levels in ovaries of both lean and obese treated groups compared to their respective control groups (Fig. 3C). Relative to lean DMBA treated mouse ovaries; obesity resulted in a greater increase (P < 0.01) in EPHX1 protein levels, relative to the obese sesame oil-treated mouse ovaries (Fig. 3C).
FIG. 3.
Effect of DMBA on ovarian GSTM1 protein in lean and obese mice. Eighteen-wk-old lean and obese female mice were treated with sesame oil or DMBA (95%; 1mg/kg; i.p) for 14 days. Three days after dosing, ovaries were collected, trimmed of excess fat, and total ovarian protein was isolated (n = 3 per treatment group) from lean control (LC), lean DMBA (LD), obese control (OC), and obese DMBA (OD). GSTM1(A), GSTP1(B), or EPHX1 (C) proteins were quantified by Western blot analysis followed by densitometric quantification of the protein band using Carestream molecular imaging software version 5.0. Bars represent means ± SEM. Different letters indicate significant difference from respective pairs (P < 0.05; n = 3).
Obesity and DMBA Have Opposing Effects on Ovarian Akt1/2 mRNA Expression
Ovaries from obese females displayed higher (P < 0.05) Akt1 (Fig. 4A) and Akt2 (Fig. 4B) mRNA levels than lean females; however, DMBA exposure significantly reduced ovarian Akt1/2 mRNA levels in both lean (P < 0.001) and obese (P < 0.05) treated groups (Fig. 4). Akt1 and Akt2 mRNA levels were not found to differ between genotype in ovaries from 6-wk-old mice (preobese) (Fig. 4C).
FIG. 4.
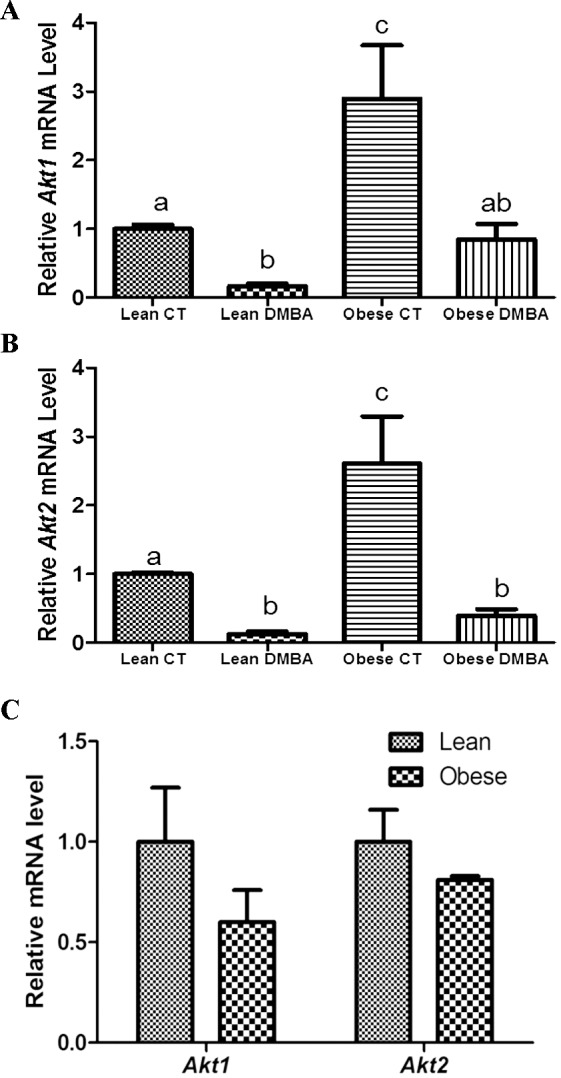
Effects of DMBA on ovarian Akt1/2 expression in lean and obese mice. At 18 wk of age, lean and obese mice were intraperitoneally (i.p.) dosed with sesame oil or DMBA (95%; 1mg/kg) for 14 days. Ovaries were collected 3 days after the end of dosing, trimmed of excess fat, and total ovarian RNA isolated (n = 3 per treatment group). Relative mRNA expressions of (A) Akt1 and (B) Akt2 were normalized using Gapdh as a housekeeping gene and quantified using quantitative RT-PCR. C) Ovarian Akt1 and Akt2 mRNA levels in 6-wk-old lean and obese mice. Bars represent means ± SEM. Different letters indicate significant difference from respective pairs (P < 0.05; n = 3).
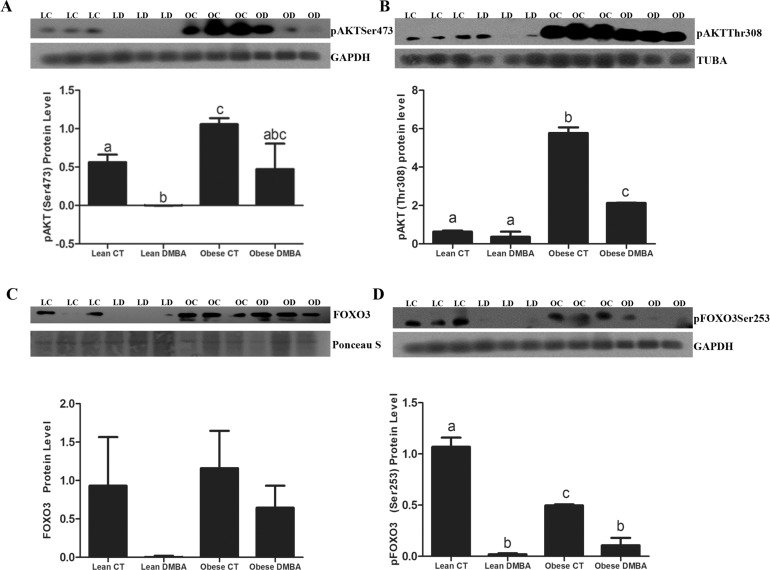
Obesity Increases Ovarian Phosphorylated AKT Without Affecting Phosphorylated FOXO3 Protein Levels
DMBA decreased (P < 0.05) ovarian pAKTSer473 protein in lean mice, while obesity increased (P < 0.05) basal pAKTSer473 levels, and the reduction observed in the lean mice was absent from ovaries of obese mice. In lean mice, there was no impact of DMBA on pAKTThr308 protein, however, obesity increased (P < 0.05) basal ovarian pAKTThr308 protein levels (Fig. 5). In contrast to lean ovaries, those from obese mice had a decrease in pAKTThr308 when exposed to DMBA. Lean and obese females did not differ in FOXO3 protein level, and although there was a visible reduction in DMBA-treated lean mice, this was not significant due to variation. pFOXO3Ser253 protein expression was reduced by DMBA in both lean and obese ovaries, and there was a lower level basally (P < 0.05) of pFOXO3Ser253 due to obesity.
FIG. 5.
Effect of DMBA on ovarian PI3K proteins in lean and obese mice. Eighteen-wk-old lean and obese female mice were treated with sesame oil or DMBA (95%; 1mg/kg; i.p) for 14 days. Three days after dosing, ovaries were collected, trimmed of excess fat, and total ovarian protein was isolated (n = 3 per treatment group) from lean control (LC), lean DMBA (LD), obese control (OC) and obese DMBA (OD). Western blot analysis was performed to measure (A) pAKTSer473, (B) pAKTThr308, (C) FOXO3, and (D) pFOXO3Ser253 levels. Densitometry of the appropriate bands was performed using Carestream molecular imaging software version 5.0. Bars represent means ± SEM. Different letters indicate significant difference from respective pairs (P < 0.05; n = 3).
DISCUSSION
Obesity is associated with elevated blood glucose and insulin levels, altering insulin's action on a number of organs, including the ovary [2, 43–46]. Recently, it has been demonstrated that the ovary maintains insulin sensitivity during obesity even though other classical tissues, including the liver, muscle, and adipose tissue, develop insulin resistance [43, 81]. Insulin has been previously shown to regulate hepatic xenobiotic metabolism [73, 74, 82, 83], and in the ovary, insulin can activate the PI3K/AKT signaling, a pathway that has been demonstrated to play critical roles in metabolism and reproduction [84]. Previous work in rodent models has shown the involvement of PI3K signaling in insulin-mediated hepatic regulation of xenobiotic metabolism [83, 85]. Recently, the involvement of PI3K signaling in ovarian metabolism of chemicals, including DMBA, has been demonstrated [60, 75, 86]. Because obesity is associated with elevated plasma insulin levels, we hypothesized that obesity-induced increased insulin could increase PI3K signaling and alter xenobiotic gene expression leading to accelerated DMBA-induced ovotoxicity. To test this hypothesis, we used the lethal yellow mouse, an excellent model for human obesity [87], to investigate whether obese females have increased susceptibility to DMBA-induced ovotoxicity. We designed our experiments on the impact of obesity on chemical metabolism when mice were 18 wk of age because elevated insulin has been shown in this mouse model at this time point [88]. In addition, we confirmed that the obese mice had elevated basal glucose and had a compromised response to a glucose tolerance test (data not shown) and that there was no impact of the lethal yellow genotype on follicle numbers at 6 wk of age (data not shown). Further, to eliminate any impact of genotype outside of the obese phenotype, we characterized gene expression in 6-wk-old mice; at this point, there is no difference in body weight and the lethal yellow mice are considered prediabetic.
Relative to sesame oil, DMBA treatment did not affect body weight; however, compared to sesame oil, DMBA decreased ovarian weight in both lean and obese treated groups. Interestingly, we observed lower than expected primordial follicle numbers in ovaries of both lean and obese sesame oil-treated animals, which could be due to their being 20 wk of age, and potentially this is a time when follicle numbers are naturally beginning to decline. As expected, DMBA treatment significantly decreased the number of healthy follicles of all types in both lean and obese treated females compared to sesame-treated females. The observed decrease in ovarian weight and the number of healthy follicles in DMBA-treated lean and obese females could be as a result of increased follicle loss induced by DMBA exposure as previously reported [59, 61]. Surprisingly, though we saw no difference in ovarian weight between lean and obese sesame oil-treated groups, there was a significant reduction in ovarian weight of obese females treated with DMBA compared to their lean DMBA-treated littermates, indicating that the obese ovaries suffered greater levels of DMBA-induced ovotoxicity, outside of follicle depletion, indicating that the ovaries from obese DMBA-treated mice had greater levels of ovotoxicity than their lean counterparts that received the same DMBA exposure. Despite the lack of ovarian weight differences between lean and obese mice, ovaries from obese mice had both decreased number of healthy primordial and primary follicles but increased secondary and preovulatory follicles compared to ovaries from lean sesame-treated mice. It was surprising that larger numbers of growing follicles did not influence ovarian weight; it has previously been shown that ovaries from mice lacking the arylhydrocarbon receptor had lower numbers of follicles at every stage without any difference in ovarian weight [89]. This difference in follicle populations could indicate increased activation of follicles from the primordial follicle pool. Insulin has been shown to activate primordial to primary transition in neonatal rat ovaries [90]. Additionally, high fat diet fed rats have been recently demonstrated to have increased number of growing and reduced numbers of primordial follicles along with an increase in ovarian weight [91]. Several studies have also reported an inverse relation between body mass index and estradiol levels in premenopausal women [92–94]. Decreased estradiol levels have been associated with increased activation of primordial follicle in mice [95]. So it is possible that obesity could trigger changes in the intrinsic ovarian signals responsible for initiation of follicle activation and recruitment, and the age of animal may be important when interpreting impacts of treatments on ovarian physiology.
DMBA has been demonstrated to have both carcinogenic and ovotoxic properties in animal models [54, 59, 61]. These two properties are mediated through DMBA's metabolite 3,4-diol-1,2-epoxide, which is formed during DMBA metabolism and facilitated by CYP1B1, EPHX1, and CYP1B1/1A1 enzymes [59, 96–99]. Although the liver is the primary site for xenobiotic metabolism, the ovary has been demonstrated to have the capacity to metabolize xenobiotic compounds [32, 60, 67, 68, 75, 76], including DMBA in the absence of hepatic contributions [59, 96–99]. Several studies have confirmed that EPHX1 [59, 61, 98] required for DMBA bioactivation is expressed at high levels in the ovary and is increased following DMBA exposure. Although obesity has been reported to promote DMBA-induced tumors [100], there is a dearth of literature examining potential effects of obesity on ovarian xenobiotic metabolism, including that of DMBA.
Consistent with previous studies, ovaries from obese mice had increased ovarian Ephx1 mRNA [81] and EPHX1 protein [59, 61] levels relative to their lean counterparts. While DMBA treatment increased Ephx1 mRNA and EPHX1 protein levels in ovaries of both lean and obese treated groups compared to their respective control groups, ovaries from obese DMBA-treated females displayed a greater increase in Ephx1 mRNA and EPHX1 protein expression compared to lean DMBA-treated females. To the best of our knowledge, this is the first study to report a DMBA-obesity synergistic effect for increased ovarian Ephx1 mRNA and EPHX1 protein expression in mice. In extraovarian tissues, insulin has been demonstrated to increase hepatic EPHX1 expression [73, 74, 83] while glucagon inhibits EPHX1 expression [83]. In rats, conditions of insulin deficiency induced by either Type 1 diabetes or starvation were associated with decreased EPHX1 enzyme activity, yet on the other hand, addition of insulin or refeeding restored EPHX1 activity [74]. Taken together, these data demonstrate that insulin plays a role in induction of EPHX1 expression and activity. Because obesity is associated with elevated levels of insulin, it is more likely that one of the mechanisms by which obesity accelerates ovarian EPHX1 expression is through hyperactivation of insulin-mediated EPHX1 induction-signaling pathways such the PI3K pathway. In cultured primary rat hepatocytes, administration of insulin increased EPHX1 mRNA and protein expression in a time- and concentration-dependent manner [83]; conversely, inhibition of PI3K by Wortmannin and LY294002 or the mTOR inhibitor, rapamycin, modulated the insulin-induced increase in EPHX1 [83], supporting the involvement of PI3K signaling in insulin-induced hepatic regulation of EPHX1. Furthermore, Ki and Kim [85] confirmed the involvement of PI3K signaling in induction of EPHX1 through C/EBP transcription factors. With recent reports demonstrating the involvement of PI3K signaling in ovarian metabolism of DMBA [60, 75, 86], it is highly probable that the observed DMBA-obesity synergistic effect on ovarian Ephx1 mRNA and EPHX1 protein expression is mediated through insulin/PI3K-signaling pathway.
Just like EPHX1, regulation of GSTM1 and GSTP1 by insulin in nonovarian tissues has been reported [73, 74, 101]. Insulin administration is known to increase GST gene expression through the PI3K/AKT/mTOR pathway while glucagon decreases such gene expression [101]. In alloxan- and streptozotocin-diabetic male Fischer-344 rats, diabetes decreased the activities of rat liver soluble GSTs, yet application of insulin to alloxan-diabetic individuals approximately restored the initial enzyme activities [74]. Similarly, starvation of Fischer-344 rats, resulted in reduced activities of GST enzymes, however, refeeding restored their initial activities [74]. Taken together, these results suggest that insulin also regulates the hepatic activities of GST enzymes. Although in the current study we did not measure the activities of these enzymes, we observed increased Gstm1 mRNA as well as GSTM1 and GSTP1 protein levels in ovaries from obese sesame oil-treated females compared to lean mice, mimicking the trend observed during normal insulin activity. Taken together, these results could support the notion that despite insulin resistance in other tissues, the ovary maintains insulin sensitivity.
DMBA-induced increases in Gstp1 mRNA and GSTP1 protein levels in PND4 cultured rat ovaries have been reported [76], however, the effect of DMBA on ovarian-expressed GSTM1 has not been previously reported. In the present study, DMBA treatment had contrasting effects on GSTP1 and GSTM1 in both lean and obese treated mice. Interestingly, DMBA increased ovarian Gstm1 mRNA and GSTM1 protein levels in obese but not in lean mice yet on the other hand DMBA increased ovarian Gstp1 mRNA in both lean and obese females and GSTP1 protein levels in only lean but decreased GSTP1 protein expression in obese females. Furthermore, though there was no difference in ovarian Gstp1 mRNA and GSTP1 protein levels between lean and obese DMBA-treated females relative to lean DMBA-treated females; ovaries from obese females exhibited a greater increase in both Gstm1 mRNA and GSTP1 protein expression, following DMBA treatment. Given the divergent roles played by GST enzymes, depending on the physiological conditions and type of cells involved, changes in expression of genes encoding for these enzymes could be beneficial or have detrimental consequences for the cells. Although best known for their detoxification role in metabolism [53, 76, 102–105], GST enzymes have also been implicated in cell signaling, intracellular transport, and isomerization of steroid hormones [105–107] as well as development of chemotherapeutic-drug resistance [104, 105, 108–111] and a variety of diseases, including cancer, diabetes, and inflammatory diseases [101, 104, 110, 112–114]. In particular, overexpression of GSTP1 has been associated with inactivation of cigarette smoke carcinogens [105], including detoxification of DMBA-induced toxicity [53, 76, 102], and development of drug resistance [105, 108, 110, 111, 114, 115], susceptibility to and poor prognosis of several cancers, including breast, cervical and ovarian cancer [112]. This could be due to its ability to selectively inhibit proapoptotic p38 and C-Jun N-terminal kinase 1 (JNK1) portions of the mitogen-activated protein kinase (MAPK) signaling cascades [53, 107, 108, 110, 114]. Like GSTP1, there is a growing body of evidence supporting the role of GSTM1 in regulating apoptotic pathways through direct protein-protein interactions, with apoptosis signal-regulating kinase 1 (ASK1), which is upstream of JNK. GSTM1 sequesters ASK1 through complex formation and subsequent prevention of its induction of the proapoptotic p38 and JNK portions of the MAPK signaling cascade [77, 101, 104, 108]. It is important to note that increased expression of GSTs in obese females and during DMBA treatment in both lean and obese females did not avert DMBA-induced follicle loss. This would imply that the role of overexpressed GSTM1 and GSP1 could be not principally involved in detoxification but rather be involved in other cellular processes that are yet to be determined.
Insulin regulates xenobiotic metabolism through PI3K/AKT pathway [83, 85, 101]. Previous studies have also implicated PI3K/AKT/mTOR pathway in DMBA-induced ovotoxicity [86]. In the current study, obesity and DMBA had opposing effects on PI3K/AKT signaling pathway members' gene expression. Ovaries from sesame oil-treated obese females displayed higher Akt1 and Akt2 mRNA levels concomitant with upregulated pAKTSer473/Thr308 protein levels, without affecting total FOXO3, but reduced pFOXO3Ser253 protein expression compared to their lean counterparts. Surprisingly, DMBA treatment significantly reduced ovarian Akt1/2 mRNA levels in both lean and obese treated groups, mirrored by decreased pAKTSer473 protein in lean but not obese ovaries. A decrease in pAKTThr308 protein expression was observed in the ovaries from obese females. Interestingly, though pFOXO3Ser253 was decreased in both lean and obese DMBA-treated mice, FOXO3 protein expression was not affected by either obesity or DMBA. These alterations to the PI3K pathway may be at least partially responsible for the altered dynamics of follicle activation observed in the obese mouse ovary.
In conclusion, the ovary contains a finite number of primordial follicles, which once depleted, cannot be replaced; therefore, ovarian xenobiotic metabolism of chemical compounds like DMBA, which can deplete the primordial follicle pool, is critical for protection of the female germ cell. Because insulin regulates hepatic-expressed xenobiotic metabolism enzymes and obesity alters insulin sensitivity in a number of target tissues, understanding how obesity might influence the ovary's capacity to metabolize chemicals is critical. Our data is in agreement with previous studies demonstrating that insulin induces the hepatic activities of EPHX1 and GST enzymes [73, 74, 101]. We have shown that obesity increased mRNA and protein levels of pAKT, EPHX1, GSTM1, and GSTP1 compared to lean mice, mimicking the trend observed during insulin administration or refeeding. Therefore, our data support the notion that despite insulin resistance in other tissues, the ovary seems to maintain insulin sensitivity and that obesity-induced increased insulin could increase PI3K signaling and alter xenobiotic gene expression leading to accelerated DMBA-induced ovotoxicity.
Footnotes
Supported by award number R00ES016818 to A.F.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. Presented in part at the 46th Annual Meeting of the Society for the Study of Reproduction, 22–26 July, 2003, Montreal, Canada.
REFERENCES
- Fatani S, Itua I, Clark P, Wong C, Naderali EK. The effects of diet-induced obesity on hepatocyte insulin signaling pathways and induction of non-alcoholic liver damage. Int J Gen Med. 2011;4:211–219. doi: 10.2147/IJGM.S17376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CR. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65–74. doi: 10.1677/JOE-09-0461. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- Wild SH, Byrne CD. ABC of obesity. Risk factors for diabetes and coronary heart disease. BMJ. 2006;333:1009–1011. doi: 10.1136/bmj.39024.568738.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Artham SM, Lavie CJ, Milani RV, Ventura HO. Obesity and hypertension, heart failure, and coronary heart disease-risk factor, paradox, and recommendations for weight loss. Ochsner J. 2009;9:124–132. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:822–828. [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and women's health: an evidence-based review. J Am Board Family Med. 2011;24:75–85. doi: 10.3122/jabfm.2011.01.100076. [DOI] [PubMed] [Google Scholar]
- Obesity Lane G. and gynaecological cancer. Menopause Int. 2008;14:33–37. doi: 10.1258/mi.2007.007036. [DOI] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Collaboration MWS. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Webb F. Obesity amidst poverty. Int J Epidemiol. 2006;35:24–30. doi: 10.1093/ije/dyi204. [DOI] [PubMed] [Google Scholar]
- Baalwa J, Byarugaba BB, Kabagambe EK, Kabagambe KE, Otim AM. Prevalence of overweight and obesity in young adults in Uganda. Afr Health Sci. 2010;10:367–373. [PMC free article] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006;65:137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- Barber TM, Franks S. The link between polycystic ovary syndrome and both Type 1 and Type 2 diabetes mellitus: what do we know today? Womens Health (Lond Engl) 2012;8:147–154. doi: 10.2217/whe.11.94. [DOI] [PubMed] [Google Scholar]
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347–364. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- Dravecka I, Lazurova I, Kraus V. Obesity is the major factor determining an insulin sensitivity and androgen production in women with anovulary cycles. Bratisl Lek Listy. 2003;104:393–399. [PubMed] [Google Scholar]
- Rachoń D, Teede H. Ovarian function and obesity-interrelationship, impact on women's reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316:172–179. doi: 10.1016/j.mce.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A. Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Correa A, Botto LD, Rasmussen SA, Waller DK, Hobbs CA, Cleves MA, Riehle-Colarusso TJ., Study NBDP. Association between prepregnancy body mass index and congenital heart defects Am J Obstet Gynecol 2010. 202 51: e51 51 e10. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Rasmussen SA, Shaw GM. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res A Clin Mol Teratol. 2010;88:804–810. doi: 10.1002/bdra.20679. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Rasmussen KM, Kjolhede CL. Maternal obesity: a problem for both mother and child. Obesity (Silver Spring) 2008;16:929–931. doi: 10.1038/oby.2008.36. [DOI] [PubMed] [Google Scholar]
- Ma C, Carmichael SL, Scheuerle AE, Canfield MA, Shaw GM., Study NBDP. Association of microtia with maternal obesity and periconceptional folic acid use. Am J Med Genet A. 2010;152A:2756–2761. doi: 10.1002/ajmg.a.33694. [DOI] [PubMed] [Google Scholar]
- Bellver J, Melo MA, Bosch E, Serra V, Remohí J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88:446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13:433–444. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- Hoyer PB. Can the clock be turned back on ovarian aging? Sci Aging Knowl Environ 2004. 2004. pe11. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol Appl Pharmacol. 2012;261:227–235. doi: 10.1016/j.taap.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LD, Hirshfield AN. An overview of follicular development in the ovary: from embryo to the fertilized ovum in vitro. Md Med J. 1992;41:614–620. [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Matzuk MM. Mouse models of ovarian failure. Rev Reprod. 1998;3:183–195. doi: 10.1530/ror.0.0030183. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, McGee EA, Hayashi M, Hsu SY. Hormonal regulation of early follicle development in the rat ovary. Mol Cell Endocrinol. 2000;163:95–100. doi: 10.1016/s0303-7207(99)00245-2. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Himelstein-Braw R, Faber M. Follicular growth: the basic event in the mouse and human ovary. J Reprod Fertil. 1975;45:559–566. doi: 10.1530/jrf.0.0450559. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012;61:114–123. doi: 10.2337/db11-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra A, Nair S, Rai L. Association of obesity and insulin resistance with dyslipidemia in Indian women with polycystic ovarian syndrome. Indian J Med Sci. 2006;60:447–453. [PubMed] [Google Scholar]
- Kashyap SR, Defronzo RA. The insulin resistance syndrome: physiological considerations. Diab Vasc Dis Res. 2007;4:13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- Fatani S, Abubakari AR, Itua I, Wong C, Thomas C, Naderali EK. Effects of diet-induced obesity on protein expression in insulin signaling pathways of skeletal muscle in male Wistar rats. Int J Gen Med. 2012;5:573–582. doi: 10.2147/IJGM.S31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LJ, Morrison BD, Pessin JE. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987;262:6939–6942. [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Ovarian metabolism of xenobiotics. Exp Biol Med (Maywood) 2011;236:765–771. doi: 10.1258/ebm.2011.011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Applied Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Keating AF, Sen N, Sipes IG, Hoyer PB. Dual protective role for glutathione S-transferase class pi against VCD-induced ovotoxicity in the rat ovary. Toxicol Appl Pharmacol. 2010;247:71–75. doi: 10.1016/j.taap.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer PB, Davis JR, Bedrnicek JB, Marion SL, Christian PJ, Barton JK, Brewer MA. Ovarian neoplasm development by 7,12-dimethylbenz[a]anthracene (DMBA) in a chemically-induced rat model of ovarian failure. Gynecol Oncol. 2009;112:610–615. doi: 10.1016/j.ygyno.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist KA, Zhang Z, You M, Gunning WT, Conran PB, Steele VE, Lubet RA. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[a]anthracene (DMBA) coated suture. Carcinogenesis. 2005;26:951–957. doi: 10.1093/carcin/bgi039. [DOI] [PubMed] [Google Scholar]
- Li N, Chen X, Han C, Chen J. Chemopreventive effect of tea and curcumin on DMBA-induced oral carcinogenesis in hamsters [in Chinese] Wei Sheng Yan Jiu. 2002;31:354–357. [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Kanter EM, Walker RM, Marion SL, Brewer M, Hoyer PB, Barton JK. Dual modality imaging of a novel rat model of ovarian carcinogenesis. J Biomed Opt. 2006;11:041123. doi: 10.1117/1.2236298. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol Appl Pharmacol. 2009;234:361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF. J Mark C, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol. 2009;241:127–134. doi: 10.1016/j.taap.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase enzyme in ovotoxicity caused by 7,12-dimethylbenz[a]anthracene. Toxicol Sci. 2007;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Sowers MR, McConnell D, Yosef M, Jannausch ML, Harlow SD, Randolph JF. Relating smoking, obesity, insulin resistance, and ovarian biomarker changes to the final menstrual period. Ann N Y Acad Sci. 2010;1204:95–103. doi: 10.1111/j.1749-6632.2010.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35:3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Jick H, Porter J. Relation between smoking and age of natural menopause. Report from the Boston Collaborative Drug Surveillance Program, Boston University Medical Center. Lancet. 1977;1:1354–1355. doi: 10.1016/s0140-6736(77)92562-4. [DOI] [PubMed] [Google Scholar]
- Mattison DR. Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol Appl Pharmacol. 1980;53:249–259. doi: 10.1016/0041-008x(80)90424-x. [DOI] [PubMed] [Google Scholar]
- Weitzman GA, Miller MM, London SN, Mattison DR. Morphometric assessment of the murine ovarian toxicity of 7,12-dimethylbenz(a)anthracene. Reprod Toxicol. 1992;6:137–141. doi: 10.1016/0890-6238(92)90115-a. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Otto S, Bhattacharyya KK, Jefcoate CR. Polycyclic aromatic hydrocarbon metabolism in rat adrenal, ovary, and testis microsomes is catalyzed by the same novel cytochrome P450 (P450RAP) Endocrinology. 1992;131:3067–3076. doi: 10.1210/endo.131.6.1332854. [DOI] [PubMed] [Google Scholar]
- Miyata M, Kudo G, Lee YH, Yang TJ, Gelboin HV, Fernandez-Salguero P, Kimura S, Gonzalez FJ. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J Biol Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- Miyata M, Motoki K, Tamura E, Furukawa M, Gonzalez FJ, Yamazoe Y. Relative importance of maternal and embryonic microsomal epoxide hydrolase in 7,12-dimethylbenz[a]anthracene-induced developmental toxicity. Biochem Pharmacol. 2002;63:1077–1084. doi: 10.1016/s0006-2952(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Becedas L, Romert L, Toft E, Jenssen D, DePierre JW, Ahlberg MB. Metabolism of polycyclic aromatic hydrocarbons to mutagenic species by rat and porcine ovarian granulosa cells: detection by cocultivation with V79 Chinese hamster cells. Reprod Toxicol. 1993;7:219–224. doi: 10.1016/0890-6238(93)90227-x. [DOI] [PubMed] [Google Scholar]
- Bengtsson M, Hamberger L, Rydström J. Metabolism of 7,12-dimethylbenz(a)anthracene by different types of cells in the human ovary. Xenobiotica. 1988;18:1255–1270. doi: 10.3109/00498258809042249. [DOI] [PubMed] [Google Scholar]
- Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Schladt L, Knehr M, Oesch F. Effect of diabetes and starvation on the activity of rat liver epoxide hydrolases, glutathione S-transferases and peroxisomal beta-oxidation. Biochem Pharmacol. 1989;38:4291–4297. doi: 10.1016/0006-2952(89)90528-5. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Sen N, Hoyer PB, Keating AF. Ovarian expressed microsomal epoxide hydrolase: role in detoxification of 4-vinylcyclohexene diepoxide and regulation by phosphatidylinositol-3 kinase signaling. Toxicol Appl Pharmacol. 2012;258:118–123. doi: 10.1016/j.taap.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Protective role for ovarian glutathione S-transferase isoform pi during 7,12-dimethylbenz[a]anthracene-induced ovotoxicity. Toxicol Appl Pharmacol. 2012;260:201–208. doi: 10.1016/j.taap.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Madden JA, Sen N, Hoyer PB, Keating AF. Glutathione S-transferase class mu regulation of apoptosis signal-related kinase 1 protein during VCD-induced ovotoxicity in neonatal rat ovaries. Toxicol Appl Pharmacol. 2013;267:49–56. doi: 10.1016/j.taap.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta. 2013;1830:3267–3288. doi: 10.1016/j.bbagen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Nteeba J, Ross JW, Perfield JW, II, , Keating AF. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reproductive Toxicology. 2013;42:68–77. doi: 10.1016/j.reprotox.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Novak RF. Insulin and glucagon regulation of glutathione S-transferase expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther. 2003;305:353–361. doi: 10.1124/jpet.102.045153. [DOI] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Kim SG, Novak RF. Insulin and glucagon signaling in regulation of microsomal epoxide hydrolase expression in primary cultured rat hepatocytes. Drug Metab Dispos. 2003;31:1260–1268. doi: 10.1124/dmd.31.10.1260. [DOI] [PubMed] [Google Scholar]
- Acosta-Martínez M. PI3K: an attractive candidate for the central integration of metabolism and reproduction. Front Endocrinol (Lausanne) 2011;2:110. doi: 10.3389/fendo.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Kim SG. Phase II enzyme induction by alpha-lipoic acid through phosphatidylinositol 3-kinase-dependent C/EBPs activation. Xenobiotica. 2008;38:587–604. doi: 10.1080/00498250802126920. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci U S A. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Norwood KA, Smith JE, Kerl JG, Wood JR. Genes involved in the immediate early response and epithelial-mesenchymal transition are regulated by adipocytokines in the female reproductive tract. Mol Reprod Dev. 2012;79:128–137. doi: 10.1002/mrd.22006. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, Flaws JA. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68:1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/s0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17:718–726. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause. 2008;15:857–861. doi: 10.1097/gme.0b013e318165981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J Steroid Biochem Mol Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29:1176–1182. [PubMed] [Google Scholar]
- Shimada T, Sugie A, Shindo M, Nakajima T, Azuma E, Hashimoto M, Inoue K. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187:1–10. doi: 10.1016/s0041-008x(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Rajapaksa KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol Appl Pharmacol. 2007;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkak R, Holley A, MacLeod S, Simpson P, Fuchs G, Jo C, Kieber-Emmons T, Korourian S. Obesity promotes 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in female Zucker rats. Breast Cancer Research. 2005;7:R627–R633. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci U S A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, McLaren AW, Moffat GJ, Bacon EJ, Wolf CR. Pi-class glutathione S-transferase: regulation and function. Chem Biol Interact. 1998;111–112:69–82. doi: 10.1016/s0009-2797(97)00176-2. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Listowsky I, Abramovitz M, Homma H, Niitsu Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab Rev. 1988;19:305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010;17:1373–1380. doi: 10.1038/cdd.2010.80. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D, Tew K. Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am J Pharmacogenomics. 2003;3:157–172. doi: 10.2165/00129785-200303030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew KD, Townsend DM. Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab Rev. 2011;43:179–193. doi: 10.3109/03602532.2011.552912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew KD. Drug resistance in cancer. Editorial. Biochem Pharmacol. 2012;83:985–986. doi: 10.1016/j.bcp.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Green JA, Robertson LJ, Clark AH. Glutathione S-transferase expression in benign and malignant ovarian tumours. Br J Cancer. 1993;68:235–239. doi: 10.1038/bjc.1993.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Yan H, Huang G, Wang B. Null genotypes of GSTM1 and GSTT1 contribute to increased risk of diabetes mellitus: a meta-analysis. Gene. 2013;518:405–411. doi: 10.1016/j.gene.2012.12.086. [DOI] [PubMed] [Google Scholar]
- Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, Townsend DM. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med. 2011;51:299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CS, Diah S, Smitherman PK, Schneider E, Townsend AJ. Multidrug resistance protein and glutathione S-transferase P1-1 act in synergy to confer protection from 4-nitroquinoline 1-oxide toxicity. Carcinogenesis. 1998;19:109–115. doi: 10.1093/carcin/19.1.109. [DOI] [PubMed] [Google Scholar]