Abstract
Influenza virus infections represent a big issue for public health since effective treatments are still lacking. In particular, the emergence of strains resistant to drugs limits the effectiveness of anti-influenza agents. For this reason, many efforts have been dedicated to the identification of new therapeutic strategies aimed at targeting the virus-host cell interactions. Oxidative stress is a characteristic of some viral infections including influenza. Because antioxidants defend cells from damage caused by reactive oxygen species induced by different stimuli including pathogens, they represent interesting molecules to fight infectious diseases. However, most of the available studies have found that these would-be panaceas could actually exacerbate the diseases they claim to prevent, and have thus revealed "the dark side" of these molecules. This review article discusses the latest opportunities and drawbacks of the antioxidants used in anti-influenza therapy and new perspectives.
Keywords: Anti-influenza therapy, Antioxidants, Antivirals, Influenza virus, Oxidative stress, Redox state.
1. INTRODUCTION
Influenza virus is a respiratory pathogen contagious to humans, belonging to Orthomyxoviridae family that contains three types of viruses (A, B, C). In particular, influenza A virus represents a great serious human pathogen since it causes large recurrent epidemics with high mortality and periodic, unpredictable pandemics.
Influenza virus is an enveloped negative-sense RNA virus and its genome possesses eight segments encoding 10 proteins, and other novel proteins discovered in the last years [1]. The envelope comprises two glycoproteins, hemagglutinin (HA) and neuraminidase (NA), and the ion channel matrix 2 (M2), that project from the viral surface. Under the envelope, there is the matrix (M1) protein that coats the core of the virus, composed by the ribonucleoprotein (RNP) complex, consisting of the viral genome, the polymerases (PB1, PB2 and PA) and the nucleoprotein (NP). Nonstructural proteins NS1 and NS2 /NEP (Nuclear Export Protein) also constitute the viral particle.
Currently, two options are available to fight influenza: vaccination and antiviral drugs. Vaccination is a key component of defense strategies against influenza, although effective vaccines cannot be produced quickly enough to deal with emerging threats. Anti-influenza drugs include the adamantanes, which target the M2 and inhibit viral uncoating, and NA inhibitors, which block the release of virions from infected cells. Unfortunately, the emergence of strains resistant to antiviral agents highlights the need for drugs that act on new molecular targets, furnishing safe and effective protection against influenza [2]. In this context, targeting of interactions between virus and host cell has been proposed as a novel antiviral strategy that could reduce both viral replication and lung inflammation, as resistance is less likely to occur. Influenza viruses are able to modulate intracellular redox sensitive signaling pathways involved in several cellular functions in order to promote viral replication and pathogenesis [3-8]. Oxidative stress has been described as a characteristic of viral infections that can be caused by several factors, among which the decrease in antioxidant defenses [9, 10] i.e. intracellular glutathione [11-13] and/or the increase in reactive oxygen species (ROS) production [14, 15]. In particular, several papers have reported that ROS and RNS (reactive nitrogen species) contribute to the development of influenza virus-induced pathogenesis in the lung [16-18]. Physiological levels of ROS play a key role in mediating cell signaling, while high levels of ROS can lead to oxidative damage to cellular components and activate several cell death pathways [19]. An “antioxidant defense network” exists inside the host cell to control ROS levels so as to allow useful functions whilst minimizing oxidative damage [reviewed in 20]. For this reason, antioxidants represent interesting molecules that have been proposed for the treatment of influenza. In 2006, Friel et al. [21] proposed the prophylactic use of a carefully designed formulation as nutritional supplement that could counteract the major pathogenic mechanism underlying avian H5N1 influenza in humans.
It would be reasonable to increase antioxidant capacity of the cell using exogenous compounds derived from the diet, thus enhancing cell defenses against the free radical formation. Natural antioxidants present in fruit and vegetables, including vitamins C and E, carotenoids and polyphenols (e.g. flavonoids), are currently considered to be beneficial. In particular, their antioxidant properties are often claimed to be responsible for the protective effects of food components against cardiovascular diseases, some forms of cancer and diseases related to photosensitivity [22]. Other natural compounds have been shown to have antioxidant activity far exceeding the existing antioxidants, i.e. astaxanthin a xanthophyll carotenoid present in different microorganisms and marine animals [23]. Antioxidant enzyme levels, like superoxide dismutase (SOD), catalase, and glutathione peroxidase, were significantly increased in rats after oral dosage of astaxanthin [24, 25]. Furthermore, after dietary supplementation with astaxanthin enhanced antibody production was reported in older animals, suggesting that carotenoid supplementation can be beneficial in restoring humoral immune response [26]. However, many controversies surround the effects of these compounds in experimental models, and their real benefits are still a matter of debate.
This review describes the structure and mechanism of action of the main antioxidants studied as antiviral agents (the relevant in vitro and in vivo studies are summarized in Table 1). In particular, this paper discusses the advantages and drawbacks of these compounds used in anti-influenza therapy and focuses on new perspectives.
Table 1.
Relevant in vitro and in vivo findings on antioxidants proposed as anti-influenza agents
| Thiol Compounds and Pro-Drugs | Effect on Different Models of Influenza Virus Infection | |
|---|---|---|
| In vitro | In vivo | |
| N-acetyl-L-cysteine (NAC) | Twenty-four h pre-incubation (5 to 15 mM): reduction of H5N1 virus-induced cytopathogenic effects, apoptosis and viral titer; reduction of pro-inflammatory molecules [39, 40]. Pre- and post-treatment: decrease of pro-inflammatory cytokine production; weak decrease of viral titer; correlation with antioxidant activity [41]. Post-treatment (15 mM): partial protection against H1N1 (2009) virus [42]. |
Oral or IP treatment (1g/Kg per day): reduction of influenza virus-induced lethal effects (alone or in combination with anti-viral) [35-38]. Oral administration (100 mg/Kg): no protection against H1N1 (2009) [42]. |
| Glutathione (GSH) | Post-treatment (10 mM): strong reduction of viral titer and protein expression; increase of intracellular GSH levels in infected cells [12, 13]. | Oral administration (50 mM): decrease of viral titer in both lung and trachea homogenates [13]. |
| GSH-C4 | Post-treatment(7.5-10 mM): strong reduction of viral titer; impairment of influenza HA maturation; increase of intracellular GSH levels [11]. |
IP treatment (370 mg/Kg): reduction of mortality, viral titer in lungs and virus-induced inflammation [11]. |
| PDTC | Post-treatment: inhibition of HA viral RNA, virus-induced apoptosis and ROS overproduction [72]. | IP pre-treatment (75, 150, 200 mg/Kg): strong increase of survival; decrease of viral titer in lungs [71]. |
| Polyphenols | ||
| Resveratrol (RV) and analogue | Post-treatment (20 mg/ml): strong decrease of viral titer; no correlation with antioxidant activity [90]. Post treatment with analogue (10 mg/ml): impairment of vRNP traffic; partial restore of virus-induced GSH depletion [95]. |
IP treatment (1 mg/kg/day): significant improve of survival; decrease of pulmonary viral yields [90]. |
| Curcumin and analogue | Pre-treatment (30 mM): strong reduction of virus yields [96]. Post-treatment (20 mg/ml) with analogue: block of NP in the nucleus; inhibition of late protein synthesis; impairment of HA maturation; restore of reducing condition in the cells [95]. |
N.A. |
| Hydroxytyrosol | Post-treatment with catechol derivatives of hydroxytyrosol (IC50=30 mM): reduction of viral titer [97]. | N.A. |
| Tocopherols | ||
| Vitamin E | N.A. | IP pre-treatment (60, 120, 240 mg/Kg): protection from increased virus induced-lipid peroxidation [113, 114]. Aged-mice fed with 500 ppm (supplemented diet): increase of antiviral activity; enhancement of Th1 response [115, 116] |
| Trolox | Post-treatment: decrease of ROS overproduction; no inhibition of viral titer and virus-induced apoptosis [72]. | N.A. |
| Vitamin C | Weak inhibition of viral replication; Treatment with dehydroascorbic acid (40 mM): strong antiviral activity due to toxic effects [108]. |
N.A. |
| Enzymes | ||
| NADPH oxidase (NOX) | Treatment with NOX4 inhibitor or NOX4 silencing: reduction of viral titer; inhibition of ROS production [14]. | NOX2-deficient mice: significant reduction in lung injury; improvement in lung function; lower airway inflammation and alveolar epithelial apoptosis [134, 137]. |
| Superoxide dismutase (SOD) | N.A. | Modified Cu/Zn SOD: reduction of lethality of infection [141]. Naturally glycosylated Cu/Zn SOD in combination with rimantadine or polyphenol-rich extract: decrease of mortality rates and lung viral titer [142, 143]. Aerosol of MnSOD in combination with ribavirin: inhibition of infection [144]. |
IP: intraperitoneal administration
N.A.: Not available
2. ANTIOXIDANTS AS ANTI-INFLUENZA AGENTS
2.1. Thiol-Based Antioxidants
2.1.1. N-Acetyl-L-Cysteine and Pro-Drugs
N-Acetyl-L-cysteine (NAC) is thiol acting directly as a free radical scavenger and as a precursor of reduced glutathione (GSH). NAC is used as a mucolytic agent and in the treatment of several disorders, including paracetamol intoxication, acute respiratory distress syndrome, bronchitis, AIDS, or psychiatric disorders such as schizophrenia and bipolar disorder reviewed in 27. NAC effects are generally attributed to the antioxidant ability of scavenging ROS and increasing intracellular GSH content [28-32], although it has been reported that treatment (oral or intra-peritoneal) with thiols may be associated with increased cysteine levels without concomitant rise in GSH synthesis [33], especially when GSH pools are normal [34]. With regard to viral infections, the protecting activity of NAC was shown in mice infected with influenza A/PR/8 virus, and in in vitro models using avian H5N1, a highly pathogenic strain. Indeed, treatment with NAC (1 g/kg per day, orally) significantly decreased the mortality of influenza virus-infected animals [35], and a combination of NAC (1 g/kg) and ribavirin (0.1 g/kg, i.p.) for 4 days reduced the lethal effects induced by influenza virus [36]. Moreover, a combined addition of NAC and oseltamivir, the antiviral usually utilized in the treatment and prevention of influenza, synergistically reduced the lethal effect of influenza virus-infected mice [37]. Recently, NAC was successfully used for a therapy of H1N1 (2009) influenza pneumonia in combination with oseltamivir [38]. These data sustain the notion that combination of antioxidant therapy with available drugs can improve the treatment of influenza.
In in vitro studies, the continuous treatment with NAC, starting by 24 h pre-incubation, reduced cytopathic effects and apoptosis induced by H5N1 strain, as well as viral titer 24 h post-infection [39]. NAC also decreased production of pro-inflammatory cytokines CXCL8, CXCL10, CCL5 and interleukin (IL)-6 in alveolar type II epithelial (A549) cells infected with H5N1 influenza virus. Specifically, the antiviral and anti-inflammatory mechanisms of NAC included inhibition of redox-sensitive pathways activation, among which transcription nuclear factor (NF)-κB and p38 mitogen activated protein kinase (MAPK) [39]. Proteomic studies performed by Wu and collaborators [40] demonstrated that NAC was able to protect PR8 infected lung epithelial cells from apoptosis induced by the virus. In addition, Mata et al. [41] demonstrated that administration of NAC inhibited the production of mucin (MUC5AC) and the expression of pro-inflammatory cytokine in A549 cells infected with different viruses, including influenza A, B or respiratory syncytial virus (RSV). In these experiments, NAC decreased the hydrogen peroxide (H2O2) production and restored intracellular total thiol levels depleted by viruses. These effects were associated with significant but weak decrease in viral titer by 10.31%, 12.99% and 30.12% for RSV, influenza A and B viruses, respectively.
Although these positive results, Garigliany and Desmetch [42] reported that NAC lacks universal anti-influenza activity. Indeed, these authors showed that NAC was not able to change the course of a fatal influenza pneumonia caused by infection with a murinized swine H1N1 influenza virus. Moreover, NAC treatment inhibited the swine viral replication in vitro but lesser than reported for other strains. Therefore, the authors suggested that anti-influenza activity of NAC seemed to be strain-dependent, and for this reason NAC could not be universally used for influenza pneumonia. Our preliminary in vitro results in lung epithelial cells indicate that despite NAC was able to restore the intracellular redox state perturbed by viral infection, its antiviral activity was very limited when the treatment was done after viral challenge. Surprisingly, although the compound was able to inhibit virus-induced p38 MAPK phosphorylation, it exacerbated the pro-inflammatory cytokines production (Sgarbanti, personal communication). In conclusion, the beneficial effects of NAC on cytokine production and on pathological conditions are still controversial [43-47]. Recently, it has been demonstrated that supplementing the diet with NAC and vitamin E markedly increased the progression of tumor in mice and reduced survival of B-RAF– and K-RAS–induced lung cancer by disrupting the ROS-p53 axis. This may be relevant to patients with chronic obstructive pulmonary disease, who are often smokers with increased risk of developing lung cancer, and they ingest high amounts of NAC to relieve mucus production [48]. Despite the large use of NAC, its administration has some drawbacks such as low systemic bioavailability; for this reason some pro-drugs of
NAC were synthesized. An example is represented by I-152, a pro-drug of NAC and cysteamine. Upon esterase activation, it can release both products and NAC can be used to increase intracellular GSH levels [49]. Antiviral activity of I-152 was demonstrated in in vitro and in vivo models [49, 50].
2.1.2. Glutathione (GSH) and Analogues
Influenza virus induces a depletion of GSH [11-13], the most abundant intracellular antioxidant, which plays a key role in maintaining the redox state [51] and in scavenging ROS [52]. Many authors demonstrated that the replenishment of intracellular GSH obtained with the administration of GSH, GSH derivative or GSH precursor inhibited the viral replication of several viruses in vitro and in vivo [3, 53]. Cai et al. [13] demonstrated that GSH administration inhibited viral matrix protein expression, caspase activation and Fas upregulation in influenza virus-infected MDCK cells. The GSH addition in the drinking water decreased viral titer in lung and trachea homogenates, 4 days after infection with a mouse-adapted influenza A/X-31 strain. Since the GSH is a molecule not readily transported into most cells or tissues, the n-butanoyl GSH derivative (GSH-C4) was tested for its antiviral activity against different viruses [54, 55].
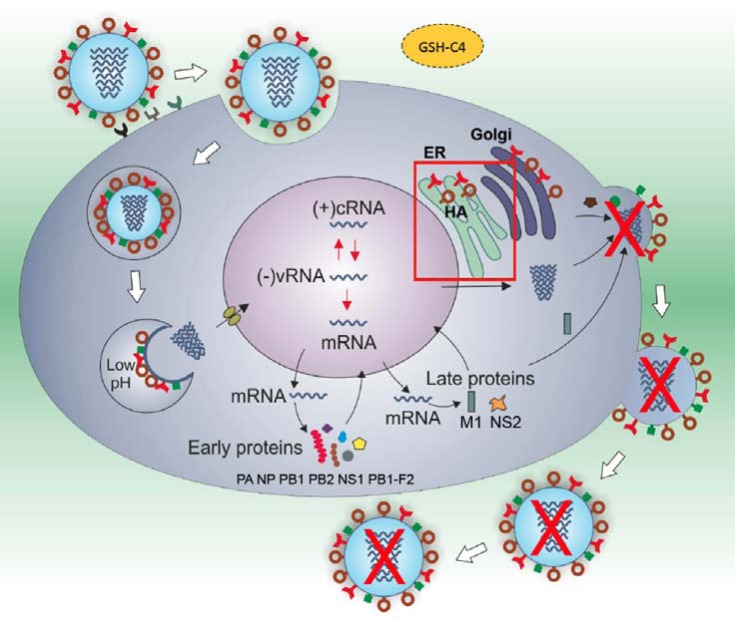
Sgarbanti et al. [11] reported that GSH-C4 is effective against influenza virus. In particular, it interfered with the viral glycoprotein HA maturation, a process mediated by redox-sensitive activity of protein disulfide isomerase (PDI), a cell oxidoreductase in the endoplasmic reticulum (ER) (Fig. 1). In fact, the oxidative environment established by GSH depletion during viral infection, is needed to increase the expression and oxidation of PDI, thus accelerating disulfide bonding and enhancing HA maturation. Accordingly, treatment with oxidant compounds, including buthionine sulfoximine BSO (an inhibitor of GSH synthesis), cadmium, morphine or cocaine, significantly decreased intracellular GSH content and increased HA expression and viral yields measured in supernatants of infected cells [11, 12, 56-58]. The protective effect of GSH-C4 has been demonstrated in a mouse model of influenza virus infection, where it reduced lung damage and mortality [11]. Since the thiol moiety of GSH is important not only in the antioxidant defense but also in the selective inhibition of viral protein disulfide bond formation (acting as “chemical blocker” of folding process), we suggest that the research should address more about the metabolic processes that contribute to the maintenance of this tripeptide within cells. It is useful to taken into account that treatment with pro-GSH molecules, including GSH-C4, increases in vitro and in vivo the intra-macrophage thiol content and it regulates the shift of immune response towards T helper (Th) 1-type response, which plays a key role in antiviral immunity [59]. In fact, in Ovalbumin-immunized mice the increased intra-macrophage thiol pool after pro-GSH molecules addition, modulated the Th1/Th2 balance in favor of a Th1 response [60]. These results together with those obtained in animals immunized with HIV Tat (Trans-activator of transcription) [61] suggest that pro-GSH molecules could be useful for modulating the immune response towards different antigens and could be further exploited in new vaccination protocols for inducing specific Th1 immune responses against intracellular pathogens or as immunomodulators in some diseases where Th1 response patterns are compromised in favor of Th2. Other GSH analogues were used to enhance macrophage Th1 cytokine production. Given the important role of GSH content in the antigen presenting cells in inducing differentiation of Th population into either Th1 or Th2 phenotype, some authors used GSH mono ethyl ester to enhance the Th1 cytokines production, such as IL-12 and IL-27, in macrophages and dendritic cells [62, 63]. To the same aim, other authors used NAC [64]. As already observed elsewhere regarding the pro-oxidant or antioxidant effect of antioxidants during viral infection, the same molecules may specifically induce different sets of genes controlling Th1 cytokine expression depending on the type and redox status of the cell; furthermore, the same molecules can exert different effects according to the concentration used [64].
Fig. (1).
Proposed molecular mechanism of GSH-C4 in inhibiting influenza virus replication. GSH-C4 arrested the folding of viral hemagglutinin (HA): this disulfide-rich glycoprotein remained in the endoplasmic reticulum (ER) as a reduced monomer, instead of undergoing oligomerization. As a consequence, its insertion into the cell plasma-membrane was strongly reduced and the virus release was blocked. The mechanism underlying GSH-C4 antiviral effect is related to the host-cell oxidoreductase, protein disulfide isomerase (PDI). This protein works in ER by helping the formation of disulphide bond during glycoprotein maturation [11].
2.1.3. Pyrrolidine Dithiocarbamate
Pyrrolidine dithiocarbamate (PDTC) is a thiol-containing molecule that can function either as pro- or anti-oxidant compound depending on the experimental conditions. The antioxidant properties of PDTC are attributed to its ability to scavenge radicals, to chelate ions and to alter ROS metabolism. In addition, PDTC regulates antioxidant enzyme gene expression, including manganese superoxide dismutase (MnSOD) [65], heme oxygenase-1 [66] and γ-glutamyl-cysteine synthetase [67] but, conversely, it may act as a pro-oxidant and modulator of free thiol groups [68, 69]. PDTC has been shown to be an inhibitor of NF-κB, due to its ability to traverse the cell surface and its prolonged stability at physiological pH [70]. For this reason, PDTC has been proposed for the treatment of acute and chronic inflammatory conditions in which NF-κB activation plays a major role [71].
It has been reported that PDTC was able to suppress ROS accumulation induced by influenza virus infection in chorion cells [72]. PDTC inhibited both apoptosis induction and viral replication in the infected cells, whereas no such inhibitory effect was observed after Trolox treatment. For this reason, the authors suggested that ROS production could not be responsible for influenza virus induced-programmed cell death. On the contrary, they suggested that this inhibition could be attributable to the antiviral activity of the compound rather than its antioxidant properties. Other authors demonstrated that PDTC inhibited the cytopathic effect of influenza virus infection on other types of cells, such as A549 and murine macrophage J774.1 cells [73-75]. Recetly, Wiesener et al. [76] described the protective effect of PDTC in mice infected with a mouse-adapted strain of A/PR/8/34 (H1N1) simultaneously treated with PDTC [75,150, 200 mg/kg body weight (b.w.), intraperitoneally]. The treatment increased survival up to 80% and reduced weight loss and virus titer in lung tissue in a dose-dependent manner. The efficacy was less pronounced, if the treatment started later during influenza A virus infection. Moreover, simultaneous treatment with PDTC limited infiltration of immune cells as well as local interferon (IFN)-γ expression in lung tissue.
Although PDTC is an anti-oxidant, some authors suggest that this characteristic can not be responsible for its ability of inhibiting NF-κB in tubular epithelial cells [77]. On the contrary, the pro-oxidant and metal-chelating properties of PDTC could paradoxically be involved in its ability of inhibiting the transcription factor [78]. Accordingly, PDTC seems to act catalytically at micromolar concentrations and cause the oxidation of several hundred molar equivalents of GSH [68, 78].
2.2. Polyphenols
Polyphenols are largely diffused in the plant kingdom, especially in fruits (like berries, pomegranate, and apple), nuts and vegetables as well as beverages, including green tea and red wine [79, 80]. They have a plethora of therapeutic health effects for many diseases including cancer, neurodegenerative diseases, diabetes, cardiovascular diseases and infectious diseases [81-83]. Particularly, polyphenols and their semi-synthetic derivatives have been shown to exert anti-inflammatory as well as anti-influenza activity [81, 84-86]. The anti-influenza activity of polyphenols has been related to their antioxidant property. Indeed, a polyphenolic extract from the medicinal plant “Geranium sanguineum L.” exerts anti-influenza activity and antioxidant and radical scavenging properties [87]. Later the same authors demonstrated that these extracts could act not only as antioxidants, but also as pro-oxidants, thus indicating a dual characteristic of some polyphenols. In fact, the biological properties of polyphenols may be both antioxidant and/or pro-oxidant based upon the structure of the particular polyphenol and the cellular redox context that may include increased levels of oxidant scavenging proteins or decreased levels of oxidized proteins and lipids [83]. Among polyphenols, resveratrol (trans-3,4,4’-trihydroxy-stilbene, RV) is a stilbene-like phytoalexin present in more than 72 plant species, among which grape skin and other fruits, with high antioxidant activity [88]. It plays a relevant role in several diseases including viral infections [89]. We previously demonstrated that RV inhibited influenza A virus replication by acting on cellular pathways involved in the regulation of specific steps of virus life-cycle, the nuclear-cytoplasmic traffic of viral RNP complex and the late viral protein synthesis [90]. We also confirmed RV’s antiviral effects in vivo model. In fact, treatment of influenza virus-infected mice markedly increased survival and decreased lung virus yields. We suggested that different mechanisms could underlie the in vivo efficacy of RV, including inhibition of both virus titer and NF-κ B-induced inflammation. In fact, it is known that RV inhibits several cell pathways that are involved in the inflammatory airway damage, characteristic of influenza [91]. Furthermore, it has been reported that (+)-vitisin A, a tetramer of RV, potently inhibited RANTES production by interfering with Akt- and STAT1-related signal pathways during influenza virus infection [92]. Finally, RV might be involved in the regulation of innate response. In fact, Xie et al. [93] reported that RV decreased IL-6 production and partially inhibited RSV replication in cell cultures, and this might be related to an inhibitory effect on TIR-domain-containing adapter-inducing interferon-β (TRIF) complex. Interestingly, in our model no correlation between RV’s antioxidant and antiviral activity was observed. Indeed, during influenza virus infection, RV treatment was not able to restore GSH depletion and, in mock-infected cells, it decreased the intracellular GSH content (compared with untreated cells) [90]. These results can be explained because RV may produce in vivo anti-oxidant or pro-oxidant effects depending on its oxidative status, that in turn mirrors the redox potential of the microenvironment. Thus, while RV can quench reactive free radicals by donating hydrogen atoms, this process generates phenoxyl radicals that can oxidize GSH to GS•. Furthermore, oxidation of the RV-phenoxyl radical produces a quinone form, that can alkylate GSH, further decreasing intracellular levels of free GSH [94].
Differences in anti-influenza activity between neo synthesized analogues of RV and curcumin have been reported by our group [95]. Curcumin [diferuloyl methane, 1,7-bis-(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3,5-dione] is a natural polyphenol present in the rhizome of Curcuma longa L. known to be effective against influenza virus [96]. In our study, we demonstrated that both analogues inhibited viral replication by impairing vRNP traffic, nevertheless while RV analogue partially interfered with redox state of host cell, curcumin analogue prevented virus-induced GSH depletion. As a consequence, redox-sensitive pathways involved in viral HA maturation and localization on cell surface could be impaired [95]. Interestingly, curcumin analogue brought two catechol groups, usually known to contribute to the antioxidant activity of compounds. Bozzini et al. [97] demonstrated that catechol derivatives with lipophilic properties exerted different anti-influenza activity depending on their length of carbon alkyl side-chain. The best compounds were catechol derivatives of hydroxytirosol, with significant antioxidant activity and relatively long carbon alkyl side-chain, suggesting that the anti-influenza activity could be due to two main factors, the antioxidant property of catechols and the presence of a lipophilic side chain (more than two carbon atoms).
Accordingly, our previous data showed that GSH-C4, a lipophilic GSH derivative, was able to enter into the cells more easily than GSH, thus inhibiting more effectively the replication of DNA and RNA viruses [11, 54].
2.3. Ascorbic Acid
Ascorbic acid, or vitamin C, has been widely suggested as antiviral agent, especially against influenza virus. High doses of vitamin C for the prevention and treatment of colds have been proposed in 1970 when the Nobel Linus Pauling published the book “Vitamin C And The Common Cold”.
In 1999, controlled clinical trial demonstrated that vitamin C in megadoses (1g, 3 doses daily) given before or after the appearance of cold and flu symptoms mitigated and prevented the symptoms in the population compared with the control group [98]. However, the role of vitamin C in the prevention and treatment of the common cold has been a subject of debate for at least 70 years.
Cochrane review [99] on this topic disclosed the failure of vitamin C supplementation in reducing the incidence of colds in the general population thus indicating that routine vitamin C addition is not justified, even if vitamin C can be useful for people exposed to brief periods of severe physical exercise. A discordance of the use of this vitamin comes from some trials that show that regular supplementation of vitamin C reduced the duration of colds, while other trials did not show the same results.
As reviewed in Yuan [100], high amounts of vitamin C would be helpful for patients during a severe avian influenza. Thorson et al. [101] demonstrated that in different public places, such as Vietnam, about 50% of the humans infected with avian flu (H5N1) did not die. Ely [102] proposed that their survival could be occurred because infection could be moderate enough to be counteracted by vitamin C, accidentally acquired from diet. It is known that 5 mM of vitamin C (about 4.4 g for about 5 L of human blood) is the effective dose to inhibit viral replication and apparent symptom alleviation usually requires over. However, it should be considered that over 1 g of vitamin C by oral administration can cause diarrhea, nausea, vomiting, stomach cramps and other side effects [reviewed in 100]. The protective effect of ascorbic acid is probably due to i) its potent scavenging and antioxidant property and ii) its accumulation in millimoles per liter in neutrophils, lymphocytes and monocytes [103, 104]. It has also been demonstrated that Vitamin C is an essential factor on the antiviral immune response at the early time of infection, through increased anti-viral cytokine IFN-α/β production. This effect has been suggested in vitamin C- insufficient Gulo (-/-) mice infected with influenza virus (H3N2/Hongkong). These animals died within 1 week after intranasal infection with influenza virus, viral yields in the lung were definitely increased and production of IFN-α/β was decreased. Moreover, the inflammatory cell infiltration into the lung and pro-inflammatory cytokines, tumor necrosis factor (TNF)-α and IL-1α/β, production were increased [105].
Vitamin C is considered a powerful antioxidant and intervenes in several physiological processes, but it can also act as a pro-oxidant when it reacts with iron or copper, which in turn reduces hydrogen peroxide to hydroxyl radicals [106, 107]. Furuya et al. [108] demonstrated that ascorbic acid weakly inhibited viral replication of several viruses, including influenza. A much stronger antiviral activity was observed by dehydroascorbic acid, an oxidized form of ascorbic acid, thus indicating that the antiviral activity was probably due to cytotoxic effects than that to its antioxidant property.
2.4. Vitamin E and Analogues
Vitamin E (α-tocopherol) is the common term given to a group of fat-soluble compounds, which possess different antioxidant activities essential for human health [109]. The human diet contains eight different vitamin E-related molecules including α, β, γ, δ-tocopherols and tocotrienols which are synthesized by plants. Although these molecules are peroxyl radical scavengers, the human body prefers α-tocopherol [109]. As concern the tocopherols, the α- and γ-tocopherols are found in the serum and red blood cells, with the α-tocopherol present in the highest concentration [110]. The α-tocopherol form accumulates particularly at sites where free radical production is greatest, such as in the membrane of mithocondria and ER in the heart and lungs [111]. It acts as the first line of defense against lipid peroxidation, protecting the cell membranes from radical attack. In particular, α-tocopherol mainly inhibits the production of new free radicals. Since oxidation has been linked to numerous diseases including viral infections, vitamin E might represent a good tool for the treatment of ROS-associated diseases. It is known that influenza virus infection in mice causes a decrease in levels of the antioxidant nutrients [112-114]. In particular, it has been reported that influenza virus causes a marked increase of lipid peroxidation products in the liver, blood and lung of infected mice accompanied by a decrease of vitamin E content [113, 114]. Supplementation with exogenous vitamin E (60, 120, 240 mg/Kg b.w.) before virus infection protects mice against lipid peroxidation. Indeed, in these conditions, a decrease of lipid peroxidation products and an increase in vitamin E content were established. The effect of vitamin E was dose-dependent in blood and liver while in lung tissues it was dose-independent, probably due to their different fatty acid and phospholipid composition [113, 114]. Aged-mice supplemented with 500 parts per million (ppm) of vitamin E had significantly reduced lung viral titers, with respect to old mice fed with a diet containing adequate levels of vitamin E (30 ppm) [115]. Interestingly, vitamin E was more effective in reducing viral titer in old mice than in young mice, probably due to the fact that aging is associated with increased oxidative stress. Therefore, the authors suggested that influenza virus-induced oxidative stress in aged-mice might require higher levels of antioxidant nutrients to control viral replication to the same level as in young animals. The antiviral effect of vitamin E was mediated by an improvement of the Th1 response, which was impaired in influenza virus-infected old mice [116]. The effect of natural tocopherols on the regulation of redox balance in the cells depends on the presence of the corresponding tocopherylquinones. Indeed, quinones can act as potent electrophiles altering the internal redox potential of the cells. Saladino et al. [117] evaluated the antiviral effect of natural tocopherols and the corresponding tocopherylquinones and cathecols in in vitro model of influenza virus infection. Interestingly, only the reduced form of tocopherols was able to inhibit viral replication indicating a key role of the oxidation state of the molecule on the antiviral activity. Importantly, in some trials using vitamin E supplementation there was an increased mortality [118]. As reviewed in Villanueva et al. [119], antioxidants become “unstable” and “reactive” when they lose or receive electrons in the presence of reactive species. In particular, α-tocopherol produces α-tocopheroxyl radical when it reacts with reactive species like peroxynitrite [120] or superoxide [121], and it is converted to α-tocopherol by other antioxidants among which vitamin C and GSH [122-124]. When ascorbic acid recycles vitamin E, it is transformed to the ascorbyl radical, which is less reactive than α-tocopherol [122]. Therefore, the authors suggest that vitamin E should be provided with other antioxidants. Trolox (6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid) is a cell-permeable, water-soluble vitamin E analogue with potent antioxidant properties. The carboxyl group present within the structure gives water solubility, which renders the use of Trolox more advantageous respect to other active antioxidants (e.g. vitamin E) that are only lipid-soluble [125]. Trolox has been used for antioxidant therapy in different models in which ROS are formed, including myocardial injury and diabetic retinopathy.
In primary cultured chorion cells isolated from human fetal membranes infected with influenza A PR8 virus, Trolox (500 µM) was able to inhibit the virus-induced ROS production but it did not inhibit DNA fragmentation and viral replication [72]. Therefore, Trolox seems to act as antioxidant but not as antiviral. In MDCK cells infected with influenza virus A H3N2, Trolox added in combination with rimantadine did not show any pronounced protective effect [126]. Our preliminary experiments on human lung epithelial cells infected with PR8 virus demonstrated that Trolox treatment after viral adsorption did not block the release of viral particles from infected cells compared to untreated infected ones, and exacerbated the virus-induced pro-inflammatory cytokines production (Sgarbanti, personal communication). The mechanism of this increased cytokine production could be explained by the fact that Trolox may be oxidized by a variety of free radicals to the phenoxyl radical [127], then the cells’ environment may remain oxidized thus favoring the progression of influenza virus infection.
2.5. New Strategies for Inhibiting ROS Production
2.5.1. NOX Inhibitors
As previously described, physiological levels of ROS interact with redox state and play a role in activating signaling cascades involved in several cell functions including growth and differentiation for [review see 128]. On the contrary, excessive cellular generation of ROS is pathological and potentially destructive and can result in oxidative damage to cellular components [19]. Several enzymes in the cells are able to produce ROS among which xanthine oxidase [129], cytochrome P450 oxidase [130], uncoupled nitric oxide synthase [131], NADPH (nicotinamide adenine dinucleotide phosphate) oxidases [132], and the mitochondrial electron transport chain [133]. However, only NADPH oxidases produce ROS as their primary and unique function. These enzymes are multi-protein complexes consisting of a catalytic, transmembrane-spanning subunit (NOX), as well as various structural and regulatory proteins localized on the membrane and in the cytosol. NOX family comprises seven members, NOX1-5, and two dual oxidases (Duox), Duox1 and Duox2 functionally expressed in different tissues and organs [132]. NOX enzymes differ in enzymatic composition, modes of activation, and the products of their enzymatic reaction. NOX isoforms regulate different physiological process but they are also implicated in several diseases including viral infections [132, 134, 135]. Regarding influenza virus, it is known that Nox2-derived superoxide production is responsible for the pathogenesis of infection [136]. In particular, Snelgrove et al. [134] showed a significant reduction in lung injury and improvement in lung function after influenza virus infection in NOX2-deficient mice. At the same time, Vlahos et al. [137] demonstrated that influenza A virus infection increases the NOX2-derived superoxide production that is responsible for increasing of peroxynitrite formation in the lung, thus contributing to the lung injury. Moreover, NOX2-deficient mice showed lower airway inflammation and alveolar epithelial apoptosis after infection with influenza viruses at low and high pathogenicity [137]. Besides the implication of NOX2 in inducing lung inflammation in infected mice, we have reported a role for NOX enzymes also in viral replication by controlling specific steps of influenza virus life-cycle [14]. In particular, we demonstrated that influenza A virus infection transiently increased intracellular ROS in lung epithelial cells. This process led to the activation of the p38 and ERK1-2 MAPK pathways that, in turn, supported the nucleo-cytoplasmic traffic of vRNP, a key event for viral assembly and release. In human pulmonary cell lines and in murine primary airway epithelial cells, NOX4 was the prime actor in the virus-induced oxidative stress and, as a consequence, in favouring viral replication. NOX4 expression was up-regulated during infection, while chemical inhibition or knockdown of NOX4 significantly impaired the release of viral particles from infected cells. Because of the lack of specificity of antioxidants toward a certain ROS at a specific site and the clinical failure of antioxidant treatments, NOX enzymes may represent a good strategy for the treatment of diseases associated with oxidative stress including viral infections. Several NOX inhibitors are currently available, but they lack clear NOX isoform selectivity [138]. Therefore, future NOX inhibitors characterized by selectivity for specific isoforms would be of great interest.
2.5.2. Superoxide Dismutase
Superoxide dismutases (SODs) are metalloproteins that dismutate the superoxide radical (O2-) into H2O2 and molecular oxygen (O2).
Mammalian cells are characterized by a SOD enzyme in the mitochondria that contains active site manganese (MnSOD) and a SOD with active site copper and zinc (CuZnSOD) largely present in the cytosol [139]. Recently, high levels of oxygen free radicals (OFRs) and decreased SOD activity were found in lungs of mice infected with avian H5N1 strain. Thus, the authors suggested a role of OFRs in acute lung injury caused by this virus [140]. Akaike et al. [141] demonstrated the pathogenic role of O2- induced by a cascade of adenosine catabolism in influenza virus-infected mice. Specifically, it was generated in Broncho-alveolar lavage fluid of influenza virus-infected mice because of an elevated xanthine oxidase (XO) and its substrate, as a result of increased levels of adenosine catabolites such as hypoxanthine and xanthine. The elimination of oxygen radicals through the treatment of infected mice with allopurinol (a XO inhibitor) and with chemically modified SOD (CuZn SOD conjugated with a pyran copolymer) had therapeutic effects by reducing the lethality of infection. Free CuZn SOD did not exhibit protective activity, because of its short pharmacokinetic clearance time. The effect of a naturally glycosylated CuZn SOD, produced by the fungus Humicula lutea (HL-SOD) strain 103, was evaluated in combination with rimantadine hydrochloride in protecting mice by influenza virus [142]. While the single treatment did not significantly protect mice against the infection, HL-SOD and rimantadine combination decreased lung viral titers, lung weights and mortality rates, and prolonged survival times. Interestingly, similar results were obtained with the combined application of HL-SOD with a polyphenol-rich extract, isolated from Geranium sanguineum L. [143]. An inhibitory effect on influenza virus infection has been demonstrated by the treatment of infected mice with MnSOD an enzyme with a longer plasma clearance time (half-life [t1/2], about 6 h in mice), in combination with ribavirin each administered with small-particle aerosol [144]. However, the authors reported that MnSOD effects were virus dose-dependent. Indeed, weak inhibition of mortality was observed in mice infected with high doses, while strong inhibition occurred in animals infected with low doses. Finally, Suliman et al. [145] reported that the enhancement of extracellular SOD in the conducting and distal airways in lung of transgenic mice minimized lung injury caused by influenza virus by reducing inflammation and impairing oxidative stress.
2.5.3. Mitochondrially Targeted Compounds
Among the potential sources of ROS discussed above, mitochondrial ROS (mROS) have attracted attention since it has been recently discovered that they contribute to inflammatory cytokine production and innate response [146] by activation of specific intracellular pathways [reviewed in 147]. However, at the present antioxidants are not selective for mitochondria and this fact may hamper their effectiveness [148]. The “ideal” antioxidant should be specifically targeted to mitochondria where ROS are produced and it should effectively remove not all the ROS but just their excess. It is also important for an antioxidant not to be toxic and not to be recognized and eliminated by cell enzymes, as described in the SkQ project - organized in participation with several research groups and aimed at the synthesis of a new type of compounds (SkQs) including plastoquinone (an antioxidant moiety), a penetrating cation, and a decane or pentane linker [148]. Between potential mitochondrial protective drugs, it should be taken into account mitochondrial antioxidant Mitoquinone (MitoQ), a compound designed to deliver ubiquinone into mitochondria, and antioxidants of SkQ-type. MitoQ has been tested successfully in human diseases such as Hepatitis C induced liver disease and skin photo damage as well as a number of experimental animal models including ischemia-reperfusion, neurodegenerative diseases, diabetes, and alcohol-induced hepatosteatosis [reviewed in 149, 150]. In addition, Rodriguez-Cuenca et al. [151] have demonstrated that the antioxidants targeting mitochondria can be safely administered long-term to wild-type mice.
Recently, mitochondria targeted antioxidants of SkQ-type seem to be very promising compounds aimed at eliminating mitochondrial ROS excess caused by aging. These compounds are chimeric molecules composed of penetrating lipophilic cations and plastoquinone, a powerful antioxidant and a component of the photosynthetic electron transport chain in chloroplasts of plants and in cyanobacteria, that is in structures that produce oxygen and therefore are under constant oxidative stress [reviewed in 152]. Plastoquinone derivatives, such as SkQ1 (plastoquinonyl-decyl- triphenylphosphonium) and SkQR1 (plastoquinonyl-decyl-rhodamine 19), protect mitochondria from oxidative damage and decrease mitochondrial damage in in vivo models of oxidative stress [148, 153].
SkQ1 enhanced the median lifespan of organisms and retarded, arrested, and even reversed development of several age-related pathological traits. Interestingly, despite the higher dose of NAC used, the effects of SkQ1 were of the higher magnitude compared to those with NAC [154]. Since mitochondrial oxidative damage may be one of the main reasons for influenza virus-induced cell death [155] these compounds could be suggested as anti-influenza agents.
CONCLUSION
Increasing evidence has demonstrated that altered intracellular redox state occurs during influenza virus infection. These redox changes versus an oxidized state play a key role in the activation of numerous cell pathways that are hijacked by virus to assure its replication and/or that control inflammatory response and the fate of infected cells [3]. Then, in the last years, antioxidant therapy has been proposed to decrease viral load and to counteract lung tissue damage caused by an overproduction of ROS induced by the virus [156]. Some antioxidants are effective in this protection against infection and represent promising therapeutic molecules that could be employed in the treatment of influenza. However, other molecules caused harmful effects in experimental models or clinical trials by highlighting the “dark side” of some antioxidants. Indeed, in some cases antioxidants could act as oxidants or produce stress, if the antioxidants overcome the physiological production of reactive species [119]. Moreover, supplementation with antioxidants may provide little if any unequivocal benefit to disease prevention in humans and may potentially impair health span [157, 158]. For example, consumption of physiological amounts of vitamin C and E abrogated the capacity of physical exercise to render insulin more effective in lowering blood sugar concentrations [159, 160]. Physical exercise by generating large numbers of ROS creates the oxidative redox potential needed to oxidize the free sulphhydryl groups of cysteine into the disulphide bonds, used to stabilize the 3D conformation of physiologically active protein. Therefore, as postulated by Watson [161], an oxidative environment delays if not prevents the occurrence and severity of type 2 diabetes.
In conclusion, there are open questions that the academic community should figure out about the efficacy of each antioxidant: the dose, its actual ability to affect redox-regulated pathways as well as the redox state of the microenvironment in which it must function. Thus, all these issues should be considered in recommending their use in the therapy of influenza.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was partially supported by the Italian Ministry of Instruction, Universities, and Research (Projects PON01-01802, FIRB Internazionale and PRIN 2010-2011), Institute Pasteur Cenci-Bolognetti Foundation grants 2012, and Ateneo grants 2012. The authors thank Dr. Cristian Ripoli for technical assistance.
REFERENCES
- 1.Shaw ML, Palese P. Orthomyxoviridae: The Viruses and Their Replication Fields virology. In: Knipe DM, Howley PM, editors. 6th ed. Lippincott williams & wilkins: Philadelphia; 2013. pp. 1648–1698. [Google Scholar]
- 2.De Clercq E. Human viral diseases: what is next for antiviral drug discoveryκ. Curr. Opin. Virol. 2012;2:572–579. doi: 10.1016/j.coviro.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Nencioni L, Sgarbanti R, Amatore D, Checconi P, Celestino I, Limongi D, Anticoli S, Palamara AT, Garaci E. Intracellular redox signaling as therapeutic target for novel antiviral strategy. Curr. Pharm. Des. 2011;17(35):3898–904. doi: 10.2174/138161211798357728. [DOI] [PubMed] [Google Scholar]
- 4.Go YM, Kang SM, Roede JR, Orr M, Jones DP. Increased inflammatory signaling and lethality of influenza H1N1 by nuclear thioredoxin-1. PLoS One. 2011;6(4):e18918. doi: 10.1371/journal.pone.0018918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo JK, Kim TS, Hufford MM, Braciale TJ. Viral infection of the lung: host response and sequelae. J. Allergy Clin. Immunol. 2013;132(6):1263–1276. doi: 10.1016/j.jaci.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. U S A. 2014;111(10):3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Börgeling Y, Schmolke M, Viemann D, Nordhoff C, Roth J, Ludwig S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J. Biol. Chem. 2014;289(1):13–27. doi: 10.1074/jbc.M113.469239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, Ly H, Liang Y. PLC-κ1 signaling plays a subtype-specific role in postbinding cell entry of influenza A virus. J. Virol. 2014;88(1):417–424. doi: 10.1128/JVI.02591-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada Y, Limmon GV, Zheng D, Li N, Li L, Yin L, Chow VT, Chen J, Engelward BP. Major shifts in the spatio-temporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS One. 2012;7(2):e31494. doi: 10.1371/journal.pone.0031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J. Nutr. 2007;137(6):1466–1471. doi: 10.1093/jn/137.6.1466. [DOI] [PubMed] [Google Scholar]
- 11.Sgarbanti R, Nencioni L, Amatore D, Coluccio P, Fraternale A, Sale P, Mammola CL, Carpino G, Gaudio E, Magnani M, Ciriolo MR, Garaci E, Palamara AT. Redox regulation of the influenza hemagglutinin maturation process: a new cell-mediated strategy for anti-influenza therapy. Anti.Redox Signal. 2011;15(3):593–606. doi: 10.1089/ars.2010.3512. [DOI] [PubMed] [Google Scholar]
- 12.Nencioni L, Iuvara A, Aquilano K, Ciriolo MR, Cozzolino F, Rotilio G, Garaci E, Palamara AT. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003;17(6):758–60. doi: 10.1096/fj.02-0508fje. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 2003;34:928–936. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 14.Amatore D, Sgarbanti R, Aquilano K, Baldelli S, Limongi D, Civitelli L, Nencioni L, Garaci E, Ciriolo MR, Palamara AT. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell Microbiol. 2014 doi: 10.1111/cmi.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr. 1997;127:S962–5. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 16.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. U S A. 1996;93:2448–53. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Vliet A, Eiserich JP, Cross CE. Nitric oxide: a pro-inflammatory mediators in lung diseaseκ. Respir Res. 2000:167–72. doi: 10.1186/rr14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev. Health. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B. Are polyphenols antioxidants or pro-oxidantsκ What do we learn from cell culture and in vivo studiesκ. Arch. Biochem. Biophys. 2008;476(2):107–12. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Friel H, Lederman H. A nutritional supplement formula for influenza A (H5N1) infection in humans. Med. Hypotheses. 2006;67(3):578–87. doi: 10.1016/j.mehy.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Rietjens I, Boersma M, de Haan L, Spenkelink B, Awad HM, Cnubben NH, van Zanden JJ, Woude Hv, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2001;11:321e33. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 23.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 24.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications-A review Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao RA, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013;61:3842–3851. doi: 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- 26.Jyonouchi H, Zhang L, Gross M, Tomita Y. Immunomodulating actions of carotenoids: Enhancement of in vivo and In vitro antibody production to T-dependent antigens. Nutr. Cancer. 1994;21:47–58. doi: 10.1080/01635589409514303. [DOI] [PubMed] [Google Scholar]
- 27.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830(8):4117–29. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Hoffer E, Baum Y, Tabak A, Taitelman U. N-acetylcysteine increases the glutathione content and protects rat alveolar type II cells against paraquat-induced cytotoxicity, Toxicol. Lett. 1996;84:7–12. doi: 10.1016/0378-4274(95)03446-3. [DOI] [PubMed] [Google Scholar]
- 29.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen induced hepatotoxicity by N-acetyl-L-cysteine in vivo - studies with N-acetyl-Dcysteine inmice, J. Pharmacol. Exp. Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- 31.Laurent T, Markert M, Feihl F, Schaller MD, Perret C. Oxidant-antioxidant balance in granulocytes during ARDS - effect of N-acetylcysteine. Chest. 1996;109(1):163–166. doi: 10.1378/chest.109.1.163. [DOI] [PubMed] [Google Scholar]
- 32.De Flora S, Bennicelli C, Camoirano A, Serra D, Romano M, G.A. Rossi GA, Morelli A, De Flora A. in vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and or mutagenic compounds. Carcinogenesis. 1985;6:1735–1745. doi: 10.1093/carcin/6.12.1735. [DOI] [PubMed] [Google Scholar]
- 33.Ball CR. Estimation and identification of thiols in rat spleen after cysteine or glutathione treatment - relevance to protection against nitrogen mustards. Biochem. Pharmacol. 1966;15:809–816. doi: 10.1016/0006-2952(66)90157-2. [DOI] [PubMed] [Google Scholar]
- 34.Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur. J. Clin. Pharmacol. 1989;36:127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- 35.Ungheri D, Pisani C, Sanson G, Bertani A, Schioppacassi G, Delgado R, Sironi M, Ghezzi P. Protective effect of N-acetylcysteine in a model of influenza infection in mice. Int. J. Immunopathol. Pharmacol. 2000;13:123–128. [PubMed] [Google Scholar]
- 36.Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int. J. Immunopathol. Pharmacol. 2004;17:99–102. doi: 10.1177/039463200401700114. [DOI] [PubMed] [Google Scholar]
- 37.Garozzo A, Tempera G, Ungheri D, Timpanaro R, Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int. J. Immunopathol. Pharmacol. 2007;20:349–354. doi: 10.1177/039463200702000215. [DOI] [PubMed] [Google Scholar]
- 38.Lai KY, Ng WY, Osburga Chan PK, Wong KF, Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann. Intern. Med. 2010;152(10):687–8. doi: 10.7326/0003-4819-152-10-201005180-00017. [DOI] [PubMed] [Google Scholar]
- 39.Geiler J, Michaelis M, Naczk P, Leutz A, Langer K, Doerr HW, Cinatl J ., Jr N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010;79:413–20. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Song W, Gao X, Liu N, Wang P, Chen H, Cai Z. Proteomics study of N-acetylcysteine response in H1N1-infected cells by using mass spectrometry. Rapid. Commun. Mass Spectrom. 2014;28(7):741–9. doi: 10.1002/rcm.6840. [DOI] [PubMed] [Google Scholar]
- 41.Mata M, Morcillo E, Gimeno C, Cortijo J. N-acetyl-L-cysteine (NAC) inhibits mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV) Biochem. Pharmacol. 2011;82(5):548–55. doi: 10.1016/j.bcp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Garigliany MM, Desmecht DJ. N-acetylcysteine lacks universal inhibitory activity against influenza A viruses. J. Negat. Results Biomed. 2011;10:5. doi: 10.1186/1477-5751-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viora M, Quaranta M, Straface E, Vari R, Masella R, Malorni W. Redox imbalance and immune functions: opposite effect of oxidized low-density lipoproteins and N-acetylcysteine. Immunol. 2001;104:431–8. doi: 10.1046/j.1365-2567.2001.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddad JJ. The involvement of L-κ-glutamyl-l-cysteinyl- glycine (glutathione/GSH) in the mechanism of redox signalling mediating MAPK p38-dependent regulation of pro-inflammatory cytokine production. Biochem. Pharmacol. 2002;63:305–20. doi: 10.1016/s0006-2952(01)00870-x. [DOI] [PubMed] [Google Scholar]
- 45.Peristeris P, Clark BD, Gatti S, Faggioni R, Mantovani A, Mengozzi M, Orencole SF, Sironi M, Ghezzi P. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell Immunol. 1992;140:390–399. doi: 10.1016/0008-8749(92)90205-4. [DOI] [PubMed] [Google Scholar]
- 46.Kim do Y, Jun JH, Lee HL, Woo KM, Ryoo HM, Kim GS, Baek JH, Han SB. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch. Pharm. Res. 2007;30:1283–92. doi: 10.1007/BF02980269. [DOI] [PubMed] [Google Scholar]
- 47.Stanislaus R, Gilg AG, Singh AK, Singh I. N-acetyl-L- cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. J. Autoimmune Dis. 2005;2:4–15. doi: 10.1186/1740-2557-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 49.Smietana M, Clayette P, Mialocq P, Vasseur JJ, Oiry J. Synthesis of new N-isobutyryl-L-cysteine/MEA conjugates: Evaluation of their free radical-scavenging activities and anti-HIV properties in human macrophages. Bioorg Chem. 2008;36(3):133–140. doi: 10.1016/j.bioorg.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Fraternale A, Paoletti MF, Casabianca A, Orlandi C, Schiavano GF, Chiarantini L, Clayette P, Oiry J, Vogel JU, Cinatl J , Jr, Magnani M. Inhibition of murine AIDS by pro-glutathione (GSH) molecules. Anti. Res. 2008;77(2):120–127. doi: 10.1016/j.antiviral.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem. Pharm. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 52.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid. Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraternale A, Paoletti MF, Casabianca A, Nencioni L, Garaci E, Palamara AT, Magnani M. GSH and analogs in antiviral therapy. Mol. Aspects Med. 2009;30(1-2):99–110. doi: 10.1016/j.mam.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Palamara AT, Brandi G, Rossi L, Millo E, Benatti U, Nencioni L, Iuvara A, Garaci E, Magnani M. New synthetic glutathione derivatives with increased antiviral activities. Antiv. Chem. Chemother. 2004;15:83–91. doi: 10.1177/095632020401500204. [DOI] [PubMed] [Google Scholar]
- 55.Fraternale A, Schiavano GF, Paoletti MF, Palma L, Magnani M, Brandi G. Effect of the N-butanoyl glutathione (GSH) derivative and acyclovir on HSV-1 replication and Th1 cytokine expression in human macrophages. Med. Microbiol. Immunol. 2014;203(4):283–9. doi: 10.1007/s00430-014-0335-4. [DOI] [PubMed] [Google Scholar]
- 56.Checconi P, Sgarbanti R, Celestino I, Limongi D, Amatore D, Iuvara A, Alimonti A, Garaci E, Palamara AT, Nencioni L. The environmental pollutant cadmium promotes influenza virus replication in MDCK cells by altering their redox state. Int. J. Mol. Sci. 2013;14(2):4148–62. doi: 10.3390/ijms14024148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palamara AT, Di Francesco P, Ciriolo MR, Buè C, Lafavia E, Rotilio G, Garaci E. Cocaine increases Sendai virus replication in cultured epithelial cells: critical role of the intracellular redox status. Biochem. Biophys. Res. Commun. 1996;228(2):579–85. doi: 10.1006/bbrc.1996.1701. [DOI] [PubMed] [Google Scholar]
- 58.Macchia I, Palamara AT, Buè C, Savini P, Ciriolo M, Gaziano R, di Francesco P. Increased replication of Sendai virus in morphine-treated epithelial cells: evidence for the involvement of the intracellular levels of glutathione. Int. J. Immunopharmacol. 1999;21:185–93. doi: 10.1016/s0192-0561(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 59.Fraternale A, Crinelli R, Casabianca A, Paoletti MF, Orlandi C, Carloni E, Smietana M, Palamara AT, Magnani M. Molecules altering the intracellular thiol content modulate NF-kB and STAT-1/IRF-1 signalling pathways and IL-12 p40 and IL-27 p28 production in murine macrophages. PLoS One. 2013;8(3):e57866. doi: 10.1371/journal.pone.0057866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fraternale A, Paoletti MF, Dominici S, Caputo A, Castaldello A, Millo E, Brocca-Cofano E, Smietana M, Clayette P, Oiry J, Benatti U, Magnani M. The increase in intra-macrophage thiols induced by new pro-GSH molecules directs the Th1 skewing in ovalbumin immunized mice. Vaccine. 2010;28(48):7676–82. doi: 10.1016/j.vaccine.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 61.Fraternale A, Paoletti MF, Dominici S, Buondelmonte C, Caputo A, Castaldello A, Tripiciano A, Cafaro A, Palamara AT, Sgarbanti R, Garaci E, Ensoli B, Magnani M. Modulation of Th1/Th2 immune responses to HIV-1 Tat by new pro-GSH molecules. Vaccine. 2011;29:6823–9. doi: 10.1016/j.vaccine.2011.07.101. [DOI] [PubMed] [Google Scholar]
- 62.Utsugi M, Dobashi K, Koga Y, Shimizu Y, Ishizuka T, Iizuka K, Hamuro J, Nakazawa T, Mori M. Glutathione redox regulates lipopolysaccharide-induced IL-12 production through p38 mitogen-activated protein kinase activation in human monocytes: role of glutathione redox in IFN-κ priming of IL-12 production. J. Leukocyte Biol. 2002;71(2):339–47. [PubMed] [Google Scholar]
- 63.Kamide Y, Utsugi M, Dobashi K, Ono A, Ishizuka T, Hisada T, Koga Y, Uno K, Hamuro J, Mori M. Intracellular glutathione redox status in human dendritic cells regulates IL-27 production and T-cell polarization. Allergy. 2011;66(9):1183–1192. doi: 10.1111/j.1398-9995.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 64.Alam K, Ghousunnissa S, Nair S, Valluri VL, Mukhopadhyay S. Glutathione-redox balance regulates c-rel-driven IL-12 production in macrophages: possible implications in antitubercolosis immunotherapy. J. Immunol. 2010;184:2918–2929. doi: 10.4049/jimmunol.0900439. [DOI] [PubMed] [Google Scholar]
- 65.Borrello S, Demple B. NF-kB-independent transcriptional induction of the human manganous superoxide dismutase gene. Arch. Biochem. Biophys. 1997;348:289–294. doi: 10.1006/abbi.1997.0355. [DOI] [PubMed] [Google Scholar]
- 66.Hartsfield CL, Alam J, Choi AMK. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 1998;12:1675–/1682. doi: 10.1096/fasebj.12.15.1675. [DOI] [PubMed] [Google Scholar]
- 67.Wild AC, Mulcahy RT. Pyrrolidine dithiocarbamate up-regulates the expression of the genes encoding the catalytic and regulatory subunits of g-glutamylcysteine synthetase and increases intracellular glutathione levels. Biochem. J. 1999;338:659–665. [PMC free article] [PubMed] [Google Scholar]
- 68.Nobel CSI, Kimland M, Lind B, Orrenius S, Slater AFG. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J. Biol. Chem. 1995;270:26202–26208. doi: 10.1074/jbc.270.44.26202. [DOI] [PubMed] [Google Scholar]
- 69.Kim CH, Kim JH, Hsu CY, Ahn YS. Zinc is required in pyrrolidine dithiocarbamate inhibition of NF- kB activation. FEBS Lett. 1999;449:28–32. doi: 10.1016/s0014-5793(99)00390-7. [DOI] [PubMed] [Google Scholar]
- 70.Topping RJ, Jones MM. Optimal dithiocarbamate function for immunomodulator action. Med. Hypoth. 1988;27:55–57. doi: 10.1016/0306-9877(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 71.Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br. J. Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Uchide N, Ohyama K, Bessho T, Yuan B, Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56(3):207–17. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 73.Knobil K, Choi AM, Weigand GW, Jacoby DB. Role of oxidants in influenza virus-induced gene expression. Am. J. Physiol. 1998;274:L134–L142. doi: 10.1152/ajplung.1998.274.1.L134. [DOI] [PubMed] [Google Scholar]
- 74.Lowy RJ, Dimitrov DS. Characterization of influenza virus-induced death of J774. macrophages. Exp. Cell Res. 1997;234:249–258. doi: 10.1006/excr.1997.3602. [DOI] [PubMed] [Google Scholar]
- 75.McKinney LC, Galliger SJ, Lowy RJ. Active and inactive influenza virus induction of tumor necrosis factor-a and nitric oxide in J774 murine macrophages: Modulation by interferon-? and failure to induce apoptosis. Virus Res. 2003;97:117–126. doi: 10.1016/j.virusres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Wiesener N, Zimmer C, Jarasch-Althof N, Wutzler P, Henke A. Therapy of experimental influenza virus infection with pyrrolidine dithiocarbamate. Med. Microbiol. Immunol. 2011;200:115–26. doi: 10.1007/s00430-010-0182-x. [DOI] [PubMed] [Google Scholar]
- 77.Woods JS, Ellis ME, Dieguez-Acuna FJ, Corral J. Activation of NF-kB in normal rat kidney epithelial (NRK 52E) cells is mediated via a redox-insensitive calcium- dependent pathway. Toxicol. Appl. Pharmacol. 1999;154:219–227. doi: 10.1006/taap.1998.8583. [DOI] [PubMed] [Google Scholar]
- 78.Pinkus RL, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kB and glutathione S-transferase gene expression. J. Biol. Chem. 1996;271:13422–13429. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- 79.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 80.Corcoran MP, McKay DL, Blumberg JB. Flavonoid basics: chemistry, sources, mechanisms of action, and safety. J. Nutr. Geront. Geriat. 2012;31:176–189. doi: 10.1080/21551197.2012.698219. [DOI] [PubMed] [Google Scholar]
- 81.Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, hearth disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 82.Saladino R, Gualandi G, Farina A, Crestini C, Nencioni L, Palamara AT. Advances and challenges in the synthesis of highly oxidised natural phenols with antiviral, antioxidant and cytotoxic activities. Curr. Med. Chem. 2008;15:1500–1519. doi: 10.2174/092986708784638889. [DOI] [PubMed] [Google Scholar]
- 83.Kim H-S, Quon MJ, Kim J-a. New insights into the mechanisms of polyphenols beyond antioxidant properties, lessons from the green tea polyphenol, epigallocatechin3-gallate. Red. Biol. 2014:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saladino R, Barontini M, Crucianelli M, Nencioni L, Sgarbanti R, Palamara AT. Current advances in anti-influenza therapy. Curr. Med. Chem. 2010;17:2101–40. doi: 10.2174/092986710791299957. [DOI] [PubMed] [Google Scholar]
- 85.Uchide N, Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules. 2011;16:2032–52. doi: 10.3390/molecules23100000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sokmen M, Angelova M, Krumova E, Pashova S, Ivancheva S, Sokmen A, Serkedjieva J. Protective effect of polyphenol-rich extract on acute lung injury in influenza virus infected mice. Life Sci. 2005;76:2981–2993. doi: 10.1016/j.lfs.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 87.Murzakhmetova M, Moldakarimov S, Tancheva L, Abarova S, Serkedjieva J. Antioxidant and Prooxidant Properties of a Polyphenol-rich Extract from Geranium sanguineum L In vitro and in vivo. Phytother. Res. 2008;22:746–751. doi: 10.1002/ptr.2348. [DOI] [PubMed] [Google Scholar]
- 88.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 89.Campagna M, Rivas C. Antiviral activity of resveratrol. Biochem. Soc. Transact. 2010;38:50–53. doi: 10.1042/BST0380050. [DOI] [PubMed] [Google Scholar]
- 90.Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo M R, Garaci E. Resveratrol inhibits Influenza A virus replication In vitro and in vivo. J. Infect. Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 91.Koeberle A, Werz O. Multi-target approach for natural products in inflammation. Drug Discov. Today. 2014 doi: 10.1016/j.drudis.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Huang YL, Loke SH, Hsu CC, Chiou WF. (+)-Vitisin A inhibits influenza A virus-induced RANTES production in A549 alveolar epithelial cells through interference with Akt and STAT1 phosphorylation. Planta Med. 2008;74:156–62. doi: 10.1055/s-2007-993786. [DOI] [PubMed] [Google Scholar]
- 93.Xie X-H, Zang N, Li S-M, Wang L-J, Deng Y , He Y , Yang -Q, Liu E-M. Resveratrol Inhibits Respiratory Syncytial Virus-Induced IL-6 Production, Decreases Viral Replication, and Downregulates TRIF Expression in Airway Epithelial Cells. Inflamm. 2012;35(4):1392–1401. doi: 10.1007/s10753-012-9452-7. [DOI] [PubMed] [Google Scholar]
- 94.Galati G, Sabzevan O, Wilson JX, O’Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicol. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 95.Fioravanti R, Celestino I, Costi R, Cuzzucoli Crucitti G, Pescatori L, Mattiello L, Novellino E, Checconi P, Palamara A T, Nencioni L, Di Santo R. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorg. Med. Chem. 2012;20:5046–5052. doi: 10.1016/j.bmc.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 96.Chen D-Y, Shien J-H, Tiley L, Chiou S-S, Wang S-Y, Chang T-J, Lee Y-J, Chan K-W, Hsu W-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119:1346–1351. [Google Scholar]
- 97.Bozzini T , Botta G, Delfino M, Onofri S, Saladino R, Amatore D, Sgarbanti R, Nencioni L, Palamara AT. Tyrosinase and Layer-by-Layer supported tyrosinases in the synthesis of lipophilic catechols with antiinfluenza activity. Bioorg. Med. Chem. 2013;21:7699–7708. doi: 10.1016/j.bmc.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 98.Gorton HC, Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J. Manipulative Physiol. Ther. 1999;22(8):530–3. doi: 10.1016/s0161-4754(99)70005-9. [DOI] [PubMed] [Google Scholar]
- 99.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan S. Drugs to cure avian influenza infection--multiple ways to prevent cell death. Cell Death Dis. 2013;4:e835. doi: 10.1038/cddis.2013.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thorson A, Petzold M, Nguyen TK, Ekdahl K. Is exposure to sick or dead poultry associated with flulike illnessκ: a population-based study from a rural area in Vietnam with outbreaks of highly pathogenic avian influenza. Arch. Intern. Med. 2006;166:119–123. doi: 10.1001/archinte.166.1.119. [DOI] [PubMed] [Google Scholar]
- 102.Ely JT. Ascorbic acid role in containment of the world avian flu pandemic. Exp. Biol. Med. 2007;232:847–851. [PubMed] [Google Scholar]
- 103.Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br. J. Nutr. 1982;47:473–482. doi: 10.1079/bjn19820059. [DOI] [PubMed] [Google Scholar]
- 104.Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J. Biol. Chem. 1990;265:2584–2587. [PubMed] [Google Scholar]
- 105.Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM, Hwang YI, Kang JS, Lee WJ. Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-a/ß at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013;13(2):70–4. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidantκ A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005;39(7):671–86. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 107.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 108.Furuya A, Uozaki M, Yamasaki H, Arakawa T, Arita M, Koyama AH. Antiviral effects of ascorbic and dehydroascorbic acids In vitro. Int. J. Mol. Med. 2008;22(4):541–5. [PubMed] [Google Scholar]
- 109.Niki E, Traber MG. A history of vitamin E. Ann. Nutr. Metab. 2012;61(3):207–12. doi: 10.1159/000343106. [DOI] [PubMed] [Google Scholar]
- 110.Chow CK. Distribution of tocopherols in human plasma and red blood cells. Am. J. Clin. Nutr. 1975;28(7):756–760. doi: 10.1093/ajcn/28.7.756. [DOI] [PubMed] [Google Scholar]
- 111.Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ Med J. 2014;14:e157–e165. [PMC free article] [PubMed] [Google Scholar]
- 112.Hennet T, Petherans E, Stocker R. Alteration in antioxidant defences in lung and liver of mice infected with influenza A virus. J Gen Virol. 1992;73:39–46. doi: 10.1099/0022-1317-73-1-39. [DOI] [PubMed] [Google Scholar]
- 113.Mileva M, Tancheva L, Bakalova R, Galabov A, Savov V, Ribarov S. Effect of vitamin E on lipid peroxidation and liver monooxygenase activity in experimental influenza virus infection. Toxicol Lett. 2000;114:39–45. doi: 10.1016/s0378-4274(99)00265-9. [DOI] [PubMed] [Google Scholar]
- 114.Mileva M, Bakalova R, Tancheva L, Galabov A, Ribarov S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp. Immunol. Microbiol. Infect. Dis. 2002;25(1):1–11. doi: 10.1016/s0147-9571(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 115.Hayek MG, Taylor SF, Bender BS, Han SN, Meydani M, Smith DE, Eghtesada S, Meydani SN. Vitamin E Supplementation decreases lung virus titers in mice infected with influenza. J. Infect. Dis. 1997;176(1):273–276. doi: 10.1086/517265. [DOI] [PubMed] [Google Scholar]
- 116.Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, Meidani SN. Vitamin E Supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunol. 2000;100(4):487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saladino R, Neri V, Farina A, Crestini C, Nencioni L, Palamara AT. A Novel and Efficient Synthesis of Tocopheryl Quinones by Homogeneous and Heterogeneous Methyltrioxorhenium/Hydrogen Peroxide Catalytic Systems. Adv. Synth. Catal. 2008;350:321–331. [Google Scholar]
- 118.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 119.Villanueva C, Kross RD. Antioxidant-induced stress. Int. J. Mol. Sci. 2012;13:2091–2109. doi: 10.3390/ijms13022091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Botti H, Batthyany C, Trostchansky A, Radi R, Freeman BA, Rubbo H. Peroxynitrite-mediated alpha-tocopherol oxidation in low-density lipoprotein: A mechanistic approach. Free Radic. Biol. Med. 2004;36:152–162. doi: 10.1016/j.freeradbiomed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 121.Maguire JJ, Wilson DS, Packer L. Mitochondrial electron transport-linked tocopheroxyl radical reduction. J. Biol. Chem. 1989;264:21462–21465. [PubMed] [Google Scholar]
- 122.Damiani E, Astolfi P, Carloni P, Stipa P, Greci L. Antioxidants: How They Work Oxidants in Biology. In: Valacchi G, Davis PA, editors. Springer Science + Buisness Media. USA: New York NY ; 2008. pp. 251–266. [Google Scholar]
- 123.Gvozdjáková A, Duracková Z. Springer Science + Business Media. USA: New York NY ; 2008. Oxidants, Antioxidants and Oxidative Stress Mitochondrial Medicine; pp. 19–54. [Google Scholar]
- 124.Liu C, Russell RM, Wang XD. Alpha-tocopherol and ascorbic acid decrease the production of beta-apo-carotenals and increase the formation of retinoids from beta-carotene in the lung tissues of cigarette smoke-exposed ferrets In vitro. J. Nutr. 2004;134:426–430. doi: 10.1093/jn/134.2.426. [DOI] [PubMed] [Google Scholar]
- 125.Poljsak B, Raspor P. The antioxidant and pro-oxidant activity of vitamin C and trolox In vitro: a comparative study. J. Appl. Toxicol. 2008;28:183–8. doi: 10.1002/jat.1264. [DOI] [PubMed] [Google Scholar]
- 126.Mlu E, Gudkova TM, Konovalova NI, Shchekanova SM, Iagoslkaia IB, Eropkina EM, Kiselev OI. Antiviral action of some antioxidants/antihypoxants and their combinations with remantadine against human influenza A (H3N2) virus studied in In vitro models. Eksp. Klin. farmakol. 2007;70:33–7. [PubMed] [Google Scholar]
- 127.Davies MJ, Forni LG, Wilson R. Vitamin E analogue Trolox C Esr and pulse-radiolysis studies of free-radical reactions. Biochem. J. 1988;255:513–522. [PMC free article] [PubMed] [Google Scholar]
- 128.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999;31(2):53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 129.McNally JS, Davia ME, Giddens DP, Saha A, Hwang J, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2290–7. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 130.Fleming I. Cytochrome P450 and Vascular Homeostasis Circ. Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 131.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA ., Jr Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors Proc. Natl. Acad. Sci. U S A. 1998;95:9220–5. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bedard K, Krause K. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 133.Skulachev VP. Why are mitochondria involved in apoptosisκ Permeability transition pores and apoptosis as selective mechanism to eliminate superoxide-producing mitochondria and cell. FEBS Lett. 1996;397:7–10. doi: 10.1016/0014-5793(96)00989-1. [DOI] [PubMed] [Google Scholar]
- 134.Snelgrove RJ, Edwards L, Rae AJ, and Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- 135.Boudreau HE, Emerson SU, Korzeniowska A, Jendrysik MA, Leto TL. Hepatitis C Virus (HCV) Proteins Induce NADPH Oxidase 4 Expression in a Transforming Growth Factor ß-Dependent Manner: a New Contributor to HCV-Induced Oxidative Stress. J. Virol. 2008;83:12934–46. doi: 10.1128/JVI.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vlahos R, Stambas J, Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol. Sci. 2012;33:3–8. doi: 10.1016/j.tips.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 137.Vlahos R, Stambas J, Bozinovski S, Broughton BRS, Drummond GR, Selemidis S. Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation. Plos Pathogens. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vlahos R, Selemidis S. NADPH oxidases as novel pharmacological targets against influenza A virus infection. Mol. Pharmacol. 2014;9 doi: 10.1124/mol.114.095216. [DOI] [PubMed] [Google Scholar]
- 139.Fridovich L. Superoxide dismutases. Methods Enzymol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 140.He G, Dong C, Luan Z, McAllan BM, Xu T, Zhao L, Qiao J. Oxygen free radical involvement in acute lung injury induced by H5N1 virus in mice. Influen. Other Respir. Viruses. 2013;7:945–953. doi: 10.1111/irv.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Akaike T, Ando M, Oda T, Doi T, Ijiri S, Araki S, Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J. Clin. Invest. 1990;85(3):739–45. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serkedjieva J, Roeva I, Angelova M, Dolashka P, Voelter WG. Combined protective effect of a fungal Cu/Zn-containing superoxide dismutase and rimantadine hydrochloride in experimental murine influenza a virus infection. Acta Virol. 2003;47(1):53–56. [PubMed] [Google Scholar]
- 143.Serkedjieva J, Stefanova T, Krumova E. A fungal Cu/Zn-containing superoxide dismutase enhances the therapeutic efficacy of a plant polyphenol extract in experimental influenza virus infection. Z. Naturforsch C. 2010;65:419–28. doi: 10.1515/znc-2010-5-616. [DOI] [PubMed] [Google Scholar]
- 144.Sidwell RW, Huffman JH, Bailey KW, Wong MH, Nimrod A, Panet A. Inhibitory effects of recombinant manganese superoxide dismutase on influenza virus infections in mice. Antimicrob. Agents Chemother. 1996;40:2626–2631. doi: 10.1128/aac.40.11.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suliman HB, Ryan LK, Bishop L, Folz RJ. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am. J. Physiol. Lung. Cell Mol. Physiol. 2001;280(1):L69–78. doi: 10.1152/ajplung.2001.280.1.L69. [DOI] [PubMed] [Google Scholar]
- 146.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB. An attempt to prevent senescence: a mitochondrial approach. Biochim. Biophys. Acta. 2009;1787:437–461. doi: 10.1016/j.bbabio.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Smith RAJ, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N.Y. Acad. Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 150.Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013;1:86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA, Pope S, Heales SJ, Lam BY, Neogi SG, McFarlane I, James AM, Smith RA, Murphy MP. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic. Biol. Med. 2010;48:161–72. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 152.Isaev NK, Stelmashook EV, Stelmashook NN, Sharonova IN, Skrebitsky VG. Brain aging and mitochondria-targeted plastoquinone antioxidants of SkQ-type. Biochemistry (Mosc) 2013;78:295–300. doi: 10.1134/S0006297913030127. [DOI] [PubMed] [Google Scholar]
- 153.Skulachev MV, Antonenko YN, Anisimov VN, Chernyak BV, Cherepanov DA, Chistyakov VA, Egorov MV, Kolosova NG, Korshunova GA, Lyamzaev KG, Plotnikov EY, Roginsky VA, Savchenko AY, Severina II, Severin FF, Shkurat TP, Tashlitsky VN, Shidlovsky KM, Vyssokikh MY, Zamyatnin AA, Zorov DB, Skulachev VP. Mitochondrial-targeted plastoquinone derivatives Effect on senescence and acute age-related pathologies. Curr. Drug Targets. 2011;12:800–826. doi: 10.2174/138945011795528859. [DOI] [PubMed] [Google Scholar]
- 154.Kolosova NG, Stefanova NA, Muraleva NA, Skulachev VP. The mitochondria-targeted antioxidant SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in rats. Aging (Albany NY) 2012;4:686–94. doi: 10.18632/aging.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Case AJ, McGill JL, Tygrett LT, Shirasawa T, Spitz DR, Waldschmidt TJ, Legge KL, Domann FE. Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic Biol Med. 2011;50:448–58. doi: 10.1016/j.freeradbiomed.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fraternale A, Paoletti MF, Casabianca A, Oiry J, Clayette P, Vogel JU, Cinati J , Jr, Palamara AT, Sgarbanti R, Garaci E, Millo E, Benatti U, Magnani M. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr. Med. Chem. 2006;13:1749–55. doi: 10.2174/092986706777452542. [DOI] [PubMed] [Google Scholar]
- 157.Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose-Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ristow M. Mitohormesis explains ROS induced health benefits. Nat. Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 159.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez-Cabrera MC, Ristow M, Viña J. Antioxidant Supplements in exercise: worse than uselessκ. Am. J. Physiol. Endocrinol. Metab. 2012;302:E476–77. doi: 10.1152/ajpendo.00567.2011. [DOI] [PubMed] [Google Scholar]
- 161.Watson JD. Type 2 diabetes as a redox disease. Lancet. 2014;383:841–43. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]