Abstract.
An increasing number of integrated optical and acoustic intravascular imaging systems have been developed and hold great promise for accurately diagnosing vulnerable plaques and guiding atherosclerosis treatment. However, in any intravascular environment, the vascular lumen is filled with blood, a high-scattering source for optical and high-frequency ultrasound signals. Blood must be flushed away to provide clearer images. To our knowledge, no research has been performed to find the ideal flushing agent for combined optical and acoustic imaging techniques. We selected three solutions as potential flushing agents for their image-enhancing effects: mannitol, dextran, and iohexol. Testing of these flushing agents was performed in a closed-loop circulation model and in vivo on rabbits. We found that a high concentration of dextran was the most useful for simultaneous intravascular ultrasound and optical coherence tomography imaging.
Keywords: imaging system, optical coherence tomography, attenuation, ultrasound
1. Introduction
Integrated intravascular ultrasound and optical coherence tomography (IVUS-OCT) has the potential to provide better visualization of coronary lesions1–4 and to improve the accuracy of atherosclerotic plaque characterization.5 Great progress has been made in developing a fully integrated IVUS-OCT system and catheter. However, to our knowledge, no research has been performed to find optimal flushing agents that provide both the necessary clarity for OCT and IVUS.
Blood is a high-scattering source for OCT signals and high definition IVUS (HDIVUS). Either blood occlusion or continuous flushing is needed for intravascular OCT imaging. Although no flushing agents are needed in 40-MHz IVUS imaging, some OCT flushing agents may hinder the transmission of IVUS signals, such as perfluorocarbon,6 when simultaneously using IVUS and OCT functions. Thus, it is critical to identify flushing agents that are effective for IVUS-OCT imaging. This research will also benefit acousto-optics (AO), photoacoustic (PA) imaging and spectroscopy, acoustic radiation force optical coherence elastography, and other imaging techniques that simultaneously use light and ultrasound (US).
X-ray contrast agents, such as iohexol and iodixanol, are commonly used for intravascular imaging to clear blood for OCT.7 However, the use of contrast agents in some patients may lead to renal function disorder8 or life-threatening reactions, such as cardiotoxic effects and seizures.9 It was reported that one dominant reason that physicians avoid using intravascular OCT is the injection of extra contract agents.10 Although combined light and sound-based techniques are very promising for improving health outcomes, the wide clinical utility of these techniques will not be achieved until the challenge of safe and effective flushing is addressed.
Dextran11,12 and oxygen-carrying blood substitute perfluorodecalin (PFD; a type of perfluorocarbon)13 have been previously studied as alternative flushing agents for OCT imaging. These have minimal toxicity compared to contrast agents.12,13 Dextran reduces scattering of red blood cells by matching refractive indices between blood plasma and blood cells.14 PFD, which has high viscosity, can displace blood and clear OCT images. However, PFD can cause significant reduction of the US signal.6 In addition, PFD has not been approved by the Food and Drug Administration (FDA) and cannot be used in patients.15 Accordingly, PFD was excluded from our quantitative experiments. We also considered using mannitol solution as a flushing agent, based on mechanisms of optical flushing. Mannitol injection is approved by the FDA and is typically used to promote diuresis and reduce intracranial/intraocular pressure. Last but not least, iohexol is a commonly used contrast agent for OCT flushing. Its toxicity is lower than iodized contrast agents.16
To pave the road for new imaging techniques with both acoustic and optical functions, we studied the principle of flushing and experimentally evaluated the attenuation characteristics of flushing agents using an integrated IVUS-OCT system. We selected three solutions as representative flushing agents for testing because of their image-enhancing effects and relatively low toxicities: mannitol, dextran, and iohexol.
While previous testing on flushing agents has been performed in static baths,14,17,18 we chose to mimic complex, dynamic in vivo human coronary arteries using an in vitro circulatory system model, and live rabbits.
2. Methods and Materials
2.1. In Vitro Circulatory System Model
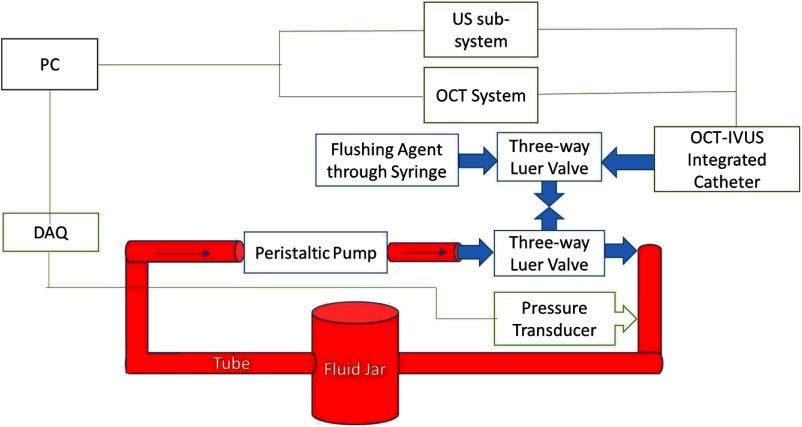
To avoid unknown variables as in animal experiments, a well-controlled phantom test was first performed for comprehensive quantitative analyses of different flushing agents. An in vitro circulation model was built (as shown in Fig. 1) according to the dimensions of a human arterial system, to best mimic the circulation system in vivo. This model was a closed-loop system, simulating the heart-artery-vein-heart closed-loop circulation, and made from Masterflex L/S® tubes, tube adapters, three-way luer valves, a glass jar, and a peristaltic pump. Similar to the middle of the human left anterior descending coronary artery, where intravascular imaging is usually performed, the tubing had an inner diameter of 2.4 mm. The total length of all tubes with blood flow was 3 m, which simulated the blood circulation through the entire arterial and venous system. Since the distance between the imaging region and the guiding catheter influences the efficiency of flushing,19 this distance was also controlled to be similar to the in vivo distance. The peristaltic pump’s parameters were then set to mimic the pressure profile of human circulation. The fluid reservoir was physically located 1 m above the imaging region which raised the minimal blood pressure to 78 mm Hg, approximately the diastolic pressure of a healthy human. The peak pressure during pumping was approximately 40 mm Hg higher than minimum, mimicking a systolic pressure of 120 mm Hg. The pump was then set to , mimicking the normal heart rate.
Fig. 1.
Schematic of the in vitro circulatory system model with signal acquisition devices. The personal computer (PC) acquired the optical coherence tomography (OCT) signal, intravascular ultrasound (IVUS) signal, and fluid pressure signal. Three-way luer valves were used to provide access to the closed-loop tube system and allow the imaging probe and chemicals to enter. Red areas denote where blood circulates.
Three flushing agents, dextran [40,000 molecular weight (MW)], 20% mannitol in normal saline, and iohexol (, NOVAPLUS), were examined in our quantitative experiments. Because the concentration of dextran solution was reported to affect the enhancement of OCT image quality,14 we tested the effect of dextran with different concentrations at 1%, 3%, and 5%. Although dextran with high MW, such as MW 500,000 or 70,000, may provide better blood optical clearing,20 high MW dextran takes a longer time to be extract from human body.21 Thus, we used dextran MW 40,000 in our experiments.
The maximal injection speed of a flushing agent commonly used in patients is at the coronary artery proximal.12,22 To keep the volumetric delivery of flushing agent comparable to the in vivo setting, we chose as the maximal testing speed, because the inner diameter of our tube is one-half of the inner diameter of the coronary artery proximal end, where flushing is usually performed. All three flushing agents were tested at flushing speeds 0.1, 0.5, and .
During the experiment, 500 ml EDTA-added (anti-coagulated) porcine whole blood was circulated inside the circulation model. Imaging was performed using our previously published IVUS-OCT system23 with a 1310-nm OCT system and 40-MHz IVUS transducer. We obtained 1000 A-lines in each frame.
An ideal flushing agent should provide minimal attenuation with the lowest possible delivery; therefore, we measured the attenuation during imaging for each agent at each speed setting. In each A-line, the attenuation coefficients (ACs) of each flushing agent at each speed setting were calculated in the equations provided next using MATLAB:
where is the intensity of the signal obtained in US or OCT system, is the intensity of the outgoing sound signal or the incident light intensity. is the reflectivity of the sample as a function of scanning depth, is the ultrasound AC, and is the optical AC.24
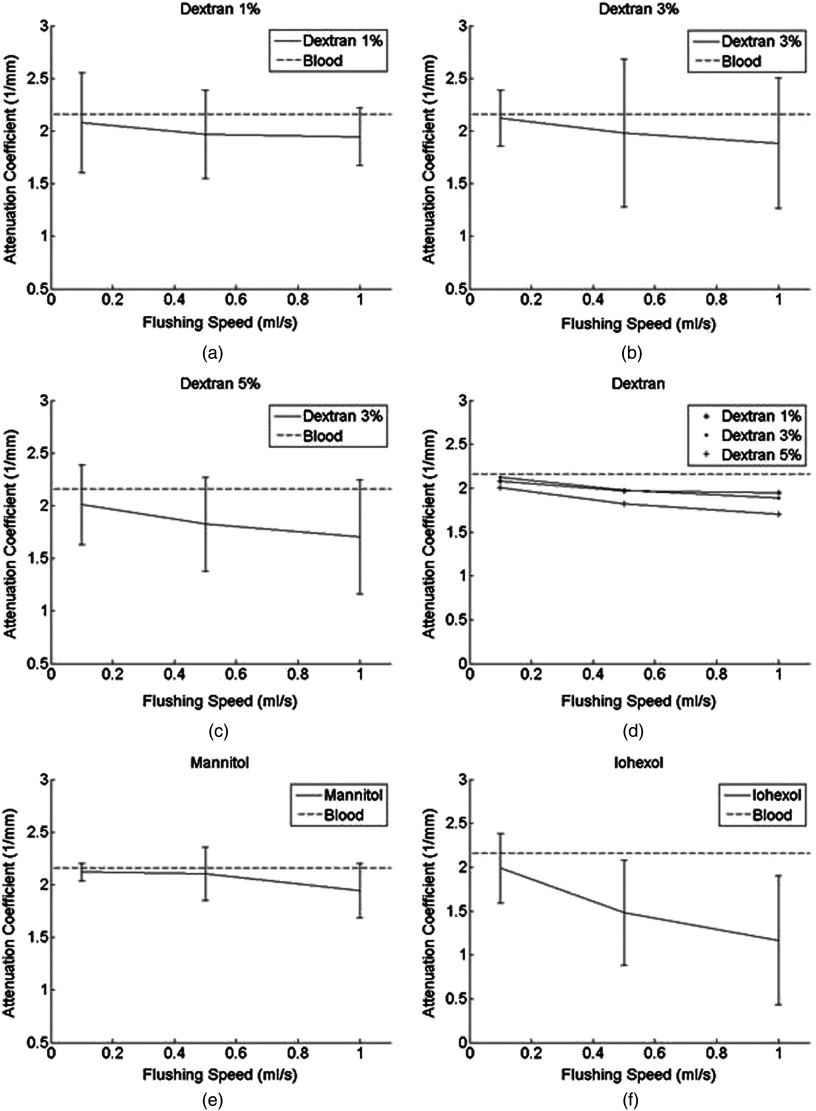
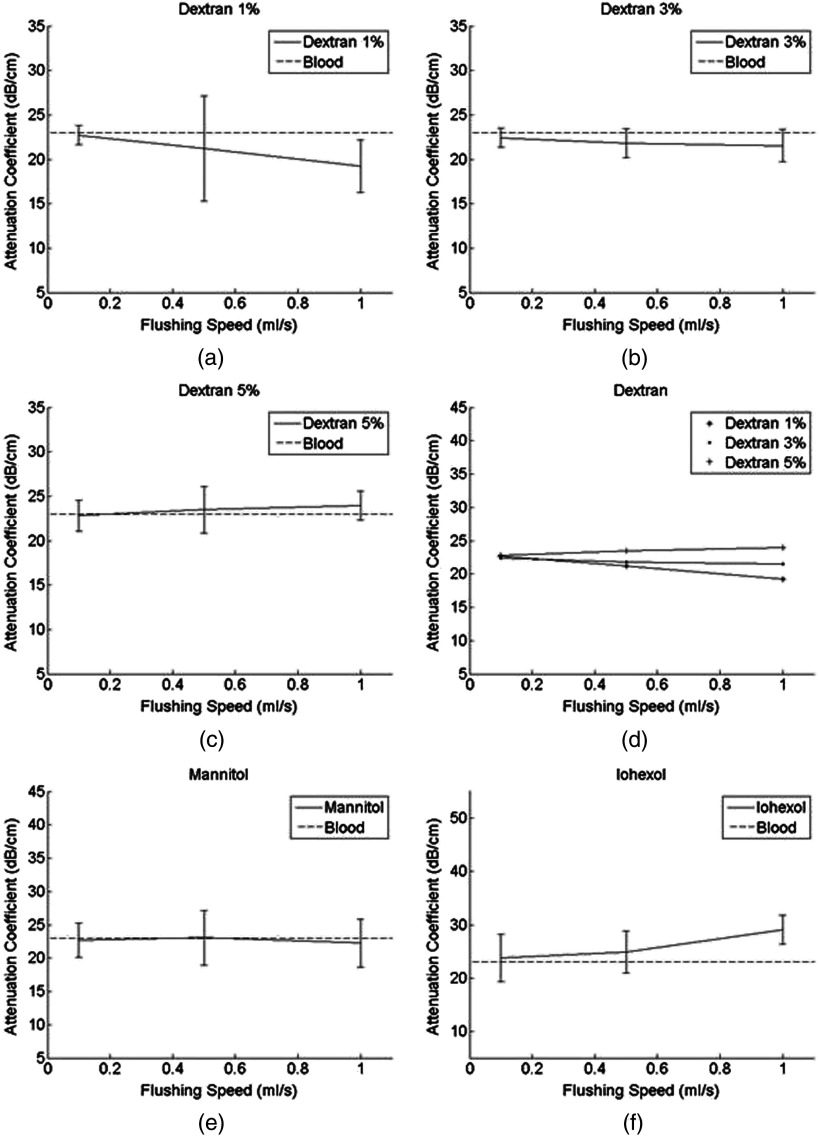
To minimize the effects of tube heterogeneity and speckles in images, pixel windows were averaged. The means and standard deviations of the AC for all 1000 A-lines were then calculated. The AC of an adjacent three-image unit was then averaged to obtain the final AC value of the corresponding flushing agent at its corresponding speed. Summaries of these final values for each setting are shown in Figs. 2 and 3.
Fig. 2.
OCT attenuation coefficients (ACs) using different flushing agents (a) dextran 1% (b) dextran 3% (c) dextran 5% (d) dextran 1%, 3%, and 5% (e) mannitol (f) iohexol. Dashed lines represent the AC of blood at .14 The error bars represent the standard deviation of ACs encountered when rotating the imaging probe along the tube wall.
Fig. 3.
IVUS ACs using different flushing agents (a) dextran 1% (b) dextran 3% (c) dextran 5% (d) dextran 1%, 3% and 5% (e) mannitol (f) iohexol. The AC of blood is (shown as dashed lines).25
2.2. In Vivo Studies
To demonstrate the clinical applicability of these flushing agents, simultaneous optical and acoustic imaging of rabbit abdominal aortas was performed in vivo. The experimental protocol was approved by the University of California, Irvine, Institutional Animal Care and Use Committee. All animals were treated in accordance with federal and state regulatory guidelines. During the imaging procedures, rabbits were anesthetized, intubated, and mechanically ventilated. Laparotomy was performed. The abdominal aorta was then isolated and exposed. At this opening, a 6-F arterial catheter was inserted into the aorta.26 The IVUS-OCT catheter was advanced through the 6-F arterial catheter and into the abdominal aorta. We examined three flushing agents, 10% dextran (40,000 Da MW) in normal saline, 20% mannitol in normal saline, and iohexol (, NOVAPLUS), in the abdominal aortas of three anesthetized rabbits, which mimic the diameter of human coronaries.13 To evaluate the effect of different flushing agents in the in vivo experiment, a clear image frame (CIF) was used as a criteria. A CIF is defined as the image frame where over 270 deg continuous arc of artery wall can be visualized, similar to previously published concepts.11,12
3. Results
3.1. Phantom Result
OCT image quality increases as the flushing speed increases. Dextran at and flushed at had strong effects on both OCT and IVUS signals (10.3% decrease, 4.16% increase). Mannitol at had marginal effects on both OCT and IVUS signals (4.6% decrease, 3.37% decrease). Iohexol at dramatically improved OCT signals (23% decrease) but hindered IVUS signals (17% increase). Iohexol had the best effect for OCT flushing. The high concentration of dextran, when flushing at a high speed, can provide a flushing effect similar to that of low-speed iohexol.
3.2. In Vivo Result
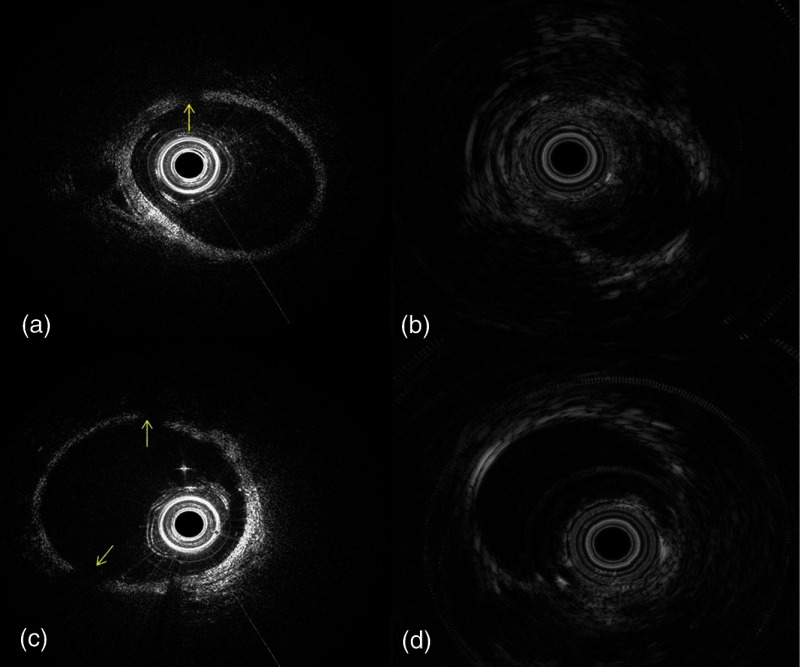
From the acquired OCT images, CIFs are 99%, 97.5%, and 50% for iohexol, dextran, and mannitol flushing, respectively. There were many image frames acquired with mannitol flushing that were partially clear, see Fig. 4(c), but most image frames acquired with dextran were clear. Based on the semi-quantitative criteria of CIFs, different flushing agents showed a similar effect on IVUS images.
Fig. 4.
Representative images acquired with alternative flushing agents: (a, b) dextran (c, d) and mannitol. (a, c) are the OCT images of rabbit artery. (b, d) are the corresponding IVUS images. Arrows denote areas where the artery wall is not visible in the image.
4. Discussion
From our phantom testing and in vivo experiments, we found that dextran at a high concentration worked well for both IVUS and OCT signals, as measured by a low optical and US attenuation and a high CIF rate. Mannitol was not very effective for OCT flushing while iohexol reduced the US signal. Here, we attempt to explain these results by the mechanisms behind OCT and US flushing.
4.1. Mechanisms of Improving Optical Coherence Tomography Image Quality
Two mechanisms are associated with the increase of OCT imaging quality: index matching and the displacing of blood.
Attenuation of optical signals is caused by absorption and scattering. At 1310 nm, where water absorption is negligible compared with scatterings, and where the absorption of hemoglobin is also low, scattering is the dominant source of attenuation. Scattering of light is generated by the difference in the index of refraction between the scatterer and surrounding medium.14 By employing index matching chemicals and raising the refractive index of blood plasma () to match that of blood cells (),11 the OCT light experiences a lesser change in the refractive index at the plasma–cell interface. During the in vivo experiment, 10% dextran (), mannitol (), and iohexol (), which have higher refractive indices than plasma (), increased the refractive index of the mixed solution toward that of RBC (1.40). Extensive studies of index matching and optical clearing were investigated for reducing scattering in the stagnant blood imaging.14,18
In flowing blood, however, there is another mechanism associated with reducing scattering. As shown in our results, 1% dextran, which has a refractive index (1.334) similar to that of blood plasma (1.33), can still improve OCT image quality. A flushed chemical may completely displace blood in which case refractive index matching does not matter because no plasma–cell interface exists. In a completely displaced medium with a constant refractive index, very little scattering occurs. Flushing low concentration dextran displaces some blood, decreasing the number of plasma–cell interfaces and thus reducing scattering. This explains why flushing low concentration dextran improved the OCT signal despite its refractive index similar to that of blood plasma (Table 1).
Table 1.
Comparison of viscosities and refractive indices.
4.2. Mechanisms of Improving IVUS Image Quality
Similar to light attenuation, the attenuation of sound is caused by acoustic absorption and scattering. The strength of absorption is related to the viscosity of material and the velocity of the flow. More friction and thermal consumption of energy will be induced when sound propagates through high viscosity media at a high speed.37 The higher the viscosity is, the stronger the resistance to shearing flow and thus the larger friction that is generated. In addition, a higher flushing speed in a high viscosity medium causes a larger speed difference between the medium flowing over the surface of the tube/artery wall and the medium flowing in the center of the tube/artery wall. As a result, a high speed flushing may generate stronger friction. Iohexol, which has the highest viscosity, caused a stronger US signal reduction than other tested chemicals (see Fig. 3), and a high flushing speed of iohexol induced an even stronger reduction than at a lower speed [see Fig. 3(f)].
High viscosity is a double-edged sword for acoustic-optical imaging. Chemicals with higher viscosity most likely displace more blood because they have stronger internal “stiffness” in pushing blood forward. This hypothesis is consistent with our results that both dextran 5% and iohexol had higher viscosities than mannitol and that they improved OCT signals better than mannitol. However, higher viscosities are known to cause more acoustic attenuation due to the increased damping of mechanical energy.25 As revealed in Figs. 2(d) and 3(d), increasing the dextran concentration (and thus viscosity) deteriorated the IVUS signal and improved OCT image quality. Similarly, iohexol (a high viscosity chemical) increased IVUS attenuation but reduced OCT attenuation. In the context of simultaneous IVUS-OCT imaging, the medium’s viscosity may serve as a critical parameter in determining the optimal balance between OCT and IVUS signal strengths. Dextran at a high concentration, with an intermediate viscosity, worked well for both IVUS and OCT. It is also significantly less toxic than iohexol, which may also enable us to perform IVUS-OCT imaging even on patients with renal insufficiency.12 Thus, we believe dextran is a good contrast agent for simultaneous IVUS-OCT imaging.
4.3. Effect of Blood Aggregation
Occurring when blood cells “stick” onto one another through fibrinogen protein interactions, aggregation alters the blood’s state and hence its AC. More specifically, aggregation is hypothesized to affect the OCT AC because it reduces the number of interfaces with different refractive indices.14 Additionally, aggregation is believed to affect the ultrasound AC because of sound reflectivity’s dependence on particle size.25 Aggregation, however, typically occurs in stagnant blood or flowing blood with very low shear rates. For our experiment, the average shear rate across the pump’s tube diameter was at 56 inverse second (), which was highly relative to lower shear rates at 0 to .38 Thus, aggregation was assumed to not occur. In addition, we did not observe any aggregation or sedimentation processes in the experimental images, as shown in Fig. 4, so changes in ACs must be caused by the introduced chemicals and their interactions with blood, not the blood interacting with itself to form cellular aggregates.
4.4. Outlook
Flushing, or optical clearing, is not only necessary in OCT but also in other optical-based imaging methods, such as optical elastography, acoustic-optics, and photoacoustic imaging.39 For example, stronger intravascular photoacoustic signals were detected in a vessel without blood than the case with blood, which indicate the necessity for effective flushing agents.40 In addition, optical clearing has also proven to be useful for enhance imaging performance and sensitivity in photoacoustic microscope41,42 and flow cytometry.43 HDIVUS (with a center frequency of over 50 MHz) recently received FDA approval and has caught physicians’ attention at prestigious medical conferences due to its remarkable resolution.44,45 However, unlike common IVUS, HDIVUS also suffers from reduction in image quality from blood scattering and performs better with a flushing agent.46 With the system setup mentioned in this paper, the ideal nontoxic flushing agent for HDIVUS can easily be found. This study will also potentially benefit the clinical adoption of HDIVUS. Based on our research, we would argue that the optimal IVUS-OCT flushing agent should have an index of refraction over 1.34 and a viscosity lower than 15 cp.
In this paper, we quantitatively investigated three representative flushing agents. However, more optical clearing agents, such as glucose solutions, propylene glycol,17 and hemoglobin,47–49 can be investigated using the methods proposed in this paper. Before in vivo experiments, a preliminary theoretical analysis and low-cost phantom testing would be useful for initial screening.
5. Conclusion
This paper analyzed the effects of three flushing agents (dextran, mannitol, and iohexol) in a blood-filled closed-loop tube system and in vivo on rabbits. Dextran improved intravascular OCT signals, with higher concentrations leading to stronger signals, but it had variable effects on IVUS signals depending on concentration. Mannitol had a marginal impact on both OCT and IVUS signals. Iohexol significantly improved OCT signal but also significantly deteriorated the IVUS signal due to its high viscosity. From in vitro results, flushing high concentration dextran at was most useful for simultaneous IVUS-OCT imaging. This conclusion confirms previous reports12,14 that dextran performs well in intravascular OCT which can replace harmful contrast agents and thus reduce the side effects from intravascular imaging. In vivo result also validated the effectiveness of dextran for simultaneous IVUS-OCT imaging.
Acknowledgments
This work was supported by the National Institutes of Health under Grant Nos. R01-HL-125084, R01-HL-12727, R01-HL-105215, R01-EY-021529, P41-EB002182, and P41-EB-015890. We also received financial support from the University of California, Irvine’s Undergraduate Research Opportunities Program. We would like to thank Dr. Matthew Brenner for his suggestion regarding the selection of flushing agents. Dr. Claire Robertson is currently working at Lawrence Berkeley National Lab.
Biographies
Jiawen Li received her BS degree in optical engineering from Zhejiang University in China in 2010. Currently, she is a PhD candidate at the University of California, Irvine. She has published more than 9 high-profile journal papers and given more than 16 talks at prestigious international conferences in the past 4 years. In 2015, she won the best technical poster award at the Optics in Cardiology conference. Her research interests include multimodality intravascular imaging, optical coherence tomography, and ultrasmall optical fiber endoscopes.
Hataka Minami is an undergraduate student in biomedical engineering at the University of California, Irvine. Currently, he conducts biophotonics and biocomputational research at the Beckman Laser Institute, investigating the interaction of NIR/IR light in OCT systems with biological tissue. His research interests include optical imaging, multimodality imaging, image and signal processing, and applications of these techniques in cardiology, neurology, and ophthalmology.
Earl Steward is an instructor with the UC Irvine School of Medicine. He is the lab director for the Department of Surgery overseeing and managing department research programs that also include cardiothoracic/cardiovascular surgery. Additionally, he manages research projects for other School of Medicine departments and a cardiovascular project with the Department of Biomedical Engineering. He has served as a lab director, lab manager or lab supervisor for surgery departments at the Boston University School of Medicine, Dartmouth Medical School, and the Thomas Jefferson University and co-authored numerous papers over the span of his evolving career.
Teng Ma received his BSE degree from the University of Michigan, Ann Arbor, Michigan, in 2011, majoring in biomedical engineering. He joined the NIH Resource Center for Medical Ultrasonic Transducer Technology as a research assistant and PhD candidate under the supervision of Dr. Kirk Shung and Dr. Qifa Zhou. In 2013, two of his papers were selected as best student paper finalists and were featured at the 2013 Joint UFFC, EFTF, and PFM symposium. His research interests include medical ultrasound technology and multimodality intravascular imaging.
Kirk Shung obtained his BS degree in electrical engineering from Cheng-Kung University in Taiwan in 1968 and his PhD degree in electrical engineering from the University of Washington, Seattle, Washington, in 1975. He has been the director of the NIH resource on medical ultrasonic transducer technology since 1997. He is a life fellow of the IEEE and a fellow of the Acoustical Society of America and the American Institute of Ultrasound in Medicine. He is a founding fellow of the American Institute of Medical and Biological Engineering.
Qifa Zhou received his PhD degree from the Department of Electronic Materials and Engineering of Xi’an Jiaotong University, China, in 1993. Currently, he is a research professor at the NIH Resource on Medical Ultrasonic Transducer Technology and the Department of Biomedical Engineering and Industry and System Engineering at University of Southern California (USC), Los Angeles, California. Before joining USC in 2002, he worked at the Department of Physics at Zhongshan University in China, the Department of Applied Physics, Hong Kong Polytechnic University, and the Materials Research Laboratory, Pennsylvania State University.
Pranav Patel is currently the chief of the division of cardiology at the University of California, Irvine. He has also been appointed the director of the Cardiac Catheterization Laboratory and clinical associate professor of medicine. He is also the associate director of the cardiovascular medicine and interventional cardiology fellowship programs at UC Irvine. He received his medical degree from the St. Louis University School of Medicine (1999); from 2002–2006 he completed his general cardiology and interventional cardiology fellowships at the Brown University School of Medicine.
Zhongping Chen is a professor of biomedical engineering and the director of the F-OCT Laboratory at the University of California, Irvine. He received his BS degree in applied physics from Shanghai Jiao Tong University in 1982, his MS degree in electrical engineering from Cornell University in 1987, and his PhD degree in applied physics from Cornell University in 1993. He is a fellow of the American Institute of Medical and Biological Engineering (AIMBE), a fellow of SPIE, and a fellow of the Optical Society of America.
Biographies for the other authors are not available.
References
- 1.Li J., et al. , “Miniature optical coherence tomography-ultrasound probe for automatically coregistered three-dimensional intracoronary imaging with real-time display,” J. Biomed. Opt. 18(10), 100502 (2013). 10.1117/1.JBO.18.10.100502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., et al. , “Integrated IVUS-OCT for real-time imaging of coronary atherosclerosis,” JACC Cardiovasc. Imaging 7(1), 102 (2014). 10.1016/j.jcmg.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B. H., et al. , “Hybrid intravascular ultrasound and optical coherence tomography catheter for imaging of coronary atherosclerosis,” Catheterization and Cardiovasc. Interventions 81(3), 495–507 (2013). 10.1002/ccd.24295 [DOI] [PubMed] [Google Scholar]
- 4.Yin J., et al. , “Integrated intravascular optical coherence tomography ultrasound imaging system,” J. Biomed. Opt. 15(1), 010512 (2010). 10.1117/1.3308642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., et al. , “Diagnostic accuracy of integrated intravascular ultrasound and optical coherence tomography (IVUS-OCT) system for coronary plaque characterization,” Proc. SPIE 8926, 892635 (2014). 10.1117/12.2037886 [DOI] [Google Scholar]
- 6.Strohm E. M., Kolios M. C., “Sound velocity and attenuation measurements of perfluorocarbon liquids using photoacoustic methods,” in Proc. IEEE Int. Ultrasonics Symp., pp. 2368–2371 (2001). [Google Scholar]
- 7.Bezerra H. G., et al. , “Intracoronary optical coherence tomography: a comprehensive review: clinical and research applications,” J. Am. Coll. Cardiol. Intervention 2(11), 1035–1046 (2009). 10.1016/j.jcin.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCullough P. A., “Contrast-induced acute kidney injury,” J. Am. Coll. Cardiol. 51(15), 1419–1428 (2008). 10.1016/j.jacc.2007.12.035 [DOI] [PubMed] [Google Scholar]
- 9.Dawson P., “Cardiovascular effects of contrast agents,” Am. J. Cardiol. 64(9), E2–E9 (1989). 10.1016/0002-9149(89)90727-3 [DOI] [PubMed] [Google Scholar]
- 10.Waksman R., et al. , “Intravascular ultrasound versus optical coherence tomography guidance,” J. Am. Coll. Cardiol. 62(17), S32–S40 (2013). 10.1016/j.jacc.2013.08.709 [DOI] [PubMed] [Google Scholar]
- 11.Brezinski M., et al. , “Index matching to improve optical coherence tomography imaging through blood,” Circulation 103(15), 1999–2003 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Ozaki Y., et al. , “Comparison of contrast media and low-molecular-weight dextran for frequency-domain optical coherence tomography,” Circ. J. 76(4), 922–927 (2012). 10.1253/circj.CJ-11-1122 [DOI] [PubMed] [Google Scholar]
- 13.Hoang K. C., et al. , “Use of an oxygen-carrying blood substitute to improve intravascular optical coherence tomography imaging,” J. Biomed. Opt. 14(3), 034028 (2009). 10.1117/1.3153895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X., et al. , “Effect of dextran-induced changes in refractive index and aggregation on optical properties of whole blood,” Phys Med. Biol. 48, 1205–1221 (2003). 10.1088/0031-9155/48/9/309 [DOI] [PubMed] [Google Scholar]
- 15.Henkel-Honke T., Oleck M., “Artificial oxygen carriers: a current review,” Am. Assoc. Nurse Anesth. 75(3), 205–211 (2007). [PubMed] [Google Scholar]
- 16.Thomson K., Varma D., “Safe use of radiographic contrast media,” Aust. Prescr. 33(1), 19–22 (2010). [Google Scholar]
- 17.Tuchin V. V., Xu X., Wang R. K., “Dynamic optical coherence tomography in studies of optical clearing, sedimentation, and aggregation of immersed blood,” Appl. Opt. 41(1), 258–271 (2002). 10.1364/AO.41.000258 [DOI] [PubMed] [Google Scholar]
- 18.Tuchin V. V., “Optical clearing of tissues and blood using the immersion method,” J. Phys. D Appl. Phys. 38(15), 2497 (2005). 10.1088/0022-3727/38/15/001 [DOI] [Google Scholar]
- 19.Bouma B. E., et al. , “Evaluation of intracoronary stenting by intravascular optical coherence tomography,” Heart 89(3), 317–320 (2003). 10.1136/heart.89.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X., Yu L., Chen Z, “Optical clearing of flowing blood using dextrans with spectral domain optical coherence tomography,” J. Biomed. Opt. 13(2), 021107 (2008). 10.1117/1.2909673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loftsson T., Phusocochemical Properties and Pharmacokinetics. Essential Pharmacokinetics: A Primer for Pharmaceutical Scientists, p. 101, Academic Press, London: (2015). [Google Scholar]
- 22.Gonzalo N., et al. , “Second-generation optical coherence tomography in clinical practice. High-speed data acquisition is highly reproducible in patients undergoing percutaneous coronary intervention,” Rev. Esp. Cardiol. 63, 893–903 (2010). 10.1016/S0300-8932(10)70201-3 [DOI] [PubMed] [Google Scholar]
- 23.Li Xiang, et al. , “Integrated IVUS-OCT imaging for atherosclerotic plaque characterization,” IEEE J. Sel. Topics Quantum Electron. 20(2), 1–8 (2014). 10.1109/JSTQE.2013.2265302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R. K., Tuchin V. V., “Optical coherence tomography. Light scattering and imaging enhancement,” in Handbook of Coherent-Domain Optical Methods: Biomedical Diagnostics, Tuchin V. V., Ed., pp. 665–742, Environmental Monitoring and Material Science 2, Springer-Verlag, New York: (2013). [Google Scholar]
- 25.Treeby B. E., et al. , “Measurement of the ultrasound attenuation and dispersion in whole human blood and its components from 0-70 MHz,” Ultrasound Med. Biol. 37(2), 289–300 (2011). 10.1016/j.ultrasmedbio.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 26.Hoang K. C., et al. , “Use of an oxygen-carrying blood substitute to improve intravascular optical coherence tomography imaging,” J. Biomed. Opt. 14(3), 034028 (2009). 10.1117/1.3153895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y.-H., Hu Y.-F., Zhang Z.-X., “Viscosity and density of the nonelectrolyte system mannitol + sorbitol + sucrose + H2O and its binary and ternary subsystems at 298.15 K,” J. Chem. Eng. Data 51, 438–442 (2006). 10.1021/je0503608 [DOI] [Google Scholar]
- 28.Bashkatov A. N., et al. , “Glucose and mannitol diffusion in human dura mater,” Biophys. J. 85(5), 3310–3318 (2003). 10.1016/S0006-3495(03)74750-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatham A. J., Prydal J., “Do non-ionic contrast media temporarily improve corneal transparency?” J. Ophthalmic. Vis. Res. 8(4), 400–402 (2013). [PMC free article] [PubMed] [Google Scholar]
- 30.Cinar Y., Senyol A. M., Duman K., “Blood viscosity and blood pressure: role of temperature and hyperglycemia,” Am. J. Hypertens. 14, 433–438 (2001). 10.1016/S0895-7061(00)01260-7 [DOI] [PubMed] [Google Scholar]
- 31.Késmárky G., et al. , “Plasma viscosity: a forgotten variable,” Clin. Hemorheol. Microcirc. 39(1–4), 243–246 (2008). [PubMed] [Google Scholar]
- 32.Sydoruk O., et al. , “Refractive index of solutions of human hemoglobin from the near-infrared to the ultraviolet range: Kramers-Kronig analysis,” J. Biomed. Opt. 17(11), 115002 (2012). 10.1117/1.JBO.17.11.115002 [DOI] [PubMed] [Google Scholar]
- 33.Tycko D. H., et al. , “Flow-cytometric light scattering measurement of red blood cell volume and hemoglobin concentration,” Appl. Opt. 24(9), 1355–1365 (1985). 10.1364/AO.24.001355 [DOI] [PubMed] [Google Scholar]
- 34.Park Y., et al. , “Refractive index maps and membrane dynamics of human red blood cells parasitized by plasmodium falciparum,” PNAS 105(37) 13730 (2008). 10.1073/pnas.0806100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L., et al. , “Low molecular weight gelator – dextran composites,” Chem. Commun. 46, 6738–6740 (2010). 10.1039/C0CC01842B [DOI] [PubMed] [Google Scholar]
- 36.Snyder C. F., et al. , “Optical rotations, refractive indices, and densities of dextran solutions,” J. Res. Natl. Bur. Stand. 53(3), 131–137 (1954). 10.6028/jres.053.016 [DOI] [Google Scholar]
- 37.Stokes G. G., “On the theories of the internal friction in fluids in motion, and of the equilibrium and motion of elastic solids,” Trans. Cambridge Philos. Soc. 8(22), 287–342 (1849). [Google Scholar]
- 38.Schmid-Schönbein H., Gaehtgens P., Hirsch H., “On the shear rate dependence of red cell aggregation in vitro,” J. Clin. Invest. 47(6), 1447–1454 (1968). 10.1172/JCI105836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L. V., Photoacoustic Imaging and Spectroscopy, CRC Press, Boca Raton: (2009). [Google Scholar]
- 40.Yeager D., et al. , “Intravascular photoacoustic imaging of exogenously labeled atherosclerotic plaque through luminal blood,” J. Biomed. Opt. 17(10), 106016 (2012). 10.1117/1.JBO.17.10.106016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Yao J., Wang L. V., “Optical clearing-aided photoacoustic microscopy with enhanced resolution and imaging depth,” Opt Lett. 38(14), 2592–2595 (2013). 10.1364/OL.38.002592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., et al. , “Optical clearing agents improve photoacoustic imaging in the optical diffusive regime,” Opt. Lett. 38(20), 4236–4239 (2013). 10.1364/OL.38.004236 [DOI] [PubMed] [Google Scholar]
- 43.Menyaev Y. A., et al. , “Skin optical clearing for in vivo photoacoustic flow cytometry,” Biomed. Opt. Express 4(12), 3030–3041 (2013). 10.1364/BOE.4.003030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka S., et al. , “Plaque assessment with a novel high-definition 60-MHz IVUS imaging system: comparison with conventional 40MHz IVUS and optical coherence tomography,” J. Am. Coll. Cardiol. 61(10_S) E1878 (2013). [Google Scholar]
- 45.Yuhei H., et al. , “Precision of a novel high-definition 60MHz IVUS in quantitative measurement: comparison with conventional 40MHz IVUS and optical coherence tomography,” J. Am. Coll. Cardiol. 64(11_S) (2014). [Google Scholar]
- 46.Ma T., et al. , “Multi-frequency intravascular ultrasound (IVUS) imaging,” IEEE Trans. Ultrason. Ferroelect. Freq. Control 62(1), 97–107 (2015). 10.1109/TUFFC.2014.006679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuchin V. V., et al. , “Theoretical study of immersion optical clearing of blood in vessels at local hemolysis,” Opt. Express 12, 2966–2971 (2004). 10.1364/OPEX.12.002966 [DOI] [PubMed] [Google Scholar]
- 48.Zhernovaya O., Tuchin V. V., Leahy M. J., “Blood optical clearing studied by optical coherence tomography,” J. Biomed. Opt. 18(2), 026014 (2013). 10.1117/1.JBO.18.2.026014 [DOI] [PubMed] [Google Scholar]
- 49.Popescu G., et al. , “Erythrocyte structure and dynamics quantified by Hilbert phase microscopy,” J. Biomed. Opt. 10(6), 060503 (2005). 10.1117/1.2149847 [DOI] [PubMed] [Google Scholar]