Abstract
Background
Levodopa-associated motor fluctuations are common complications observed in Parkinson’s disease (PD) patients. Although nonmotor fluctuations are a significant cause of morbidity, they frequently are not properly identified. Few studies have characterized the nonmotor emotional fluctuations and their relation to motor fluctuations.
Aims
The objective of the present study is to analyze the occurrence of fluctuations in anxiety and depression symptoms, as well as in cognitive function (memory, language, executive function, and attention), and their relation to motor fluctuations in PD patients presenting wearing-off phenomenon.
Methods
Twenty-four patients were assessed during the wearing on-off periods. The State-Trait Anxiety Inventory (STAI-State) and Beck Depression Inventory (BDI) were used to assess anxiety and depression, respectively, and the Wisconsin Card Sorting Test (WCST), Stroop Test, Rey Auditory Verbal Learning Test (RAVLT), Weschler Memory Scale - digits (WMS) and Controlled Oral Word Association (COWA) for assessing executive functions, verbal memory, attention and work memory and verbal fluency, respectively.
Results
Patients presented higher depression and anxiety scores in the wearing-off period (P<0.05). Differences were also found in the semantic verbal fluency (P=0.017) and executive function (P=0.008) tests performance.
Conclusions
Nonmotor symptoms such as anxiety and depression, verbal fluency, and executive function performance are influenced by motor fluctuations.
Keywords: Parkinson’s disease, wearing-off, neuropsychological assessment, depression, anxiety
Introduction
Parkinson’s disease (PD) is characterized by tremor, rigidity, bradykinesia, and postural instability. Most PD patients receiving long-term treatment with levodopa will develop motor fluctuations. Motor fluctuation is one of the most difficult problems encountered in PD management.1 Frequently, the first motor complication to appear is the wearing-off phenomenon, where the symptomatic benefit of a certain dose of antiparkinsonian medication is not maintained until the next dose.
The clinical manifestations that occur during motor fluctuations are not restricted to motor symptoms, although these have been the most commonly studied. Few studies have assessed the relation of nonmotor phenomena to wearing-off phenomenon. It has been suggested that all patients with motor fluctuations associated with the levodopa therapy experience nonmotor fluctuations simultaneously.2 Sleep disorder, neuropsychiatric manifestations such as depression and anxiety, as well as autonomic, gastrointestinal, sensorial, and cognitive-deficit disorders are among the nonmotor PD manifestations.3
Depression is the most common neuropsychiatric disorder in PD, with a prevalence rate of 7%–90%. Such a wide range can be explained by several methodologies and the populations studied.4–7 Among the tools used to assess depression in PD patients, the Beck Depression Inventory (BDI) has been widely used in many studies.7 Distinct moments of clinical evaluation (in on or off periods) may have contributed to the wide range of differences between studies.
Anxiety symptoms have a high prevalence rate in PD as well.8,9 The mechanisms involved in PD anxiety have not been well clarified. The relation with motor fluctuations is not completely known, with some studies showing and others not showing,12 a relation with motor fluctuation.10,11 The study that evaluated the state of anxiety in the on and off periods suggests that patients have higher anxiety scores in the off period.11 In another study that assesses the variations in anxiety scores, it was observed that lower anxiety scores were related to high levodopa doses.13
Like anxiety and depression, there is a high prevalence of cognitive deficit in PD. Even in patients with no dementia, a great range of cognitive deficits has been described, such as executive deficits, deficit in the capacity of temporal ordination and sequencing of ideas and facts, memory strategies, attention and concentration, initiative and spontaneous interest, capacity of visual–spatial interpretation, as well as verbal fluency decrease.14
The deficits associated with the early stages of the disease involve the pre-frontal cortex specifically, such as: difficulty in maintaining context, serial and temporal ordination, initiate responses, strategy management, cognitive, slowness and productivity decrease.15
In spite of being little known, nonmotor fluctuations can be significantly dysfunctional. The present study aims at analyzing the differences between the on and off periods regarding symptoms of depression and anxiety, as well as cognitive performance, in PD patients presenting the wearing off phenomenon.
Methods
Participants
Twenty-four patients from the Hospital de Clínicas de Porto Alegre Movement Disorder Clinic who had been diagnosed with PD by a movement-disorder neurologist, according to the London Brain Bank,16 and who presented wearing-off motor fluctuations, were included in the study. These individuals should have at least 4 years of schooling and a performance score >24 on the Mini Mental State Exam.17 Patients with psychotic symptoms or other associated neurological diseases were excluded from the study, pursuant to a structured clinical assessment. All patients agreed to participate in the study and signed the informed consent forms. The study was approved by the Ethics Council of the Hospital de Clínicas de Porto Alegre.
The evaluation was carried out in four stages, split over 2 days. The same patient was assessed at different moments, so that each patient was his or her own control. On the first day, the scales to assess anxiety and depression, and an executive function test, were applied. On the second day, cognitive tests to assess memory, attention, working memory, verbal fluency, and executive function were applied.
Assessment
A survey was carried out regarding demographic and clinical data, such as motor manifestations assessed through the Unified Parkinson’s Disease Rating Scale (UPDRS-III),18 duration of the disease, stage of the disease,19 daily life activities,20 medication in use, and comorbidities. The depression symptoms were assessed through the BDI,21 and anxiety was assessed through the State–Trait Anxiety Inventory (STAI-State).22 Memory was assessed through the Rey Auditory–Verbal Learning Test,23 verbal fluency through Controlled Oral Word Association,23 executive function through Wisconsin Card Sorting Test23 and Stroop Test,23 and attention and working memory through the Weschler Memory Test Inverted and Direct Digit Subtest.23 The same protocols were assessed in both periods. Both assessments were done in the same day; patients were randomized to be assessed in the on, or in the wearing-off, period initially, in order to avoid test-learning bias. “Wearing-off” was considered as the predictable emergence of one or more motor PD signs or symptoms before the application of the next scheduled antiparkinsonian medication dose.
On the basis of the UPDRS-III scores, the patients were divided into three groups according to PD type: the tremor-dominant type (TDT) group; the akinetic rigid type (ART) group; and the mixed type (MT) group. A score for each group was calculated as previously described by Spiegel et al.24 The presence of dyskinesia dose peak was controlled. Of the 24 individuals who participated in the first stage, seven refused to participate in the second.
Statistical methods
Clinical and demographic characteristics were analyzed through descriptive statistics. In order to verify if there was any difference in depression and anxiety symptoms between the wearing on-off periods, Student’s t-test for paired samples was applied with Bonferroni correction. The correlations between UPDRS-III variation, BDI, and STAI-State variation and cognitive tests were measured by Spearman correlation coefficients. The existence of correlation between these data and the demographic data (age and sex), and the duration of the disease was evaluated. In order to verify the differences between the on and wearing-off periods of the assessed cognitive aspects, Student’s t-test for paired samples was used.
Results
The clinical and demographic characteristics of the patients involved in the study are shown in Table 1. From the 24 patients, eleven (44%) were males and, according to Hoehn and Yahr (HY) staging,19 seven patients were in stage II PD and 17 in stage III PD. According to the motor manifestation predominance, seven were ART and 17 were MT, and nine patients presented dyskinesias in the on period.
Table 1.
Patient characteristics
| Baseline characteristics | Mean | Standard deviation | Average |
|---|---|---|---|
| Age (years) | 60.7 | 9.6 | 62.0 |
| Educational level (years) | 9.2 | 4.6 | 8.0 |
| Duration of the disease (years) | 10.0 | 4.7 | 9.0 |
| DLAs (Schwab and England scale) | 77.9 | 11.4 | 80.0 |
| UPDRS-III (on) | 13.1 | 6.7 | 14.0 |
| UPDRS-III (off) | 36.2 | 12.0 | 33.5 |
| BDI (on) | 13.9 | 10.7 | 11.5 |
| BDI (off) | 17.0 | 12.6 | 14.0 |
| STAI-State (on) | 40.0 | 7.7 | 40.0 |
| STAI-State (off) | 45.3 | 12.3 | 42.5 |
Abbreviations: BDI, Beck Depression Inventory; DLAs, daily life activities; STAI-State, State–Trait Anxiety Inventory; UPDRS-III, Unified Parkinson’s Disease Rating Scale.
A significant difference in the depression scores assessed by BDI was observed between the on and off periods when Bonferroni correction was carried out F(1.23)=6.35, P=0.019. Patients showed higher depression scores in the wearing-off period; the mean value of the difference in the depression scores was −3.1 (95% confidence interval [CI] = −5.6 to −0.5).
When the BDI symptoms were analyzed individually, four out of the 21 symptoms checked by the scale presented statistically significant differences when the on and wearing-off periods were compared. These were success perception (P=0.016), capacity to feel pleasure (P=0.036), work capacity (P=0.009), and health concerns (P=0.043).
When the patients with and without dyskinesia were compared, no statistically significant difference was noticed between the groups regarding depression; however, as can be seen in Figure 1, the patients with dyskinesia presented lower variation in the depression scores.
Figure 1.
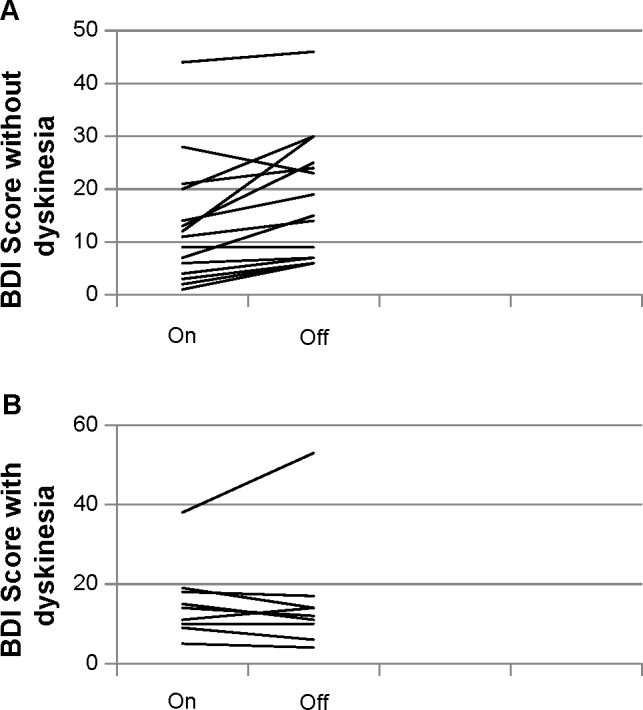
Beck Depression Inventory in patients without and with dyskinesia.
Notes: (A) BDI without dyskinesia. (B) BDI with dyskinesia.
Abbreviation: BDI, Beck Depression Inventory.
With respect to anxiety, there was also a statistically significant difference (P=0.003) between the on and wearing-off periods, according to the STAI-State, with higher scores in the off period, when Bonferroni correction was carried out F(1.23)=10.75, P=0.003. The mean value was −5.3 (95% CI = −8.7 to −2.0).
Two symptoms out of the 20 presented in the STAI-State showed a statistically significant difference when scores were compared between the on and wearing-off periods: tranquility perception (P=0.0001) and concern with the future (P=0.003).
There was no statistically significant difference with regards to anxiety in the on and wearing-off periods between patients with and without dyskinesia; however, the patients with dyskinesia showed noticeably lower variation in the anxiety scores (Figure 2).
Figure 2.
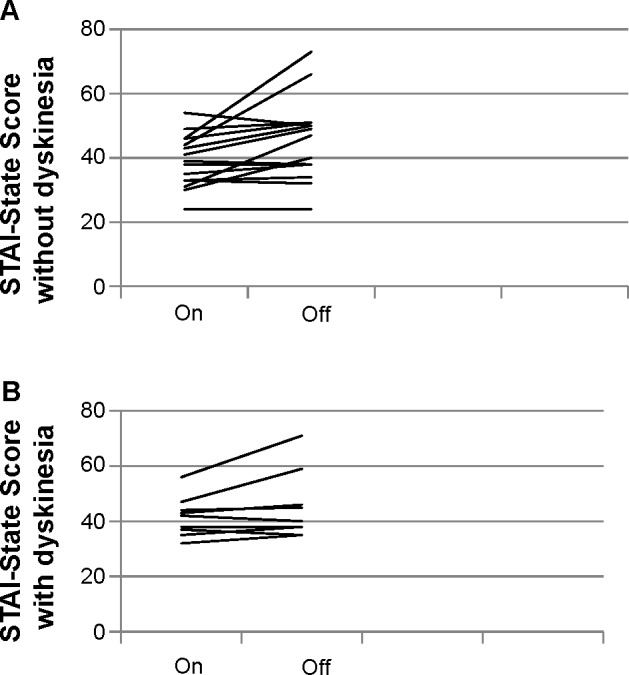
STAI-State (anxiety) results in patients with and without dyskinesia.
Notes: (A) STAI-State without dyskinesia. (B) STAI-State with dyskinesia.
Abbreviation: STAI-State, State–Trait Anxiety Inventory.
No correlation has been found between the UPDRS-III variability from the wearing on-off periods, with the BDI variability, nor with the STAI-State variability. Variability of BDI and STAI-State scales was not related to clinical type (ART, TDT, or MT groups).
With respect to the cognitive tests data, a statistically significant difference was obtained between the on and off periods in the Stroop Test (time) and in the Controlled Oral Word Association Test. The other cognitive tests assessed presented no differences, as shown in Table 2.
Table 2.
Student’s t-test for paired samples
| Mean | Standard deviation | 95% CI | P-value | |
|---|---|---|---|---|
| BDI | −3.1 | 5.9 | −5.6 to −0.5 | 0.19 |
| STAI-State | −5.3 | 7.9 | −8.7 to −2.0 | 0.003 |
| Stroop Test (time) | −8.4 | 14.2 | 2.4–14.4 | 0.008 |
| Stroop Test (errors) | −1.3 | 5.3 | 0.96–3.5 | 0.25 |
| RAVLT (total) | 1.8 | 8.1 | 2.4–6.0 | 0.37 |
| RAVLT (auditory) | −0.2 | 3.7 | 1.6–2.1 | 0.79 |
| RAVLT (delayed recall) | −0.9 | 3.3 | 0.8–2.6 | 0.29 |
| RAVLT (recognition) | 0.5 | 2.1 | 0.5–1.6 | 0.31 |
| Digits (direct) | 0.06 | 1.3 | 0.6–0.7 | 0.85 |
| Digits (inverted) | 0.0 | 1.3 | 0.7–0.7 | 1.00 |
| Semantic verbal fluency | 1.3 | 2.0 | 0.3–2.3 | 0.017 |
| Phonemic verbal fluency | 2.2 | 6.7 | 1.2–5.7 | 0.19 |
| WCST (errors) | −0.5 | 10.4 | 4.8–5.9 | 0.84 |
| WCST (categories) | −0.06 | 1.2 | 0.6–0.7 | 0.84 |
| WCST (faults) | 0.18 | 1.6 | 0.7–1.0 | 0.66 |
Abbreviations: BDI, Beck Depression Inventory; CI, confidence interval; STAI-State, State–Trait Anxiety Inventory; RAVLT, Rey Auditory–Verbal Learning Test; WSCT, Wisconsin Card Sorting Test.
No correlation between UPDRS-III variability with the cognitive tests, BDI, and STAI-State variation results was found. No correlation between these results and demographic data (age and sex), and duration of the disease was found. Statistically significant correlation was obtained between the cognitive data, and between them and the anxiety and depression variabilities (Table 3). Analysis of variance was calculated in order to verify if the differences found in the error results in the Stroop Test could be explained by the delta in the depression scores where F=7.16; P=0.14 were obtained.
Table 3.
Spearman’s correlation coefficients between the cognitive data, anxiety, and depression variabilities
| Stroop Test variability (rs) | RAVLT auditory variability (rs) | RAVLT delayed recall variability (rs) | STAI-State variability (rs) | |
|---|---|---|---|---|
| BDI variability | 0.5 | 0.6 | 0.5 | NS |
| Total RAVLT variability | NS | 0.5 | NS | 0.7 |
| RAVLT recognition | NS | NS | 0.5 | NS |
Abbreviations: BDI, Beck Depression Inventory; STAI-State, State–Trait Anxiety Inventory; NS, not significant; RAVLT, Rey Auditory–Verbal Learning Test; rs, Spearman correlation coefficient.
Discussion
The present study identified differences between the on and wearing-off periods regarding the PD anxiety and depression symptoms. The presence of anxiety and depression symptoms is associated with worse quality of life and incapacity related to those patients’ disease.
Many studies4–13 examined the prevalence of depression in the different PD stages. A study that has stratified PD patients according to the HY staging system9 has identified the occurrence of depressive symptoms in 40% of the individuals in stage I PD, 19% in stage II PD, 36% in stage III PD, 65% in stage IV PD, and 50% in stage V PD. Anxiety symptoms have also been widely studied in PD. Some authors attribute the high prevalence of anxiety in these patients to a psychological response to physical overload associated with chronic disease. It must be highlighted that anxiety scores are higher in individuals with PD than in patients with other kinds of chronic diseases.25 Besides, a significant association between anxiety and disease severity has been demonstrated.11,26 In the present study, the severity of the disease did not correlate with the variation in the depression or anxiety in the on and off periods. The patients who present wearing off can show insecurity about the near future with regards to the difficulty in dealing with the motor limitations imposed by the disease, especially in the case of the off period.25
Although this study has some limitations in assessing the depressive state, these findings highlight the importance of knowing in which state (on or off) the patient is for an adequate assessment of the intensity of depression and anxiety symptoms. The patients with dyskinesia have presented lower variability between on and off periods, in relation to both depression and anxiety symptoms.27 The reasons for the lower variability in patients with dyskinesia is not clear. One possibility could be due to their perception of the limitations imposed by the disease that persist in the on period.
Besides mood alterations that vary according to the above, alterations in cognitive functions, such as executive function, verbal fluency, and memory, also occur in the PD individuals. The present study found differences between the on and off periods in verbal fluency and in the capacity for context change. The first interferes in the patient’s capacity of expressing him/herself appropriately through spontaneous verbal fluency; and the second, in making the patients have some difficulty in changing their perception with new demands. Such limitations can lead to avoidance behavior and harm the patient’s work and social life. Although correlation between the BDI variation and the Stroop Test have been identified, it was verified, through logistic regression, that the depression symptoms are not sufficient to explain the Stroop Test results even if there is statistically significant differences between the on and off periods, to the perception of success, capacity to experiment pleasure and work capacity that could justify a lower performance in the cognitive tasks. The variations between the on and wearing-off periods in the Stroop Test and in verbal fluency may not be attributed to a motor function once there was no correlation between the UPDRS-III variation and the tests variation, suggesting that the verbal speed is not influenced by the motor issue involved in the test.
This study had some limitations, including the small sample size. Another aspect is that the participants belonged to just the HY II and HY III stages of the disease. In future studies, the variable analysis studied in this paper could be considered in other stages of the disease. In addition, most of the patients corresponded to the MT type, which limits the data extrapolation to the other stages and statistically analyzes the MT group behavior in relation to ART or TDT.
However, in spite of the limitations, it must be highlighted that, as far as the authors know, no other study has been carried out using such methodology to assess the differences between the on and wearing-off periods in the anxiety and depression symptoms, as well as in the cognitive functions. These findings highlight the importance of knowing in which state (on or off) the patient is, for an adequate assessment of the intensity of the depression and anxiety symptoms. The findings must also call the attention of health professionals to the fluctuation of non-motor symptoms and the relevance of optimizing drug treatment and thus, reducing the off period.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stacy M, Bowron A, Guttman M, et al. Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord. 2005;20(6):726–733. doi: 10.1002/mds.20383. [DOI] [PubMed] [Google Scholar]
- 2.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59(3):408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 4.Hantz P, Caradoc-Davies G, Caradoc-Davies T, Weatherall M, Dixon G. Depression in Parkinson’s disease. Am J Psychiatry. 1994;151(7):1010–1014. doi: 10.1176/ajp.151.7.1010. [DOI] [PubMed] [Google Scholar]
- 5.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53(2):175–179. doi: 10.1001/archneur.1996.00550020087019. [DOI] [PubMed] [Google Scholar]
- 6.Meara J, Mitchelmore E, Hobson P. Use of the GDS-15 geriatric depression scale as a screening instrument for depressive symptomatology in patients with Parkinson’s disease and their carers in the community. Age Ageing. 1999;28(1):35–38. doi: 10.1093/ageing/28.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Schrag A, Jahanshahi M, Quinn NP. What contributes to depression in Parkinson’s disease? Psychol Med. 2001;31(1):65–73. doi: 10.1017/s0033291799003141. [DOI] [PubMed] [Google Scholar]
- 8.Nègre-Pagès L, Grandjean H, Lapeyre-Mestre M, et al. Anxious and depressive symptoms in Parkinson’s disease: the French cross-sectionnal DoPAMiP study. Mov Disord. 2010;25(2):157–166. doi: 10.1002/mds.22760. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer RB, Kurlan R, Rubin A, Boer S. Evidence for atypical depression in Parkinson’s disease. Am J Psychiatry. 1988;145(8):1020–1022. doi: 10.1176/ajp.145.8.1020. [DOI] [PubMed] [Google Scholar]
- 10.Silberman CD, Laks J, Capitão CF, Rodrigues CS, Moreira I, Engelhardt E. Recognizing depression in patients with Parkinson’s disease: accuracy and specificity of two depression rating scale. Arq Neuropsiquiatr. 2006;64(2B):407–411. doi: 10.1590/s0004-282x2006000300011. [DOI] [PubMed] [Google Scholar]
- 11.Starkstein SE, Robinson RG, Leiguarda R, Preziosi TJ. Anxiety and depression in Parkinson’s disease. Behav Neurol. 1993;6(3):151–154. doi: 10.3233/BEN-1993-6306. [DOI] [PubMed] [Google Scholar]
- 12.Stein MB, Heuser IJ, Juncos JL, et al. Anxiety disorder in patients with Parkinson’s disease. Am J Psychiatry. 1990;147(2):217–220. doi: 10.1176/ajp.147.2.217. [DOI] [PubMed] [Google Scholar]
- 13.Maricle RA, Nutt JG, Valentine RJ, Carter JH. Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease: a double-blind, placebo-controlled study. Neurology. 1995;45(9):1757–1760. doi: 10.1212/wnl.45.9.1757. [DOI] [PubMed] [Google Scholar]
- 14.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55(5):674–680. doi: 10.1001/archneur.55.5.674. [DOI] [PubMed] [Google Scholar]
- 15.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S, Elton RL, Members of the UPDRS Development Committee . Unifed Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 19.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 20.Schwab RS, England AC. Newer preparations in the treatment of Parkinsonism. Med Clin North Am. 1957;41(2):369–379. doi: 10.1016/s0025-7125(16)34443-1. [DOI] [PubMed] [Google Scholar]
- 21.Levin BE, Llabre MM, Weiner WJ. Parkinson’s disease and depression: psychometric properties of the Beck Depression Inventory. J Neurol Neurosurg Psychiatry. 1988;51(11):1401–1404. doi: 10.1136/jnnp.51.11.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene RE, et al. Manual for the state-trait anxiety inventory. Consulting Psychologist Press; Palo Alto: 1970. [Google Scholar]
- 23.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd ed. New York, NY: Oxford University Press Inc; 1998. [Google Scholar]
- 24.Spiegel J, Hellwig D, Samnick S, et al. Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J Neural Transm. 2007;114(3):331–335. doi: 10.1007/s00702-006-0518-2. [DOI] [PubMed] [Google Scholar]
- 25.Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE. Anxiety and motor fluctuations in Parkinson’s disease: a cross-sectional observational study. Parkinsonism Relat Disord. 2012;18(10):1084–1088. doi: 10.1016/j.parkreldis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Menza MA, Roberston-Hoffman DE, Bonapace AS. Parkinson’s disease and anxiety: comorbidity with depression. Biol Psychiatry. 1993;34(7):465–470. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- 27.Schrag A, Quinn N. Dyskinesias and motor fluctuation in Parkinson’s disease: a community-based study. Brain. 2000;123(Pt 11):2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]


