Abstract
A 13-year-old boy with metastatic rhabdomyosarcoma presented with biliary obstruction requiring ERCP for stent placement. Re-evaluation after recurrence of symptoms revealed external migration of the stent with formation of a contralateral wall bleeding duodenal ulcer. Endoscopic therapy was not feasible and bleeding persisted despite medical therapy and embolization. Given his life-threatening condition, approval for the use of Hemospray (Cook Medical, Winston-Salem, NC, USA) was obtained. Hemospray was applied to the visualized bleeding sites with subsequent hemostasis. Patient tolerated the procedure well. This is the first use of Hemospray in the United States outside of a clinical trial.
Introduction
Acute upper gastrointestinal hemorrhage in the United States accounts for greater than 300,000 annual hospital admissions and a mortality rate of up to 10%.1 At the current time, there are various treatment modalities approved for the management of upper gastrointestinal bleeding, which include injection (saline, epinephrine, sclerosants), cautery (heat probes, lasers, argon plasma coagulation, electrocautery), and mechanical therapy (clips, band ligation).1 In some cases, hemostasis is difficult to achieve and patients may have persistent or recurrent bleeding. Additionally, there are some instances in which the patient's anatomy, the location of a lesion, or severe bleeding makes it difficult to achieve hemostasis with currently available methods. Hemospray (Cook Medical, Winston-Salem, North Carolina, USA) is an alternative method that is currently undergoing trials and the process of FDA approval. Hemospray is a granular, mineral blend nanopowder with clotting abilities that increase the concentration of clotting factors, activate platelets, and form a mechanical plug on an injured blood vessel.2 According to a trial conducted by Sung et al, it has been indicated that Hemospray can be used for hemostasis of nonvariceal confirmed peptic ulcer bleeding.3 In that study, 20 patients were recruited and acute hemostasis was achieved in 19 of the 20 patients. The other patient required arterial embolization of a pseudoaneurysm. Additionally, 2 patients had recurrent bleeding within 72 hours, indicated by a hemoglobin drop. No active bleeding was identified on repeat upper endoscopy.
Case Report
We report a case in which hemostatic powder was used to treat a patient with profound thrombocytopenia and recurrent gastrointestinal bleeding secondary to duodenal ulcer disease. The patient was a 13-year-old male with a medical history significant for metastatic rhabdomyosarcoma with pancreatic head mass. He was referred to adult gastroenterology in 2010 for a biliary stricture requiring endoscopic retrograde cholagiopancreatography with sphincterotomy and placement of a biliary stent. Since that time, the patient had undergone multiple stent exchanges including placement of a fully covered self-expandable biliary metal stent in August 2011. This was complicated by acute cholecystitis requiring the placement of a cholecystostomy tube. Since the original diagnosis of metastatic rhabdomyosarcoma, the patient had undergone multiple courses of chemotherapy over a 4-year time period and was in remission for 3 months after undergoing his most recent course of chemotherapy. However, the patient developed recurrent jaundice, vomiting, and thrombocytopenia. Repeat endoscopic evaluation in February 2012 revealed that the biliary stent had migrated distally and was embedded in the contralateral wall of the duodenum. A large bleeding ulcer was identified where the stent was embedded (Figure 1). There was also diffuse erythema and edema with oozing proximal to this area. Endoscopic therapy with conventional measures, including epinephrine injection, clip placement, and cautery, was not feasible secondary to the extent of bleeding, mucosal edema, and anatomic changes.
Figure 1.
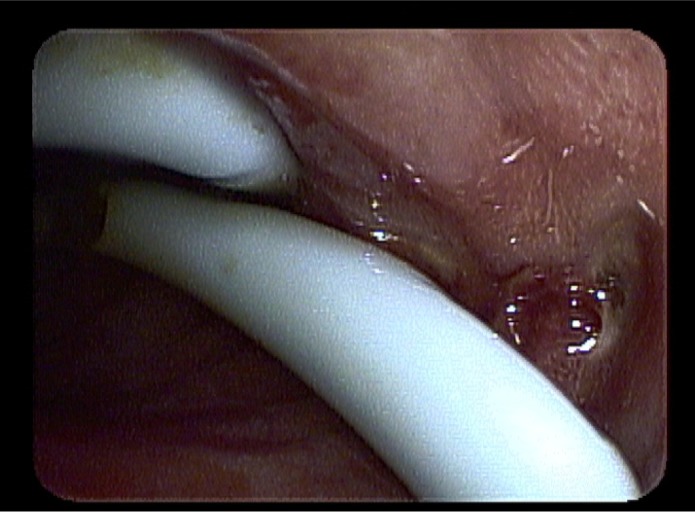
Bleeding duodenal ulcer.
The patient was started on intravenous drips of pantoprazole and octreotide. He underwent urgent embolization of the gastroduodenal artery per interventional radiology. He was transferred to the pediatric intensive care unit for further monitoring and treatment. Over the next week, the patient developed recurrent bleeding despite medical and interventional therapy, requiring multiple blood transfusions. Additionally, he developed worsening thrombocytopenia requiring platelet transfusions. Surgery evaluated the patient and deemed him a non-surgical candidate. Given his life-threatening condition with transfusion dependency, persistent active bleeding and no feasible medical, endoscopic, or surgical option, we attempted to obtain approval for the use of hemostatic powder.
An investigational device exemption was obtained to use hemostatic powder through the appropriate process involving clearance through our institution's IRB chairperson and chairman of the Department of Medicine. An additional gastroenterologist, who was not involved in the patient's care, reviewed the case to determine whether obtaining an investigational device exemption for use of hemostatic powder was appropriate. Once approved, a Cook Medical representative was flown out to train us on the use of the Hemospray device. The entire process to obtain approval from the time the letters were written to the time of endoscopy was five days. Upper endoscopy was then performed revealing erythematous, edematous, friable antral mucosa with a mosaic pattern. An adherent clot was seen at the pyloric channel and removed with irrigation and aspiration, revealing an oozing ulcer. The duodenum was also erythematous and edematous. There was diffuse oozing in the antrum and duodenum along with re-identification of the duodenal ulcer. A total of 25–30 g of hemostatic powder was applied to the duodenal ulcer site, the proximal duodenum, the antrum, and the pyloric channel site. After application, there was no further evidence of oozing or active bleeding (Figure 2). The patient tolerated the procedure well and he was discharged home 6 days later with no further episodes of bleeding. This is the first use of hemostatic powder for the management of upper gastrointestinal bleeding outside of a clinical trial in the United States.
Figure 2.
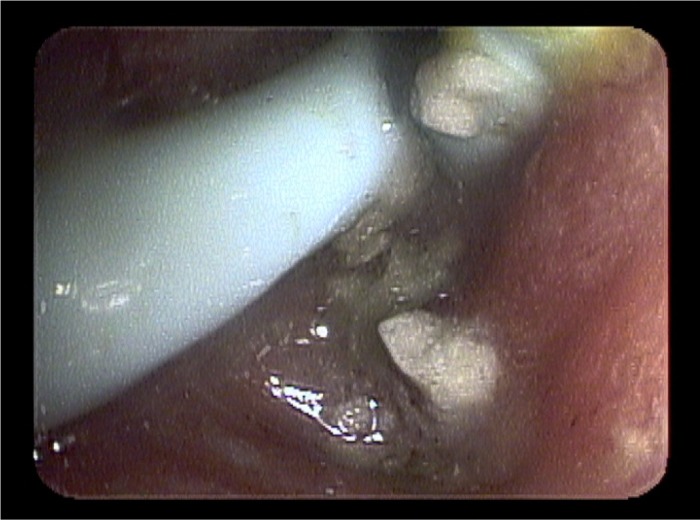
Hemostasis of bleeding duodenal ulcer after application of Hemospray.
Discussion
Hemostatic powder is approved for use in Europe and Canada for non-variceal ulcer bleeding and is currently undergoing FDA approval for the same indication in the United States. At this time, there are no major complications or adverse events associated with the use of hemostatic powder. There has been concern for embolization if used in variceal bleeding or larger vessels; however, this has not yet been encountered. The difficulty that we encountered in using hemostatic powder was clumping in a moist or wet environment. As part of the standard procedure, the biopsy port of the endoscope would have to be flushed with an air-filled syringe to avoid clumping within the catheter. However, we still experienced clumping within the catheter if it touched fluid or mucosa that was not fully dry. One can imagine that it may be difficult to have a completely dry environment in a patient with upper gastrointestinal hemorrhage requiring aggressive irrigation.
Based on our review of the literature and personal experience with using the device, there will likely be certain scenarios and circumstances where hemostatic powder will be most helpful. Anatomic location of the lesion may dictate use of hemostatic powder, such as in patients with pyloric channel ulcers, which are difficult to treat with conventional methods. Additionally, large areas of bleeding where it is difficult to identify lesions can be sprayed with the hemostatic powder without the need to target the lesion directly. Applications outside of non-ulcer bleeding will also need to be investigated. At this time, there are no published trials evaluating use of hemostatic powder in variceal bleeding or other non-ulcer etiology of bleeding.
Holster et al recently published a case series in which 16 consecutive patients on antithrombotic therapy with upper gastrointestinal hemorrhage were treated with hemostatic powder at the Erasmus MC University Medical Centre in Rotterdam, The Netherlands. Hemostasis was initially achieved in 5 of the 8 (63%) patients on antithrombotic therapy and 8 of the 8 (100%) patients not on antithrombotic therapy (P=0.20). Rebleeding rate was similar in both groups; however, in 4 of the 5 total patients with rebleeding, hemostatic powder was applied to arterial spurting bleeding.4 It would be beneficial to have future trials investigating the use of hemostatic powder with combination therapy as well as trials investigating its use in non-ulcer bleeding, including variceal bleeding.
Disclosures
Author contributions: P.J. Sargon wrote and edited the manuscript, and is the article guarantor; T.J. Laurie edited the manuscript.
Financial disclosure: No competing financial interests to disclose.
References
- 1.Adler DA, Leighton JA, Davila RE, et al. . ASGE guideline: The role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastroinest Endosc. 2004;60(4):497–504. [DOI] [PubMed] [Google Scholar]
- 2.Giday SA. Preliminary data on the nanopowder hemostatic agent TC-325 to control gastrointestinal bleeding. Gastroenterol Hepatol. 2011;7(9):620–622. [PMC free article] [PubMed] [Google Scholar]
- 3.Sung JJ, Luo D, Wu JC, et al. . Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43(4):291–295. [DOI] [PubMed] [Google Scholar]
- 4.Holster IL, Kuipers EJ, Tjwa ET. Hemospray in the treatment of upper gastrointestinal hemorrhage in patients on antithrombotic therapy. Endoscopy. 2013;45(1):63–66. [DOI] [PubMed] [Google Scholar]


